Abstract
Fungi are ubiquitous lifeforms that colonize a wide range of diverse environments. Many of them have a saprophytic lifestyle and are principal decomposers of our ecosystem. Fungi play an important role in our food and pharmaceutical industries, but their appearance can also be harmful to us. They are responsible for many diseases in humans and animals, ranging from allergies to life-threatening intoxications and mycoses. The infection of plants and the contamination of crops lead to high economic losses and pose a threat to food supply and safety. Fungal developmental programs, including the formation of infection structures, are closely linked to the production of specific chemicals, called secondary metabolites. This chapter describes these interrelated processes and their regulation, including the genetic networks linking transcriptional to epigenetic control of gene expression, protein degradation machineries, and the autophagy membrane trafficking pathway.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
I. Introduction
Fungi are ubiquitous lifeforms that can grow in a wide range of diverse environments. Based on current phylogenetic analyses, the fungal kingdom is divided into 8 phyla with 12 subphyla and 46 classes (Spatafora et al. 2017). The two monophyletic groups of Ascomycota and Basidiomycota form together the subkingdom of Dikarya. Many fungi have a saprophytic lifestyle. They live on decaying organic matter as principal decomposers of our ecosystem. Fungi play important roles in our food industry to make cheese, ferment soybeans, or brew beer and in the pharma industry to produce drugs (Gerke and Braus 2014). But their appearance can also be harmful to us. Fungal spores are dispersed through the air and can be inhaled into our lungs where most of them are inactivated as long as the immune system is not compromised (Shlezinger et al. 2017). Fungi are responsible for many diseases in humans and animals, ranging from allergies to life-threatening intoxications and mycoses. The infection of plants and contamination of harvest products by fungi lead to high economic losses and are a threat for food supply and safety (Meyer et al. 2016). In fungi, differentiation processes, including the formation of infection structures, are closely linked to the production of specific chemicals. These interconnected processes and their regulations are the main focus of this chapter.
A. Fungal Differentiation
Filamentous fungi of the Dikarya germinate from single spores and initially grow in long, tubular vegetative hyphae by tip extension (Riquelme et al. 2018). The Spitzenkörper, which is located at the hyphal tip, acts as a dynamic center for the organization and supply of the vesicles required for material transport. Hyphae fuse through anastomosis tubes at the tips and form branched two dimensional networks, called mycelia. When a certain state of developmental competence is reached, the fungus can respond to external signals to proliferate through either asexual or sexual differentiation (Fig. 8.1a). In asexual reproduction, the fungus produces spore-forming structures called conidiophores, which carry the uninuclear, mitotic-derived asexual spores called conidia (Adams et al. 1998). These airborne spores are distributed by the wind. In sexual reproduction, ascomycetes produce sexual spores called ascospores in a sac-shaped ascus.Footnote 1 In most species, each ascus carries eight spores which are formed by meiosis and subsequent mitotic division. The asci usually form within the protecting fruiting bodies, which can be closed and spherical (cleistothecia), closed and flask-like (perithecia), or open and cup-shaped (apothecia) and which serve as overwintering structures in the soil (Pöggeler et al. 2018). Whether asexual or sexual structures are formed depends strongly on the environmental conditions, such as nutrients, light, temperature, or oxygen availability. Fungi change their lifestyle also during the infection process. Whereas some fungi can gain entry into the host without forming specialized structures, there are many plant pathogenic fungi that produce adhesion and penetration structures, such as appressoria and hyphopodia. These penetration organs form tiny infection hooks and penetrate the host using turgor pressure and/or by secreting large amounts of plant cell wall-degrading enzymes (Lo Presti et al. 2015).
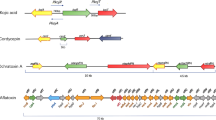
Functions of fungal secondary metabolites during life of an ascomycetous filamentous fungus living in the soil. (a) Examples of secondary metabolites contributing to growth, differentiation, communication, competition, protection, and survival of Aspergillus nidulans . (b) Classification of fungal secondary metabolites into the most common classes
B. Fungal Secondary Metabolism
Whether a fungus initiates a differential program depends not only on environmental conditions but also on endogenous factors such as the formation of primary or secondary metabolites including pheromones. Metabolic programs are tightly connected with morphogenic differentiation through sophisticated signal sensing and transduction mechanisms as well as transcriptional networks. Whereas primary metabolites (also called central metabolites) are essential for the growth of an organism, secondary metabolites (also called specialized metabolites or natural products) are dispensable but offer advantages in the natural habitat of their producer. Usually, primary metabolites are the precursors for the biosynthesis of secondary metabolites, and the biosynthesis occurs during developmental or aging processes (Bayram et al. 2016). Several secondary metabolites directly related to development are known and show the close connection between these two processes in fungi (Fig. 8.1a). These secondary metabolites are involved, for example, in the initiation and regulation of development and in protection and survival. They are also required for communication, competition, and defense against other microorganisms as well as for virulence in plant and animal infections (Macheleidt et al. 2016; Künzler 2018).
