Abstract
Renal replacement therapy (RRT) is an important component in the care of critically ill children with acute kidney injury (AKI), inborn errors of metabolism, and certain intoxications that respond inadequately to conservative measures. There are several modalities of RRT including peritoneal dialysis (PD), continual flow peritoneal dialysis (CFPD), hemodialysis (HD), sustained low-efficiency dialysis (SLED), and continuous renal replacement therapy (CRRT). Each of these modalities has its own inherent advantages and risks, and the clinical situation will help guide the most appropriate approach for the individual patient. Peritoneal dialysis allows for both solute clearance and ultrafiltration. However, PD is suboptimal therapy for patients with life-threatening hyperkalemia, severe volume overload, or intoxications that would benefit from rapid ultrafiltration or solute clearance. In those settings, intermittent HD would provide a more effective modality. CRRT is a common mode of RRT utilized in the pediatric intensive care unit. There are three primary forms of CRRT including continuous veno-venous hemofiltration (CVVH; convective clearance), continuous veno-venous hemodialysis (CVVHD; diffusive clearance), and continuous veno-venous hemodiafiltration (CVVHDF) which is a combination of convective (CVVH) and diffusive (CVVHD) clearance. CRRT is frequently used in conjunction with extracorporeal membrane oxygenation (ECMO) therapy. Sustained low-efficiency dialysis (SLED) represents a hybrid between HD and CRRT. Although it has been used commonly in adults for over two decades, there is very little experience in pediatrics. A sound understanding of the various forms of RRT and their use in critical illness is essential.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
To recognize the main indications for renal replacement therapy
-
To identify the various modalities of renal replacement therapy
-
To understand the fundamental working principles of each modality of renal replacement therapy
-
To understand the advantages and the associated risks of each individual modality of renal replacement therapy
-
To appreciate the appropriate access needed for renal replacement therapy
-
To understand the various forms of continuous renal replacement therapy and their appropriate application to clinical situations
1 Introduction
Renal replacement therapies (RRT) have been used for more than three decades in children. The primary indications for RRT include acute kidney injury (AKI), inborn errors of metabolism, and intoxications (◘ Table 32.1). This book chapter will describe the modalities used throughout the world and address the risk and benefits of each one. Additionally, insight will be offered regarding the optimal modality of therapy based on the underlying indication for RRT. The modalities of RRT described in this chapter include peritoneal dialysis (PD), continual flow peritoneal dialysis (CFPD), hemodialysis (HD), sustained low-efficiency dialysis (SLED), and continuous renal replacement therapy (CRRT).
2 Peritoneal Dialysis (PD)
Peritoneal dialysis (PD) is considered a standard throughout the world for AKI therapy. PD has been used for more than three decades in children. The equipment required for PD includes peritoneal access for PD delivery, dialysate solutions, and tubing.
2.1 PD Access
PD access can be cuffed or non-cuffed. Cuffed access can classically be single cuffed or double cuffed, with either a straight configuration or a swan neck configuration with a curl at the end. These catheters are typically placed by surgical colleagues with the openings of the catheter positioned in the pelvis area for maximum flow. An omentectomy is commonly performed in this setting in order to avoid adherence of the omentum to the peritoneal dialysis catheter.
Acute access is typically non-cuffed. There are many companies that manufacture catheters that can be placed using the Seldinger technique at the bedside. These catheters are usually placed below the umbilicus and can be positioned in the left or right lower quadrant of the peritoneum. These catheters can be placed by physical exam or by ultrasound guidance based on the experience of the individual placing the catheter. In other parts of the world, sterile catheters including chest tubes, feeding catheters, as well as intravenous catheters have been used for acute peritoneal dialysis.
2.2 Solutions for PD
Solutions for peritoneal dialysis in the United States are lactate based. Outside of the United States, options for PD solutions include bicarbonate-based solutions. The disadvantage of a lactate-based solution primarily occurs in the patient with compromised hepatic metabolism in which an acidosis and/or an elevated lactate level may occur as the liver is not able to sufficiently metabolize the lactate to bicarbonate. Outside of the United States, bag technology has allowed for improvement in the delivery of bicarbonate-based solutions at the bedside. Classically, the components of PD solutions are mildly hyponatremic (Na of 132 mEq/dL) and contain no potassium or phosphorous. Additional electrolytes can be added based on the individual needs of the patient.
2.3 PD Tubing
Peritoneal dialysis can be performed using either a manual or an automated system. The automated system (e.g., Fresenius and Baxter) allows for the delivery of PD solutions via a machine. Automated systems may be complicated by a large “dead space” in the PD tubing, thereby affecting dialysis efficiency. Manual PD systems such as the Pedialyte (Gesco Utah Medical) can be used easily at the bedside. These systems utilize the buretrol infusion and drainage system so accurate inputs and outputs can be determined. The advantage of this system is that there is only 6 mL of dead space as opposed to the other PD systems which may have dead space three to four times larger.
2.4 Heating Units in PD
Heaters are incorporated into the automated systems. In contrast, there is no heater in the manual system. Therefore, it is critical to assure that hypothermia does not occur at the time of PD.
2.5 Initiation of PD
At the initiation of PD, it is not unusual that the fluid coming out of the peritoneal cavity may be full of fibrin or blood. Therefore, the standard practice is to place 250–500 units of heparin per liter into the PD solution. This will allow for diminished fibrin formation without resulting in systemic heparinization.
