Abstract
A wealth of behavioral evidence suggests that performance improves more following intervals containing wake compared to intervals containing sleep. This sleep benefit reflects the consolidation of memories that takes place over sleep. Just as memory encoding is associated with distinct neural representations, as laid out in this review, the physiology of sleep associated with sleep-dependent memory consolidation also varies with the type of memory. As revealed by recent neuroimaging studies, behavioral changes over sleep are also associated with reorganization of the neural representation of the memory at a systems level and with changes in plasticity at a cellular and molecular level as revealed by studies in animal models. While the reviewed work provides a clear picture of the function of sleep on memory consolidation, we present critical questions that will be essential to address regarding the extent of and limitations on sleep-dependent memory consolidation. Understanding the role of sleep on memory and cognition represents an important step to understanding sleep function more broadly.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The functions of sleep are not fully understood, a surprising fact given the vast amount of time that this state takes from our lives. One of the most exciting and contentious hypotheses is that sleep makes an important contribution to learning and memory processing. Over the last decade, a large number of studies, from a range of disciplines, have begun to provide a substantive body of evidence supporting this role of sleep in what is becoming known as sleep-dependent memory processing. While this renaissance is relatively recent in the annals of sleep research, the topic itself has a surprisingly long history. The earliest reference to a relationship between sleep and memory is from the Roman rhetorician Quintillian, stating “It is a curious fact, of which the reason is not obvious, that the interval of a single night will greatly increase the strength of the memory” and suggesting that “the power of recollection…undergoes a process of ripening and maturing” (Quintillian; first century ad). This is striking not only for the level of insight at a time when knowledge of brain function was so anemic, but also considering that it represented the first suggestion of memory requiring a time-dependent process of development, resulting in improved memory recall. Perhaps what is most surprising, however, is that these two fields of research (sleep and memory) then remained separate for almost two millennia.
In the mid-eighteenth century, the British psychologist David Hartley proposed that the processes of dreaming might alter the strength of associative memory links within the brain [1]. Yet the fields of sleep and memory research remained independent until 1924 when Jenkins and Dallenbach [2] performed the first systematic studies of sleep and memory to test Ebbinghaus’ theory of memory decay [3]. Jenkins and Dallenbach [2] showed that memory retention was better following a night of sleep than after an equivalent amount of time awake. However, they concluded that the memory benefit following sleep was simply a passive one, due to a lack of sensory interference in contrast to wake. They did not consider that the physiologic state of sleep itself could actively orchestrate these memory modifications. It is only in the last half-century, following the discovery of rapid eye movement (REM) and non-REM (NREM) sleep, that researchers began testing the hypothesis that specific aspects of sleep physiology may actively participate in memory processing.
This chapter explores what has become known as sleep-dependent memory processing, and its associated brain basis, sleep-dependent plasticity. It is divided into three primary sections: (1) an overview of memory categories and the unique stages of memory development; (2) a review of the specific relationships between sleep and memory, both in humans and in animals; and (3) a brief survey of the wide range of evidence describing sleep-dependent brain plasticity, including human brain imaging studies as well as animal studies of cellular neurophysiology and molecular biology. We close with a consideration of unanswered questions that are the focus of ongoing research.
Delineations and Definitions
Before discussing interactions between sleep and memory, we must first understand what these terms represent and encompass. The process of sleep, with its varied stages and equally diverse physiology and biology, has already been described in earlier chapters in this section, clearly demonstrating that sleep itself cannot be treated as a homogeneous state that either does or does not affect memory. Instead, sleep possesses a range of physiologic and neurochemical mechanisms that can contribute to memory consolidation. Moreover, just as sleep cannot be considered homogeneous, the spectrum of memory processes believed to exist in the human brain, and the unique stages that create and sustain memory, are likewise diverse.
Memory Categories
Although often used as a unitary term, “memory” is not a single entity. Human memory has been subjected to several different classification schemes, the most popular being based on the distinction between declarative versus nondeclarative memory [4, 5] (Fig. 13.1a).
Memory systems and memory stages. a Memory systems. Human memory is most commonly divided into declarative forms, with further subdivisions into episodic and semantic, and nondeclarative forms, subdivided into an array of different types including procedural skill memory. b Developing stages of memory. Following initial encoding of a memory, several ensuing stages are proposed, beginning with consolidation, as well as integration of the memory representation, and translocation of the representation or erasure of the memory. Also, following later recall, the memory representation is believed to become unstable once again, requiring periods of reconsolidation
Declarative memory is the consciously accessible memories of fact-based information (i.e., knowing “what”). Several subcategories of the declarative system exist, including episodic memory (memory for events of one’s past) and semantic memory (memory for general knowledge, not tied to a specific event) [5]. Current neural models of declarative memory formation emphasize the critical importance of structures in the medial temporal lobe, predominantly the hippocampus [6, 7], a structure that is thought to form a contextual retrieval code for neocortically stored information.
In contrast, nondeclarative memory can be regarded as unconsciously acquired learning. The nondeclarative category includes procedural memory (i.e., knowing “how”), such as the learning of habits and motor skills (e.g., playing a piano, athletic sports, surgical skills, etc.), as well as classical conditioning and non-associative learning. The neural basis for nondeclarative learning appears to be more diverse, varying with task characteristics (e.g., motor versus non-motor) [7].
While these categories offer convenient and distinct separations, they rarely operate in isolation in real life. For example, language learning requires a combination of memory sources, ranging from nondeclarative memory for procedural motor programs to articulate speech, to memory of grammatical rules and structure, to aspects of declarative memory for the source of word selection. Such interactions must be kept in mind as we consider the role of sleep in learning and memory.
Memory Stages
Just as memory cannot be considered monolithic, similarly, there does not appear to be one sole event that creates or sustains it. Instead, memory appears to develop in several unique stages over time (Fig. 13.1b). For example, memories can be initially encoded by engaging with an object or performing an action, leading to the formation of a representation of the object or action within the brain. In a subsequent recall stage, memories can be actively recalled or recognized by comparison with the representation. However, the memory continues to evolve between encoding and recall through the process of consolidation. Classically, the term memory consolidation refers to a process whereby a memory becomes increasingly resistant to interference from competing or disrupting factors in the absence of further practice, through the simple passage of time [8]. That is to say, the memory becomes more stable even with time away from the task.
Important, memory stabilization can occur over periods of both wake [9, 10] and sleep [11, 12]. Sleep-dependent consolidation refers to the fact that consolidation is greatest over sleep, compared to wake, for most tasks [13–15]. From this perspective, the enhancement phase of consolidation causes either the active retention of a memory instead of its decay, or the enhancement of a memory over and above its simple maintenance. There is also accumulating evidence that memory consolidation is accompanied by a process of memory integration and generalization [e.g., 16, 17]. Thus, consolidation can be expanded to include more than one phase of post-acquisition memory processing, with each phase occurring in specific brain states such as wake or sleep, or even specific stages of sleep [18].
Although this chapter focuses primarily on the effects of sleep on post-acquisition memory stabilization and generalization, it is important to note that there are additional post-acquisition stages of memory processing that perhaps should also fall under the rubric of consolidation. These include the anatomic reorganization of memory representations (memory translocation), reconsolidation of memory representations following conscious recall (memory reconsolidation), and even the active erasure of memory representations, all of which appear to occur outside of awareness and without additional training or exposure to the original stimuli. It is interesting to note that, while not reviewed here, sleep has been implicated in all of these steps [19–22].
Summary
There are a number of stages of memory processing, which use distinct brain mechanisms to perform separate functions. When multiple classes of memories and the several stages of sleep are combined, one is faced with a truly staggering number of possible ways that sleep might affect memory consolidation.
Behavioral Studies of Sleep and Memory
Evidence of sleep-dependent memory processing has been found in numerous species using a variety of behavioral paradigms. Here, we provide an overview of these studies. The reader is referred to recent reviews for more detail than is provided here [23–25].
Human Studies of Declarative Memory
Much of the early work investigating sleep and memory in humans focused on declarative learning tasks. These studies offered mixed conclusions, some arguing for sleep-dependent memory processing and others against it. For example, De Koninck et al. [26] demonstrated significant increases in post-training REM sleep after intensive foreign language learning, with the degree of successful learning correlating with the percentage increase of REM sleep. Such findings suggest that REM sleep plays an active role in memory consolidation, and that post-training increases reflect a homeostatic response to the increased demands for REM-dependent consolidation.