Although the biological benefit of most secondary metabolites has not yet been understood, their biological activities have been used for a very long time. Already our ancestors used the healing extracts of fungi and plants as medicine and nowadays many fungal compounds serve as lead structures for the synthesis of new drugs (Newman and Cragg 2012). Examples of secondary metabolites with pharmaceutical relevance from ascomycetes are the antibiotic penicillin, the anticancer drug taxol, the cholesterol-lowering drug lovastatin, or the immunosuppressant ciclosporin. However, the biological activities of secondary metabolites can also be harmful for us. Fungi synthesize strong carcinogens and mycotoxins such as aflatoxins produced by Aspergillus and trichothecenes produced by Fusarium (Gerke and Braus 2014).
1. Secondary Metabolite Categories
Fungal secondary metabolites are categorized according to their biosynthesis in the four major groups polyketides, non-ribosomal peptides, terpenes, and indole alkaloids but comprise also mixed forms or smaller groups (Fig. 8.1b; Keller et al. 2005).
Polyketides are the most abundant secondary metabolites in fungi and are synthesized by type I polyketide synthases (PKS). Type I PKS are multi-domain enzymes similar to eukaryotic fatty acid synthases, consisting of the essential ketoacyl CoA synthase (KS), acyltransferase (AT), acyl carrier protein (ACP) domains, and a termination domain (Keller et al. 2005). Additionally, they can harbor variable domains like dehydratase, ketoreductase, enoylreductase, or methyltransferase domains. The domains are arranged in a module. Whereas bacterial PKS usually have multiple modules, the fungal PKS typically carries only one module, which can be used iteratively. The precursors for the biosynthesis of polyketides are usually acetyl coenzyme A (acetyl-CoA) and malonyl-CoA, but also propionyl-CoA or methylmalonyl-CoA are used. During the starting stage of polyketide synthesis, the precursors are loaded onto the starter domains KS and ACP, catalyzed by the AT domain. In the elongation stage, these loaded precursors are condensed by decarboxylative condensation similar to the fatty acid synthesis, resulting in a polyketide chain. By loading of another starter unit, the next cycle starts, and the polyketide chain is elongated by the new starter unit similarly. The control of how many cycles are performed is not yet understood. Finally, the polyketide chain is released from the enzyme by the termination domain (Keller et al. 2005).
Among the class of polyketides are several products that demonstrate the tight link between developmental processes and secondary metabolism by fulfilling survival or longevity tasks, inducing sexual development as hormones or conferring virulence. Typical examples are melanins, zearalenone, and T-toxin. Melanins are pigments that can be incorporated into the fungal cell wall or secreted to the environment. They strengthen the cell wall, protect the fungal spores from UV light, or can inhibit hydrolytic enzymes produced by other microorganisms (Toledo et al. 2017). Zearalenone, an estrogenic polyketide produced exclusively by different Fusarium species, is a sex hormone. Whereas loss of zearalenone prevents sexual reproduction, its addition can positively or negatively affect sexual development, depending on the applied concentrations (Wolf and Mirocha 1977). T-toxin is a polyketide produced by Cochliobolus heterostrophus , a necrotrophic fungal plant pathogen that causes southern corn leaf blight in maize. It is connected with high virulenceFootnote 2 in maize cultivars carrying the “Texas male sterile cytoplasm”, but it is not required for pathogenicityFootnote 3 itself. It contributes to virulence by disrupting the mitochondrial activity (Stergiopoulos et al. 2013).