2.6 Antibiotics in PD
Antibiotics may be given intraperitoneally for peritonitis and be maintained systemically if necessary. In a patient with no residual renal function, the only loss of antibiotics will occur either via hepatic metabolism or by loss via dialysis. In those patients with renal function, the urine output will also affect the clearance of antibiotics. Classically, intraperitoneal antibiotics such as cephalosporins, vancomycin, and aminoglycosides are used for local installation into the abdomen for peritonitis. In patients who are anuric, a single dose of antibiotics may be given intravenously, and the same antibiotic may be placed intraperitoneally to maintain adequate levels in both the peritoneum and systemically in the body.
2.7 Complications of PD
Complications of PD include peritoneal leak at the site of the catheter, peritonitis, impairment of diaphragmatic movement decreasing ventilation, and drainage or movement of the peritoneal dialysis fluid from the peritoneum into the thoracic cavity. Additionally, over time, PD will result in a negative nitrogen balance, removing albumin as part of the solute clearance. Attention to these potential risk factors may minimize complications and improve outcomes. In the face of a leak at the site of the catheter, it is helpful to decrease the volume of the PD solution and to consider “gluing” the exit site of the catheter access. In the case of peritonitis, appropriate antimicrobials need to be given intraperitoneally. If fungal peritonitis occurs, optimal therapy requires removal of the PD catheter and the establishment of alternative forms of dialysis. The effect of PD upon diaphragmatic excursion as well as respiratory function is important to understand. As the abdomen fills with dialysate, the diaphragm will move upward, decreasing total pulmonary and functional residual capacity and diminishing respiratory integrity. Therefore, close monitoring of respiratory function is essential during PD therapy. Finally, there may be an efflux of peritoneal solution from the peritoneal cavity into the pleural space. If a pleural effusion develops in a child receiving PD, a simultaneous tap of the pleural effusion and an analysis of PD solution instilled in the abdomen should be performed. A similar composition in both (i.e., glucose) suggests efflux of dialysis fluid into the pleural cavity. In the face of such a hydrothorax, discontinuation of PD or a longer drain time should be considered to minimize the ongoing fluid collection in the thorax.
2.8 Solute Clearance in PD
Solute clearance in PD is based on the volume per pass, the components of the PD solution, as well as the number of cycles.
At the start of PD, each pass typically consists of only 10 mL/kg. However, over time (usually measured in weeks) that volume increases to 40–50 mL/kg per pass; solute clearance will improve with the larger volumes with better clearance of phosphorus, urea, and potassium. The number of cycles per day will also improve the clearance. Therefore, 12 hours per day will provide less clearance than 24 hours per day. Finally, the components of the PD solution will be important. For example, if the patient has a high potassium concentration, the PD solution should contain little to no potassium. In contrast, if the potassium level is low or decreasing, potassium may be added to the PD solution in order to minimize excess clearance.
2.9 Ultrafiltration of PD
Ultrafiltration of PD is classically affected by the frequency of passes, by the osmolality of the solution (as measured by the dextrose content), as well as by the length of the pass. Solute clearance is best accomplished with longer passes while ultrafiltration is best attained with shorter passes in order to maintain a high osmolality. Therefore, for a patient with fluid overload, pulmonary edema, or other systemic findings of fluid excess, a high dextrose concentration such as 4.25% dextrose will be more proper to use to facilitate ultrafiltration. In contrast, in patients with normal or hypovolemia, a 1.5 or 2.5% dextrose solution may be more appropriate to use.
3 Continuous-Flow Peritoneal Dialysis
Continuous-flow peritoneal dialysis (CFPD) was first identified by Amerling and colleagues over a decade ago. Recent and pediatric-specific work by Nourse has identified that CFPD can be performed easily at the bedside, negating some of the problems associated with standard PD. CFPD is performed using the same solutions of PD. The difference with CFPD is that two catheters are placed in the abdomen. One is an inflow and the other is an outflow catheter. By using this technique, the patient can be loaded with 10 mL/kg of dialysate and have that fluid held in the abdomen. A continuous dialysate flow of 10 mL/kg/hour on the infusion side is then initiated, while at the same time, drainage of the PD solution occurs via the outflow catheter. Clearance can be improved by increasing the intraperitoneal infusion rate. Advantages of CFPD compared to standard PD include less pulmonary effect (less diaphragmatic changes) and substantially better clearance with CFPD.
The disadvantage of CFPD is that it requires placement of two catheters in the abdomen. In theory, a double-lumen 7 Fr, non-cuffed vascular catheter may be placed in the peritoneum, and both ports of that catheter may be used to perform CFPD. Similar to standard PD, CFPD may cause problems with amino acid losses, immunoglobulin losses, hypothermia, and electrolyte disturbances. Therefore, attention to these potential complications is essential to effective dialysis and quality outcomes.
4 Hemodialysis
Hemodialysis may be performed at the bedside. It requires vascular access that can be cuffed or non-cuffed as well as the equipment for hemodialysis. It may be performed as standard or high flux. The difference between these two modalities is that standard HD removes lower-molecular-weight molecules as opposed to high-flux or high-efficiency HD which results in the removal of higher-molecular-weight products.
