Abstract
A wide variation in renal replacement therapy practices for critically ill patients with acute kidney injury exists across the world, with continuous renal replacement therapy (CRRT) predominating in developed countries and peritoneal dialysis in developing countries. Sustained low efficiency dialysis is a technical hybrid, combining the ease of use and low costs of intermittent hemodialysis with the hemodynamic stability of CRRT. It is performed using conventional hemodialysis machines and dialyzers, without anticoagulation where necessary. Current studies have shown similar efficacy results with SLED and CRRT. The use of ultrapure water and sterile dialysate produced by cold sterilization have enabled the addition of a convective clearance to diffusion with improved survival in small studies. As a hybrid therapy, great flexibility has been shown in coupling SLED with other extracorporeal treatments.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Definition and History of Prolonged Intermittent Renal Replacement Therapy
In 1946, Wilhem Kolff first carried out a successful 690 min dialysis session on a patient of acute kidney injury and severe hyperkalemia. Blood flow was 116 mL/min, urea clearance 87 mL/min, and Kt/V of 1.4 [1]. This prolonged session with low blood flow and clearances could be described as the first prolonged intermittent renal replacement therapy (PIRRT), a term employed by Fealy, Baldwin, and Bellomo in 2004 [2]. Although continuous renal replacement therapy (CRRT) was first used by Kramer in 1979, the large majority of adult patients with acute kidney injury continued to be treated with intermittent hemodialysis similar to those with chronic kidney disease. In the late 1980s and 1990s Schlaper et al. extended the length of intermittent hemodialysis sessions and by 2000 the terms extended dialysis(ED), extended daily dialysis(EDD), slow low efficiency daily dialysis(SLEDD), sustained low efficiency dialysis(SLED) had been coined. Early sessions varied from 8 to 12 h on single pass machines, to 18 h on the Genius machine. The continuous mode with sessions lasting 24 h was described by Salahudeen [3] in 2009, and the criteria became more variable with some workers using sessions as short as 6 h.
The technical difficulties of performing intermittent hemodialysis in small children and infants have meant that peritoneal dialysis and CRRT have been the mainstay of renal replacement therapy in developed and developing countries, respectively. A recent survey including 60 centers from the USA and 48 from the Indian subcontinent and Latin America revealed a marked geographical variation in RRT practices. Limited availability, expertise, and high costs limit CRRT in developing countries. SLEDD however was used in 25% of centers in developing countries as compared to 20% in developed countries [4].
Sustained low efficiency dialysis is a conceptual and technical hybrid of continuous renal replacement therapy (CRRT) and intermittent hemodialysis (IHD), combining the desirable properties of each modality [5]:
-
1.
A reduced rate of ultrafiltration for optimized hemodynamic stability.
-
2.
Low-efficiency solute removal to minimize solute disequilibrium.
-
3.
Sustained treatment duration to maximize dialysis dose.
-
4.
Intermittent nature for convenient access to patients for out-of-unit diagnostic and therapeutic procedures during scheduled downtime.
