Abstract
It has become increasingly evident that the diversity of adipose tissue types may play distinct and potentially important roles in human physiology. White adipose tissue (WAT), the primary energy storage tissue, is important in homeostasis but when found in excess can predispose individuals to severe insulin resistance and diabetes. Human life is compatible with WAT composing between 4 and 60% of total body mass, pointing to its incredible adaptability. In contrast, brown adipose tissue (BAT), typically thought to occur only in hibernating animals and babies, is becoming recognized for its role in human energy expenditure, hormone production, immune regulation, among others. BAT usually comprises around 0–2% of total body mass; however, recent scientific advances in noninvasive imaging and molecular biology have allowed to explore unknown functions of this dynamic tissue. We discuss here the anatomy and physiology of WAT and BAT in the lean and obese human, and potential mechanisms regarding the interplay within obesity and Type 2 diabetes. Later, we review the current methodology for measuring and detecting WAT and BAT, how to experimentally modulate BAT activity, as well as future investigations to yield greater insight into BAT’s functional role in human health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
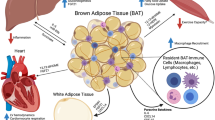
Distribution and Functions of Human Adipose Tissue
It is estimated that in 2025, global obesity rates will reach 18% in men and 21% in women (NCD Risk Factor Collaboration 2016), with diabetes prevalence reaching nearly 400 million people globally (Nathan 2015). Obesity, defined clinically by a body mass index (BMI) of greater than 30 kg/m2, is accompanied by an excess of white adipose tissue (WAT). Obesity is the result of long-term energy intake exceeding energy expenditure, being one of the main comorbidities Type 2 Diabetes (T2D). A diagnosis of T2D is most often made with a glycosylated hemoglobin (HbA1C) of ≥6.5% or a fasting plasma glucose of ≥126 mg/dl (Association, A. D. 2 2019). T2D is more likely to be obesity-related and can progress to loss of pancreatic beta-cell insulin secretion, often in the background of insulin resistance. Because the risk for T2D is increased substantially by obesity, the mechanisms that we will discuss throughout the chapter will pertain to both obesity and T2D, unless stated otherwise.
White Adipose Tissue
Humans have several types of adipose tissue, although the most studied are white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is normally distributed subcutaneously and in the visceral compartments, the latter known as visceral adipose tissue (VAT). The amount of WAT in humans varies a lot between individuals, with minimal levels sustainable with life around 2% of body mass for men and 6% of body mass for women, and maximal levels reaching over 60% of body mass. In simple terms, the higher the BMI, the higher the WAT accumulation in both compartments. Subcutaneous WAT is found in all areas beneath the skin surface but has a propensity to be stored in central, or proximal, locations. The distribution of subcutaneous WAT differs between men, who typically have an “android” distribution and women, with a “gynoid” distribution (Min and Min 2015).
VAT is also found in central locations, however within the abdominal cavity, surrounding vital organs. VAT contributes a much smaller percentage to total body fat (~5% in men and ~3% in women) (Sasai et al. 2015), yet the accumulation of VAT confers greater risk for T2D and cardiovascular disease than subcutaneous WAT. This phenomenon partly explains the occasional metabolically unhealthy, normal weight phenotype, where individuals that do not have BMIs classified as obese suffer from metabolic syndrome comparable to individuals with much higher body mass. Some data show that physical exercise can selectively reduce VAT, even in the absence of significant weight loss.
As WAT is a tissue for energy storage, it is evolutionarily designed with the ability to expand to a great extent, so that high energy density lipids can be available even in times of food shortage. In the modern era with food shortage being less of a concern, this expansion has occurred without periods of energy deficit. Though WAT exists in excess in patients with obesity, WAT in itself is not causative of insulin resistance (the inability for insulin to perform its usual functions). Lessons from patients with lipodystrophy demonstrate this fact, as they have nearly nonexistent WAT, but often develop severe T2D. The contributions of excess adiposity to diabetes may be a result of adipose-derived cytokines (adipokines) or accumulation of ectopic lipids in skeletal muscle and the liver that disrupt insulin signaling. Much has been learned since the discovery of WAT as an endocrine organ (the discovery of leptin, as an adipokine) (Zhang et al. 1994), yet there is still much to be investigated.
Brown Adipose Tissue
The main function of BAT is to generate heat (thermogenesis), principally through dissipation of the proton gradient in the inner mitochondrial membrane by way of uncoupling protein 1 (UCP1) (Cannon and Nedergaard 2004). Thus, as fuel sources including glucose and fatty acids are utilized by the brown adipocyte, rather than the generation of adenosine triphosphate (ATP), heat is released, resulting in energy-depleting thermogenesis. BAT is extremely important in maintaining euthermia in the hibernating animal during prolonged periods of fasting in cold temperatures.