4.1 Vascular Access
Data to date have suggested that in the acute setting, a cuffed or non-cuffed catheter is equally effective. However, for outpatient chronic hemodialysis, a cuffed catheter is more appropriate. The vascular access, whether cuffed or non-cuffed, is proportional to the size of the child. For example, a 10 kg child may require a 7 Fr catheter while a larger child such as a 60–70 kg child would need at least an 11 Fr catheter (see recommendations at ► www.pcrrt.com and ◘ Table 32.2).
4.2 Blood Flow Rate for Hemodialysis
The blood flow rate (BFR) for HD is often started at approximately 5 mL/kg/min. This can be increased as necessary to maintain patency of the catheter as well as to minimize clotting. A contraindication of a rapid BFR is in those patients who are hyperosmolar (e.g., elevated blood urea nitrogen (BUN), sodium, or glucose concentration) and at high risk for a rapid drop in osmoles that will result in dialysis disequilibrium. Dialysis disequilibrium occurs when there is too brisk of drop in the serum osmoles of the patient potentially resulting in seizures or cerebral demyelination.
The classic scenario for dialysis disequilibrium occurs in a child with a sodium of 140 mmoles/L, a glucose of 100 mg/dL, and a BUN of 150 mg/dL. In standard or high-flux hemodialysis, the BUN may be cleared by two-thirds in the first 2–3 hours of dialysis. Consequently, if the BUN decreases acutely by 100 mg/dL, the osmoles would decrease by approximately 35 mOsm/L which would place the patient at risk for disequilibrium. Recall, plasma osmolality can be calculated with the formula 2 Na (mOsm/L) + (urea [mg/dL])/2.8 + (glucose [mg/dL])/18, where 2.8 and 18 are used to convert mg/dL to mOsm/L based on the molecular weight. Therefore, a lower BFR would be indicated in this situation and in any situation where there is a risk for dialysis disequilibrium. However, in most other settings , a higher BFR is reasonable.
4.3 Dialysate Flow Rate
The dialysate flow rate can range anywhere from 300 to as high as 800 mL/min. This is somewhat HD machine dependent. Higher dialysate flow rates, especially with a high-porosity membrane (high-flux membrane), provide greater solute clearance. In smaller children, excess solute clearance may occur, and thus, the dialysate flow rate may need to be decreased in order to have less clearance. Some children (e.g., neonates with anuria) may require dialysis 4–5 days/week due to the fact that their nutrition is liquid based. In this setting, the dialysate flow rate may need to be decreased in order to avoid hypophosphatemia.
The temperature in HD can range from 34.0 to 39.5 degrees Fahrenheit. This is important for infants particularly those having a fair amount of bedside cooling. Therefore, meticulous attention to body temperature must occur to assure that hypothermia does not occur in this setting. In those patients that are febrile, one can promote normothermia by decreasing the core temperature via the dialysis. However, the practitioner must recognize that the patient would otherwise be febrile and evaluate and treat the etiology of the hyperthermia accordingly (e.g., sepsis).
4.4 Extracorporeal Blood Volume in Hemodialysis
In performing hemodialysis , particularly in the infant and small child, it is essential to assess the extracorporeal blood volume of the circuit relative to the patient. For example, a 10 kg infant will have an intravascular blood volume of approximately 80 mL/kg or 800 mL. Therefore, if one accepts the “10% rule” that extracorporeal blood volume should not exceed 10% of the total blood volume, it would be prudent to have no more than 80 mL of blood volume extracorporeal to the patient. If the required extracorporeal blood volume is excessive, a blood prime of the circuit at the initiation of hemodialysis may be required.
However, the use of a blood prime has two problems associated with it; the first is the component of the blood itself, and the second is the potential impact upon transplantation in the future. In an acute setting, a blood prime is less concerning for transplant issues because the focus is for survival and potential reversibility, not for transplantation. In terms of blood composition, it is important to recognize that blood blank blood has a pH of 6.4, an ionized calcium 0.02 mmoles/L, and a potassium concentration that may be as high as 40 mEq/L. Therefore , it is necessary to be aware that in the first 5–10 min of initiation of blood prime in a child with AKI, a metabolic acidosis, hypocalcemia, and/or hyperkalemia may result from the components of the blood bank blood.
4.5 The Standard Prescription for Hemodialysis
A standard prescription for hemodialysis in a non-acute setting is a duration of 3–4 hours with a goal rate of ultrafiltration based on the volume status of the patient as assessed by the blood pressure as well as by the physical exam. The components of the dialysate are standard with a sodium concentration of 140 mEq/L, bicarbonate of 35 mEq/dL, a calcium level that would reflect a physiologically normal value approximating 10.5 mg/dL, and a potassium concentration that may range from 2 to 5 mEq/L. Most hemodialysis solutions contain no phosphorus, but phosphorus may be added in order to avoid hypophosphatemia during dialysis. In an acute setting, HD may be performed for a shorter or longer period of time based on the needs of the patient.
Solute clearance in HD is the greatest of all the dialysis modalities and can occur with or without ultrafiltration. Electrolyte and blood chemistry values as well as the need for medications and nutrition will determine the need for solute clearance, while the daily weight and the physiological parameters of blood pressure, oxygen requirements, and heart rate will determine ultrafiltration goals. Typically, the goal is to remove the entire historical weight gain since the last HD session over a 3- to 4-hour period of time if physiological parameters allow for that. If this amount of fluid cannot be removed in this relatively short period of time, then an alternative RRT modality may be required.
