Abstract
The treatment of ureteral strictures is a challenging procedure because of the variability of the aetiology. Traditionally, this procedure has been done using an open access. Regarding the site and the extension of ureteral strictures, many different techniques can be used: end-to-end anastomosis or reimplantation into the bladder with a Boari flap or psoas hitch. The biggest game changer in the management of ureteric strictures and vesicoureteral reflux was the invention of robot-assisted technology that is now performed safe and routinely with good functional postoperative outcomes.
This book chapter is focused on robot-assisted approach for ureteral reimplantation and describes the preoperative assessment, patient positioning, trocar placement, different surgical techniques for this surgery. Available intraoperative tools that are useful to detect the ureteral stricture, postoperative outcomes, and complications related to this kind of surgery are also assessed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Background
Traditionally the treatment of ureteric strictures (pelvic ureteric junction obstruction [PUJO], proximal and distal ureteric strictures) and vesico-ureteral reflux (VUR) has been managed with complex open reconstructive techniques. The invention of traditional laparoscopic techniques allowed for a minimally invasive approach to the treatment of these conditions. With the increase in laparoscopic skills amongst the urological community, more and longer segment strictures were treated laparoscopically. However, the reconstruction is technically challenging and requires considerable expertise in laparoscopic suturing.
The biggest game changer in the management of ureteric strictures and VUR was the invention of robotic technology. The Da Vinci surgical system (Intuitive Surgical, Sunny Vale, California) has been used for more than 15 years. It has several major advantages compared to conventional laparoscopy—the surgeon is in control of the surgical instruments and camera at the same time, the vision is three dimensional (3D) and magnified 10 times, the instruments allow for a greater dexterity facilitating intracorporeal suturing in a small and confined spaces with tremor elimination.
The incidence of ureteral strictures is rising due to the increased use of endoscopic instruments for the treatment of calculi and laparoscopic gynaecological and colorectal procedures [1, 2].
2 Indications
The indications for the management of VUR in children have evolved over the years. Nowadays more therapeutic options have become available and the guidelines have been updated, respectively. According to the EAU guidelines [3,4,5,6,7], the first treatment of choice is conservative management, because 80% of reflux grades I–II and 30–50% of grades III–V resolve spontaneously within 4–5 years of follow-up. Spontaneous resolution however is low for bilateral high-grade reflux. The next step in management is continuous antibiotic prophylaxis (CAP). Decision-making may be influenced by the presence of risk factors for UTI, such as young age, high-grade VUR, status of toilet training/LUTS, female sex and circumcision status.
Subureteric injections with bulking agents might be an alternative to the aforementioned measures. In a meta-analysis of 5527 patients, the reflux resolution rate (by ureter) following one treatment for grades I and II reflux was 78.5%, 72% for grade III, 63% for grade IV, and 51% for grade V. Subsequent injections improved the resolution of reflux further—second treatment by 68% and third by 34%. The combined success rate with one or more injections was 85% [8].
Surgical intervention is indicated in children who have failed conservative management—breakthrough UTI while on CAP, renal scarring and worsening or unresolved VUR.
In adults the aetiology of ureteric strictures is different compared to children. These can be divided into two main groups—strictures associated with benign (including iatrogenic) and malignant conditions. The benign group of ureteric strictures incorporates strictures related to other surgical procedures; for further details and incidence please refer to Table 101.1. Iatrogenic ureteral trauma can result from various mechanisms: ligation or kinking with a suture, crushing from a clamp, partial or complete transection, thermal injury, or ischemia from devascularisation [12, 16, 17]. It usually involves damage to the lower ureter [9, 16,17,18]. Ureteral injuries are often missed intra-operatively and only discovered in the postoperative period, which may result in severe sequelae. Other treatment modalities apart from surgery are also known to cause ureteric strictures, radiotherapy being the most prevalent; but also radiofrequency ablation (RFA) and cryotherapy for small renal masses can have similar complications in the proximal ureter. Other benign conditions such as ureteric calculi, obstetric complications, endometriosis, trauma, inflammatory and autoimmune diseases (appendicitis, Crohn’s disease, acute diverticulitis, etc.) can also lead to stricture formation.