Case Vignette
An 8-year-old child, weighing 19 kg, admitted in the Pediatric Intensive Care Unit, following a blunt trauma, had a distended, tender abdomen with guarding, and anuria for 8 h after the event. He had received around 1000 mL of crystalloid at the time of surgical evacuation. At ICU admission his blood pressure (BP) was 90/40 mm of Hg, RR 26/min, heart rate 170/min, and CVP 4 cm H2O. As further fluid resuscitation and dopamine infusion did not improve urine output, a CT scan of the abdomen was done. This revealed a solitary left kidney, a non-visualized renal artery, splenic hematoma, multiple lacerations in the body and tail of pancreas, and numerous large and small intraperitoneal collections. Exploratory laparotomy showed hemoperitoneum with anterior abdominal wall necrosis, widespread intra- and retroperitoneal fat necrosis, liquefaction of the jejunal wall, edematous pancreas with slough collection, and omentum covered with slough, a thrombosed left renal artery with cyanotic kidney and splenic hematoma. Left nephrectomy with de-sloughing, drain insertion, splenectomy, and feeding jejunostomy was done. Postoperatively, he received antibiotics, noradrenaline, intravenous fluids, elective ventilation, and hemodialysis. On the third postoperative day, he developed sudden bleeding from the peritoneal drain and his hemoglobin dropped to 2.6 g%, platelet count to 47,000/mm3, prothrombin and activated thromboplastin time were prolonged. Despite transfusions he developed shock with a systolic BP of 60 mm of Hg, hypoxia, bradycardia, and a cardiac arrest requiring CPR. The following day his circulation was stabilized with noradrenaline and vasopressin. His blood reports showed pH of 7.00, bicarbonate of 7.3 mmol/L, blood urea of 167 mg/dL, creatinine of 7.3 mg/dL, and serum potassium of 6.5 mmol/L. He received 41 h of continuous veno-venous hemofiltration with a blood flow of 100 mL/min, replacement fluid (Prismasol) at 600 mL/h, and ultrafiltration of 50 mL/h. Regional heparinization was used with monitoring of the patient’s and extracorporeal circuit aPTT individually. The procedure was stopped when the filter clotted, by which time the noradrenaline dose had decreased to 0.12 mcg/kg/min.
He required amphotericin B for candidemia. His coagulopathy continued to require blood products. He was taken for sustained low efficiency daily dialysis for 10 h daily using the ArrT plus machine at a blood flow of 135 mL/min, citrate bicarbonate dialysate at 200 mL/min with 800 mL of ultrafiltration per session and no anticoagulant. After three sessions he was extubated, and noradrenaline discontinued after the seventh session. He was discharged from the ICU on the 29th postoperative day, a cuffed tunneled catheter was inserted in the left internal jugular vein and he was placed on a maintenance hemodialysis program.
2 Introduction
The case described above highlights the indications, and the problems with providing renal replacement therapy to the critically ill child. What is unique about these patients is that multiple indications for renal replacement therapy may exist simultaneously and a balance may have to be created between providing biochemical correction of hyperkalemia, acidosis and uremia, fluid removal for pulmonary edema, and maintenance of blood pressure in a patient with increased extravascular volume, inotrope need and increased permeability of capillaries and veins.
Box1: Indications for SLED
-
Clinically significant fluid overload of more than 10% above baseline in AKI not responding to high dose diuretics.
-
Severe metabolic acidosis with other signs of decreased end-organ perfusion.
-
Hyperkalemia (plasma K+ > 6.5 meq/L or rapidly rising).
-
Azotemia (BUN >100 mg/dL).
-
Uremic organ involvement (pericarditis, encephalopathy, neuropathy, myopathy).
-
Progressive severe dysnatremia (Na+ > 160 or < 115 meq/L).
-
Nonobstructive oliguria (<0.5 mL/kg/h for more than 6 h).
-
Malignant hyperthermia.
-
Overdose with a dialysable drug.
-
Neonatal hyperammonemia and other inborn errors of metabolism.
-
Coagulopathy requiring large amounts of blood products in patients at risk of pulmonary edema or ARDS.
3 Procedure of Sustained Low Efficiency Dialysis
SLED is typically carried out using equipment used for conventional hemodialysis, easily available in dialysis units and familiar to dialysis nurses. While many centers rely on dialysis nurses or technicians to initiate the procedure with troubleshooting from ICU personnel, Marshall reported a study in which the entire procedure of SLEDD-f was carried out by ICU nurses trained for this purpose [5]. In our own unit the process has evolved from a largely nocturnal procedure where machines shifted from the dialysis unit were operated by dialysis personnel after the daily maintenance dialysis was over, to stationing of machines permanently in the ICU and operated by ICU nurses and technicians. The responsibility and treatment objectives, goals, and plan are shared jointly by nephrologists and intensivists. An overview of machines available for SLEDD with their specifications is shown in Table 19.1. Dialyzers can be low flux for SLEDD, but either high flux dialyzers or hemofilters are required if online hemodiafiltration (SLEDD-f) is prescribed. The dialyzer needs to be appropriately chosen for the patient’s size, a slightly smaller dialyzer with lower clearance and KoA is appropriate as it reduces the risk of disequilibrium and hypotension further, while dose delivery is compensated for, by the increased session length. Similarly blood tubings for pediatric treatments may require adjustment of the blood pump occlusion diameter if the same machine is used with standard 6.4 mm sets.