4.6 Anticoagulation in Hemodialysis
Anticoagulation in hemodialysis is usually achieved using heparin. Heparin can be initiated with a bolus of 20 U/kg followed by an infusion of 10 U/kg/hour for the duration of HD titrating to a bedside activated clotting time (ACT) of 180–200 seconds to avoid system clotting. Although this range of dosing should have minimal risk of systemic bleeding, the child needs to be monitored for this risk.
5 Sustained Low-Efficiency Dialysis
Although sustained low-efficiency dialysis (SLED) has been used commonly in adults for over two decades, there is very little experience in pediatrics. There are two pediatric studies, one out of Taiwan and one out of India, demonstrating its utility in pediatrics.
SLED is a hybrid between HD and CRRT. In the United States, SLED may only be performed in the dialysis mode, but outside of the United States, it may be used with filtration or so-called SLEDF. Dialysis and filtration can both occur in the setting of SLEDF for maximum benefit of large and small molecule removal.
SLED shares many similarities with HD. First , it requires the same vascular access. Next, the extracorporeal blood volume of SLED, much like HD, needs to be calculated carefully. Therefore, utilizing the “10% rule,” no more than 10% of the blood volume should be extracorporeal to the patient in either modality. Presently, both a low volume system for pediatrics and adult volume systems are commercially available. Additionally, the membranes used in HD and in SLED are very similar. Finally, the blood flow rate in SLED is similar to HD as well as the dialysate constituents and temperature control.
However, the two approaches differ in the duration of dialysis as well as in the dialysate flow rate. In classic HD, dialysis will run for 3–4 hours; in contrast, it is continued for 6–12 hours in SLED. In terms of the dialysate flow rate, the standard HD dialysate flow rate is approximately 500 mL/min or 30 L/hour, while in SLED or SLEDF, the dialysate will flow at only 100 mL/min or 6 L/hour.
As described above, data regarding the use of SLED in pediatrics are limited. However, data from Taiwan and India suggest that SLED is very effective in delivering the therapies needed to address AKI. However, data to date have not established a role for the use SLED in the treatment of either inborn errors of metabolism or intoxication, but those data are being generated.
6 Continuous Renal Replacement Therapy (CRRT)
Continuous renal replacement therapy (CRRT) has been used for more than two decades in children. There are three primary forms of CRRT including continuous veno-venous hemofiltration (CVVH; convective clearance), continuous veno-venous hemodialysis (CVVHD; diffusive clearance), and continuous veno-venous hemofiltration with dialysis (also referred to as continuous veno-venous hemodiafiltration (CVVHDF)) which is a combination of convective (CVVH) and diffusive (CVVHD) clearance (◘ Fig. 32.1).
The diagrams depict the setup and circuitry of the three modes of continuous renal replacement therapy. a Illustrates the setup for continuous veno-venous hemofiltration (CVVH). Blood is accessed from the patient (red) and powered through the membrane filter using a motorized blood pump. As the blood moves through the filter, the ultrafiltrate (yellow) passes through and is channeled into a collecting bag. The ultrafiltration rate must be closely monitored and adjusted based on the hemodynamic status of the patient and the fluid intake. As depicted in purple, replacement fluid is infused either pre- or post-filter. Pre-filter administration serves to dilute the blood prior to passing through the filter and thereby holds the theoretical advantage of extending filter lifetime. b Illustrates the setup for continuous veno-venous hemodialysis (CVVHD). In this mode, a dialysate solution (green) is run countercurrent to the blood flow, and replacement fluid is not used. c Illustrates the setup for continuous veno-venous hemodiafiltration (CVVHDF). As depicted in the diagram, this mode of continuous renal replacement therapy utilizes both replacement fluid and a dialysate solution
Similar to hemodialysis, CRRT machines are marketed by only a few companies. Features common to CRRT machines include an accurate ultrafiltration monitor as well as a venous and an arterial pressure monitor. Despite the provision of an internal blood warmer, maintaining normothermia can be a challenge with the CRRT machine especially in smaller children because of the disproportionate large surface area and relatively large extracorporeal volume. The use of external warming may be necessary in some instances to maintain normothermia of the patient.
CRRT extracorporeal blood volumes can range from 60 mL up to 200 mL. This is an important issue given the “10% rule” of permissible extracorporeal blood volume . Consequently, blood priming of the extracorporeal CRRT circuit is often required especially in hemodynamically compromised patients. As mentioned in the HD section, blood bank blood carries its own inherent risks of acidosis, hypocalcemia, and hyperkalemia. In addition, episodes of anaphylaxis have been reported to occur with blood priming and certain CRRT membranes as a result of the acidotic blood triggering a bradykinin reaction when it interfaces with these membranes. Although protocols to mitigate this side effect have been established, the risk can also be obviated by the use of polysulfone membranes as these have not been associated with the bradykinin reactions.
6.1 Selection of CRRT Modality
The choice of CVVH , CVVHD, or CVVHDF is, in large part, a style of practice. Work by Maxvold and colleagues identified that similar prescriptions in convection (CVVH) or diffusion (CVVHD) result in similar, if not identical, clearance of small-molecular-weight solutes such as urea or citrate. However, large-molecular-weight solutes (e.g., vancomycin) have a preferential clearance in the convective mode.