The close proximity of the distal ureter to other organs in the pelvis makes it susceptible to involvement from malignancies arising from adjacent organs, such as advanced prostate cancer, and colorectal and gynaecological malignancies. Primary urothelial malignancies can also lead to strictures and obstruction.
3 Preoperative Assessment
All patients undergoing robotic ureteral surgery will require thorough assessment with history and examination. The diagnosis of ureteral trauma is challenging; therefore a high index of suspicion should be maintained. Imaging plays a crucial role in the decision-making process. Depending on the aetiology of ureteral pathology different imaging modalities can be utilized. In children—renal USS, micturating cystourethrogram, nuclear medicine scans, in adults—CT urogram, MRI, retrograde and antegrade ureterogram provide sufficient anatomical details to establish the location and length of the stricture. Diuretic renogram is useful to assess residual renal function on the affected side. In cases with long-standing ureteral obstruction and minimal kidney function, one can omit the ureteral reimplantation. If iatrogenic injury is suspected during the primary procedure, intravenous dye (e.g. indigo carmine) can be administered to help to detect an injury.
The following signs are characteristic of delayed diagnosis—flank pain, urinary incontinence, vaginal or drain urinary leakage, haematuria, fever, uraemia or urinoma. Earlier recognition of injuries facilitates their repair and provides better surgical outcomes [11, 19].
4 Intraoperative Localization of the Ureteral Stricture
Identifying the course of the ureter and the location of the ureteric stricture can be quite difficult in cases where previous surgery has taken place, where there is severe inflammation or large pelvic masses are distorting the normal anatomy. Successful surgical outcomes on the other hand are highly dependent on accurate localization and excision of the entire strictured segment, which will minimize the risk of recurrence and, at the same time, limiting the unnecessary resection of healthy tissue, which can present challenges with anastomosis and tension. In the robotic setting, surgeons must rely on visual cues in the absence of tactile feedback. Several techniques are available to help in this scenario. Firstly, the insertion of ureteric stent or catheter preoperatively may allow for easier identification of the ureter. Administration of diuretics will cause distention proximal to the stricture which can also be helpful. Some surgeons use concurrent ureteroscopy, allowing the light of the scope to guide them to the level of the stricture.
The use of the robotic Firefly™ technology is also helpful in assessing the location and the extension of the ureteral obstruction. Indocyanine Green (ICG) is a dye which can be visualized under near-infrared fluorescence (NIRF). ICG is prepared in the operating theatre on demand by dissolving 25 mg of ICG in 10 mL of distilled water. This solution can then be injected into the ureter (Fig. 101.1) retrogradely, via a ureteric stent, or antegradely via a nephrostomy tube. Intravenous injection of ICG can be used to evaluate the viability of the ureter too (Fig. 101.2). The normal ureter appears green, while the stricture remains dark [20]. The major drawback with intraureteral ICG is that care should be taken to avoid its spillage out of the ureter [21]. This will make difficult to differentiate the ureter from the surrounding structures. Alternatively, intraoperative ureteroscopy may be used to identify the stricture. The ureteroscope is placed up to the distal extent of stricture, at which point the NIRF modality of the da Vinci robot allows visualization of the ureteroscope light (Fig. 101.3) [22]. The proximal extent of the stricture can be identified with the ureteroscope if it passes through the distal stricture. If not, ICG is injected intravenously to confirm the proximal margin of the healthy tissue.
Intraureteral ICG: (a) Intraoperative ureteral stricture under white light and (b) under NIRF. (Reproduced with permission [20])
Intravascular ICG: (a) Intraoperative ureteral stricture under white light and (b) under NIRF showing a poorly perfused section of ureter (dotted line). (Reproduced with permission [21])
Intraoperative ureteroscopy: (a) The ureter in white light and (b) the ureter in fluorescence mode; the ureteroscope light can easily be seen in fluorescence mode. (Reproduced with permission [22])
5 Surgical Techniques
In this section we will cover the surgical techniques for the treatment of VUR in children and also the different approaches for the management of distal ureteric strictures in adults.