Non-ribosomal peptides are peptides that are produced from proteinogenic and non-proteinogenic amino acids by enzymes called non-ribosomal peptide synthetases (NRPS) without the use of ribosomes. NRPS are multi-domain and multi-modular enzymes. Each module contains an adenylation domain for the recognition of the amino acid, a peptidyl carrier domain for the activation and covalent binding of the amino acid to the phosphopantetheine transferase cofactor (PPTase) and a condensation domain for peptide formation. The final peptide is usually released by a thioesterase domain at the C-terminus of the enzyme. The diversity of non-ribosomal peptides derives from cyclization or branching of peptides. Among them are, for instance, the antibiotic penicillin, siderophores, and gliotoxin. Siderophores are iron-chelating compounds produced for the uptake and storage of iron ions. Usually they contain hydroxamate groups that form strong iron(III) binding bidentates. Most filamentous fungi and some yeasts, except for the model organisms S. cerevisiae or C. albicans , produce siderophores. A fine-tuned system between iron uptake and storage is necessary to prevent the fungus from iron starvation or toxicity (Haas 2014). Gliotoxin is another prominent example of non-ribosomal peptides. It is a mycotoxin produced by human pathogens such as Aspergillus fumigatus , Trichoderma spp., and Penicillium spp. It inhibits the proteasome and prevents NF-κB activation (see Sect. V.A) as presumed virulence factor of A. fumigatus by targeting primarily the activity of neutrophils or other phagocytes of the host immune system (Scharf et al. 2016).
Terpenes are built up from isoprene units by terpene synthases (terpene cyclases). The subgroup of oxygenated forms of terpenes is called terpenoids. Most of known terpenes derive from plants, for example, the major constituents of essential oils. In terpene synthesizing fungi, they are produced by the mevalonate pathway. Prominent examples are the carotenoid pigments, the gibberellin phytohormones or the trichothecene mycotoxins (Schmidt-Dannert 2014).
Indole alkaloids are nitrogen-containing secondary metabolites incorporating one or more indole or indoline moieties (Xu et al. 2014). Mostly, the indole precursors are tryptophan or related indole-3-glycerol-phosphate. Tryptophan is prenylated with dimethylallyl pyrophosphate by dimethylallyl tryptophan synthases (DMATS). Ergotamine from Claviceps purpurea represents a prominent example for an indole alkaloid. It works as vasoconstrictor with structural similarity to several neurotransmitters and is medicinally used against acute migraine attacks (Schardl et al. 2006).
Besides the four main groups, there are additional classes of fungal secondary metabolites (Fig. 8.1b). Metabolites consisting of combinations of peptides and polyketides (peptide-polyketide hybrids), such as emericellamides or aspyridone, are formed by PKS-NRPS hybrids that contain all typical domains of the single PKS and NRPS (Du et al. 2001). Meroterpenoids are products with a partial terpenoid structure plus a polyketide or a peptide moiety (Matsuda and Abe 2016). An example for a meroterpenoid with a polyketide moiety is dehydroaustinol produced by A. nidulans . The addition of dehydroaustinol together with the polyketide diorcinol restores spore formation of A. nidulans mutants defective in sporulation, suggesting that these two compounds or derivatives serve as developmental signal for asexual sporulation (Rodríguez-Urra et al. 2012; Fig. 8.1a). Oxylipins (collectively termed psi-factor (precocious sexual inducer)) are oxygenated polyunsaturated fatty acids produced by oxygenases (Brodhun and Feussner 2011). In Aspergillus nidulans , three psi factor producing oxygenases PpoA, PpoB, and PpoC have been characterized producing mainly hydroxylated oleic (18:1) and linoleic (18:2) acid. A careful regulated system of oxylipins is necessary for a proper balance between asexual and sexual development (Tsitsigiannis et al. 2004). Besides these non-volatile oxylipins, also volatile oxylipins exist. They share an eight-carbon scaffold and are suggested to be involved in fungus-invertebrate interactions (Holighaus and Rohlfs 2018). Isocyanides , which function as chalkophores, contain nitrile groups and are synthesized by isocyanide synthases that can also occur as hybrids with NRPS. The first characterized isocyanide was xanthocillin, which is produced, e.g., by Penicillium notatum and by A. fumigatus (Lim et al. 2018).