Thus, the decision of convection versus diffusion should not be based solely on style of practice but should also incorporate the sieving coefficient of the toxin or the medication to be removed. For example, vancomycin is approximately 1500 KDa and 75% protein bound. The sieving coefficient of vancomycin with CVVH is approximately 0.85, while with CVVHD, it approximates 0.75. The sieving coefficient is the ratio of the concentration of solutes in the ultrafiltrate to that of plasma. A sieving coefficient of one reflects complete permeability, while a sieving coefficient of zero reflects complete impermeability. Thus, vancomycin is more effectively cleared with CVVH rather than CVVHD.
A classic CRRT prescription includes a blood flow rate of approximately 5 mL/kg/min (although this is vascular access dependent) and a dialysate or replacement fluid rate approximating 40 mL/kg/hour or 2000–2500 mL/1.7 M2 corrected for body surface area per hour. The amount of fluid removal is based on the hemodynamic status of the patient. Although there is a tendency to try to remove as much fluid as possible in patients on CRRT, the amount and rate of fluid removal should be based on the hemodynamics of the child including the blood pressure and heart rate.
6.2 Anticoagulation in CRRT
Anticoagulation in CRRT can be accomplished using heparin, citrate, or, in some programs, prostacyclin. Examples of anticoagulation protocols can be found at the website (► www.pcrrt.com). Brophy and colleagues reported that performing CRRT without anticoagulation results in a circuit life of only 24 hours. Although their study demonstrated no difference in circuit life between citrate and heparin anticoagulation, more recent data published by Zaoral and others have suggested that citrate use is associated with longer circuit life than heparin.
Prostacyclin has also been suggested as an alternate anticoagulant for CRRT in those settings when heparin and citrate cannot be used, when they may be associated with increased risk, or when they may be ineffective, particularly in patients with an underlying coagulopathy and hepatic insufficiency. The risk of bleeding with heparin therapy is exacerbated in children with hepatic failure. The risk of citrate toxicity, with its inherent potential for metabolic, calcium, and acid-base imbalances, is increased in the setting of liver dysfunction as citrate metabolism is largely dependent on liver function. Citrate toxicity, or “lock” as it has been termed, occurs when the level of citrate exceeds the metabolism and clearance of citrate. The characteristic laboratory findings are a rising patient total calcium with a stable or even decreasing patient ionized calcium. In theory, this has the potential to impact muscle and cardiac metabolism. The treatment for citrate lock is to decrease the citrate infusion rate as well as to increase the replacement fluid and/or dialysate rate. Prostacyclin therapy is also not without risk as it can result in vasodilatation and a risk of hypotension and/or ventilation perfusion mismatching.
6.3 CRRT Use with Extracorporeal Membrane Oxygenation (ECMO)
CRRT use in conjunction with extracorporeal membrane oxygenation (ECMO) is now quite common. In this setting, CRRT may be utilized in one of two ways. In the first approach, a hemofilter is simply placed “in line” to perform slow continuous ultrafiltration (SCUF). This approach will allow for fluid clearance but will have little to no effect upon solute clearance. It may be accomplished by attaching the arterial limb to the post-ECMO pump stopcock and the venous limb to the pre-pump bladder. However, because the resistance of the oxygenator of the ECMO circuit is greater than the resistance of the hemofiltration membrane, adjustments may be needed to prevent shunting of blood away from the oxygenator. The second approach involves the use of a standard CRRT machine “in line” with the ECMO circuit. The advantage of this approach is that it has the added benefit of solute clearance as well as more accurate ultrafiltration control.
7 Nutrition Losses in Renal Replacement Therapy
Classically, PD and CFPD result in large losses of protein, amino acids, and albumin in the setting of chronic dialysis. Hemodialysis and SLED have a higher risk of mineral and vitamin losses. CRRT has been found to remove approximately one-third of the amino acids delivered as well as trace elements. Therefore, attention to nutrition and maximization of nutrition delivery in these patients are important.
8 Medication Clearance
Medication clearance is affected by the dialysis prescription, the protein binding, the molecular weight, and the distribution of the medication. In patients on CRRT, it is not unusual that vasoactive medications (e.g., epinephrine, norepinephrine, dopamine, and dobutamine) are cleared easily because they are small-molecular-weight compounds with low protein binding. Therefore, it is essential to monitor for hemodynamic compromise with the initiation of CRRT as the process may result in removal of these vasoactive medications. In particular, the proximity of the dialysis catheter to the infusion site of the vasoactive medication is important to assess. If the RRT access is in the immediate proximity of the infusion site of the vasoactive medications, the likelihood that these medications will be cleared is enhanced and the likelihood of hemodynamic compromise increased.
In comparison to CRRT, PD has less effect upon drug clearance. HD is considered the optimal therapy for drug clearance particularly in cases of intoxication (see below). Little data exist regarding medication clearance with SLED; however, it would seem to be similar to HD, but less efficient.