In the paediatric population two main approaches exist—intravesical and extravesical.
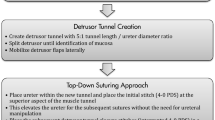
The intravesical approach of robotic-assisted laparoscopic ureteral reimplantation (RALUR) is composed of the following surgical steps: creation of pneumovesicum by the instillation of CO2 gas into the bladder, intravesical ureteral mobilization, submucosal tunneling, and intravesical advancement of the mobilized ureters within the newly created submucosal tunnels. This requires meticulous dissection and suturing in a limited working space. Peters and Woo [23] first described this procedure using a cross-trigonal or Cohen method of reimplantation. This approach is not very popular and is more technically challenging which explains why since its description only two other groups have reported their results [24, 25].
The extravesical approach for RALUR is based on the open Lich-Gregoir technique [26, 27]. This technique was first described by Peters et al. [28] in 2004 and since has been adopted by many paediatric centres for RALUR. Currently there is no consensus on which surgical techniques for RALUR should be used in order to maximize outcomes and minimize complications. Some centres have reported better results with the so called “top-down approach” [29], compared to the “bottom to top” technique [30, 31]. The “top-down technique” uses interrupted sutures starting at the superior aspect of the detrusor tunnel. This first stich elevates the ureter, facilitating the placement of subsequent sutures without the need for a stent or additional traction or manipulation of the ureter. In any case some of the principles for successful surgical outcomes after VUR surgery include—allowing for sufficient detrusor tunnel (4–5 cm), no touch rule for handling the ureter, low cautery settings, careful use of the instruments, avoiding inadvertent ureteric or bladder injury.
In adults the management of distal ureteric strictures requires the use of complex reconstructive techniques. Traditionally these have been performed via an open approach. The introduction of robotic technology eliminated some of the technical challenges of conventional laparoscopy and allowed for more of these procedures to be performed via a minimally invasive approach.
Distal ureteric strictures 4–5 cm in length can be successfully managed with ureterocystostomy. Longer segments 4–8 cm require mobilization of the bladder and use of a psoas hitch. The most challenging are strictures longer than 8–10 cm which require bladder mobilization, psoas hitch and creation of a Boari flap to bridge the gap between bladder and proximal healthy ureteric tissue [32].
The patient is positioned in lithotomy position with an 18–20 F 3-way indwelling catheter in situ, which will allow bladder drainage but also filling when required during the dissection. Lithotomy position is not necessary when side docking is utilized with the new Da Vinci Xi system. A steep Trendelenburg of 25° or more is maintained throughout the case in order to keep the bowels out of the pelvis. Entry into the abdomen is gained using Hasson’s technique via a midline incision 2–3 cm superior to the umbilicus (camera port) and pneumoperitoneum is established. 8 mm robotic trocars are inserted five finger breadths (approx. 8 cm) to the left and right from the camera port. A third 8 mm robotic trocar is placed three fingers medial and 2–3 cm superior to the left anterior superior iliac spine. A 12 mm Airseal™ port for the assistant is placed using the same landmarks but on the right side of the patient. Thanks to the Airseal™ technology a low intra-abdominal pressure of 8 mmHg is maintained throughout the case. This results in less postoperative pain, quicker recovery and less hypercapnia in our experience. Only three robotic instruments can be used in order to successfully complete the procedure and minimize costs—monopolar HotShears™, ProGrasp™, Large needle driver.
The procedure begins with the identification of the ureter proximally to the level of the stricture and its dissection distally to the strictured segment. For distal strictures the ureter can be easily identified at the level of its crossing with the iliac vessels. As discussed previously the use of ICG facilitates this process in difficult cases.