The vascular access should be chosen according to the size of the child and the blood flow requirement [6]. Care should be taken while siting the vascular access for dialysis and central venous access for giving inotropes, antibiotics, etc. as the proximity of these two accesses can cause siphoning of drugs given through the central access by the negative pressure applied to the dialysis catheter. A brief overview of dialyzers for SLEDD is given in Table 19.2. Although the hemofilters from the AV series have been used in SLEDD-f in adults, they appear to be too large for use in children and an equally efficient treatment can be carried out using high flux dialyzers.
4 Fluid Removal
Large volumes of fluid for maintaining adequate nutrition and hydration, blood products to combat DIC and coagulopathy, antibiotic infusions, inotropes and pressors all increase the total daily administered fluid. In an anuric patient, this mandates fluid removal to prevent pulmonary edema. An increasing body of evidence indicates that volume overload which is invariably an unavoidable consequence of resuscitation is associated with increased mortality, morbidity, and hospital stay [7, 8]. In fact, in children an increase in body weight of 10%, and definitely of 20%, is associated with increased mortality and considered an indication for dialysis [6, 8]. In a study from India, an even lower degree of fluid overload increased mortality [9].
Continuous therapies achieve a better reduction in fluid accumulation compared to intermittent ones, underscoring the importance of extending the time available for ultrafiltration to maintain hemodynamic stability [7, 10]. Many patients with AKI diagnosed as having acute respiratory distress syndrome (ARDS) actually have pulmonary edema, as cardiac function can also be profoundly impaired by fluid overload. Such patients achieve normal lung compliance and improved oxygenation with fluid removal by dialysis. Systemic hemodynamics remain stable as long as the rate of fluid removal does not exceed the rate of mobilization of interstitial fluid into the vascular compartment. Kumar et al. [11] achieved a median UF of 3000 mL/day with both 7.5 h EDD sessions and 19.5 h CRRT sessions in adults, with excellent maintenance of MAP and similar hypotensive episodes in the two therapies. This permitted obligatory fluid administration, even in anuric patients, though the patients did not necessarily achieve overall negative fluid balance. While patients with chronic kidney disease (CKD) with hypertension were shown in the HEMO study [12] to tolerate ultrafiltration of 10–12 mL/kg/h, the patient with high pressor support, increased capillary permeability and hypoalbuminemia would simply not tolerate such rates. Most studies on SLED in adults report total ultrafiltration achieved but not the hourly rate. In a multicenter study in children from India [9], the reported rate was 9.28 ± 6.67 mL/kg/h. However in 30% of these sessions performed in patients who were not on inotropes, intradialytic hypotension was seen in 31 of 211 sessions with termination in 20. It may be prudent to restrict ultrafiltration rates to a lower level in the interest of hemodynamic stability, probably around 4–5 mL/kg/h. This entails increasing the duration of the session and thus the desired ultrafiltration will drive the session length. The dialysate flow rate is reduced both to decrease the consumption of dialysate and the biochemical clearance. Lonemann et al. [13] using the Genius machine with blood and dialysate flow both set at 70 mL/min carried out 18 h sessions in adult patients with 4000 mL of ultrafiltration and an increase in mean arterial pressure from 69 to 81 mm of Hg. A longer and slower dialysis reduces the rate of change of the plasma osmolality, which also enhances hemodynamic stability and reduces the incidence of disequilibrium. Additionally, use of sodium profiling, cooled dialysate and increased dialysate calcium or potassium can also be used although their role in the critically ill patient is less clear. In the recently conducted RESCUE study [14], the mean duration of daily SLEDD session was 14.9 h and CRRT session was 15.9 h daily, while 24 h SLEDD sessions have also been conducted [3]. Once the duration and dialysate flow have been chosen, blood flow can be adjusted according to the desired clearance and the dialyzer specifications. The flexibility of varying the dialysate flow rate, composition, ultrafiltration rate, blood flow and dialyzer to adapt a treatment on a standard dialysis machine for this patient subset are some of the unique features of SLEDD.