9 Indications
As previously described, RRT can be used to treat three primary conditions including AKI, inborn errors of metabolism, and intoxications (◘ Table 32.1). The decision of what modality to use is based, in large part, on the experience of the center and clinician as well as on the clinical condition of the patient. PD or CFPD is often utilized for the treatment of infants. Hemodynamically stable patients with minimal oxygen requirements can effectively be treated with HD or SLED. Additionally, postoperative cardiac patients frequently have a PD catheter placed in the peritoneum especially in the setting of a long cardiac pump bypass time; these infants may accumulate ascites, and the PD catheter can be used for RRT or to drain ascites. However, PD would be a suboptimal modality for patients with life-threatening hyperkalemia, severe volume overload, or intoxications that would benefit from rapid ultrafiltration or solute clearance. In those settings, intermittent HD would provide a more effective modality for rapid ultrafiltration and solute clearance. In patients with hemodynamic compromise or those who are inflamed such as hematopoietic cell transplant patients or those with sepsis, CRRT may be beneficial over other modalities of RRT. In addition, the optimal RRT modality for AKI is based on the experience of the clinician, available resources, and the experience of the bedside staff.
9.1 Inborn Errors of Metabolism
Picca has identified that all modalities of RRT including PD, HD, and CRRT can be used as clearance for ammonia. However, work by our group has identified that maximum clearance of ammonia can be achieved by HD. If the need of clearance is ongoing, the use of sequential HD followed by CRRT is the optimal way to address elevations of ammonia in infants with inborn errors of metabolism.
Although HD is challenging to perform in small infants, with close monitoring of hemodynamics as well as meticulous thermic control, HD has been performed in patients down to 2 kg. Given that these children with inborn errors of metabolism usually have normal renal function, components of the dialysate bath must be adjusted to ensure that normal physiological concentrations of electrolytes are maintained.
9.2 Intoxications
Several factors must be considered in applying RRT in the treatment of a toxic ingestion. First, it is necessary to determine if the drug is primarily impacted by renal or hepatic metabolism. In the setting of hepatic or renal dysfunction, the drug itself (or the by-product of the drug) may be retained resulting in toxicity. If systemic toxicity is observed, particularly if it is impacting neurologic and/or cardiac function, then RRT is often indicated. Other issues that must be considered when determining the role for RRT include whether the drug is a short-acting or sustained-release product, as well as the volume of distribution, molecular weight, and protein binding of the drug. ◘ Table 32.3 illustrates these characteristics for common medications that may require RRT in settings of intoxication.
A high-flux or high-efficiency HD membrane provides the largest and most effective form of medication clearance.
This often requires a large membrane, a high blood flow rate, and a very high dialysate flow rate. For some medications (e.g., vancomycin), clearance is impacted by a “two-compartment” model. In these situations, after an initial clearance of the medication by a course of HD, it is not unusual for there to be a rebound in the serum level 4–6 hours after dialysis as medication in the tissues moves back to the vascular space in response to the lowered serum concentration. Therefore, and similar to the case of the inborn errors of metabolism, the use of sequential HD followed by CRRT (preferably in a convective mode) may be necessary to provide optimal and continual clearance of these medications or toxins. Also, and again similar to the situation of an inborn error of metabolism, these children may have normal renal function, and attention to the components of the dialysate bath is necessary to ensure normal physiological levels of electrolytes.
10 Summary
In summary, RRT is a useful therapy for many critically ill children, particularly those with AKI, those with inborn errors of metabolism, and those experiencing some forms of intoxication. It may also be useful in the setting of extracorporeal membrane oxygenation. There are several forms of RRT, and the choice of RRT modality is based on a combination of factors including the clinical state of the patient (particularly the hemodynamics), the experience of the practitioner, and local resources. Data to date have not demonstrated one modality to be superior or inferior to any other for all indications and clinical conditions. ◘ Table 32.4 compares five modalities of dialysis, including the need for anticoagulation, the dialysate or replacement flow rate, as well as the impact upon nutrition, drug clearance, and hemodynamics.
Review Questions
-
1.
Which of the following statements is correct regarding peritoneal dialysis?
-
A.
Increasing the dextrose concentration of the dialysate solution may enhance solute clearance but will decrease ultrafiltration.
-
B.
Peritoneal dialysis is the most effective dialysis modality for treating life-threatening hyperkalemia.
-
C.
Solute clearance of phosphorus, urea, and potassium are enhanced using larger exchange volumes approximating 50 mL/kg per pass.
-
D.
Ultrafiltration with peritoneal dialysis is enhanced with longer pass times.
-
A.
-
2.
A 17-year-old adolescent is transferred to the intensive care unit with severe hemolytic anemia secondary to a brown recluse spider bite. On clinical exam, he is somnolent but arousable. He is tachypneic but easily oxygenated with nasal cannula oxygen. His heart rate is 105 bpm, and his blood pressure is 129/79 mm Hg. His distal pulses are 1+. His ECG rhythm strip is depicted below. He has become severely oliguric (<0.2 mL/kg/min).