The bladder is mobilized from its peritoneal attachments to allow mobility in cranial direction. In some cases, division of the contralateral superior vesical pedicle (or bilateral superior vesicle pedicles) is required to achieve adequate bladder mobilization and tension free anastomosis.
The bladder can then be filled with 200–300 mL normal saline. This helps to identify the location of the new anastomosis between ureter and bladder, ensuring that the bladder will reach the proximal healthy ureter. The ureter can be then clipped distally and divided proximal to the stenosed segment. The proximal ureter is spatulated up to 2 cm, and its proximal patency can be checked prior to anastomosis by inserting a 6F ureteric catheter or stent.
The bladder is then opened at the desired location, the anastomosis is completed using two running 4.0 Monocryl sutures starting from the apex of the spatulated ureter over a 6F J-J stent. Additional sutures can be placed on the perivesical fat to reinforce the anastomosis.
In order to minimize traction on the newly created anastomosis, a psoas hitch can be used. This is usually achieved by using 2–3 interrupted 2.0 Vicryl sutures between the posterior bladder wall on the side of the anastomosis and the psoas muscle. If it is suspected that there might be tension on the anastomosis, it is better to apply the psoas hitch first which is easier and in turn will facilitate the anastomosis itself.
The most challenging reconstructive cases are the ones requiring the formation of a Boari flap. Similar preparation of the ureter and bladder is required. The bladder however has to be mobilized bilaterally and as distally as possible. With a distended bladder the flap is marked out with diathermy on the bladder surface. The base of the flap is on the dome, and on the side of the affected ureter, the width is approximately 4 cm. The flap then continues and stops just proximal to the bladder neck on the contralateral side. Care should be taken not to make the tip of the flap to narrow to avoid subsequent ischemia. A width of the flap tip of approximately 3 cm should be aimed for. The use of ICG to assess the viability of the Boari flap is an important step in this procedure. The ureter is divided proximal to the pathological area and spatulated. The posterior surface of the bladder flap is secured using a psoas hitch as described earlier in the text. A sub-mucosal tunnel is created at the cranial aspect of the Boari flap, and the spatulated ureter is tunneled through it into the bladder. Alternatively, the mucosa can be split longitudinally and then folded over the ureter in order to serve as an antireflux mechanism. The ureter is then sutured to the mucosa of the flap using interrupted sutures as per the technique described by Politano and Leadbetter [33]. A 6F J-J stent is inserted and the flap is tubularized using continuous absorbable sutures in two layers. The bladder is also closed in two layers in similar fashion. The repair can be leak tested and any reinforcements made if necessary. A pelvic drain is left at the end of the procedure. The indwelling catheter remains in situ for 2 weeks and the stent for 4–6 weeks. (See Fig. 101.4, 101.5, 101.6, 101.7, 101.8, 101.9, 101.10, 101.11, 101.12, 101.13, 101.14, 101.15, 101.16, 101.17, 101.18, and 101.19).
6 Discussion of Outcomes
Traditionally open ureteral reimplantation (OUR) is considered the “gold standard” surgical intervention for VUR. According to Bowen et al. [34], the number of all VUR ureteral reimplantations has decreased between 2002 and 2012 in the United States. However, the minimally invasive approaches have increased during the same period from 0.3 to 6.3% and 81.2% of those were done robot assisted. This increase coincides with the introduction and adoption of robotic technology worldwide. Despite that, the procedure of RALUR is still lagging behind other robotic procedures due to technical difficulties and limitations of the use of the Da Vinci system in infants and toddlers. These limitations include: the manufacturer’s recommended distance between each port is 8 cm; the larger size of the robotic instruments 8 mm, compared to laparoscopic instruments which are smaller at 3 mm [35]. Now with the introduction of the Da Vinci SP system, some of these challenges in paediatric robotic surgery will hopefully be addressed.