5 Anticoagulation
While SLEDD can be performed using standard unfractionated heparin as with intermittent hemodialysis, a single dose of Enoxaparin 1 mg/kg or regional citrate can also provide adequate and safe anticoagulation for a 8 h session. Perhaps the greatest advantage of SLEDD over CRRT is the ability to conduct a complete session without the need for any anticoagulation. Kumar et al. [11] found that patients on EDD required significantly less heparin than those treated with CVVH. 117 EDD treatment days (31.9%) were done without heparin compared to only three CVVH treatment days (2.7%). Of over 3000 sessions of SLEDD in our unit, almost half were performed without anticoagulation. We generally increase the blood flow rate by 20–25% for anticoagulant free sessions. Since 2014 we have successfully used dry citrate bicarbonate dialysate concentrate for all SLEDD sessions, as described by Suhail Ahmed [15], which produces a dialysate citrate concentration of 3–4 mmol/L, protecting the dialyzer from clotting without producing systemic anticoagulation. It is important to confirm that the machine proportioning system is configured for the compatible citrate concentrate, that the contribution of the acid concentrate to the final conductivity is at least 9.4 mS/cm and that the dialysate flow is switched on during the priming procedure.
A sample algorithm, taking into account the need for ultrafiltration, fluid intake, anticoagulation, correction of metabolic parameters, and patient size for the patient described, is provided in Table 19.3 and also in a recent review [16].
6 Solute Clearance and Control
The solute clearances in SLED are low compared to conventional hemodialysis because of the low blood flow and dialysate flow. This decreases the rapid osmolar shifts induced by hemodialysis, the risk of disequilibrium, fluid shifts from intravascular to interstitial and intracellular space and maintains blood pressure, permitting ultrafiltration (Fig. 19.1). As the session length is greater, the net solute removal is enhanced despite the lower clearance and the equivalent solute clearance may be expected to be higher than for intermittent hemodialysis (Fig. 19.2). This has actually been shown by Marshall et al. [5] who obtained an equivalent renal urea clearance of 35.7 mL/min with 8 h SLEDD-f sessions and a single pool Kt/V of 1.43 for urea and 1.02 for vitamin B12. In fact various SLEDD regimens have shown EKR levels of 25.1–36.8 mL/min, and with daily 8 h sessions, the overall efficacy may be equivalent to or slightly higher than CRRT prescribed at 30–35 mL/kg/h [5]. Wu et al. also found a higher dialysis dose in SLED than CVVH after 8-h dialysis (equivalent urea clearance, 62.7 ± 4.4 vs. 50.2 ± 3.9 mL/min, p = 0.002) [15].
The extended sessions allow solute equilibration between intracellular and extracellular fluid with more efficient removal of phosphates, which may have to be replaced to prevent deficiency. The serum ET-1 level increased after CVVH (p = 0.019) but not after SLED treatment in a study by Wu which also found equivalent effects on intracranial pressure by both modalities [17].
While no renal replacement therapy has been shown to clear lactate, SLEDD provides an almost unlimited source of bicarbonate buffer which corrects metabolic acidosis and may improve mean arterial pressure, tissue perfusion and decrease pressor requirement. This is our own observation, and is also described in the retrospective pediatric study from India [16] and in C-SLEDD [3], where pH normalized at 12 h and was sustained up to 48 h.