Though the depots of human BAT were extensively defined nearly 50 years ago by autopsy (Heaton 1972), advances in noninvasive imaging have brought a resurgence in the interest in BAT. Classically thought only to exist in the interscapular adipose tissue region of human newborns, 18F-Fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) combined with X-ray computed tomography (CT) studies have shown that active BAT exists in a large portion of the adult population, and can be reliably activated by cold (Cypess et al. 2009; van Marken Lichtenbelt et al. 2009) and by other means. Indeed, over 450 publications on PubMed have studied adult BAT in humans since just 2008. BAT’s functions include substrate metabolism, immune system modulation, endocrine functions, and others. To begin a thorough understanding of BAT function in adults, we have characterized the depots of adult men and women with and without obesity (Leitner et al. 2017; Martinez-Tellez et al. 2019a).
Anatomical Sites
Generally, BAT lines visceral locations surrounding major vasculature in the abdomen, neck, and axilla, vital organs including the kidneys and adrenal glands, and alongside the sympathetic trunk. The highest proportion of BAT exists in the supraclavicular region, making it the most commonly studied BAT depot across various studies, followed by the paraspinal, cervical, abdominal (mostly retroperitoneal), axillary, and mediastinal (Leitner et al. 2017). Previous evidence showed that newborns have huge amounts of subcutaneous BAT in the dorsocervical area, as adequate skeletal muscle has not yet been developed for shivering or activity thermogenesis. It has been thought that this subcutaneous BAT disappeared when the newborn grew older; however, recent evidence suggests that it could be still present in young healthy adults, mostly in women (Martinez-Tellez et al. 2019a; Kim et al. 2019). The discovery of this new depot will boost research.
Beige Adipose Tissue
Beige and/or brite adipose tissue has been discovered as an inducible form of brown adipose tissue, with morphological features between those of white adipocytes and brown adipocytes, however physiological functions (thermogenesis) of BAT (Wu et al. 2012; Jespersen et al. 2013) remain under intense investigation. In humans, very few studies have been able to characterize anatomical differences throughout the human body, with both functional and biochemical confirmation (Cypess et al. 2013). We have also shown that depots outside of these listed likely have very little capacity for browning or the conversion of white to brown adipocytes; however, depots currently harnessing metabolically active adipocytes comprise up to 5% of total body fat, thus making BAT an organ of appreciable size in adult humans.
Though anatomical characterization has come to higher resolution in recent years, it is unclear whether or not there are differential functions harbored by white adipocytes or brown adipocytes that exist in different regions. As most functions seem to operate at an organism-wide level, impactful studies can still be conducted without this knowledge. Yet, as noninvasive imaging and other biochemical techniques become more advanced, perhaps soon we will be able to create a functional atlas of human WAT and BAT. It is interesting that most anatomy textbooks do not have a chapter that maps adipose tissue. Maybe that points to our need to better understand adipocytes in all forms (Zwick et al. 2018).
White Adipose Tissue and Insulin Resistance
WAT can account for as little as 4% of total body mass in patients with congenital generalized lipodystrophy (Lima et al. 2016), a disease characterized by the absence of WAT, and as much as nearly 60% of body mass in patients with morbid obesity (Shah and Braverman 2012). Adipose tissue expansion by hypertrophy (increase in adipocyte size) or hyperplasia (increase in adipocyte number) is frequently followed by unfavorable metabolic consequences, such as insulin resistance and inflammation, and as such, WAT plays a major role in the development of insulin resistance.
Insulin, a major anabolic hormone, mediates an integrated metabolic response in the presence of nutrients, and insulin signaling is highly active in the adipocytes of lean and obese humans. Most importantly, insulin’s role in the white adipocyte is to suppress lipolysis and stimulate glucose uptake. In the lean individual, these functions are tightly regulated to ensure no excessive freeing of fatty acids and the disposal of glucose. However, leading up to the development of and in the setting of T2D, insulin resistance ensues, manifesting as both blunted insulin signaling in adipocytes, and reduced insulin receptor content (Petersen and Shulman 2018). Functionally, insulin’s main roles in the adipocyte, to stimulate glucose uptake and to suppress lipolysis are less effective. The effects of insulin resistance lead to an excess of plasma insulin, non-esterified fatty acids, and glucose levels, and implicates WAT as a major player in the pathogenesis of insulin resistance.
Interestingly, WAT plays a small role in whole-body glucose disposal (less than 5%), however has a significant impact on the development of insulin resistance. Several mechanisms have been proposed for WAT’s role in the pathogenesis of T2D, despite a seemingly minor participation in glucose disposal. Most convincingly, WAT dysfunction includes altered crosstalk with skeletal muscle and liver at maintaining energetic homeostasis and chronic low-grade inflammation.
Glucose Homeostasis Crosstalk
There is substantial coordination between the skeletal muscle, liver, and WAT to maintain normoglycemia in the fed and the fasted states. As humans transition to the fasted state (hours after the previous feeding), leptin and the hypothalamic–pituitary–adrenal (HPA) axis become activated to regulate WAT lipolysis, in order to free fatty acids to the plasma and to the liver for gluconeogenesis (Perry et al. 2018). This integrated response causes a whole-body shift from carbohydrate to fat oxidation. Studies have shown that this coordinated response may be altered in obese men, suggesting that nonesterified fatty acids are not released as readily, resulting in a net storage of fat. In addition, insulin’s roles in suppressing WAT lipolysis and endogenous glucose production (from the liver) become less effective, which has been suggested as a link between WAT inflammation and the development of hyperglycemia (Roden and Shulman 2019).