A total of six studies containing more than 7000 children with primary VUR were included in a recent meta-analysis of robotic vs. OUR from 2017 [36]. The results showed significantly longer operative time for RALUR than OUR (median difference (MD) 66.69 min, 95% CI 41.71–91.67, P < 0.00001). On the contrary the RALUR group had significantly fewer days of hospital stay (MD −17.80 h, 95% CI −21.18 to −14.42, P < 0.00001) and shorter duration of postoperative catheter placement (MD −0.32 days, 95% CI −0.57 to −0.07, P = 0.01). No significant differences were found in estimated blood loss (EBL), success rate, complications, and postoperative analgesia usage between the two groups. In subgroup analyses, a significantly higher rate of short-term postoperative complications in RALUR was found compared with OUR (OR 3.17, 95% CI 1.72–5.85, P = 0.0002) [24, 34, 37,38,39,40].
Evidence to support minimally invasive robotic surgery for ureteral reimplantation has gained wide acceptance in recent years. However most of the published studies report a relatively low volume of patients undergoing RALUR.
The recently published paper from Buffi et al. [41] in 2017 reports their multi-institutional and multinational experience of robotic surgery for upper and lower ureteric pathology. Overall, 21 out of 183 underwent RALUR. The median operative time was 165 min (range 90–255). The median length of stay (LOS) was 5 days (range 4–30), the median drain removal 3 days (range 1–29) and median catheter removal was 4 days [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Two patients (9.5%) had high-grade complication (Clavien-Dildo >III). The median follow-up of the entire cohort was 24 months, with a success rate of >90%.
On the same direction Hemal et al. [42] reported 18 distal ureteral procedures. The mean operative time was 137.9 min (range: 70–240). Mean blood loss was 98.2 mL (range: <50–400). There were no urine leaks. Mean drain tube removal time was 1.4 days (range: 1–2.5) and mean LOS 2.4 days (range: 1–6). Complications included 2 cases both infection related. Average follow-up period was 13.5 months. Operative success as defined by symptom resolution and imaging was 100%.
Patil et al. [43] reported the results of three multinational institutions. A total of 12 patients were included. The mean operative time was 208 min (80–360 min), the mean EBL was 48 mL (45–100 min) and the mean LOS was 4.3 days (2–8 days). After a mean follow-up of 15.5 months the patients were symptoms free.
The largest comparative study including open, laparoscopic and robotic case was reported in 2014 by Elsamra et al. [44]. There were 130 cases in the series, of those 20 cases were robotic, 85 were laparoscopic and 25 were open. Operative time was similar across all cohorts (235–257 min, p = 0.123); EBL was significantly lower in the robotic and laparoscopic groups relative to open approach (100 vs. 150 vs. 300 mL, respectively p = 0.001). No intraoperative complications or conversions were identified in the robotic or open groups. Conversely, the rate of intraoperative complications and conversion in the laparoscopic group was 4.7% and 2.4%, respectively. Median LOS was significantly shorter in the minimally invasive cohorts compared to open (p < 0.002). The open group showed the highest rate of major postoperative complications (10–20%) and failure rates (5.9–16%), but these were without statistical significance in these series.
In conclusion the robotic platforms have provided an impetus for innovation in surgery without compromising patient safety and surgical outcomes.
In the field of distal ureteral reimplantation, it has provided the benefits of minimal blood loss, shorter length of hospital stay, less pain and better cosmetic results. However, this is at the expense of robust robotic surgical training and significant expertise.
References
Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839–43.
Smith AD. Management of iatrogenic ureteral strictures after urological procedures. J Urol. 1988;140:1372–4.
Leonardo CR, Filgueiras MFT, Vasconcelos MM, Vasconcelos R, Marino VP, Pires C, et al. Risk factors for renal scarring in children and adolescents with lower urinary tract dysfunction. Pediatr Nephrol. 2007;22:1891–6.
Colen J, Docimo SG, Stanitski K, Sweeney DD, Wise B, Brandt P, et al. Dysfunctional elimination syndrome is a negative predictor for vesicoureteral reflux. J Pediatr Urol. 2006;2:312–5.