2. Secondary Metabolite Clusters Are Often Silenced and Have to Be Activated for Analysis in the Laboratory
The large diversity of secondary metabolites is due to modifications that are performed after the initial step of building the skeleton. These include oxidations, cyclizations, methylations, dehydrations, or reductions. Fungal genes that are necessary for the biosynthesis of the final secondary metabolites as well as genes encoding proteins necessary for regulation of the synthesis or transport of the final metabolite product are usually, and similarly to genes for specialized or accessory primary metabolism, clustered together on one chromosomal locus (Rokas et al. 2018). The gene clusters enable an economical production of secondary metabolites due to transcriptional co-regulation. Most secondary metabolite biosynthetic gene clusters are silenced and are only expressed under very specific conditions. Especially under laboratory conditions, where the fungus does not naturally meet environmental triggers such as stressors or nutrient limitations, the analysis of secondary metabolism is difficult. The rapidly growing number of genome studies has shown that there are many more secondary metabolite clusters in the genome of fungi than metabolites have been identified so far (Andersen et al. 2013; Inglis et al. 2013; van der Lee and Medema 2016). In order to eliminate this discrepancy and exploit the full potential of fungal metabolite production, strategies have been developed to activate silenced gene clusters and thus enable their analysis in the laboratory (Gerke and Braus 2014; Ren et al. 2017).
The easiest way to induce secondary metabolite production is to change the growth parameters as described by the OSMAC (one strain many compounds) approach (Bode et al. 2002). Parameters such as temperature, media components, pH, air supply, and light can be varied to force the fungus to adapt its secondary metabolite repertoire. Since secondary metabolites are often used in nature for communication, competition, and defense, their production can also be stimulated by co-cultivation with other microorganisms (Netzker et al. 2015). Additionally, genes involved in the metabolite biosynthesis can be heterologously introduced and expressed in suitable hosts. Heterologous expression systems for fungal biosynthetic genes have been designed, e.g., for A. niger (Gressler et al. 2015), A. nidulans (Chiang et al. 2013), or A. oryzae (Sakai et al. 2012). Silenced biosynthetic gene clusters can also be awakened by manipulating the transcriptional machinery, the epigenetic status, or the degradation machinery of the cell.
In this chapter we focus on the molecular regulatory links of the coordinated and specific formation of secondary metabolites in different phases of fungal developmental programs, with a particular focus on the mold Aspergillus nidulans . This includes the control and interaction of different genetic networks, which can be organized chronologically and hierarchically and can contain several feedback functions. Genetic transcriptional control is linked to post-translational histone modifications as epigenetic control and specific signal transduction pathways. Several additional post-translational control mechanisms, such as the attachment and removal of ubiquitin, link fungal differentiation to the corresponding secondary metabolism by altering protein function and cellular localization and by controlling protein stability through the ubiquitin 26S proteasome as well as autophagy degradation pathways.
II. Transcriptional Networks Linked to Signal Transduction Pathways Control Development and Secondary Metabolism
Fungal growth and differentiation and the concomitant secondary metabolism occur in response to internal and external signals that are sensed through receptors and transported by highly controlled signal transduction pathways. This leads to a choreography of changes in transcription, translation, post-translational histone modifications and protein stability followed by proteomic changes. Several examples of well-studied signal transduction pathways and responding transcriptional regulatory circuits are summarized in the following section.
A. Transcriptional Networks Interact in Fungal Morphogenic Transitions
Transcriptional reprogramming plays a crucial role in the morphological transition from vegetative fungal growth to developmental programs. Besides chromatin modifications (see Sect. III), changes in transcriptomes are mediated by approximately 80 families of currently classified fungal DNA binding transcription factors including a few examples of dual factors with two distinct DNA binding domains. Ascomycetous transcription factors of the largest group carry a Zn2Cys6 zinc cluster domain. This domain is generally found in all fungi but also in a few additional non-fungal organisms. In contrast, four types of transcription factors carrying an APSES-type DNA binding domain (named after fungal developmental transcription factors Asm1, Phd1, Sok2, Efg1, and StuA), mating-type MAT α1, copper fist DNA-binding, or velvet domains are fungal specific. Notably, the mating-type protein is exclusively present in ascomycetes (Ahmed et al. 2013; Shelest 2017). The velvet domain is structurally similar to the immunoglobulin-like Rel homology domain of the mammalian transcription factor NF-κB p50 (Ahmed et al. 2013). The four velvet domain proteins of Aspergilli steer large hierarchical networks and interconnect developmental programs with secondary metabolism triggered by chemical, oxidative, light, or temperature sensing and subsequent signal transduction pathways (Bayram et al. 2008b; Bayram and Braus 2012; Lind et al. 2016).