Inflammatory Adiposopathy
Inflammation in WAT has been characterized by inadequate tissue vascularization, fibrosis, and hypoxia, which develop in the setting of increased WAT mass and cell size. Thus, obesity and the accompanying excess of WAT has been characterized as a state of chronic low-grade inflammation, with possible impaired immune function and activation of stress pathways. Much research has been dedicated to elucidate the mechanisms of WAT inflammation, including upregulation of the inflammatory cytokines tumor necrosis factor (TNF alpha), interleukin (IL)-1B, and IL-6 within adipose depots. Evidence for treatability of obesity-induced inflammation came from a clinical trial in obese humans, where Amlexanox, an anti-inflammatory drug typically used in the treatment of asthma, improved glycemic control in a subset of obese, T2D (Oral et al. 2017). Clearly, excess WAT and obesity are accompanied by multiple mechanisms that factor into the development of insulin resistance and diabetes, yet it is important to mention that obesity is not required for the development of these conditions.
Ectopic Fat Depots
WAT is not the only player in the development of T2D. Patients with lipodystrophy lacking adipocytes, and thus all adipose-derived hormones including leptin, often become hyperphagic due to the absence of leptin’s hunger-suppressing effect. With the incapacity to store excess food intake in adipocytes, ectopic lipids are stored in skeletal muscle and the liver, sometimes to extreme extents. In the setting of massively increased ectopic lipid storage, patients without treatment often develop severe insulin resistance and poorly controlled diabetes, which highlights the role of ectopic lipids rather than excess WAT in the causal pathway of insulin resistance (Grundy 2015). It is possible that this same mechanism could underlie non-lipodystrophic patients who develop T2D, with contributions from genetics, epigenetics, and other factors.
Brown Adipose Tissue and Insulin Resistance
Preclinical models have suggested that BAT is a great tool to combat adiposity and T2D. Some of these studies have demonstrated that the transplantation of interscapular BAT from lean to obese mice, induced a significant weight loss and improved levels of HbA1C, alleviating insulin resistance. This is the main reason why this tissue has aroused a great interest in human physiology. Although human clinical studies have developed interventions, based on cold exposure, to activate and recruit the amount of BAT in obese and T2D individuals, they have failed to replicate the positive effects of preclinical models (Jensen 2015). The main reason could be that human BAT can represent as little as 1% of total body mass, whereas in preclinical models BAT reaches up to 30–40% of the total body mass.
Moreover, it is important to note that BAT consumes glucose and fatty acids, and brown adipocytes have glucose transporter 1 (GLUT1, non-insulin-dependent) and 4 (GLUT4, insulin-dependent). The most used technique to quantify BAT in humans is 18F-FDG-PET/CT scan, which is based on a glucose tracer. This technique is also used to quantify BAT in T2D. As one can imagine, in the scenario of insulin resistance this technique is far less accurate for the quantification of such tissue, which hampers understanding of the role of BAT in insulin resistance in humans.
Activation of Human Brown Adipose Tissue
The interest in BAT lies in its previously mentioned ability to uncouple ATP production from the electron transport chain, providing a means to “waste” energy in the form of heat. Studies that have attempted to examine the maximal energy expending capacity for BAT have been difficult to perform in humans, but have ranged from 5 to 730 kcal/day at maximal activation (Ruiz et al. 2018; Marlatt et al. 2018). These measures range from clinically meaningless, to having a measurable effect on reducing weight.
Because BAT is inactive in thermoneutral resting conditions, maximal thermogenesis must be measured in the active state. Researchers have attempted many approaches to BAT activation, including cold exposure, exercise, and dietary/pharmacological modulation (Ruiz et al. 2018) (see Fig. 5.1). Most often, cold exposure is used to study active BAT. This is a reasonable method to use, as it highlights BAT’s most obvious role in nature: non-shivering thermogenesis to defend against the cold. Cold is sensed by free nerve endings in the skin, and the hypothalamus begins a coordinated whole-body response to defend core body temperature: intense vasoconstriction occurs at the distal extremities to shunt warm blood to vital organs, contributing to a slowed heart rate.
This schematic figure summarizes the main issues discussed in the present chapter. 18F-FDG 18F-fluorodeoxyglucose, BAT brown adipose tissue, CT computed tomography, DEXA Dual-energy X-ray absorptiometry, FFA free fatty acids, H20 water, MRI magnetic resonance imaging, PET positron emission tomography. This image is courtesy by The Voice Of Science
Even at temperatures too low to activate detectable shivering, metabolic rate increases (up to 17% above resting in lean men and 6% above resting in obese men) (Brychta et al. 2019). The blunted response in non-shivering thermogenesis in obese men is likely due to a thicker “shell,” and perhaps greater insulation from cold exposure. This insulation requires less heat production in response to the same cold stimulus, as the heat is trapped in the core, and loss is prevented. If this is the sole mechanism for reduced BAT activity in obese men, it may be expected that pharmacological agents may yield more success for energy expenditure than what cold exposure can reveal.