Dias CS, Silva JMP, Diniz JSS, Lima EM, Marciano RC, Lana LG, et al. Risk factors for recurrent urinary tract infections in a cohort of patients with primary vesicoureteral reflux. Pediatr Infect Dis J. 2010;29:139–44.
Hodson EM, Wheeler DM, Vimalchandra D, Smith GH, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2007. https://doi.org/10.1002/14651858.CD001532.pub3.
Williams G, Wei L, Lee A, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD001534.pub2.
Elder JS, Diaz M, Caldamone AA, Cendron M, Greenfield S, Hurwitz R, et al. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. J Urol. 2006;175:716–22.
Visco A. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97:685–92.
Gilmour DT, Das S, Flowerdew G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet Gynecol. 2006;107:1366–72.
Wu HH, Yang PY, Yeh GP, Chou PH, Hsu JC, Lin KC. The detection of ureteral injuries after hysterectomy. J Minim Invasive Gynecol. 2006;13:403–8.
Delacroix SE, Winters JC. Urinary tract injures: recognition and management. Clin Colon Rectal Surg. 2010;23:104–12.
Pokala N, Delaney CP, Kiran RP, Bast J, Angermeier K, Fazio VW. A randomized controlled trial comparing simultaneous intra-operative vs sequential prophylactic ureteric catheter insertion in re-operative and complicated colorectal surgery. Int J Color Dis. 2007;22:683–7.
Johnson DB, Pearle MS. Complications of ureteroscopy. Urol Clin North Am. 2004;31:157–71.
Jhaveri JK, Penna FJ, Diaz-Insua M, Jeong W, Menon M, Peabody JO. Ureteral injuries sustained during robot-assisted radical prostatectomy. J Endourol. 2014;28:318–24.
Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94:277–89.
Chou MT, Wang CJ, Lien RC. Prophylactic ureteral catheterization in gynecologic surgery: a 12-year randomized trial in a community hospital. Int Urogynecol J. 2009;20:689–93.
Elliott SP, McAninch JW. Ureteral injuries: external and iatrogenic. Urol Clin N Am. 2006;33:55–66.
Lucarelli G, Ditonno P, Bettocchi C, Grandaliano G, Gesualdo L, Selvaggi FP, et al. Delayed relief of ureteral obstruction is implicated in the long-term development of renal damage and arterial hypertension in patients with unilateral ureteral injury. J Urol. 2013;189:960–5.
Lee Z, Moore B, Giusto L, Eun DD. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur Urol. 2015;67:291–8.
Bjurlin MA, Gan M, McClintock TR, Volpe A, Borofsky MS, Mottrie A, et al. Near-infrared fluorescence imaging: emerging applications in robotic upper urinary tract surgery. Eur Urol. 2014;65:793–801.
Zhao LC, Weinberg AC, Lee Z, Ferretti MJ, Koo HP, Metro MJ, et al. Robotic ureteral reconstruction using buccal mucosa grafts: a multi-institutional experience. Eur Urol. 2018;73:419–26.
Peters CA, Faap F, Woo R. Intravesical robotically assisted bilateral ureteral reimplantation. J Endourol. 2005;19:618–22.
Marchini GS, Hong YK, Minnillo BJ, Diamond DA, Houck CS, Meier PM, et al. Robotic assisted laparoscopic ureteral reimplantation in children: case matched comparative study with open surgical approach. J Urol. 2011;185:1870–5.
Chan KWE, Lee KH, Tam YH, Sihoe JDY. Early experience in robotic-assisted laparoscopic bilateral intravesical ureteral reimplantation for vesicoureteral reflux in children. J Robot Surg. 2012;6:259–62.
Gregoir W. Congenital vesico-ureteral reflux. Urol Int. 1964;18:122–36.
Riedmiller H, Gerharz EW. Antireflux surgery: Lich-Gregoir extravesical ureteric tunnelling. BJU Int. 2008;101:1467–82.