7 Complications
Both technical and medical complications occur in SLEDD. Studies investigating complications have largely focused on hypotension, an inevitable occurrence in this population of already hemodynamically unstable patients, and the frequency has been estimated at around 50% [18]. However in a recent randomized study comparing two different session lengths of SLEDD, the incidence of hypotension was 82.6%, with no significant difference between 6 and 10 h sessions [19]. An attempt to decrease hypotension by increasing the session length is balanced by an increased risk of circuit clotting and filter loss which was encountered in 25.3% of sessions.
We counter hypokalemia by using a dialysate potassium of 4 mmol/L in patients at risk, and monitor phosphate levels every 3 days, after starting SLEDD.Hypophosphatemia and hypomagnesemia may be minimized by intravenous or oral supplementation, or by increasing the dialysate content, a flexibility which is unique to SLEDD.
Concerns have also been raised about the microbiological purity of the dialysate. With the use of the Genius machine and the online replacement fluid in SLEDD-f (see below), these have been addressed in studies by Dhondt and Lonnemann [13, 20].
8 Outcomes with SLEDD
Controversy continues to surround the utility of SLEDD and its advantages/disadvantages compared to CRRT. Till date only two observational studies on SLEDD in children have been published, a single center study from Taiwan [21] and a multicenter study from India [9]. The mortality was 28.6 and 42.6%, respectively, however these studies appear to have included a heterogeneous population and in the Taiwanese study the sickest patients were not considered for SLEDD, but taken for CRRT.
An increasing number of studies in adults have compared CRRT and SLEDD in recent years. In a non-randomized study Kitchlu [22] compared 158 patients undergoing CRRT with 74 patients undergoing SLEDD for an average duration of 7.11 h. All-cause mortality at 30 days was 54% and 61% among SLED and CRRT-treated patients respectively (adjusted OR 1.07, 95% CI 0.56–2.03). Secondary outcomes including fluid removal after 7 days, was also similar between the two therapies.
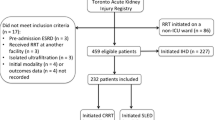
In a longitudinal study between 1995 and 2005, involving 1347 patients from ICUs of three different hospitals, Marshall et al. observed that the switch from CRRT to PIRRT use was not associated with any increase in mortality rate, with an adjusted IRR of 1.02 (0.61–1.71). The IRR was virtually identical in the three ICUs (P-value = 0.63 for the difference in the IRR between ICUs) [23]. In the largest RCT comprising 232 patients, the RESCUE study [14], the duration of CRRT and SLEDD barely differed by 1 hour (14.9 vs. 15.9 h, despite the SLED prescription being 12 h), and the 90-day mortality was also similar (49.6 vs. 55.6%, p = 0.43). Systolic blood pressure improved after SLED, but not CRRT without increase in the vasopressor doses and with similar ultrafiltration. There was also a significantly lower ICU stay, duration of mechanical ventilation, and RRT requirement with SLED compared with CRRT. This underscores the point that CRRT for technical and logistic reasons is almost never continuous and that SLEDD needs to be more than 12 h long to allow optimum ultrafiltration without compromising hemodynamics and yet maintain the flexibility of allowing out of ICU procedures.
In a recent meta analysis, involving 18 studies, only four were of high quality. In the primary outcome analysis of renal recovery no difference was found between CRRT and SLEDD (OR = 0.87, 95% CI = 0.63–1.20), but a small mortality benefit was actually found with SLEDD (OR for mortality with CRRT = 1.21, 95% CI = 1.02–1.43) [24] The analysis included both randomized controlled and observational studies and the difference was not present in a sensitivity analyses for only RCTs. A similar finding was noted in an earlier analysis of 17 studies, indicating that a marked selection bias may exist in observational studies [25]. In conclusion, SLEDD appears to offer similar survival benefits as CRRT, lower anticoagulation requirements, technical ease and lower costs. Future trials, designed like the RESCUE study and importantly more studies in children are needed to address these issues.