Cold Exposure Techniques
Methods for studying cold-induced thermogenesis and BAT in humans include ambient cold air exposure, the wearing of liquid-perfused cooling suits, and placing the feet on ice blocks. It is uncertain whether all of these methods are comparable, yet studies suggest that the degree of BAT activation is likely reproducible when maintaining a standardized protocol (Fraum et al. 2019). All methodologies considered, BAT is quickly activated in response to cold exposure (Leitner et al. 2018), it can contribute to cold-induced energy expenditure in the absence of shivering, and BAT metabolizes a combination of both fatty acids and glucose when active.
In addition, cold-acclimation studies have demonstrated that BAT volume and metabolic activity can be increased over the course of a week in both lean and obese individuals (Lee et al. 2014; Hanssen et al. 2016). Detectable BAT is found in most obese patients, though consistently to a lesser extent than their lean counterparts. One of the only studies that has rigorously measured BAT in type 2 diabetic patients has shown that an improvement in insulin sensitivity following chronic cold exposure, can be mostly attributed to skeletal muscle adaptation rather than BAT glucose uptake (Hanssen et al. 2015), thus the utility of chronic BAT activation for energy expenditure must certainly be considered carefully. Importantly, nearly all studies that have examined subcutaneous WAT for features of BAT in response to chronic cold exposure, have yielded negative results.
Recently, a new study nicely shows that human BAT is rapidly activated upon cold exposure, achieving its maximum activity after 35 min of cold exposure (Oreskovich et al. 2019). This study was performed by a dynamic magnetic resonance imaging (MRI) scan. After 2 h of cold exposure, a warm-up phase during 30 min followed, and no replenishment of the lipid content of the BAT depots ensued. This study revealed that BAT is only active during the first part of the cooling protocols, questioning much evidence published so far. To date the most used cooling protocols to activate BAT applied a total duration of 2 h; therefore, they have quantified BAT when it was not truly activated.
Exercise Activation
Exercise is a non-pharmacological tool that induces a myriad of physiological adaptations, enhancing human cardiovascular health and insulin sensitivity in almost every single human being. Therefore, it is biologically plausible that exercise will affect BAT function, although it is unknown in which direction. Preclinical studies have suggested that exercise could increase BAT function and induce browning in almost all WAT depots (Stanford et al. 2015). However, it seems that most of these studies were not performed under strictly thermoneutral conditions, rendering it impossible to discriminate whether this browning effect is caused by the exercise, by the temperature, or by the combination of both stimuli (McKie et al. 2019).
Regarding humans, the evidence is even more contradictory. There are only a few cross-sectional studies that showed that trained-individuals had lower levels of BAT 18F-FDG uptake in comparison to sedentary controls. Moreover, we recently demonstrated that objectively measured physical activity by accelerometers and cardiorespiratory fitness protocols, were not associated with BAT 18F-FDG uptake (Acosta et al. 2019). Surprisingly, we observed that handgrip strength was positively and slightly related with BAT 18F-FDG uptake, suggesting that maybe resistance training could have a different role in terms of BAT activation in comparison to endurance training. However, much more studies are needed (Martinez-Tellez et al. 2019b). Lastly, only a few studies performed exercise interventions including BAT 18F-FDG uptake and browning markers in humans, and again the results are quite controversial.
Food Intake and Thermoregulation
Recently, we demonstrated that BAT activity is not related to energy intake assessed by an ad libitum meal and by 24-h dietary recalls (Sanchez-Delgado et al. 2020). Moreover, we did not detect the role of BAT in the regulation of appetite-related sensations either before or after the subjects ate. In a submitted study, we also observed that BAT activity was not related to the adherence to a Mediterranean diet, which is one of the healthiest diets. On the other hand, an independent study showed that the oxygen consumption of BAT depots increased after a single meal intake. It seems that BAT is not playing a role in appetite perception, energy, or nutrition intakes, although it could be playing a minimal role during meal intake.
Role of Brown Fat in Human Metabolism in Healthy, Obese, and Diabetic Individuals
One option to prevent and combat obesity and related comorbidities is to increase thermogenesis, which leads to increased energy expenditure. In mice, BAT is responsible for 20% (Blondin et al. 2017) of both resting metabolic rate and adaptive thermogenesis, i.e., cold-induced thermogenesis (CIT) and meal-induced thermogenesis (MIT) (Nathan 2015), and can account for up to 60% of total energy expenditure when fully stimulated. Human BAT becomes metabolically active upon cold exposure in most individuals. However, a recent study nicely demonstrated that the contribution to the increase observed in CIT is almost negligible (Din et al. 2016; Carpentier et al. 2018). The same research group, demonstrated that BAT was active after eating a carbohydrate-rich meal to a similar extent than during mild cold exposure, yet its contribution to the increase in MIT is quite low. These novel studies confirm that BAT is contributing to human thermogenesis, but in a lower proportion than was thought at the beginning. What is the main role of BAT in the human metabolism? After 10 years of thoughtful research in human trials and mice studies we still do not know, although several new roles have been attributed, such as a secretory organ orchestrating the shivering and non-shivering responses or immune system modulation, among others.