Peters CA. Robotically assisted surgery in pediatric urology. Urol Clin North Am. 2004;31:743–52.
Silay MS, Baek M, Koh CJ. Robot-assisted laparoscopic extravesical ureteral reimplantation in children: top-down suturing technique without stent placement. J Endourol. 2015;29:864–6.
Orvieto MA, Large M, Gundeti MS. Robotic paediatric urology. BJU Int. 2012;110:2–13.
Gundeti MS, Kojima Y, Haga N, Kiriluk K. Robotic-assisted laparoscopic reconstructive surgery in the lower urinary tract. Curr Urol Rep. 2013;14:333–41.
Schimpf MO, Wagner JR. Case report: robot-assisted laparoscopic Boari flap ureteral reimplantation. J Endourol. 2008;22:2691–4.
Politano VA, Leadbetter WF. An operative technique for the correction of vesicoureteral reflux. J Urol. 2017;197(2S):S94–S100.
Bowen DK, Faasse MA, Liu DB, Gong EM, Lindgren BW, Johnson EK. Use of pediatric open, laparoscopic and robot-assisted laparoscopic ureteral reimplantation in the United States: 2000 to 2012. J Urol. 2016;196:207–12.
Bruns NE, Soldes OS, Ponsky TA. Robotic surgery may not “make the cut” in pediatrics. Front Pediatr. 2015;3:10.
Deng T, Liu B, Luo L, Duan X, Cai C, Zhao Z, et al. Robot-assisted laparoscopic versus open ureteral reimplantation for pediatric vesicoureteral reflux: a systematic review and meta-analysis. World J Urol. 2018;36:819–28.
Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol. 2011;185:1876–81.
Schomburg JL, Haberman K, Willihnganz-Lawson KH, Shukla AR. Robot-assisted laparoscopic ureteral reimplantation: a single surgeon comparison to open surgery. J Pediatr Urol. 2014;10:875–9.
Arlen AM, Broderick KM, Travers C, Smith EA, Elmore JM, Kirsch AJ. Outcomes of complex robot-assisted extravesical ureteral reimplantation in the pediatric population. J Pediatr Urol. 2016;12:169–e1.
Kurtz MP, Leow JJ, Varda BK, Logvinenko T, Yu RN, Nelson CP, et al. Robotic versus open pediatric ureteral reimplantation: costs and complications from a nationwide sample. J Pediatr Urol. 2016;12:408–e1.
Buffi NM, Lughezzani G, Hurle R, Lazzeri M, Taverna G, Bozzini G, et al. Robot-assisted surgery for benign ureteral strictures: experience and outcomes from four tertiary care institutions. Eur Urol. 2017;71:945–51.
Hemal AK, Nayyar R, Gupta NP, Dorairajan LN. Experience with robot assisted laparoscopic surgery for upper and lower benign and malignant ureteral pathologies. Urology. 2010;76:1387–93.
Patil NN, Mottrie A, Sundaram B, Patel VR. Robotic-assisted laparoscopic ureteral reimplantation with psoas hitch: a multi-institutional, multinational evaluation. Urology. 2008;72:47–50.
Elsamra SE, Theckumparampil N, Garden B, Alom M, Waingankar N, Leavitt DA, et al. Open, laparoscopic, and robotic ureteroneocystotomy for benign and malignant ureteral lesions: a comparison of over 100 minimally invasive cases. J Endourol. 2014;28:1455–9.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wisz, P., Penkoff, P., Palagonia, E., Mottrie, A., Dell’Oglio, P. (2021). Robot-Assisted Ureteral Reimplantation. In: Gharagozloo, F., Patel, V.R., Giulianotti, P.C., Poston, R., Gruessner, R., Meyer, M. (eds) Robotic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-53594-0_101
Download citation
DOI: https://doi.org/10.1007/978-3-030-53594-0_101
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53593-3
Online ISBN: 978-3-030-53594-0
eBook Packages: MedicineMedicine (R0)