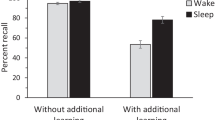
To the contrary, most recent studies suggest a critical function of slow wave sleep (SWS) in declarative memory consolidation. Perhaps most convincing are studies demonstrating the reactivation of declarative memories during SWS. Rasch et al. [27] taught participants a visuospatial task requiring learning the spatial location of a matrix of images. Learning occurred in the presence of an odor (scent of roses). The experimental odor was re-presented during subsequent SWS for a subset of the participants (Fig. 13.2a). Recall was superior following sleep when the odor present during learning was re-presented during sleep. In a similar study, Rudoy et al. [28] had participants learn a similar visuospatial task, but in this case, each image was paired with a specific sound. Half of these sounds were re-presented during subsequent SWS. Delayed recall was greater for those items for which the associated sound was re-presented during sleep relative to those items for which the associated sound was not re-presented. It is thought that cues presented during sleep, selectively trigger associated memories to be reactivated. Notably, when Rasch et al. [27] presented odors during wake and REM sleep, there was no benefit for subsequent recall beyond that seen in a no-odor (vehicle) control condition (Fig. 13.2b), supporting the specific role of SWS in declarative memory consolidation.
Declarative memory reactivation during slow wave sleep. a Subjects performed a visuospatial learning task in the presence of an experimental odor (scent of roses). During subsequent slow wave sleep, this odor or a non-odor vehicle control was presented. Memory for the visuospatial task was tested following sleep. b Participants recalled more locations following sleep when the experimental odor was re-presented during slow wave sleep compared to vehicle (first panel). Such an effect was not observed if the odor present during slow wave sleep was not also present during learning (second panel), if the odor cue was re-presented during REM (third panel), or if the odor was re-presented during waking (Permissions needed from Rasch et al. [27])
Marshall et al. [29] showed that experimentally boosting human slow oscillations, widespread oscillations between “up” and “down” states that are characteristic of SWS, results in improved memory performance the following day. Following learning of a word-pair list, a technique called transcranial direct current stimulation (tDCS) was used to induce these slow oscillation-like (in this case, 0.75 Hz) field potentials during early delta-rich sleep. The tDCS not only increased the amount of delta sleep during the stimulation period (and for some time after), but also enhanced the retention of these hippocampal-dependent factual memories, suggesting a causal benefit of delta sleep neurophysiology.
Memory enhancement reported in this study is striking in the face of earlier studies that showed memory protection but not enhancement. Notably, this study used a classic paired associates task (semantically related word-pair learning). This task tends to show sleep-dependent memory enhancements [30, 31], while those using semantically unrelated word pairs report sleep-dependent memory protection [32, 33]. The nature of the learning task thus shifts from forming and retaining completely novel associations (dog-leaf) to the strengthening or tagging of well-formed associations (dog-bone) for recall at testing [34].
Sleep’s role in declarative memory consolidation, rather than being absolute, depends on several aspects of the task including task difficulty, future relevance, and emotional salience. More difficult tasks are thought to benefit most from sleep [12, 35]. This may reflect enhanced consolidation for weak compared to strong memories. For instance, learning a second list of word pairs weakens the memory for the first list. Yet, recall of the first list following sleep is greater than when recalled following an equivalent interval of wake while recall of list two differed little following sleep and wake [36].
The benefit of sleep on declarative memories may also be greatest for memories with clear future relevance. Wilhelm et al. [37] demonstrated this preferential effect of sleep by comparing recall after sleep and wake for two groups of participants: one group was informed that they would be asked to recall the learned word pairs in a subsequent session and a second group was not informed of the later recall. Sleep-dependent enhancement in performance was evident for those who expected to recall the words following the delay interval, whereas there was no difference for sleep and wake groups who performed the surprise delayed recall test. Future relevance implied by associating items with a reward (monetary or points) also yields greater performance benefits following sleep compared to unrewarded items [38, 39].
Emotional salience may cue future relevance as well, as memory for items with negative valence is important for self-preservation. A number of studies have illustrated the preferential consolidation of negative emotional memories over neutral memories. Hu et al. [40] found greater recognition of emotionally arousing images after sleep compared to wake while no condition differences were found for neutral images. Moreover, when negative items are embedded in neutral scenes, sleep preferentially enhanced recognition of the negative items relative to the neutral items while the scenes themselves, which were neutral in valence, were uninfluenced by the type of interval (sleep vs. wake) [41]. These behavioral effects have been associated with REM sleep [42]. Likewise, oversleep protection of emotional reactivity to negative images is predicted by the time spent in REM sleep [43].
While these examples highlight when sleep functions to stabilize and enhance veridical memories, as discussed earlier, sleep also functions to generalize declarative memories and integrate them into existing representations [44]. Indeed, the end goal of sleep-dependent memory processing may not be simply the enhancement of individual memories in isolation, but instead, the integration of these memories into a common schema and, by doing so, facilitation of the extraction of universal rules, which may be the basis of generalized knowledge. The most basic demonstration of this comes from studies using the Deese–Roediger–McDermott paradigm in which participants are presented with lists of semantically related words (e.g., BOWL, SPOON, MILK) that lack a critical word representing the gist of the list (e.g., CEREAL). When these lists are learned prior to sleep, recall of the list words is greater than following wake. Notably, sleep also yields greater recall of those critical, “gist” words that were never presented. This suggests that declarative memories are generalized and integrated into the existing lexicon over sleep, thereby allowing the extraction of the more general form of the memory after sleep [17, 45, 46].
Sleep-dependent generalization of declarative memories may provide other cognitive benefits of sleep such as enhancing rule learning, decision-making, and creativity. For instance, it has also been demonstrated that, following initial practice on a numeric-sequence problem-solving task, a night of sleep can trigger insight into a hidden rule and thus improve performance strategy the following morning [47]. Likewise, performance on the Iowa gambling task, a measure of affectively guided decision-making, becomes more optimal after sleep as subjects gain more insight into the statistical probabilities of the four decks of cards from which they must choose [48]. Integrating new memories with prior representations may also yield novel, creative solutions [49, 50].
Taken as a whole, these studies suggest a rich and multifaceted role for sleep in the processing of human declarative memories. While SWS is associated with learning of nonemotional veridical memory processing, REM sleep appears to contribute to changes in the emotional memory representation. How sleep facilitates memory generalization and the function of sleep in translocation and reconsolidation of declarative memories are a matter of ongoing research.
Human Studies of Procedural Memory
The benefit of sleep has been demonstrated across a wide variety of procedural tasks spanning the visual, auditory, and motor systems. In this section, we review evidence of procedural memory improvements following sleep as well as possible limitations.
Motor Skill Learning
Motor skill learning is defined as the process by which movements are more quickly and accurately executed after practice [51, 52]. Motor skills are often broadly classified into two forms: motor sequence learning (e.g., learning a piano scale) and motor adaptation (e.g., learning to use a computer mouse) [53].
The movement sequencing task, a common task for measuring sequence learning, requires that subjects repeatedly tap a sequence of response keys as quickly and accurately as possible. With training, movement time decreases, indicating that learning has occurred. A night of sleep can trigger significant performance improvements in speed and accuracy on a sequential finger-tapping task, while equivalent periods of time awake provide little to no benefit [15, 33]. These sleep-dependent benefits appear to be specific to both the motor sequence learned [54, 55] and the hand used to perform the task [56]. Furthermore, the amount of overnight learning expressed the following morning correlated positively with the amount of stage 2 NREM sleep, particularly late in the night (Fig. 13.3) [15]. This late-night stage 2 NREM window corresponds to a time when sleep spindles, a defining electrophysiologic characteristic of stage 2 sleep, reach peak density [57]. Interestingly, spindles have been shown to increase following training on a motor task [58, 59] and are associated with cellular plasticity as discussed later in this chapter.
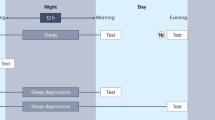
Sleep-dependent motor skill learning in the human brain. a Wake 1st: After morning training (10 am, light blue bar), subjects showed no significant change in performance when tested after 12 h of wake time (10 pm, light blue bar). However, when tested again following a night of sleep (10 am, dark blue bar), performance had improved significantly. b Sleep 1st: After evening training (10 pm, light blue bar), subjects displayed significant performance improvements just 12 h after training following a night of sleep (10 am, dark blue bar), yet expressed no further significant change in performance following an additional 12 h of wake time (10 pm, dark blue bar). c Sleep stage correlation: The amount of overnight improvement on the motor skill task correlated with the percentage of stage 2 NREM sleep in the last (fourth) quarter of the night (stage 2 NREM4). Asterisk, significant improvement relative to training; error bars, standard error of the mean (SEM)
A more detailed analysis of the performance improvements on the motor sequencing task after sleep provides insight into how improvements come about [60]. Prior to sleep, individual key-press transitions within the sequence are uneven (Fig. 13.4a, light circles), with some transitions seemingly easy (fast) and others problematic (slow), as if the entire sequence was being parsed into smaller subsequences during initial learning (a phenomenon termed chunking [61]). Surprisingly, after a night of sleep, the problematic slow transitions are preferentially improved, while transitions that were already mastered prior to sleep did not change (Fig. 13.4a, dark circles). In contrast, if subjects were retested after an 8-h waking interval across the day, no such improvement in the profile of key-press transitions, at any location within the sequence, was observed (Fig. 13.4b). These changes suggest that the sleep-dependent consolidation process may involve the unification of smaller motor memory units into one single memory element by selectively improving problem regions of the sequence. This overnight process would therefore offer a greater degree of performance automation and effectively optimize skill speed throughout the entire motor program.