9 Adaptations of SLEDD
Drug dose modifications—The increased solute removal with SLEDD over the longer duration means greater drug removal. This is particularly critical for the septic shock patient receiving antibiotics which are cleared to a level intermediate between intermittent hemodialysis and CRRT. Extensive studies are still lacking except for a few drugs like Meropenem [26], which should be dosed either side of an extended dialysis session. In a review by Sinha et al. [16] recommendations are provided for drug dosing based on adult studies as no specific data is available in children.
9.1 Adding a Convective Component (SLEDD-f)
Sustained low efficiency daily diafiltration (SLEDD-f) is possible with the new generation of machines (Table 19.1). They possess the feature of cold sterilization or ultrafiltration of the dialysate through ultrafilters having a pore size of 0.05μm, which results in a 4-log reduction of bacteria and a 2-log reduction in endotoxin, producing almost unlimited quantities of a sterile, pyrogen-free fluid suitable for intravenous infusion. The process of cold sterilization by ultrafiltration was validated by Lebedo [27], for the Gambro system and Vasalaki [28], for the Fresenius system. One dry powder bicarbonate cartridge generally allows around 160–200 L of dialysate generation. With a dialysate flow of 100 mL/min and a replacement of 30 mL/kg/h for a 20-kg child, a treatment could actually be run for 24 h. A complete description of the process of obtaining and ensuring ultrapure water is beyond the scope of this chapter, but should confirm to the EU standards for generation and monitoring.
SLEDD-f enables adding a convective component to the predominant diffusion clearance of the standard HD machines. Ultrapure water and sterile dry powder concentrates usually dedicated for individual machines are required for the dialysate preparation. High flux dialyzers or hemofilters having UF coefficients >20 mL/mmHg/h, are mandatory. At least a part of the replacement fluid should be delivered prefilter to reduce the viscosity of the blood caused by the high ultrafiltration rates and the blood flow has to be proportionally higher than usual in order to avoid a filtration fraction >25%, which predisposes to circuit clotting and filter loss. The ultrafiltration actually carried out is the sum of the replacement fluid rate and the desired ultrafiltration and is calculated by the machine software.
Several small series have demonstrated encouraging results with Marshall et al. using the technique in 24 critically ill patients, while Holt and White noted a 30-day survival of 100% in the SLEDD-f group, despite higher APACHE II scores, against 38% in the SLEDD group. Furthermore, all patients in the SLEDD-f group recovered significant renal function to allow discontinuation of RRT [29]. In another small study from Eucador, Dario et al. showed a small non-significant survival benefit from online hemodiafiltration, and a significantly shorter pressor requirement time and ICU stay [30].
Coupling SLEDD with hemoperfusion and plasma exchange has been successfully carried out (Figs. 19.3 and 19.4) using both the charcoal hemoperfusion device and cytokine removal cartridges. In a case of fulminant drug overdose with liver and kidney injury, we have used an external roller pump at a speed of 50 mL/min to perform coupled plasma filtration adsorption with SLEDD. The blood pump of the dialysis machine drives blood from the access to a plasma filter with an external pump to control plasma removal rate and perfuse the charcoal hemoperfusion cartridge before it is reinfused into the venous bubble chamber, while the blood exiting the plasma filter is dialyzed using a low flux or high flux dialyzer. When using cytokine removal devices, we connect them in series with the dialyzer using the blood pump to drive the circuit, this may not be feasible in very small children as the extracorporeal volume is increased, in older children, priming the circuit with blood should sort out this issue.