Laboratory analysis reveals the following:
-
White blood cell count: 21,000/μL
-
Hemoglobin: 4.8 g/dL
-
Platelet count: 98,000/μL
-
Lactate dehydrogenase: 9800 U/L
-
Sodium: 132 mmol/L
-
Potassium: 7.8 mmol/L
-
Chloride: 100 mmol/L
-
Bicarbonate: 16 mmol/L
-
Blood urea nitrogen: 82 mg/dL
-
Creatinine: 4.1 mg/dL
-
pH: 7.37
-
PaCO2: 27 mm Hg
-
PaO2: 116 mm Hg
-
Base deficit: −8
-
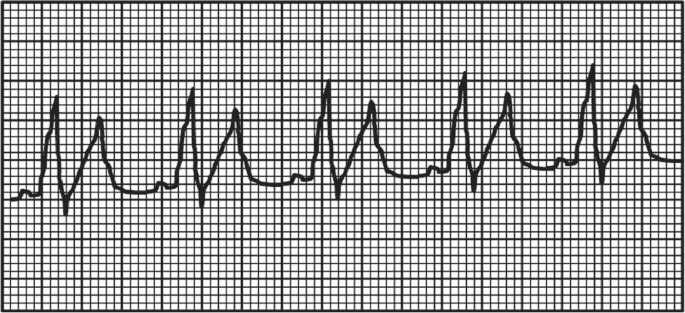
In addition to standard medical therapy, which of the following therapies should be implemented to provide the most effective treatment of his most immediate life-threatening problem?
-
A.
Continuous arteriovenous hemofiltration
-
B.
Continuous veno-venous hemofiltration
-
C.
Hemodialysis
-
D.
Peritoneal dialysis
-
-
3.
A 4-day-old male presents with multiple organ dysfunction syndrome and encephalopathy. The infant is intubated, mechanically ventilated, and started on a dopamine infusion for pulmonary and hemodynamic stabilization. Laboratory workup reveals severe hyperammonemia (ammonia level 1285 μmol/L). The Genetics service is consulted and suspects a urea cycle defect, most likely ornithine transcarbamylase deficiency. The therapy most urgently needed to improve his chance of successful outcome includes which one of the following?
-
A.
Hemodialysis
-
B.
Lactulose
-
C.
Neomycin
-
D.
Peritoneal dialysis
-
A.
-
4.
Which of the following statements is true regarding the use of continuous renal replacement therapy in children?
-
A.
Continuous veno-venous hemofiltration (CVVH) provides less clearance of large-molecular-weight solutes (e.g., vancomycin) than continuous veno-venous hemodialysis (CVVHD).
-
B.
The continuous nature of fluid removal in continuous renal replacement therapy results in more hemodynamic instability than experienced with intermittent hemodialysis.
-
C.
The use of continuous veno-venous hemodiafiltration (CVVHDF) is a combination of convective and diffusive clearance utilizing both replacement fluids and a dialysate solution.
-
D.
When the combined volume of the hemofilter and circuit is more than 10% of the patient blood volume, the continuous veno-venous hemofiltration circuit may be primed with normal saline rather than blood.
-
A.
-
5.
A 3-year-old child with resolving methicillin-resistant Staphylococcus aureus sepsis and dialysis-dependent acute kidney injury has been found to have a toxic level of vancomycin. Which of the following is most accurate regarding its clearance with renal replacement therapy?
-
A.
Continuous renal replacement therapy in a convective mode may be useful to treat rebound high levels 4 hours after initial dialysis.
-
B.
Continuous veno-venous hemodialysis is more effective than continuous veno-venous hemofiltration in clearing vancomycin.
-
C.
Dialysis is not effective in the clearance of vancomycin.
-
D.
Peritoneal dialysis is the most effective renal replacement therapy to rapidly clear toxic vancomycin levels.
-
A.
-
6.
A 12-year-old female status post allogeneic hematopoietic cell transplant is admitted to the pediatric intensive care unit with severe Streptococcus viridans sepsis, grade IV acute graft-versus-host disease, hepatic failure, and acute kidney injury requiring continuous veno-venous hemodiafiltration with citrate anticoagulation. She is noted to have a steadily increasing total calcium level but a decreasing ionized calcium level. Which of the following interventions would be most likely to resolve this clinical concern in calcium levels?
-
A.
Decrease the rate of the citrate infusion.
-
B.
Decrease the replacement fluid infusion rate.
-
C.
Increase the rate of the calcium infusion.
-
D.
Decrease the rate of the dialysate.
-
A.
-
7.
Which of the following clinical situations is most likely to be associated with dialysis disequilibrium?
-
A.
A 3-hour course of hemodialysis that resulted in a decrease in the blood urea nitrogen concentration from 153 mg/dL (pre-dialysis) to 70 mg/dL (post-dialysis).
-
B.
A 12-hour course of peritoneal dialysis with short dwell times and a 4.25% dextrose dialysate solution resulting in a blood glucose concentration increase from 83 mg/dL to 192 mg/dL.
-
C.
A 2-hour course of hemodialysis that resulted in a decrease in the systolic blood pressure from 145 mm Hg to 98 mm Hg.
-
D.
The use of citrate anticoagulation for continuous veno-venous hemofiltration that produced a rise in the patient total calcium level to 12.2 mg/dL and a fall in the ionized calcium concentration to 0.97 mmol/l (normal range 1.22 to 1.37 mmol/L).
-
A.
Answers
-
1.
C
-
2.
C
-
3.
A
-
4.
C
-
5.
A
-
6.
A
-
7.
A
Change history
28 April 2022
https://doi.org/10.1007/978-3-030-53363-2
Suggested Reading
Amerling R, Glezerman I, Savransky E, Dubrow A, Ronco C. Continuous flow peritoneal dialysis: principles and applications. Semin Dial. 2003;16:335–40.