Single-subject examples of changes in transition speeds. Within a five-element motor sequence (e.g., “4-1-3-2-4”), there are four unique key press transitions: (1) from 4 to 1, (2) from 1 to 3, (3) from 3 to 2, and (4) from 2 to 4. a The transition profile at the end of training before sleep (light circles) demonstrated considerable variability, with certain transitions being particularly slow (most difficult; “problem points”), whereas other transitions appear to be relatively rapid (easy). Following a night of sleep (dark circles), there was a specific reduction (improvement) in the time required for the slowest problem point transition. b Similarly, at the end of training before a waking interval, transition profiles were uneven (light circles), with some particularly slow transitions (“problem points”) and other relatively fast transitions (easy). However, in contrast to post-sleep changes, no change in transition profile was observed following 8 h of wakefulness (dark circles)
The role of sleep in motor adaptation is less clear. Doyon and colleagues [62, 63] compared off-line changes in motor sequence learning to off-line changes in visuomotor adaptation. To measure adaptation, subjects used a joystick to move a cursor on the screen to a target. After practice, mapping between the joystick space and the cursor on screen was inverted. Movements that were initially slow and inaccurate under inverted mapping conditions became faster and more accurate over time as participants adapted to the visuospatial shift. While replicating sleep-dependent performance changes on the motor sequence learning task, Doyon and colleagues failed to find preferential changes in performance over sleep on the motor adaptation task. Rather, motor adaptation improved equally over intervals with wake and sleep. These results are in contrast to an earlier study by Huber et al. [64]. Moreover, a rather consistent benefit of sleep has been observed for learning on a mirror tracing task which, like the visuomotor adaptation task of Doyon, requires learning of novel mapping between motor output and visual input. For example, Plihal and Born [65] found improvements in mirror tracing performance following late-night sleep, where REM and stage 2 non-REM dominate, even when early-night sleep was deprived. No such improvements were observed when participants had SWS-rich early-night sleep and late-night sleep was deprived. Moreover, Tamaki [66] found increased sleep spindle amplitude and duration after learning a mirror-tracing task, and this increase was associated with over-sleep improvements in performance.
Conflicting results across studies of motor skill consolidation may reflect limitations on sleep-dependent consolidation of motor skills. For instance, motor skill consolidation over sleep is biased by task difficulty. Kuriyama investigated the effects of increasing task complexity on sleep-dependent motor learning [60]. Subjects trained on a variety of task configurations involving either a short or long motor sequence performed either with one hand (unimanual) or coordinated between two hands (bimanual). Interestingly, the more complex the task became (long sequence, bimanual), the greater the overnight, sleep-dependent memory enhancement. This would indicate that, as task difficulty increases, the overnight sleep-dependent process responds with even greater performance improvements. Consistent with this, sleep was reported to benefit motor sequence learning for adults who were trained to an intermediate level of performance prior to sleep, whereas there was no benefit of sleep for experts [67].
Additionally, performance benefits from sleep are greater when participants are aware of the information to be learned [68]. This was demonstrated in a study comparing implicit motor sequence learning (subjects were not aware that finger responses to visual cues were in a sequence) under conditions which were either contextual—the sequence was embedded in a unique repeating context—or non-contextual. Sleep-dependent consolidation was present for the implicit contextual learning condition but not the non-contextual condition. Given that contextual learning is known to engage the hippocampus [69], we interpret these results as demonstrating the role of the hippocampus in consolidation of motor skills.
Taken together, overnight sleep has been shown to benefit motor skill performance, a benefit primarily associated with stage 2 NREM sleep and associated sleep spindles. Discrepancies across studies may be explained by limitations on this process with preferential consolidation for difficult tasks and those that engage the hippocampus [reviewed in 24].
Perceptual Learning
Karni et al. [70] have demonstrated that learning on a visual texture discrimination task improves significantly following a night of sleep. Furthermore, they established that selective REM, but not NREM sleep appears essential for these performance gains. Using the same task, Stickgold et al. [71] have shown that these enhancements are specifically sleep- and not time-dependent. Specifically, performance gains were correlated positively with the amount of both early-night SWS and late-night REM sleep, and the product of these two sleep parameters explained over 80 % of intersubject variance, an incredibly strong correlation.
Performance on a repeated visual search task also improves with sleep. In this task, participants are presented with arrays of L’s in various orientations and must find a hidden T. When displays are repeated, although unbeknownst to the observer, reaction time decreases. In spite of the implicit nature of learning, individuals with medial-temporal lobe lesions (including the hippocampus) fail to learn this task [72]. Geyer et al. [73] demonstrated sleep-dependent improvements on this task, as measured by reduced reaction times to repeated displays further supporting a role of the hippocampus in sleep-dependent consolidation even in the procedural domain.
The Weather Prediction Task is a probabilistic category-learning task in which participants learn to predict the “weather” from a set of cards, each of which has a learned probability of “sun” or “clouds” [74]. Djonlagic et al. [75] found that a group of subjects who slept after observing responses on the task made significantly more accurate weather predictions than a group who stayed awake. Here too, the performance benefits for the sleep group varied by the level of encoding: Those participants for whom the task was less difficult (high accuracy) before sleep showed less improvement than those for whom the task was more difficult (intermediate accuracy). Moreover, when the study was repeated and subjects were required to learn the probabilities through feedback, now sleep had no effect on performance. Given that observational learning is hippocampus-dependent while feedback-based learning of this task is associated with the striatum [e.g., 76], the authors interpreted these results to suggest that sleep may consolidate hippocampal-based learning but not memories encoded in the striatum.
Summary
In summary, delayed off-line learning of many perceptual and motor skills appears to develop during overnight sleep and not across equivalent time periods awake. Such changes have been associated with stage 2 NREM and sleep spindles that are predominantly found in this sleep stage. However, the function of hippocampal-dependent reactivation in SWS is suggested to play a parallel role. How these functions interact across sleep is a matter of ongoing research [e.g., 24, 77]
Animal Studies
Studies using animal models have provided evidence for the role of sleep in primarily hippocampus-dependent tasks. Training on both spatial and shock avoidance tasks triggers alterations in sleep-stage characteristics [78–82], suggesting, as in humans, a homeostatic response to increased demands on sleep-dependent consolidation mechanisms. In one such study, the magnitude of change in sleep architecture following learning demonstrated a strong relationship to initial performance during acquisition, with animals that learned quickly showing the largest change in sleep structure, while those that learned poorly showed relatively little [83].
The benefit of sleep after learning on subsequent performance has been demonstrated in a range of species including non-human primates [84], cats [85], rats [86, 87], starlings [13, 88], honeybees [89], and fruit flies [90]. In one such study, Inostroza et al. [87] examined memory for object-place pairs in rats following an 80-min interval that either contained sleep or was spent fully awake. Memory for the location of objects was greater following sleep, whereas animals performed at chance if kept awake after encoding the object location (Fig. 13.5a). Moreover, sleep did not benefit the memory for a novel object recognition task, which is thought to be encoded independent of the hippocampus (Fig. 13.5b).
Sleep-dependent learning in rats. a Animals learned an object-location task in the morning followed by normal sleep (Sleep) or sleep deprivation (S-Deprivation). The Wake condition took place in the evening when wakefulness is typical (i.e., not sleep deprived). Animals were significantly more likely to identify the misplaced object following sleep compared to both the sleep-deprivation and wake conditions as measured by the discrimination ratio (time spent at novel minus time spent at familiar divided by total exploration time). b In the novel-object recognition task, thought to be encoded independent of the hippocampus, animals were probed for their memory of a new object in the arena following sleep. There was no difference in discriminability for the novel object following sleep compared to the sleep-deprivation and wake conditions (Permissions needed from Inostroza et al. [87])
Memories are not only protected over sleep but enhanced as demonstrated in starlings, a songbird known for its ability to imitate the sounds of other birds. If two songs are learned in parallel, the memories interfere with one another as seen by impaired performance following an interval spent awake. However, when birds slept following the exposure to the interfering songs, performance improvements were observed, even when sleep followed a significant waking delay. These results suggest that sleep actively restored and enhanced the song memories. Additionally, when interference was presented following sleep, it did not impair performance for the song learned prior to sleep. Collectively, this work in song birds, similar to observations in humans [91], suggests that learning is actively consolidated and enhanced over sleep, leaving it more resistant to interference after sleep.