9.2 SLED in ECMO
The use of extracorporeal membrane oxygenation in ARDS delivers one cardiac output to an external oxygenator via a centrifugal pump, operating with a veno-venous access. In the case of low cardiac output, a veno-arterial access is used. In patients with concomitant AKI requiring SLEDD and ECMO, a portion of the blood (usually between 100 and 200 mL/min) being delivered to the oxygenator is shunted through a parallel circuit to the dialysis machine (Fig. 19.5). The return of blood is always pre-oxygenator and it should be noted that the pre-pump arterial pressure in such a SLEDD circuit is positive rather than negative and the machine software may not be able to calculate the actual blood flow.
Key Learning Points
-
SLEDD is a hybrid therapy with remarkable flexibility and has been adapted by nephrologists and intensivists to suit the needs of individual patients.
-
It provides adequate small solute clearance, correction of acidosis and fluid removal while maintaining hemodynamic stability.
-
Convective clearance can be added with the use of a sterile online substitution fluid prepared by the machine from ultrapure water and dry powder concentrates.
-
Survival, renal recovery, ICU stay and hemodynamic stability are similar to that of CRRT.
-
Future developments will include exact determination of drug dosing especially antibiotics during this therapy, and further adaptations for special situations like septic shock and ARDS requiring other extracorporeal therapies.
References
Fliser D, Kielstein JT. Technology insight: treatment of renal failure in the intensive care unit with extended dialysis. Nat Clin Pract Nephrol. 2006;2:32–9.
Bellomo R, Baldwin I, Fealy N. Prolonged intermittent renal replacement therapy in the intensive care unit. Crit Care Resusc. 2002;4:281–90.
Salahudeen AK, Kumar V, Madan N, Xiao L, Lahoti A, Samuels J, et al. Sustained low efficiency dialysis in the continuous mode (C-SLED): dialysis efficacy, clinical outcomes, and survival predictors in critically ill cancer patients. Clin J Am Soc Nephrol. 2009;4:1338–46.
Raina R, Chauvin A, Bunchman T, Askenazi D, Deep A, Ensley M, Krishnappa V, Sethi S. Treatment of AKI in developing and developed countries: an international survey of pediatric dialysis modalities. Plos One. 2017:1–9.
Marshall M, Ma T, Galler D, Patrick A, Rankin N, Williams A. Sustained low-efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: towards an adequate therapy. Nephrol Dial Transplant. 2004;19:877–84.
Vinsonneau C, Allain-Launay E, Blayau C, et al. Renal replacement therapy in adult and pediatric intensive care - recommendations by an expert panel from the French Intensive Care Society (SRLF) with the French Society of Anesthesia Intensive Care (SFAR) French Group for Pediatric Intensive Care Emergencies (GFRUP) the French dialysis society (SFD). Ann Intensive Care. 2015;5:58–76.
Ostermann M, Oudemans-van Straaten HM, Forni L. Fluid overload and acute kidney injury cause or consequence? Crit Care. 2015;19:443–5.
Schrier R. AKI: fluid overload and mortality. Nat Rev Nephrol. 2009;5:485.
Sethi S, Sinha R, Jha P, Wadhwani N, Ragunathan V, Dhaliwal M, Shyam B, Bansal S, Kher V, Lobo V, Sharma J, Raina R. Feasibility of sustained low efficiency dialysis in critically sick pediatric patients: A multicentric retrospective study. Hemodial Int. 2017;22:228–34.
Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis. 2004;44(6):1000–7.
Kumar VA, Craig M, Depner T, Jane Y, Yeun J. Extended daily dialysis: a new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis. 2000;36(2):294–300.
Eknoyan G, Beck G, Cheung A, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–9.
Lonnemann G, Jurgen Floege J, Kliem V, Brunkhorst R, Koch K. Extended daily veno-venous high-flux hemodialysis in patients with acute renal failure and multiple organ dysfunction syndrome using a single batch dialysis system. Nephrol Dial Transplant. 2000;15:1189–93.
Schwenger V, Weigand M, Hoffmann O, et al. Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury – a randomized interventional trial: the Renal Replacement Therapy Study in Intensive Care Unit PatiEnts. Crit Care. 2012;1:R14.