BAT Hormone-Like Secretions
Some specialists suggest that the role of BAT in the human metabolism is negligible because it represents only 1% of total body mass. On the other hand, humans have similarly small organs (i.e., thyroid, adrenal, pituitary) that have very important roles in metabolism, so it could be reasonably expected that BAT has unknown and relevant functions. BAT has the ability to secrete several hormones (batokines) that could coordinate/orchestrate the responses of other tissues to different stimuli. For instance, we know that when humans are exposed to cold there is an increase of the energy expenditure, which could be explained by the contribution of two thermogenic tissues (BAT and skeletal muscles) (Villarroya et al. 2017). One of the hypotheses that is open for discussion is that BAT could be releasing a set of batokines that could boost the involvement of skeletal muscle during cold exposure.
BAT has been proposed to interact with bone (via IGFBP2), WAT, brain, pancreas, heart (via FGF-21 or IL6), and liver (IGF1), and therefore, impact tissue plasticity and metabolism. A research group from Copenhagen performed the first human BAT secretome. In this particular study, they discovered that ependymin-related protein 1 (EPDR1) was a novel batokine important for brown fat commitment (Deshmukh et al. 2019). They suggest that this batokine could be involved in the regulation of human metabolism.
Correlations with Obesity and Diabetes
Originally, it has been thought that obesity is negatively related to the amount of BAT; however, we recently discovered that obesity was positively related to BAT volume in 150 young healthy adults. This controversial finding could be explained by several factors: (1) BAT normally is measured with a glucose analog. Since the individuals of our study were quite young (<25 years old), they might not have developed insulin resistance yet; (2) most studies that found a negative relation between the degree of obesity and BAT activity, were performed without cold stimulation; and (3) another possible explanation is that the prevalence of T2D or insulin resistance increases with age, and those studies that were showing the inverse relationship between the degree of obesity and BAT activity did not include age as a possible confounder.
In an alternative study, it was shown that BAT measured with glucose, in T2D patients, was diminished in comparison with a free fatty acid tracer, suggesting that BAT could be playing a role in obese and T2D individuals (Blondin et al. 2015). Moreover, there is a group of obese individuals characterized by a lower risk of obesity-related cardiometabolic complications, the so-called metabolically healthy obese (MHO) (Blüher 2010). MHO individuals have a normal metabolic profile, i.e., do not have dyslipidemia, hyperglycemia, hypertension, or T2D, whereas their counterparts that present any of these conditions are known as metabolically unhealthy obese (MUO).
In an unpublished study, we observed that MHO individuals had higher BAT activity, cold and meal-induced thermogenic responses in comparison to MUO individuals (Sánchez-Delgado 2018). We also observed that MHO perceived the temperature as colder than MUO controls (Martinez-Tellez 2018). Both groups were similar in body mass index, although they were different in terms of the amount of VAT.
Methods for Detecting Human Brown Adipose Tissue
The contribution of BAT to adult human energy expenditure, thermogenesis, and other systemic functions remains difficult to fully uncover. One major reason for this difficulty is the variety of methods used to examine BAT in vivo. In general, methods either employ a noninvasive imaging strategy or a measurement of another physiological parameter as a surrogate of BAT activity.
Monitoring of Skin Temperature
The impetus for using skin temperature as a measure of BAT activity derives from BAT’s inherent function as a thermogenic organ, which, by definition generates heat to be dissipated from the body. This heat dissipation is thought to be captured at the surface of the skin, as a reflection of heat transfer from the deeper tissues to dilated blood vessels superficial to BAT. The inherent shortcoming of this technique is that skin vasculature is impacted by a number of other systemic factors, including catecholamines and nitric oxide among others, such that the blood flow beneath a skin surface measurement may be reflecting physiological process unrelated to underlying BAT activity.
A major benefit of using skin temperature is the ability to capture physiological shifts in long-term bouts, free-living conditions, and during multiple environmental settings (such as exercise, and entering cold or warm rooms). Skin temperature is commonly measured with either infrared thermography, where photos are taken of exposed skin, or with surface temperature probes like iButton chips (www.maximintegrated.com). As supraclavicular BAT is detected in the highest proportion of individuals, and because it is the depot least obstructed by other structures such as bone and muscle, infrared photos and surface probes often capture this location on subjects.
It is important to take into account that supraclavicular fossa is a complex anatomic region that is contiguous with the neck above and axilla below (Kellman et al. 1987). There are several structures in this region, such as the scalene and omohyoid muscles, subclavian vessels, and brachial plexus. Other contents of the supraclavicular fossa include small branches of the subclavian vessels, fat, lymph nodes, and the posterior lung apex. The benefits of supraclavicular skin temperature measurements are the ease of use, minimal discomfort to study participants, the ability to be employed in a variety of settings, and relatively low cost. However, observers still deal with the inability to rule out other physiological factors that influence skin blood flow and therefore temperature (Jimenez-Pavon et al. 2019; Martinez-Tellez et al. 2019c, d).
Radiolabeled Glucose Analog
The most commonly used technique for detecting BAT is 18F-FDG PET/CT, which utilizes a radioactive glucose analog coupled with anatomic and radiodensity data from X-ray computed tomography. This technique has likely been studied most due to a number of factors including active BAT’s avidity for glucose, the current availability in many clinical settings because of its utility in cancer staging, and the largest breadth of preprocessing and image processing protocols associated with it. In general, subjects undergo a protocol in order to activate BAT (cold exposure, dietary or physical intervention, pharmacological intervention) and are then injected with the 18F-FDG glucose analog tracer. The tracer accumulates in metabolically active tissue by entering the cell and becoming phosphorylated by hexokinase, then trapped as 18F-FDG-6-phosphate.