1. Chemical Sensing and Oxidative Stress as Developmental Signal: How Fungi Smell Their Environment
Chemical sensing is accomplished by G protein-coupled receptors (GPCRs) with a common seven transmembrane domain architecture that initiate signal transduction through binding to the heterotrimeric Gα, Gβ, and Gγ protein complex. In Aspergilli, three different Gα, one Gβ, and one Gγ protein exist (de Vries et al. 2017; Brown et al. 2018). GPRCs are the largest fungal group of surface receptors important for sensing of pheromones, nutrients, and host cells in pathogenic interactions. For instance, the binding of a pheromone to the receptor initiates GDP-GTP exchange on the Gα protein that then dissociates from the βγ dimer and typically activates a three-step mitogen-activated protein (MAP) kinase cascade, which finally changes transcription. The result of this well conserved cascade in fungi is a cell cycle arrest and the cellular fusion of both mating partners (Bahn et al. 2007). Further, similar sensing systems through G protein-coupled receptors with different transcriptional outcomes exist for different macro- and micronutrients including glucose as main carbon energy source. Glucose is sensed through the sugar receptor Gpr1 (G protein-coupled receptor 1) that stimulates Gpa2 resulting in increased cyclic adenosine monophosphate (cAMP) levels through activation of adenylyl cyclase and protein kinase A (PKA). Active PKA inhibits the Atg1 (autophagy-specific gene 1) initiation kinase of autophagy degradation pathways (see Sect. V.B). Specialized sugar transporters such as yeast Snf3 (sucrose nonfermenting 3) and Rgt2 (restores glucose transport 2) act as sensors inducing signal transduction and changed transcription (Bahn et al. 2007).
The regulation of the mycotoxin sterigmatocystin in A. nidulans works through a Gα-PKA pathway. The Gα protein FadA (fluffy autolytic dominant A) activates PkaA (protein kinase A A), which in turn is a repressor of the specific transcription factor of the sterigmatocystin biosynthesis cluster, AflR (aflatoxin regulator). The activation of the Gα protein FadA is inhibited by the fluffy low brlA protein FlbA (Shimizu et al. 2003; Fig. 8.2).
Interconnection of genetic networks and subnetworks for light and developmental control of A. nidulans . Simplified model depicting examples of major molecular control lines to support sexual or asexual development and the appropriate secondary metabolism. The velvet domain protein dimer VeA-VelB supports sexual development in dark and VeA is connected to blue (LreA, B) and red (FphA) light receptors. VeA is controlled by epigenetic signal transduction (VapA, VipC-VapB) and bridges DNA binding with epigenetic control of secondary metabolism (LaeA: loss of aflR expression A). Velvet proteins (VelB-VosA, VosA-VosA) as well as NsdD and different signal transduction pathways (e.g., the cAMP PkaA pathway) inhibit the expression of brlA as part of the central conidiation pathway (with AbaA and WetA), whereas different Flb factors, SclB, and the epigenetic VapB-VipC support asexual development
Reactive oxygen species (ROS) function as signalling molecules in light perception and redox biology and thereby induce fungal development (Gessler et al. 2007). Therefore, an impact on ROS homeostasis is often linked to distorted development in filamentous fungi (Nahlik et al. 2010; Kolog Gulko et al. 2018). Fungi respond to oxidative stress induced by ROS through activation of a MAP kinase pathway (Yu and Fischer 2019). The MAP kinase Hog1 (high osmolarity glycerol response 1) of S. cerevisiae or its homologue SakA (stress activated kinase A) in A. nidulans are activated by ROS and in turn induce transcription factors needed for the induction of the oxidative stress response, such as the A. nidulans AtfA (activating transcription factor A). These transcription factors regulate different antioxidants, such as superoxide dismutases, catalases, peroxidases, glutathione peroxidases, peroxiredoxins, and antioxidative secondary metabolites. The stress-activated kinase SakA is not only induced by ROS but also by light, mediated through the red light photoreceptor FphA (fungal phytochrome A), which represses fruiting body formation in A. nidulans (Yu and Fischer 2019). SakA additionally mediates repression of the A. nidulans NADPH oxidase gene noxA, which is essential for different steps during sexual fruiting body formation (Lara-Ortíz et al. 2003).