Summary
Taken as a whole, behavioral studies in humans and animal models leave little doubt that sleep plays a critical role in post-training memory consolidation. While both declarative and procedural memories have been shown to improve following sleep compared to wake, these memory systems appear to require subtly different sleep stages or even sleep-stage time windows, for consolidation and overnight improvement. With such evidence, it is important to consider the impact of little to no sleep as measured by studies of napping and sleep deprivation.
Sleep Deprivation
Reinforcing the role of sleep in memory consolidation is a wealth of literature illustrating the converse that sleep deprivation results in impaired declarative and procedural learning [80, 89, 92–100]. For example, total sleep deprivation (38 h awake) [101] and sleep restriction (overnight sleep reduced to 4 h) [102], both yield performance impairments on a number of cognitive tasks including declarative learning.
However, many of these studies have been legitimately criticized for a failure to control for general effects of sleep deprivation on performance [103, 104]. Retesting in a sleep-deprived state may mask evidence of successful consolidation due to lowered alertness and attention. Alternatively, the increased stress of prolonged wakefulness, rather than the lack of sleep itself, may be the cause of unsuccessful consolidation.
More recent studies in both humans and animals have, however, demonstrated that impaired performance can still be seen several days after the end of sleep deprivation, when alertness and attention have returned to normal [105]. In addition, selective deprivation of specific sleep stages inhibits memory consolidation [98, 106], making arguments of sleep deprivation-induced stress relatively untenable, since the stress effects would have to be uniquely produced by deprivation of specific sleep stages during specific time windows following training.
Napping
Given this clear evidence that sleep is advantageous and sleep deprivation is disadvantageous for learning, several studies have asked the question “how much sleep is enough?” These studies have consistently revealed that a nap is sufficient for sleep-dependent changes in declarative [31, 107] and procedural memories [108–112]. Even a very brief nap of 6 min has been reported to increase recall of a declarative, word-list learning task [113]. Nonetheless, more sleep is better. Mednick and colleagues have shown that a 30- to 60-min daytime nap between repeated administrations of a texture discrimination task protects learning from the performance deterioration that is seen when subjects stay awake. If a longer nap period is introduced, ranging from 60 to 90 min and containing both REM sleep and NREM SWS, performance not only returns back to baseline but may be enhanced.
Sleep-Dependent Brain Plasticity
Memory formation depends on brain “plasticity”—lasting structural and functional changes in a neuron’s response to a stimulus. The behavioral studies described above indirectly suggest a role of hippocampal plasticity in sleep-dependent memory consolidation, but recent direct evidence of sleep-dependent plasticity greatly strengthens this claim. In this section, we consider a wealth of data describing sleep-dependent brain plasticity at a variety of different levels in both animals and humans, complementing evidence of sleep-dependent changes in behavior.
Neuroimaging Studies
Modification of Post-training Sleep and Brain Activation
Several studies have investigated whether initial daytime training is capable of modifying functional brain activation during later sleep episodes. Based on earlier animal findings (described below), neuroimaging experiments have focused on whether the signature pattern of brain activity elicited while practicing a memory task actually re-emerges, or is “replayed” during sleep.
Maquet and colleagues have shown that patterns of brain activity expressed during motor skill training reappear during subsequent REM sleep, while no such change in REM sleep brain activity occurs in subjects who received no daytime training [114] (Fig. 13.6). Furthermore, these researchers have gone on to show that the extent of learning during daytime practice exhibits a positive relationship to the amount of reactivation during REM sleep [115]. Training on a hippocampus-dependent virtual maze task during the day has similarly been associated with a subsequent increase in hippocampal reactivation in NREM SWS [116], with the magnitude of reactivation correlating with performance on the task the following day, suggesting a function for neuronal replay. Such findings suggest that it is not simply experiencing the task that modifies subsequent sleep physiology, but the process of learning itself, and that this reactivation can lead to next-day behavioral improvements.
Task-dependent reactivation of human brain activity during REM sleep. Statistical activation maps of different experimental contrasts. Maps are displayed at six different brain levels (from 16 mm below to 64 mm above the bicommissural plane), superimposed on the average magnetic resonance imaging (MRI) scan of subjects. All maps are thresholded at p < 0.001 (uncorrected), except for A, which is thresholded at voxel-level-corrected p < 0.05. a Brain regions activated during daytime performance of the motor skill task (Task–Rest). b Brain regions activated during subsequent REM sleep in subjects who received daytime training (REM Sleep Training–Rest); note considerable overlap with daytime task-dependent activity patterns. c Brain regions activated during REM sleep in subjects who did not receive any daytime training (REM Sleep No Training–Rest) (Reproduced with permission from Maquet et al. [114])
Overnight Reorganization of Memory Representations
An alternative approach to investigate sleep-dependent plasticity is to compare patterns of brain activation after a night of sleep. In contrast to measuring changes in functional activity during sleep, this approach aims to determine whether there is evidence that the neural representation of a memory has been reorganized following a night of sleep and whether this reorganization differs from that which may occur over wake.
With the behavioral characteristics of sleep-dependent motor sequence learning well established, Walker et al. were the first to examine the neural basis of sleep-dependent changes in learning by investigating differences in brain activation before and after sleep using functional magnetic resonance imaging (fMRI) [117]. Following a night of sleep, relative to an equivalent intervening time period awake, increased activation was identified in motor control structures of the right primary motor cortex (Fig. 13.7a) and left cerebellum (Fig. 13.7b)—changes that likely allow faster motor output to the trained, left hand, and more precise mapping of key-press movements post-sleep. There were also regions of increased activation in the right medial prefrontal lobe and hippocampus (Fig. 13.7c, d), structures that support improved sequencing of motor movements in the correct order. In contrast, decreased activity post-sleep was identified bilaterally in the parietal cortices (Fig. 13.7e), possibly reflecting a reduced need for conscious spatial monitoring, and throughout the limbic system (Fig. 13.7f–h), indicating a decreased emotional task burden. In total, these results suggest that sleep-dependent motor learning is associated with a large-scale plastic reorganization of memory throughout several brain regions, allowing skilled motor movements to be executed more quickly, more accurately, and more automatically following sleep.
Sleep-dependent motor memory reorganization. Subjects were trained on a sleep-dependent motor skill task and retested 12 h later, either following a night of sleep or following intervening wakefulness, during a functional MRI (fMRI) brain scanning session. Scans after sleep and wakefulness were compared (subtracted), resulting in regions showing increased fMRI activity post-sleep (in red/yellow; a–d) or decreased signal activity (in blue; e–h) post-sleep, relative to post-wakefulness. Activation patterns are displayed on three-dimensional rendered brains (top panel of each graphic), together with corresponding coronal sections (bottom panel of each graphic). Following sleep, regions of increased activation were identified in the right primary motor cortex (b), the left cerebellum (a), the right hippocampus (c), and the right medial prefrontal cortex (d). Regions of decreased activity post-sleep were expressed bilaterally in the parietal lobes (e), together with the left insula cortex (f), left temporal pole (g), and left frontopolar area (h), all regions of the extended limbic system. All data are displayed at a corrected threshold of p < 0.05
Debas et al. [62] contrasted changes in neural activation following sleep and wake for a motor skill learning task and a motor adaptation task. Consistent with their behavioral observations described above, there was an increase in activation in the primary motor cortex, the cerebellum, and the striatum for the motor sequence learning task following the off-line interval, with the activation of the striatum specifically modulated by sleep. However, there were no sleep-specific changes in neural activation patterns in conjunction with the motor adaptation task, consistent with the observation of limited over-sleep change in performance for this task.
Visual skill learning is also associated with underlying cortical plasticity. Post-sleep performance was associated with significantly increased activity not only in the primary visual cortex, but also in several downstream visual processing regions following sleep, at the occipital–temporal junction and in the medial temporal and inferior parietal lobes (regions involved in object detection and identification) [118]. These findings strengthen the claim that a night of sleep reorganizes the representation of a visual skill memory, with greater activation throughout the visual system following sleep likely offering improved identification of both the visual stimulus form and its location in space.
While most studies of over-sleep neural reorganization have focused on procedural learning, the neural basis of declarative memory consolidation has more recently been investigated. Takashima et al. [119] examined the long-term effects of a nap following the encoding phase of a picture recognition task. Picture recognition and the associated neural correlates were measured following a delay of 1, 2, 30, and 90 days. Following a delay of 1 day, the amount of time spent in SWS correlated positively with recognition performance and negatively with hippocampal activity during correct recognition. With time, recognition was associated with a decrease in the hippocampus activation coupled with a corresponding increase in ventromedial prefrontal cortex activation. This result is interpreted to suggest that SWS is associated with transfer of memories from the labile storage in the hippocampus to a more permanent representation in the cortex.