The concentration of the trapped glucose analog can be quantified as a standardized uptake value (SUV) relative to the subject’s body weight or lean mass and represents a reflection of tissue-specific glucose uptake. Static 18F-FDG PET/CTs give no information regarding metabolic flux rates, nor the fate of glucose within the tissue (whether or not the tissue utilizes the glucose for glycolysis, oxidative phosphorylation, or anaplerosis), which constitute some of the method’s largest limitations for studying in vivo physiology. Though 18F-FDG still provides rich information about whole-body metabolic activity, it is important that a CT scan accompanies the PET scan.
The CT scan is necessary to determine the density and structure of the underlying tissue.
Attempts to classify metabolically active tissue (obtained from PET) as BAT requires that the CT detects this tissue within the range of densities known to be adipose tissue. On CT alone, brown and white adipose tissue have nearly indistinguishable densities, with BAT being slightly denser due to its higher vascularization, lower lipid content, and higher mitochondrial content. Thus, CT alone cannot reliably distinguish WAT from BAT. Similarly, metabolic activity from the PET scan cannot differentiate any tissue from another, as the only output is tracer accumulation. Only the combination of the density of adipose tissue (from CT) and the metabolic activity above a certain threshold (from PET) are able to quantify active BAT (Fig. 5.1).
Tracer and Imaging Alternatives
The goal of these alternatives, such as fatty acids, is to reduce radioactivity exposure to subjects (i.e., with MRI), or examine metabolic flux rates (i.e., with dynamic PET/CT imaging). Studies from animal models have suggested that the primary fuel for BAT thermogenesis is lipid, thus a glucose tracer misses much of the information. Tracers to measure fatty acid uptake (Blondin et al. 2017) have been used, and labeled water (Muzik et al. 2013) can also be considered to examine adipose tissue perfusion. MRI has been used both on its own or in combination with PET, in order to detect BAT. MRI can be used in many ways in order to examine features of living tissue, and most commonly fat fraction has been employed, in order to distinguish WAT from BAT. Limitations include the possible presence of beige adipose tissue, long scanning duration in which physiological changes in BAT activation may impact the fat fraction and very few validation studies to date (Rasmussen et al. 2013). Dynamic PET imaging holds promise but awaits further validation. In summary, all noninvasive imaging methods have positive and negative aspects, none of which outperforming all others in accuracy.
Immunohistochemical or Gene-Expression Confirmation
Ideally, evidence from all studies should be complemented with immunohistochemical evaluation of BAT, WAT, or beige adipose tissue markers from biopsy (Cypess et al. 2013). Due to the technical complexity and discomfort of obtaining adipose tissue biopsies from deep locations, these studies are quite limited. Additionally, not all studies that have performed adipose tissue biopsies in the abdomen have found expression of thermogenic genes or presence of browning in WAT, even after a cold exposure intervention, which is the best stimulus to activate BAT.
Future Investigations into Human Brown Adipose Tissue Physiology
Since 2009 researchers have been looking for alternative strategies to activate human BAT without exposing participants to cold, which is not very comfortable (Fig. 5.1).
Dietary Components
Capsinoids are substances naturally present in chili peppers and they are particularly abundant in C.annuum L. or “CH-19 Sweet” (non-pungent red pepper). Capsinoids include capsiate, dihydrocapsiate, and nordihydrocapsiate. Although capsinoids are structurally similar to capsaicin, they are 1000 times less pungent but are as potent as capsaicin in increasing thermogenesis. The thermogenic activation pathways of capsinoids include transient receptor potential cation channel (TRPV1), which have possible mechanisms of action on BAT.
Tea catechins are polyphenolic components present in green tea. The most abundant and bioactive component is epigallocatechin gallate. The thermogenic effect of tea catechins has repeatedly been shown in humans. The mechanisms of action are similar to capsinoids, via TRP channels. However, there is no solid evidence that capsinoids and tea catechins can activate and recruit BAT in humans. Therefore, studies are needed to elucidate the role of dietary components as possible BAT activators (Osuna-Prieto et al. 2019).
Pharmaceutical Compounds
Mirabegron, an antimuscarinic drug, is a β3-adrenergic receptor agonist normally used to treat overactive bladder in adults. Cypess et al. showed that the acute ingestion of 50 or 200 mg of mirabegron was able to increase the 18F-FDG uptake by BAT in young healthy adults (Baskin et al. 2018). Chronic administration of mirabegron was also able to increase the levels of BAT 18F-FDG uptake. In an alternative study, we have observed that a single dose of 200 mg of Mirabegron was not able to induce a decrease of the fat fraction of BAT measured by magnetic resonance imaging. Moreover, we observed that mirabegron did not have any effect of the lipidomic profile in humans. However, there is still a debate ongoing about whether mirabegron is directly activating human BAT. Recently, Blondin et al. demonstrated that human brown adipocytes lacked β3-receptors, suggesting that mirabegron has specificity for others β-receptors (Blondin et al. 2019).