Brophy PD, Mottes TA, Kudelka TL, et al. AN-69 membrane reactions are pH-dependent and preventable. Am J Kidney Dis. 2001;38:173–8.
Brophy PD, Somers MJ, Baum MA, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant. 2005;20:1416–21.
Bunchman TE. Acute peritoneal dialysis access in infant renal failure. Perit Dial Int. 1996;16(Suppl 1):S509–11.
Bunchman TE, Donckerwolcke RA. Continuous arterial-venous diahemofiltration and continuous veno-venous diahemofiltration in infants and children. Pediatr Nephrol. 1994;8:96–102.
Bunchman TE, Ferris ME. Management of toxic ingestions with the use of renal replacement therapy. Pediatr Nephrol. 2011;26:535–41.
Bunchman TE, Meldrum MK, Meliones JE, Sedman AB, Walters MB, Kershaw DB. Pulmonary function variation in ventilator dependent critically ill infants on peritoneal dialysis. Adv Perit Dial. 1992;8:75–8.
Bunchman TE, Gardner JJ, Kershaw DB, Maxvold NJ. Vascular access for hemodialysis or CVVH(D) in infants and children. Dial Transplant. 1994;23:314–8.
Bunchman TE, Valentini RP, Gardner J, Mottes T, Kudelka T, Maxvold NJ. Treatment of vancomycin overdose using high-efficiency dialysis membranes. Pediatr Nephrol. 1999;13:773–4.
Bunchman TE, Barletta GM, Winters JW, Gardner JJ, Crumb TL, McBryde KD. Phenylacetate and benzoate clearance in a hyperammonemic infant on sequential hemodialysis and hemofiltration. Pediatr Nephrol. 2007;22:1062–5.
Bunchman TE, Hackbarth RM, Maxvold NJ, Winters JW, Barletta GM. Prevention of dialysis disequilibrium by use of CVVH. Int J Artif Organs. 2007;30:441–4.
Cullis B, Abdelraheem M, Abraham G, et al. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34:494–517.
Deep A, Zoha M, Dutta Kukreja P. Prostacyclin as an anticoagulant for continuous renal replacement therapy in children. Blood Purif. 2017;43:279–89.
Donckerwolcke RA, Bunchman TE. Hemodialysis in infants and small children. Pediatr Nephrol. 1994;8:103–6.
Gallieni M, Giordano A, Pinerolo C, Cariati M. Type of peritoneal dialysis catheter and outcomes. J Vasc Access. 2015;16(Suppl 9):S68–72.
Hackbarth R, Eding D, Gianoli Smith C, Koch A, Sanfilippo DJ, Bunchman TE. Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol. 2005;20:1328–33.
Hackbarth R, Bunchman TE, Chua AN, et al. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs. 2007;30:1116–21.
Katz A, Kashtan CE, Greenberg LJ, Shapiro RS, Nevins TE, Kim Y. Hypogammaglobulinemia in uremic infants receiving peritoneal dialysis. J Pediatr. 1990;117:258–61.
Lee CY, Yeh HC, Lin CY. Treatment of critically ill children with kidney injury by sustained low-efficiency daily diafiltration. Pediatr Nephrol. 2012;27:2301–9.
Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE. Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med. 2000;28:1161–5.
Nourse P, Sinclair G, Gajjar P, du Plessis M, Argent AC. Continuous flow peritoneal dialysis (CFPD) improves ultrafiltration in children with acute kidney injury on conventional PD using a 4.25% dextrose solution. Pediatr Nephrol. 2016;31:1137–43.
Olszewski AE, Daniel DA, Stein DR, et al. Teaching pediatric peritoneal dialysis globally through virtual simulation. Clin J Am Soc Nephrol. 2018;13:900–6.
Parekh RS, Bunchman TE. Dialysis support in the pediatric intensive care unit. Adv Ren Replace Ther. 1996;3:326–36.
Picca S, Dionisi-Vici C, Abeni D, et al. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol. 2001;16:862–7.
Raaijmakers R, Schröder CH, Gajjar P, Argent A, Nourse P. Continuous flow peritoneal dialysis: first experience in children with acute renal failure. Clin J Am Soc Nephrol. 2011;6:311–8.
Sethi SK, Sinha R, Jha P, et al. Feasibility of sustained low efficiency dialysis in critically sick pediatric patients: a multicentric retrospective study. Hemodial Int. 2018;22:228–34.
Zaoral T, Hladík M, Zapletalová J, Trávníček B, Gelnarová E. Circuit lifetime with citrate versus heparin in pediatric continuous venovenous hemodialysis. Pediatr Crit Care Med. 2016;17:e399–405.
Zappitelli M, Juarez M, Castillo L, Coss-Bu J, Goldstein SL. Continuous renal replacement therapy amino acid, trace metal and folate clearance in critically ill children. Intensive Care Med. 2009;35:698–706.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bunchman, T.E. (2021). Renal Replacement Therapies. In: Lucking, S.E., Maffei, F.A., Tamburro, R.F., Zaritsky, A. (eds) Pediatric Critical Care . Springer, Cham. https://doi.org/10.1007/978-3-030-53363-2_32
Download citation
DOI: https://doi.org/10.1007/978-3-030-53363-2_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53362-5
Online ISBN: 978-3-030-53363-2
eBook Packages: MedicineMedicine (R0)