Overnight reorganization of emotional declarative memories has been the focus of many recent studies [120–122]. Similar to non-emotional declarative memories, memory for negative images following sleep is associated with less diffuse hippocampal activation. Specifically, Payne and Kensinger [120] examined changes in neural activity associated with an emotional picture recognition task following retention intervals with sleep or wake. Patterns of neural activation associated with successful recognition were distinct following sleep versus wake. After an interval of daytime wake, successful recognition of the negative images was associated with activation in a broad network including the lateral prefrontal, parietal, and medial temporal lobes (including the hippocampus). In contrast, recognition performance following sleep was concentrated to a smaller network including the ventromedial prefrontal cortex, left amygdala, and cingulate gyrus.
Summary
Learning and memory are dependent on processes of brain plasticity, and sleep-dependent learning and memory consolidation must be mediated by such processes. Using brain imaging techniques, several studies have now identified changes in (1) the patterns of functional brain activity during post-training sleep periods (both REM and NREM) and (2) the reorganization of newly formed memories following a night of sleep. These plastic brain changes likely contribute to the refinement of the memory representations, resulting in improved next-day behavioral performance.
Electrophysiologic Studies
Evidence of sleep-dependent neuronal replay of waking experiences has accumulated from studies using multi-electrode recordings in the hippocampus of rodents. During waking exploration, the pattern of neural firing in hippocampal CA1 and CA3 cells, known as “place cells,” is predictable as these cells exhibit an increased firing rate when the animal is in a particular location in space [123]. Initially, Wilson and McNaughton [124] observed that place cells that fired together during waking exploration tended to also fire together during subsequent sleep. Notably, in a subsequent study, Skaggs and McNaughton [125] found that not only was the same neural ensemble active, but cells were reactivated in the same order during sleep as they were during waking behavior. Dave and Margoliash [126, 127] have also shown that waking patterns of premotor activity observed during song learning in the zebra finch are also replayed during sleep, with a temporal structural similar to that seen in wakefulness.
Similar to studies in humans using contextual cues (odors, sounds) associated with learning to reactivate memories during sleep, Bendor and Wilson [128] selectively reactivated memories in rats during sleep and measured associated hippocampal replay. Prior to sleep, rats learned to move either left or right depending on a sound cue. During subsequent non-REM sleep, neurons associated with learning of this task were reactivated in the absence of any sound cue. However, when a task-related sound cue was presented, neurons associated with movements in the direction corresponding to that sound cue were preferentially fired. These results demonstrate a direct association between recent learning and the content of hippocampal replay.
Replay occurs in conjunction with sharp wave/ripple complexes in the hippocampus. Sharp waves are fast depolarizing events that overlap with high-frequency local field potential oscillations (ripples) and originate from pyramidal neurons in the CA1 region of the hippocampus [129–132]. Ripples have been associated with long-term potentiation (LTP), believed to be a physiologic mediator of memory formation [133, 134]; the frequency of ripples is conducive for LTP [135] and experimental suppression of hippocampal ripples has been shown to impair memory [136, 137].
Sleep spindles, generated by the thalamus and spread throughout the neocortex, co-occur with sharp wave/ripple complexes [138]. Rosanova and Ulrich [139] have shown that spike trains designed to mimic sleep spindles, delivered in vivo, produce both short-term and LTP. Moreover, functional connectivity between the hippocampus and the neocortex is increased in conjunction with spindles [140]. Thus, while behavioral evidence suggests that sleep spindles are associated with sleep-dependent performance changes, physiologically, spindles have been shown to support plasticity. Consistent with this, pharmacological manipulations which enhance spindle density in humans increase memory performance [141].
Together these data indicate that sleep-dependent reactivation of temporal patterns of network activity consistently occurs following learning experiences during wakefulness, across a broad spectrum of species. Neuronal replay of recent memories in the hippocampus via ripple events co-occurs with widespread neocortical activity driven by spindles. Such a mechanism is consistent with the proposal of hippocampal–neocortical transfer of memories over sleep, making memories less resistant to interference as new memories are encoded in the hippocampus [131, 142].
Cellular Studies
Sleep-dependent plasticity at the cellular level has been elegantly demonstrated during early post-natal development of the cat visual system [143, 144]. Under normal circumstances, brief periods of monocular visual deprivation during critical periods of development lead to the remodeling of synaptic connectivity, with the deprived eye’s inputs to cortical neurons being first functionally weakened and then anatomically diminished [145]. Frank et al. [146] demonstrated that when 6 h of monocular deprivation are followed by 6 h of sleep, the size of the shift in cortical representation doubles. In contrast, if the cats are kept awake for these same 6 h (in the dark, without input to either eye), a nonsignificant reduction in the size of the shift occurs. Thus, sleep can contribute as much to developmental changes in synaptic connectivity as does visual experience, presumably by enhancing the initial changes occurring during a prior period of monocular deprivation. In contrast, sleep deprivation results in a loss of previously formed, experience-dependent synaptic change, consistent with behavioral studies in humans [71].
Shaffery et al. [147] have reported complimentary findings of sleep-dependent plasticity in the rat visual cortex, suggesting that REM sleep, in conjunction with visual experience, modulates the initial time course of visual cortex maturation. In rats under 30 days of age, electrical stimulation produces increased excitability (potentiation) in specific layers of the visual cortex, while stimulation after this early developmental stage is unable to produce such potentiation. Depriving rats of REM sleep during this period can extend the window of plasticity by as much as 7 additional days, suggesting events occurring during REM sleep normally control the duration of this period of experience-dependent plasticity.
Molecular Studies
At the molecular level, Smith et al. [148] have shown that administration of protein synthesis inhibitors to rats during REM sleep windows thought to be critical for consolidation prevents behavioral improvement following the sleep period, while rats receiving saline injections show normal sleep-dependent learning. Such protein synthesis could reflect a fundamental mechanism regulating plasticity, namely the activation of genetic cascades that produce key molecules for synaptic remodeling. Such gene inductions during sleep have been the focus of more recent investigations. In their initial studies, Cirelli and Tononi reported that several of the known “immediate early genes” (IEGs) are specifically downregulated during sleep [149–151]. These findings have been used to argue that sleep is incapable of supporting plasticity and hence memory consolidation [103]. However, ample, more recent research by Cirelli et al. [152] has described approximately 100 genes that are specifically upregulated during sleep—almost the same number that are upregulated during wakefulness.
This extensive upregulation of genes was seen in the absence of any specific learning tasks being performed prior to sleep. Insofar as this upregulation is related to learning and memory consolidation, one might expect that such gene induction would only be seen after training on tasks that undergo sleep-dependent consolidation. Indeed, Ribeiro et al. [153, 154] have found upregulation of zif-268 and other plasticity-associated IEGs [155] in rats during REM sleep following exposure to a rich sensorimotor environment or induced hippocampal LTP, but found zif-268 downregulation during both SWS and REM sleep in the absence of such exposure. Thus, there appears to be a window for increased neuronal plasticity during REM sleep periods following enriched waking experience (Fig. 13.8).
Experience-dependent upregulation of zif-268 gene expression during wakefulness (WAKE) and slow-wave sleep (SWS) and REM sleep (REM) states in the rat. Autoradiograms of frontal coronal brain sections whose gene expression levels best represent the means for each group studied. In controls, zif-268 expression decreased from WAKE (A) to SWS (A′) and REM (A″). In enriched-environment animals, zif-268 levels decreased from WAKE (B) to SWS (B′), but increased from the latter to REM (B″). This effect was particularly noticeable in the cerebral cortex and the hippocampus (Reproduced with permission from Ribeiro et al. [153])
Summary
These studies at the cellular and molecular level illustrate the physiological basis of the changes in memory over sleep observed in behavioral studies. Although initial work failed to integrate behavioral changes with physiological findings, recent studies have successfully demonstrated sleep-dependent plasticity directly associated with waking behavior. While further work is needed to continue to integrate measures of plasticity and behavioral changes over sleep, evidence is accumulating in support for this relationship.
Unresolved Questions
Over the last several years, as evidence of sleep’s role in learning and memory consolidation has grown, some researchers have raised questions concerning the nature of this relationship and specific mechanisms that are necessary to support it. Having focused our attention so far on the evidence in support of sleep’s role, we turn now to concerns and objections to this research, many of which hold clinical relevance.
Antidepressants, Sleep, and Memory
A recurring issue in the sleep and memory debate has been the REM sleep suppressant effects of monoamine oxidase inhibitors (MAOIs) and other antidepressants and the impact, or lack thereof, on memory functioning. These findings appear to suggest REM suppressants can be taken for years with no deleterious effects on learning [103, 104, 156]. First, although MAOIs appear to reduce REM sleep to a greater or lesser extent early in medication [157, 158], REM sleep re-emerges later in the course of medication [159–161], suggesting a strong REM compensatory mechanism. Furthermore, there is a potent REM rebound during periods when medication is paused—the so-called drug holidays [160, 162, 163]. As such, the claim that patients live for years without REM sleep is unfounded.