2. Light Response: The Fungal Vision
Almost all fungi are able to perceive changes in light or temperature conditions. Light is perceived as a signal and also as a stressor and induces a wide range of responses by inducing different genetic networks resulting in changes in development (photomorphogenesis), secondary metabolism, circadian clock, phototropism, or DNA repair (Bayram et al. 2016; Cetz-Chel et al. 2016; Fischer et al. 2016). The developmental light responses vary considerably between different fungi. Blue or red light can promote asexual spore formation in A. nidulans (Fig. 8.1a) but inhibit sporulation in Botrytis cinerea (Tan 1974). Similarly, A. nidulans sexual fruiting bodies are preferentially formed in darkness, but Trichoderma reesei forms its corresponding sexual structures in light (Pöggeler et al. 2018).
Typical fungal photosensory systems include light sensors specific for different wavelengths from 350 to 650 nm distributed into four types of light receptor proteins. The white collar proteins perceive blue light, phytochromes sense red light, opsins are specific for green light, and cryptochromes react to blue and ultraviolet (UV) light. The light perception occurs through binding of the photoreceptor to a chromophore, which are flavin for blue and UV light, linear tetrapyrrole for red light and retinal for green light.
White collar (WC) proteins are blue-light regulated transcription factors with a GATA-type zinc finger DNA binding domain and three PAS (Per, Arnt, Sim) domains directly controlling gene expression. One of the PAS domains is a LOV (light oxygen voltage) domain, which is able to bind the chromophore flavin. In Neurospora crassa , two WC proteins are present, WC-1 and WC-2 (Wu et al. 2014). They form a heterodimeric complex through the PAS domains, which binds to light response elements in the dark and activates light-induced transcription after phosphorylation and dissociation of WC-1. The complex regulates approximately 20% of the N. crassa genes encoding transcription factors as large hierarchical network responding to light and acting downstream of the WC complex in light and circadian clock control (Wu et al. 2014). Besides the typical WC proteins, blue light photoreceptors with only one LOV domain (vivid, VVD) exist that act in fine-tuning the light response (Malzahn et al. 2010).
N. crassa biosynthesis of carotenoid secondary metabolites and asexual spore formation are dependent on light and controlled by the circadian clock (Rodriguez-Romero et al. 2010). WC-1 comprises a flavin mononucleotide binding LOV domain for environmental sensing of light, oxygen, and voltage. The WC-1 and WC-2 zinc fingers dimerize to form the white collar complex transcription factor for DNA binding. WC-1 and WC-2 complexes are conserved in numerous fungi with sometimes several orthologs. WC-1 homologs of pathogenic species are connected to virulence, probably through secondary metabolite production (Idnurm et al. 2010; Fischer et al. 2016). The white collar complex of Aspergilli, LreA/LreB (light response ), activates the expression of the brlA (bristle A) gene encoding the central transcription factor of the conidiation pathway in response to light (Ruger-Herreros et al. 2011, Fig. 8.2). The brlA promoter is repressed by binding factors such as NsdD (never in sexual development D ) or the velvet domain protein VosA (viability of spores A) (Ni and Yu 2007). brlA must be activated through a complex cascade of early genes for proteins which produce small signal molecules (fluG: fluffy G) or act as transcription factors (flbB-E: fluffy low brlA B-E; sclB: sclerotia-like B). Activation of brlA by light then activates the downstream pathway with the genes abaA (abacus) and wetA (wet-white; Fig. 8.2; Thieme et al. 2018).