Tu & Ahmad, Dialysis & Transplantation. Citrate dialysate in advanced liver failure (Abstr); 2000.
Sinha R, Sethi S, Bunchman T, Lobo V & Raina R. Prolonged intermittent renal replacement therapy in children. Pediatr Nephrol. 2017.
Wu V, Huang T, Shiao C, NSARF Group. The hemodynamic effects during sustained low-efficiency dialysis versus continuous veno-venous hemofiltration for uremic patients with brain hemorrhage: a crossover study. J Neurosurg. 2013;119:1288–95.
Fieghen H, Friedrich J, Burns K, et al. The hemodynamic tolerability and feasibility of sustained low efficiencydialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 2010;11(1):32.
Albino B, Balbi A, Ponce D. Dialysis complications in AKI patients treated with extended daily dialysis: is the duration of therapy important? Biomed Res Int. 2014;2014:9. Article ID 153626
Dhondt A, Vanholder R, De Smet R, et al. Studies on dialysate mixing in the Genius_ single-pass batch system for hemodialysis therapy. Kidney Int. 2003;63:1540–7.
Chia-Ying L, Yeh H, Lin C-Y. Treatment of critically ill children with kidney injury by sustained low-efficiency daily diafiltration. Pediatr Nephrol. 2012;27:2301–9.
Kitchlu A, Adhikari N, , Burns K et al. Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: a cohort study; BMC Nephrol (2015) 16:127 – 134.
Marshall M, Creamer J, Foster M, et al. Mortality rate comparison after switching from continuous to prolonged intermittent renal replacement for acute kidney injury in three intensive care units from different countries. Nephrol Dial Transplant. 2011;26:2169–75.
Kovacs B, Sullivan K, Hiremath S, Patel R. Effect of sustained low efficient dialysis versus continuous renal replacement therapy on renal recovery after acute kidney injury in the intensive care unit: a systematic review and meta-analysis. Nephrology. 2017;22:343–53.
Zhang L, Yang J, Eastwood G, et al. Extended daily dialysis versus continuous renal replacement therapy for acute kidney injury: a meta-analysis. Am J Kidney Dis. 2015;66(2):322–30.
Deshpande P, Chen J, Gofran A, Murea M, Golestaneh L. Meropenem removal in critically ill patients undergoing sustained low-efficiency dialysis (SLED). Nephrol Dial Transplant. 2010;25(8):2632–6.
Ledebo I. On-line preparation of solutions for dialysis: practicalaspects versus safety and regulations. J Am Soc Nephrol. 2002;13(Suppl 1):S78–83.
Vaslaki L, Karátson A, Vörös P, Major L, Pethö F, Ladányi E, Weber C, Mitteregger R, Falkenhagen D. Can sterile and pyrogen-free on-line substitution fluid be routinely delivered? A multicentric study on the microbiological safety of on-line haemodiafiltration. Nephrol Dial Transplant. 2000;15(Suppl 1):74–8.
Holt BG, White JJ, Kuthiala A, Fall P, Szerlip HM. Sustained low-efficiency daily dialysis with hemofiltration for acute kidney injury in the presence of sepsis. Clin Nephrol. 2008;69(1):40–6.
Darío J, Manuel G, Ana A, et al. Intermittent hemodialysis low intensity vs. on line Hemodiafiltration in critically ill patients with sepsis and acute kidney injury. Choosing the best treatment in a developing country. J Nephrol Ther. 2017;7:4–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Lobo, V. (2018). Sustained Low-Efficiency Dialysis (SLED) and Hybrid Therapies in Children. In: Deep, A., Goldstein, S. (eds) Critical Care Nephrology and Renal Replacement Therapy in Children. Springer, Cham. https://doi.org/10.1007/978-3-319-90281-4_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-90281-4_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90280-7
Online ISBN: 978-3-319-90281-4
eBook Packages: MedicineMedicine (R0)