The antidiabetic agent Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor, which increases the production of insulin and decreases the production of glucagon by the pancreas. Recently we aimed to study the long-term effect (12 weeks) of 100 mg/day of sitagliptin on the levels of BAT in overweight individuals. Although we observed that insulin resistance of the participants was attenuated, BAT 18F-FDG uptake was not affected by sitagliptin (Nahon et al. 2018). Curiously, sitagliptin induced an increase in subcutaneous WAT in the abdomen, suggesting a possible increase in browning markers. We also demonstrated that sitagliptin induced upregulation of the mitochondrial gene PGC1B in skeletal muscle.
Gut Microbiota as a New Target to Combat T2D
Case–control studies have shown differences in gut microbiota composition, diversity and function between healthy people and obese individuals, and have suggested that modifying the gut microbiota composition of obese individuals toward a healthy phenotype might be a useful therapy to combat T2D. In that respect, Plovier et al. showed that fecal microbiota transplantation (FMT) from healthy donor mice to obese recipient mice reduces adiposity and energy intake, while improving lipoprotein metabolism and glucose tolerance (Plovier et al. 2017). The bacteria Akkermansia municiphila appeared to be the cause of these beneficial effects, and it was successfully used and tested in a human trial. They also showed that modifying the gut microbiota composition had a strong impact on the parameters of cardiometabolic health in humans.
Gut microbiota regularly produces short-chain fatty acids (SCFA) (e.g., butyrate or acetate), which can play an important role in the regulation of metabolic health, mitigating T2D. Butyrate also acts on the gut–brain neural circuit to improve energy metabolism, by activating brown adipose tissue (BAT) in APOE*3-Leiden.CETP male mice (Li et al. 2018). Further studies are needed to confirm this hypothesis in a human setting.
Future Perspectives
-
BAT is present in obese and T2D individuals but its role is unknown.
-
Cooling protocols for BAT activation may not be longer than 1 h based on the new evidence, which shows that BAT could be only activated during the first 35 min of cold exposure.
-
BAT depots are not easily biopsied. However, several studies suggest that humans could have a subcutaneous BAT in the dorsocervical area, which is an accessible depot. The confirmation of this finding could mean a step forward in the field.
-
Nuclear medicine techniques should be further developed in order to better quantify the thermogenic activity of BAT and WAT during dynamic processes. This new technique could be applied to cold exposure, but also to acute responses such as meal-intake, drugs, or even exercise interventions.
-
Supraclavicular skin temperature should be interpreted as the result of several thermogenic tissues and blood vessels that are activated in response to the stimulus, not only BAT activation. The fact that supraclavicular skin temperature did not decrease after a cold exposure is disturbing, and further studies are needed that help to understand which are the main tissues that could be explaining this lack of a decrease.
-
There is a lack of translational models’ studies, promoting the transition of findings from preclinical to human studies.
References
Acosta FM et al (2019) Association of objectively measured physical activity with brown adipose tissue volume and activity in young adults. J Clin Endocrinol Metab 104:223–233
Association, A. D. 2 (2019) Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 42:S13–S28
Baskin AS et al (2018) Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3-adrenergic receptor agonist. Diabetes db180462. https://doi.org/10.2337/db18-0462
Blondin DP et al (2015) Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64:2388–2397
Blondin DP et al (2017) Inhibition of intracellular triglyceride lipolysis suppresses cold-induced brown adipose tissue metabolism and increases shivering in humans. Cell Metab 25:438–447
Blondin DP et al (2019) Human BAT thermogenesis is stimulated by the β 2-adrenergic receptor. https://papers.ssrn.com/abstract=3440265
Blüher M (2010) The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 21:38
Brychta RJ et al (2019) Quantification of the capacity for cold-induced thermogenesis in young men with and without obesity. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2019-00728
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
Carpentier AC et al (2018) Brown adipose tissue energy metabolism in humans. Front Endocrinol 9:447
Cypess AM et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
Cypess AM et al (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19:635–639
Deshmukh AS et al (2019) Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab 30:963–975.e7
Din UM et al (2016) Human brown adipose tissue [(15)O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging 43:1878–1886
Fraum TJ et al (2019) Repeatability of quantitative brown adipose tissue imaging metrics on positron emission tomography with 18F-fluorodeoxyglucose in humans. Cell Metab 30:212–224.e4
Grundy SM (2015) Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest 45:1209–1217
Hanssen MJW et al (2015) Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21:863–865
Hanssen MJW et al (2016) Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 65:1179–1189
Heaton JM (1972) The distribution of brown adipose tissue in the human. J Anat 112:35–39
Jensen MD (2015) Brown adipose tissue – not as hot as we thought. J Physiol 593:489–490
Jespersen NZ et al (2013) A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17:798–805
Jimenez-Pavon D et al (2019) Infrared thermography for estimating supraclavicular skin temperature and BAT activity in humans: a systematic review. Obes Silver Spring Md 27:1932–1949