It is also important to keep in mind that most of these studies use a single test of memory which does not address the question of intact or impaired sleep-dependent learning, which requires a retest following sleep. No studies, to date, have retested performance following sleep, or recorded subjects’ sleep to determine the extent of REM suppression. As a result, current studies are not conclusive regarding the role of post-training REM sleep in memory consolidation. A systematic study focusing on the effects of antidepressant medication on memory consolidation represents an important future goal, particularly considering the implications of such sleep-dependent memory impairment as a consequence of these drugs.
State Versus Trait
Some physiological measures of sleep that have been associated with memory have high within-subject correlations overtime, suggesting that they are a stable trait or phenotype. For instance, twin studies indicate that a significant proportion of the variability in stage 2 NREM and SWS is genetically determined [164]. Likewise, Geiger et al. [165] found low intra-individual (night-to-night) variability and high inter-individual variability in sleep-stage distributions and spectral power including the spindle range (sigma power). Of note, sigma power correlated with IQ. Thus, it is possible that associations between over-sleep changes in performance with sleep physiology, particularly sleep spindles, reflects these known associations between phenotypes. While research disentangling the state and trait relationships is ongoing, Schabus et al. [166] found state-specific spindle increases following learning in addition to the baseline association between spindles and cognitive abilities. This suggests that spindles, and perhaps other sleep features, may play both a state and trait role in learning.
Contributions of Sleep Micro- and macrostructure
As reviewed here, it is apparent that sleep spindles, occurring predominately in NREM, contribute to sleep-dependent hippocampal plasticity. However, it is clear that other aspects of sleep microstructure and macrostructure contribute to memory consolidation and how these factors work independent of or in conjunction with NREM sleep spindles has yet to be elucidated. For instance, Datta has suggested that ponto-geniculo-occipital (PGO) waves present in REM sleep underlie consolidation. PGO waves increase following training on an avoidance task and the increase is proportional to the degree of retention of performance over sleep [167]. These results imply that more time in REM sleep should be associated with greater memory consolidation. Given that Datta’s observations were based on an avoidance task in which a foot shock is delivered, one possibility is that the role of PGO waves and, more generally, REM sleep may be restricted to aversive memory processing as suggested in human studies cited above. Likewise, it has been suggested that the macrostructure of sleep is important. Ficca and colleagues have reported that pre-sleep learning can result in an increase in the number of sleep cycles across the night and an increase in cycle time, and this increase is proportional to post-sleep performance [168]. Disturbing sleep cycle organization also resulted in impaired verbal recall following sleep [169]. More than likely memories evolve throughout sleep and reported associations with sleep micro and macrostructure may reflect the predominant contributing process. How the form of memory processing changes across a bout of sleep continues to provide fuel for theoretical debate [24] and future research.
Summary
Over the last 25 years, the field of sleep and memory research has grown exponentially. These reports, ranging from studies of cellular and molecular processes in animals to behavioral studies in humans, have provided a wealth of converging evidence that sleep-dependent mechanisms of neural plasticity lead to the consolidation of learning and memory across a range of animal species.
At the molecular level, a significant number of genes appear to be upregulated specifically in brain tissue during sleep. IEGs related to synaptic plasticity, including zif-286, are upregulated during REM sleep expressly in response to environmental or direct electrical stimulation of the hippocampus. In rats, patterns of neuronal activation expressed during waking exploration reappear during subsequent sleep, and, in humans, patterns of regional brain activation seen during daytime task training are repeated during subsequent REM sleep.
At the electrophysiologic level, studies have shown enhanced hippocampal plasticity in conjunction with sleep spindles. In humans, spindle density increases following training on a declarative memory task, and, again, this increase correlates with subsequent improvement on the task.
At the behavioral level, animal studies have found robust increases in REM sleep following task training and decrements in performance after REM deprivation, even when retesting is delayed up to a week after the end of deprivation. In contrast, several animal studies have failed to find evidence of either increased REM sleep or deterioration following deprivation. Most likely, this reflects a combination of methodologic problems and conditions under which consolidation is, in fact, not sleep-dependent. Similarly, human studies have provided examples in which increases in REM sleep are seen following training; REM, SWS, or stage 2 NREM deprivation diminishes subsequent performance; and overnight improvement correlates with REM, SWS, or stage 2 NREM sleep.
In the end, the question appears not to be whether sleep mediates learning and memory consolidation, but instead, how and when it does so. The future of the field is truly exciting, and the challenge to neuroscience will be to both uncover the mechanisms of brain plasticity that underlie sleep-dependent memory consolidation, and to expand our understanding of sleep’s role in memory processes beyond simple consolidation, into the constellation of additional processes that are critical for efficient memory development. Work across the neurosciences will be necessary to answer these questions, but with the current rate of growth of research in the field, the next decade should provide important advances in our understanding of this critical function of sleep. By way of this multidisciplinary approach, and with a measured appreciation that sleep plays a fundamental role in consolidating and reforming memories, we can look forward to new advances in treating disorders of memory, and perhaps even improving the capacity of our own.
References
Hartley D (1801) Observations on man, his frame, his deity, and his expectations (1749/1966). Scholars Facsimile Reprint, Gainseville, FL
Jenkins JG, Dallenbach KM (1924) Oblivescence during sleep and waking. Am J Psychol 35:605–612
Ebbinghaus H (1964) Memory: a contribution to experimental psychology. Dover, New York
Squire LR, Zola SM (1996) Structure and function of declarative and nondeclarative memory systems. Proc Nat Acad Sci 93(24):13515–13522
Tulving E (1985) On the classification problem in learning and memory. In: Archer T (ed) Perspectives on learning and memory. Erlbaum, New Jersey
Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1(1):41–50
Squire LR (2004) Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem 82(3):171–177
McGaugh JL (2000) Memory—a century of consolidation. Science 287:248–251
Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 382(6588):252–255
Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S et al (2002) Early consolidation in human primary motor cortex. Nature 415(6872):640–644
Ellenbogen JM, Hulbert JC, Jiang Y, Stickgold R (2009) The sleeping brain’s influence on verbal memory: boosting resistance to interference. PLoS ONE 4(1):e4117
Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP (2007) Human relational memory requires time and sleep. Proc Natl Acad Sci U S A 104(18):7723–7728
Brawn TP, Nusbaum HC, Margoliash D (2013) Sleep consolidation of interfering auditory memories in starlings. Psychol Sci 22(24):439–447
Tucker MA, Fishbein W (2008) Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep 31(2):197–203
Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R (2002) Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35:205–211
Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK (2009) Sleep promotes generalization of extinction of conditioned fear. Sleep 32(1):19–26
Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, Tucker MA et al (2009) The role of sleep in false memory formation. Neurobiol Learn Mem 92(3):327–334
Walker MP (2005) A refined model of sleep and the time course of memory formation. Behav Brain Sci 28:51–104
Crick F, Mitchison G (1983) The function of dream sleep. Nature 304(5922):111–114
Saletin JM, Goldstein AN, Walker MP (2011) The role of sleep in directed forgetting and remembering of human memories. Cereb Cortex 21(11):2534–2541
Stickgold R, Scott L, Rittenhouse C, Hobson JA (1999) Sleep-induced changes in associative memory. J Cogn Neurosci 11(2):182–193
Diekelmann S, Buchel C, Born J, Rasch B (2011) Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci 14(3):381–386
Diekelmann S, Wilhelm I, Born J (2009) The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev 13(5):309–321
Spencer RMC (2013) Neurophysiological basis of sleep’s function on memory and cognition. ISRN Physiol 2013:1–17
Stickgold R, Walker MP (2007) Sleep-dependent memory consolidation and reconsolidation. Sleep Med 8(4):331–343
De Koninck J, Lorrain D, Christ G, Proulx G, Coulombe D (1989) Intensive language learning and increases in rapid eye movement sleep: evidence of a performance factor. Int J Psychophysiol 8(1):43–47
Rasch B, Buchel C, Gais S, Born J (2007) Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315(5817):1426–1429