Phytochromes are multi-domain photoreceptors that bind linear tetrapyrrole as chromophore at their N-terminal photosensory domain and have red/far-red light absorbance properties. The tetrapyrrole undergoes a conformational change upon red light induction and thereby changes its spectroscopic properties by shifting the absorption maximum toward far-red light. The photosensory domain consists of a PAS, a GAF (vertebrate cGMP-specific phosphodiesterases, cyanobacterial adenylate cyclases, transcription activator FhlA) and a PHY (phytochrome-specific PAS-related) domain. The further domains are a histidine kinase domain and a response regulator domain. Phytochromes seem to be absent from Mucoromycotina fungi but might be present in some Chytridiomycetes and have been extensively studied in Aspergilli (Fischer et al. 2016). The A. nidulans phytochrome FphA interacts with the white collar orthologs LreA and LreB, corresponding to WC-1 and WC-2, and the velvet transcription factor VeA (see Sect. II.B.2) that coordinates development and secondary metabolism (Purschwitz et al. 2008; Bayram et al. 2008b; Fig. 8.2). LreA binds to its target sequences in the dark in dependency of FphA while being released after illumination with blue or red light (Blumenstein et al. 2005; Hedtke et al. 2015). LreA modulates gene expression together with FphA through modification of histone H3 by interacting with the acetyltransferase GcnE (general control nonderepressible E) and the histone deacetylase HdaA (Grimaldi et al. 2006; Hedtke et al. 2015). FphA of A. nidulans is a phytochrome with light-driven histidine kinase activity and presumably transmits the white collar and phytochrome light signal directly to the velvet protein VeA by phosphorylation (Rauscher et al. 2016). This results in an enhancement of asexual development in light and a delay in dark, where sexual development is favored. After 30 min of illumination as minimum time required to initiate conidiation, 19% of the transcriptome of competent A. nidulans mycelia reacts to light (Bayram et al. 2010, 2016; Hedtke et al. 2015; Macheleidt et al. 2016). Austinol and dehydroaustinol (see Sect. I.B.1) are secondary metabolites connected to conidiospore production that are only produced in light but not in dark (Rodríguez-Urra et al. 2012).
Cryptochromes are blue and UV light receptors with similarities to blue light-dependent DNA repairing photolyases, carrying a photolyase domain (Idnurm et al. 2010; Fischer et al. 2016). They bind non-covalently to flavin adenine dinucleotide (FAD) as well as other chromophores such as pterin or deazaflavin. The cryptochromes CryA of A. nidulans and Cry1 of T. reesei are so far the only known dual-function cryptochrome/photolyase proteins. A. nidulans CryA inhibits sexual development in UV light and has a DNA repair function (Bayram et al. 2008a), whereas T. reesei Cry1 is needed for light-induced transcription besides its DNA repair function in conidia (García-Esquivel et al. 2016). In N. crassa , Cry1 acts as transcriptional repressor of the white collar blue light complex without photolyase activity (Nsa et al. 2015).
Opsins , the green light receptors, are membrane-bound proteins associated with a retinal chromophore. They are related to bacterial and archaeal rhodopsins that, in their activated form, channel ions across the membrane (Yu and Fischer 2019). In filamentous fungi, opsins are poorly characterized. In F. fujikuroi , the opsin CarO (carotenoid O) was described as green light-driven proton pump that is involved in spore germination (García-Martínez et al. 2015). In contrast, the N. crassa NOP-1 (Neurospora opsin-1) lacks proton pump activity but is involved in the regulation of the switch between asexual and sexual development in response to light and ROS levels (Wang et al. 2018).
B. Gene Expression for Secondary Metabolite Production Is Interconnected with Morphological Differentiation
Environmental stimuli such as light, oxygen, pH, and nutrients affect fungal morphological programs as well as the tightly interconnected specific secondary metabolite production. Accordingly, defects in light control affect secondary metabolism. One example is Fusarium fujikuroi , which is impaired in secondary metabolite production when the white collar blue light sensor WC-1 is defective, and which uses its cryptochrome CryD to repress the production of the antibiotic bikaverin during growth in light (Estrada and Avalos 2008; Castrillo et al. 2013).
1. Expression of Silenced Secondary Metabolite Clusters by Specific and Global Regulators
Filamentous fungi are a vast reservoir of yet undescribed secondary metabolites, carrying dozens of usually clustered but only specifically expressed biosynthetic gene clusters. Many of these clusters are controlled by cluster-specific, poorly conserved transcription factors (Alberti et al. 2017; Keller 2018). In addition, there are several master regulators of secondary metabolism such as the originally in A. nidulans described methyltransferase LaeA or the multi-cluster regulator A (McrA). LaeA is encoded by a conserved regulatory gene with homologues in filamentous fungi such as Fusarium, Penicillium, Trichoderma, and Aspergillus (Bok and Keller 2016). Absence of laeA or overexpression of mcrA results in silencing, whereas laeA overexpression or mcrA deletion leads to increased production of several secondary metabolites (Bok and Keller 2016; Oakley et al. 2016). The fact that only some global regulators are conserved, whereas the targets of individual regulators differ considerably, correlates with the finding that secondary metabolite genes are more variable and significantly less conserved than genes of the primary metabolism (Lind et al. 2015).