Kellman GM et al (1987) MR imaging of the supraclavicular region: normal anatomy. AJR Am J Roentgenol 148:77–82
Kim K et al (2019) Whole body and regional quantification of active human brown adipose tissue using 18F-FDG PET/CT. JoVE J Vis Exp e58469. https://doi.org/10.3791/58469
Lee P et al (2014) Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63:3686–3698
Leitner BP et al (2017) Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci 114:8649–8654
Leitner BP et al (2018) Kinetics of human brown adipose tissue activation and deactivation. Int J Obes 1. https://doi.org/10.1038/s41366-018-0104-3
Li Z et al (2018) Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 67:1269–1279
Lima JG et al (2016) Clinical and laboratory data of a large series of patients with congenital generalized lipodystrophy. Diabetol Metab Syndr 8:23
Marlatt KL, Chen KY, Ravussin E (2018) Is activation of human brown adipose tissue a viable target for weight management? Am J Physiol-Regul Integr Comp Physiol 315:R479–R483
Martinez-Tellez B (2018) Role of brown adipose tissue on the thermoregulatory system and physical fitness: the Actibate study. Universidad de Granada
Martinez-Tellez B et al (2019a) Evidence of high 18F-fluorodeoxyglucose uptake in the subcutaneous adipose tissue of the dorsocervical area in young adults. Exp Physiol 104:168–173
Martinez-Tellez B, Sanchez-Delgado G, Amaro-Gahete FJ, Acosta FM, Ruiz JR (2019b) Relationships between cardiorespiratory fitness/muscular strength and 18 F-fluorodeoxyglucose uptake in brown adipose tissue after exposure to cold in young, sedentary adults. Sci Rep 9:1–9
Martinez-Tellez B et al (2019c) Concurrent validity of supraclavicular skin temperature measured with iButtons and infrared thermography as a surrogate marker of brown adipose tissue. J Therm Biol 82:186–196
Martinez-Tellez B et al (2019d) Supraclavicular skin temperature measured by iButtons and 18F-fluorodeoxyglucose uptake by brown adipose tissue in adults. J Therm Biol 82:178–185
McKie GL et al (2019) Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice. J Physiol 597:4581–4600
Min K-B, Min J-Y (2015) Android and gynoid fat percentages and serum lipid levels in United States adults. Clin Endocrinol (Oxf) 82:377–387
Muzik O et al (2013) 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54:523–531
Nahon KJ et al (2018) Effect of sitagliptin on energy metabolism and brown adipose tissue in overweight individuals with prediabetes: a randomised placebo-controlled trial. Diabetologia. https://doi.org/10.1007/s00125-018-4716-x
Nathan DM (2015) Diabetes: advances in diagnosis and treatment. JAMA 314:1052–1062
NCD Risk Factor Collaboration (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. The Lancet 387:1377–1396
Oral EA et al (2017) Inhibition of IKKɛ and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab 26:157–170.e7
Oreskovich SM et al (2019) MRI reveals human brown adipose tissue is rapidly activated in response to cold. J Endocr Soc 3:2374–2384
Osuna-Prieto FJ et al (2019) Activation of human brown adipose tissue by capsinoids, catechins, ephedrine, and other dietary components: a systematic review. Adv Nutr Bethesda Md 10:291–302
Perry RJ et al (2018) Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell 172:234–248.e17
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98:2133–2223
Plovier H et al (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23:107–113
Rasmussen JM et al (2013) Brown adipose tissue quantification in human neonates using water-fat separated MRI. PLoS One 8:e77907
Roden M, Shulman GI (2019) The integrative biology of type 2 diabetes. Nature 576:51–60
Ruiz JR et al (2018) Role of human brown fat in obesity, metabolism and cardiovascular disease: strategies to turn up the heat. Prog Cardiovasc Dis. https://doi.org/10.1016/j.pcad.2018.07.002
Sánchez-Delgado G (2018) Brown adipose tissue and exercise: implications on human energy balance and metabolism. The Actibate study. Universidad de Granada
Sanchez-Delgado G et al (2020) Brown adipose tissue volume and 18F-fluorodeoxyglucose uptake are not associated with energy intake in young human adults. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqz300
Sasai H et al (2015) Does visceral fat estimated by dual-energy X-ray absorptiometry independently predict cardiometabolic risks in adults? J Diabetes Sci Technol 9:917–924
Shah NR, Braverman ER (2012) Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One 7:e33308
Stanford KI, Middelbeek RJW, Goodyear LJ (2015) Exercise effects on white adipose tissue: Beiging and metabolic adaptations. Diabetes 64:2361–2368
van Marken Lichtenbelt WD et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Villarroya F, Cereijo R, Villarroya J, Giralt M (2017) Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13:26–35
Wu J et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376
Zhang Y et al (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425
Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV (2018) Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab 27:68–83
Acknowledgments
This chapter was made possible by support from the NIH Medical Scientist Training Program Training Grant T32GM007205 and a postdoctoral grant from the Fundación Alfonso Martin Escudero.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Leitner, B.P., Martinez-Tellez, B. (2020). White and Brown Adipose Tissue in Obesity and Diabetes. In: Faintuch, J., Faintuch, S. (eds) Obesity and Diabetes. Springer, Cham. https://doi.org/10.1007/978-3-030-53370-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-53370-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53369-4
Online ISBN: 978-3-030-53370-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)