Rudoy JD, Voss JL, Westerberg CE, Paller KA (2009) Strengthening individual memories by reactivating them during sleep. Science 326(5956):1079
Marshall L, Helgadottir H, Molle M, Born J (2006) Boosting slow oscillations during sleep potentiates memory. Nature 444(7119):610–613
Gais S, Molle M, Helms K, Born J (2002) Learning-dependent increases in sleep spindle density. J Neurosci 22(15):6830–6834
Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W (2006) A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem 86:241–247
Donohue K, Spencer RMC (2011) Continuous re-exposure to environmental sound cues during sleep does not improve memory for semantically unrelated word pairs. J Cogn Educ Psychol 10(2):167–177
Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RMC (2012) Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 33(5):991–1000
Payne JD, Tucker MA, Ellenbogen JM, Wamsley EJ, Walker MP, Schacter DL et al (2012) Memory for semantically related and unrelated declarative information: the benefit of sleep, the cost of wake. PLoS ONE 7(3):e33079
Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M et al (2006) Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci 26(35):8976–8982
Drosopoulos S, Schulze C, Fischer S, Born J (2007) Sleep’s function in the spontaneous recovery and consolidation of memories. J Exp Psychol Gen 136(2):169–183
Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Molle M, Born J (2011) Sleep selectively enhances memory expected to be of future relevance. J Neurosci 31(5):1563–1569
Fischer S, Born J (2009) Anticipated reward enhances offline learning during sleep. J Exp Psychol Learn Mem Cogn 35(6):1586–1593
Baran B, Daniels D, Spencer RMC (in press) Sleep-dependent consolidation of value-based learning. PLoS ONE
Hu P, Stylos-Allan M, Walker MP (2006) Sleep facilitates consolidation of emotional declarative memory. Psychol Sci 17(10):891–898
Payne JD, Stickgold R, Swanberg K, Kensinger EA (2008) Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci 19(8):781–788
Nishida M, Pearsall J, Buckner RL, Walker MP (2009) REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex 19(5):1158–1166
Baran B, Pace-Schott EF, Ericson C, Spencer RMC (2012) Processing of emotional reactivity and emotional memory over sleep. J Neurosci 32:1035–1042
Lewis PA, Durrant SJ (2010) Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci 15(8):343–351
McKeon S, Pace-Schott EF, Spencer RM (2012) Interaction of sleep and emotional content on the production of false memories. PLoS ONE 7(11):e49353
Diekelmann S, Born J, Wagner U (2010) Sleep enhances false memories depending on general memory performance. Behav Brain Res 208(2):425–429
Wagner U, Gais S, Haider H, Verleger R, Born J (2004) Sleep inspires insight. Nature 427:352–354
Pace-Schott EF, Nave G, Morgan A, Spencer RM (2011) Sleep-dependent modulation of affectively guided decision-making. J Sleep Res 21(1):30–39
Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC (2009) REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A 106(25):10130–10134
Walker MP, Liston C, Hobson JA, Stickgold R (2002) Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Cogn Brain Res 14(3):317–324
Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning. Neuron 72(3):443–454
Willingham DB (1998) A neuropsychological theory of motor skill learning. Psychol Rev 105(3):558–584
Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41(3):252–262
Fischer S, Hallschmid M, Elsner AL, Born J (2002) Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 99(18):11987–11991
Spencer RMC, Sunm M, Ivry RB (2006) Sleep-dependent consolidation of contextual learning. Curr Biol 16(10):1001–1005
Korman M, Raz N, Flash T, Karni A (2003) Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci U S A 100(21):12492–12497
De Gennaro L, Ferrara M, Bertini M (2000) Topographical distribution of spindles: variations between and within nrem sleep cycles. Sleep Res Online 3(4):155–160
Fogel SM, Smith CT (2006) Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res 15:250–255
Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G et al (2011) Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res 217(1):117–121
Kuriyama K, Stickgold R, Walker MP (2004) Sleep-dependent learning and motor-skill complexity. Learn Mem 11(6):705–713
Sakai K, Kitaguchi K, Hikosaka O (2003) Chunking during human visuomotor sequence learning. Exp Brain Res 152(2):229–242
Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G et al (2010) Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A. 107(41):17839–17844
Doyon J, Korman M, Morin A, Dostie V, Hadj Tahar A, Benali H et al (2009) Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Exp Brain Res 195(1):15–26
Huber R, Ghilardi MF, Massimini M, Tononi G (2004) Local sleep and learning. Nature 430(6995):78–81
Plihal W, Born J (1997) Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9(4):534–547
Tamaki M, Matsuoka T, Nittono H, Hori T (2008) Fast sleep spindle (13-15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep 31(2):204–211
Wilhelm I, Metzkow-Meszaros M, Knapp S, Born J (2012) Sleep-dependent consolidation of procedural motor memories in children and adults: the pre-sleep level of performance matters. Dev Sci. 15(4):506–515
Robertson EM, Pascual-Leone A, Press DZ (2004) Awareness modifies the skill-learning benefits of sleep. Curr Biol 14:208–212
Chun MM, Jiang Y (2003) Implicit, long-term spatial contextual memory. J Exp Psychol Learn Mem Cogn 29(2):224–234
Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D (1994) Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265:679–682
Stickgold R, James L, Hobson JA (2000) Visual discrimination learning requires sleep after training. Nat Neurosci 3(12):1237–1238
Chun MM, Phelps EA (1999) Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci 2:844–847
Geyer T, Mueller HJ, Assumpcao L, Gais S (2013) Sleep-effects on implicit and explicit memory in repeated visual search. PLoS ONE 8(8):e69953
Knowlton BJ, Squire LR, Gluck MA (1994) Probabilistic classification learning in amnesia. Learn Mem 1(2):106–120
Djonlagic I, Rosenfeld A, Shohamy D, Myers C, Gluck M, Stickgold R (2009) Sleep enhances category learning. Learn Mem 16(12):751–755
Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA (2004) Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain 127(Pt 4):851–859
Walker MP, Stickgold R (2010) Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci 11(3):218
Ambrosini MV, Mariucci G, Colarieti L, Bruschelli G, Carobi C, Giuditta A (1993) The structure of sleep is related to the learning ability of rats. Eur J Neurosci 5(3):269–275
Ambrosini MV, Sadile AG, Gironi Carnevale UA, Mattiaccio M, Giuditta A (1988) The sequential hypothesis on sleep function. I. Evidence that the structure of sleep depends on the nature of the previous waking experience. Physiol Behav 43(3):325–337
Hennevin E, Hars B (1987) Is increase in post-learning paradoxical sleep modified by cueing? Behav Brain Res 24(3):243–249
Mandai O, Guerrien A, Sockeel P, Dujardin K, Leconte P (1989) REM sleep modifications following a Morse code learning session in humans. Physiol Behav 46:639–642
Smith C, Young J, Young W (1980) Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep 3(1):67–81
Ambrosini MV, Langella M, Gironi Carnevale UA, Giuditta A (1992) The sequential hypothesis of sleep function. III. The structure of postacquisition sleep in learning and nonlearning rats. Physiol Behav 51(2):217–226
Martin-Ordas G, Call J (2011) Memory processing in great apes: the effect of time and sleep. Biol Lett 7(6):829–832
Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N et al (2012) Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol 22(8):676–682
Binder S, Baier PC, Molle M, Inostroza M, Born J, Marshall L (2012) Sleep enhances memory consolidation in the hippocampus-dependent object-place recognition task in rats. Neurobiol Learn Mem 97(2):213–219
Inostroza M, Binder S, Born J (2013) Sleep-dependency of episodic-like memory consolidation in rats. Behav Brain Res 15(237):15–22
Brawn TP, Nusbaum HC, Margoliash D (2010) Sleep-dependent consolidation of auditory discrimination learning in adult starlings. J Neurosci 30(2):609–613
Beyaert L, Greggers U, Menzel R (2012) Honeybees consolidate navigation memory during sleep. J Exp Biol 215(Pt 22):3981–3988
Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ (2011) Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332(6037):1571–1576
Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL (2006) Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol 16(13):1290–1294
Beaulieu E, Dufour LA, Beaudet R (2000) Better oral health for infants and toddlers: a community based program. J Dent Hygienists 74(2):131–134 (Spring)
Fishbein W, Kastaniotis C, Chattman D (1974) Paradoxical sleep: prolonged augmentation following learning. Brain Res 79(1):61–75
Haaland KY, Prestopnik JL, Knight RT, Lee RR (2004) Hemispheric asummetris for kinematic and positional aspects of reaching. Brain 127:1145–1158
Marti-Nicolovius M, Portell-Cortes I, Morgado-Bernal I (1988) Improvement of shuttle-box avoidance following post-training treatment in paradoxical sleep deprivation platforms in rats. Physiol Behav 43(1):93–98
Oniani TN, Lortkipanidze ND, Maisuradze LM (1987) Interaction between learning and paradoxical sleep in cats. Neurosci Behav Physiol 17(4):304–310
Shiromani P, Gutwein BM, Fishbein W (1979) Development of learning and memory in mice after brief paradoxical sleep deprivation. Physiol Behav 22(5):971–978
Smith C, Kelly G (1988) Paradoxical sleep deprivation applied two days after end of training retards learning. Physiol Behav 43(2):213–216
Smith C, Rose GM (1996) Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav 59:93–97
Rahman A, Languille S, Lamberty Y, Babiloni C, Perret M, Bordet R et al (2013) Sleep deprivation impairs spatial retrieval but not spatial learning in the non-human primate grey mouse lemur. PLoS ONE 8(5):e64493
Carskadon MA, Harvey K, Dement WC (1981) Sleep loss in young adolescents. Sleep 4(3):299–312
Carskadon MA, Harvey K, Dement WC (1981) Acute restriction of nocturnal sleep in children. Percept Mot Skills 53:103–112
Siegel JM (2001) The REM sleep-memory consolidation hypothesis. Science 294(5544):1058–1063
Vertes RP (2004) Memory consolidation in sleep: dream or reality. Neuron 44:135–148
Smith C, Smith D (2003) Ingestion of ethanol just prior to sleep onset impairs memory for procedural but not declarative tasks. Sleep 26(2):185–191
Smith C, Butler S (1982) Paradoxical sleep at selective times following training is necessary for learning. Physiol Behav 29(3):469–473
Baran B, Wilson JK, Spencer RMC (2010) REM-dependent repair of competitive memory suppression. Exp Brain Res 203:471–477
Milner CE, Fogel SM, Cote KA (2006) Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol 73(2):141–156
Backhaus J, Junghanns K (2006) Daytime naps improve procedural motor memory. Sleep Med 7:508–512
Walker MP, Stickgold R (2005) It’s practice, with sleep, that makes perfect: implications of sleep-dependent learning and plasticity for skill performance. Clin Sports Med 24(2):301–317
Mednick S, Nakayama K, Stickgold R (2003) Sleep-dependent learning: a nap is as good as a night. Nat Neurosci 6(7):697–698
Nishida M, Walker MP (2007) Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE 2:e341
Lahl O, Wispel C, Willigens B, Pietrowsky R (2008) An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res 17(1):3–10
Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C et al (2000) Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci 3(8):831–836
Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X et al (2003) Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage 20(1):125–134
Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J et al (2004) Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44(3):535–545
Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G (2005) Sleep-dependent motor memory plasticity in the human brain. Neuroscience 133(4):911–917
Walker MP, Stickgold R, Jolesz FA, Yoo SS (2005) The functional anatomy of sleep-dependent visual skill learning. Cereb Cortex 15(11):1666–1675
Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ et al (2006) Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 103(3):756–761
Payne JD, Kensinger EA (2011) Sleep leads to changes in the emotional memory trace: evidence from FMRI. J Cogn Neurosci 23(6):1285–1297
Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, Dang Vu TT et al (2009) Sleep promotes the neural reorganization of remote emotional memory. J Neurosci 29(16):5143–5152
Lewis PA, Cairney S, Manning L, Critchley HD (2011) The impact of overnight consolidation upon memory for emotional and neutral encoding contexts. Neuropsychologia 49(9):2619–2629
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34(1):171–175
Wilson MA, McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 65:676–679
Skaggs WE, McNaughton BL (1996) Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271:1870–1873
Dave AS, Margoliash D (2000) Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290(5492):812–816
Dave AS, Yu AC, Margoliash D (1998) Behavioral state modulation of auditory activity in a vocal motor system. Science 282(5397):2250–2254
Bendor D, Wilson MA (2012) Biasing the content of hippocampal replay during sleep. Nat Neurosci 15(10):1439–1444
Buzsaki G (1986) Hippocampal sharp waves: their origin and significance. Brain Res 398(2):242–252
Buzsaki G (1989) Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31(3):551–570
Buzsaki G (1998) Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res 7(Suppl 1):17–23
Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A et al (1995) Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15(1 Pt 1):30–46
Huerta PT, Lisman JE (1995) Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15(5):1053–1063
Pavlides C, Greenstein YJ, Grudman M, Winson J (1988) Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res 439(1–2):383–387
Girardeau G, Zugaro M (2011) Hippocampal ripples and memory consolidation. Current Opinion Neurobiology. 21(3):452–459
Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12(10):1222–1223
Ego-Stengel V, Wilson MA (2010) Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 20(1):1–10
Siapas AG, Wilson MA (1998) Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21(5):1123–1128
Rosanova M, Ulrich D (2005) Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci 25(41):9398–9405
Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG et al (2011) Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci 31(28):10331–10339
Kaestner EJ, Wixted JT, Mednick SC (2013) Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci 25(10):1597–1610
Marshall L, Born J (2007) The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci 11(10):442–450
Shaffery JP, Oksenberg A, Marks GA, Speciale SG, Mihailoff G, Roffwarg HP (1998) REM sleep deprivation in monocularly occluded kittens reduces the size of cells in LGN monocular segment. Sleep 21(8):837–845
Shaffery JP, Roffwarg HP, Speciale SG, Marks GA (1999) Ponto-geniculo-occipital-wave suppression amplifies lateral geniculate nucleus cell-size changes in monocularly deprived kittens. Brain Res Dev Brain Res 114(1):109–119
Antonini A, Stryker MP (1993) Rapid remodeling of axonal arbors in the visual cortex. Science 260(5115):1819–1821
Frank MG, Issa NP, Stryker MP (2001) Sleep enhances plasticity in the developing visual cortex. Neuron 30(1):275–287
Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA (2002) Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 110(3):431–443
Smith C, Tenn C, Annett R (1991) Some biochemical and behavioural aspects of the paradoxical sleep window. Can J Psychol 45(2):115–124
Cirelli C, Tononi G (1998) Differences in gene expression between sleep and waking as revealed by mRNA differential display. Brain Res Mol Brain Res 56(1–2):293–305
Cirelli C, Tononi G (2000) Gene expression in the brain across the sleep-waking cycle. Brain Res 885(2):303–321
Cirelli C, Tononi G (2000) On the functional significance of c-fos induction during the sleep-waking cycle. Sleep 23(4):453–469
Cirelli C, Gutierrez CM, Tononi G (2004) Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron 41(1):35–43
Ribeiro S, Goyal V, Mello CV, Pavlides C (1999) Brain gene expression during REM sleep depends on prior waking experience. Learn Mem 6(5):500–508
Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C (2002) Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci 22(24):10914–10923
Romcy-Pereira RN, Erraji-Benchekroun L, Smyrniotopoulos P, Ogawa S, Mello CV, Sibille E et al (2009) Sleep-dependent gene expression in the hippocampus and prefrontal cortex following long-term potentiation. Physiol Behav 98(1–2):44–52
Vertes RP, Eastman KE (2000) The case against memory consolidation in REM sleep. Behav Brain Sci 23(6):867–876 (discussion 904–1121)
Landolt HP, Raimo EB, Schnierow BJ, Kelsoe JR, Rapaport MH, Gillin JC (2001) Sleep and sleep electroencephalogram in depressed patients treated with phenelzine. Arch Gen Psychiatry 58(3):268–276
Wetzel W, Wagner T, Balschun D (2003) REM sleep enhancement induced by different procedures improves memory retention in rats. Eur J Neurosci 18(9):2611–2617
Landolt HP, de Boer LP (2001) Effect of chronic phenelzine treatment on REM sleep: report of three patients. Neuropsychopharmacology 25(5 Suppl):S63–S67
Mendelson WB, Cohen RM, Campbell IC, Murphy DL, Gillin JC, Wyatt RJ (1982) Lifetime monoamine oxidase inhibition and sleep. Pharmacol Biochem Behav 16(3):429–431
Minot R, Luthringer R, Macher JP (1993) Effect of moclobemide on the psychophysiology of sleep/wake cycles: a neuroelectrophysiological study of depressed patients administered with moclobemide. Int Clin Psychopharmacol 7(3–4):181–189
Steiger A, Benkert O, Holsboer F (1994) Effects of long-term treatment with the MAO-A inhibitor moclobemide on sleep EEG and nocturnal hormonal secretion in normal men. Neuropsychobiology 30(2–3):101–105
Steiger A, Holsboer F, Benkert O (1987) Effects of brofaremine (CGP 11 305A), a short-acting, reversible, and selective inhibitor of MAO-A on sleep, nocturnal penile tumescence and nocturnal hormonal secretion in three healthy volunteers. Psychopharmacology 92(1):110–114
Linkowski P (1999) EEG sleep patterns in twins. J Sleep Res 8(Suppl 1):11–13
Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P (2011) The sleep EEG as a marker of intellectual ability in school age children. Sleep 34(2):181–189
Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S et al (2008) Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res 29(1191):127–135
Datta S (2000) Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J Neurosci 20(22):8607–8613
Conte F, Carobbi G, Errico BM, Ficca G (2012) The effects of pre-sleep learning on sleep continuity, stability, and organization in elderly individuals. Front Neurol 3:1–9
Ficca G, Lombardo P, Rossi L, Salzarulo P (2000) Morning recall of verbal material depends on prior sleep organization. Behav Brain Res 112(1–2):159–163
Acknowledgments
This work was supported by grants from the National Institutes of Health (MH48,832, MH65,292, MH06,9935, and MH67,754 to M.P.W. and R.S. and AG040133 and HL111695 to R.M.C.S.) and the National Science Foundation (BCS-0121953 to M.P.W. and R.S.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media New York
About this chapter
Cite this chapter
Spencer, R.M.C., Walker, M.P., Stickgold, R. (2017). Sleep and Memory Consolidation. In: Chokroverty, S. (eds) Sleep Disorders Medicine. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-6578-6_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-6578-6_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-6576-2
Online ISBN: 978-1-4939-6578-6
eBook Packages: MedicineMedicine (R0)