2. The Fungal Velvet Complex Physically Connects Transcriptional and Heterochromatin Control to Coordinate Secondary Metabolism and Development
Velvet proteins with their characteristic velvet domain, such as VeA (velvet A), VelB (velvet-like B) VelC (velvet-like C), and VosA in A. nidulans , form complex regulatory networks by direct DNA binding to thousands of target gene promoters in numerous fungi (Ahmed et al. 2013; Becker et al. 2016; Fig. 8.2). The master regulator of secondary metabolism LaeA is physically linked to the heterodimer VeA-VelB to coordinate secondary metabolite biosynthesis with developmental programs. It was originally shown in A. nidulans that this VelB-VeA-LaeA complex is required for the appropriate formation of fruiting bodies and concomitant production of the aflatoxin family metabolite sterigmatocystin (Bayram et al. 2008b). LaeA is a methyltransferase and acts as epigenetic control element by counteracting the silencing heterochromatic lysine 9 methylation marks at histone H3 in secondary metabolite clusters (Strauss and Reyes-Dominguez 2011; see Sect. III.A). VeA, which provides the interphase of the trimeric complex, therefore physically links transcription to post-translational epigenetic control of histone modifications (Sarikaya-Bayram et al. 2015). Velvet proteins and LaeA contribute to the virulence of several fungi, possibly through mycotoxin production (Wiemann et al. 2010; Kumar et al. 2016; López-Díaz et al. 2018). The trimeric velvet complex is conserved in the fungal kingdom and can physically interact through VeA with the phytochrome FphA as part of the light and presumably temperature control machinery (Lind et al. 2016; Yu and Fischer 2019). The light reception through VeA-FphA-LreA-LreB is presumably less stable and rather transient in comparison to the velvet complex VelB-VeA-LaeA (Bayram et al. 2010).
3. Velvet Domain Transcription Factors Expand Their Transcriptional Networks Through Formation of Sub-networks
Velvet domain proteins control several genetic networks either acting as homo- or heterodimers (Fig. 8.2). VelB does not only support as VelB-VeA heterodimer sexual development linked to its specific secondary metabolism but also represses asexual development in combination with the velvet protein VosA as VelB-VosA together with VosA-VosA (Park and Yu 2012). Additionally, VelB-VosA is a positive regulator for spore viability (Park and Yu 2012). How the formation of the different VosA complexes is regulated is still unknown. Velvet proteins bind to promoters of hundreds of genes, including genes for additional transcription factors, which in turn control their own genetic network (Ahmed et al. 2013).
VosA directly represses the central asexual regulator gene brlA and the gene for the Zn2Cys6 zinc cluster domain transcriptional activator SclB. The SclB network induces early developmental genes including brlA to promote asexual sporulation and to support spore viability. SclB links asexual spore formation to its specific secondary metabolism, including the putative signal molecule dehydroaustinol, and to the fungal oxidative stress response (Thieme et al. 2018). This illustrates a convoluted surveillance apparatus, including sub-networks with feedback mechanisms and overlapping as well as antagonistic functions, to perform fungal development and its accurate linkage to the appropriate secondary metabolism (Fig. 8.2). Developmental functions of velvet domain proteins are rewired in different fungi. Whereas veA is dispensable for conidiation in A. nidulans , the veA homolog of N. crassa ve-1 regulates asexual sporulation (Bayram et al. 2008c). Rewiring of transcriptional regulation is presumably, together with gene mutations and gene transfer, an important driving force to evolve fungal divergences and to adapt to different lifestyles (Nocedal et al. 2017).
III. Epigenetics and Fungal Secondary Metabolism and Development
Secondary metabolite gene clusters evolved either from gene relocations with sometimes prior gene duplication or, in rarer cases, by horizontal gene cluster transfer from bacteria to fungal ancestors (Rokas et al. 2018). Clustering offers the economic advantage that these genes can be transcriptionally co-regulated by changes in the chromatin structure through covalent chromatin modifications (Fig. 8.3). These chromatin regulations can be inherited to the next generation of spores and are therefore epigenetic (epi-: “over, outside of”), which describes heritable phenotypic changes that are not caused by a change in the DNA sequence. In chromatin, the DNA is wrapped twice around eight histones (two H2A, two H2B, two H3, and two H4) to form the nucleosome. With the help of non-histone proteins, the nucleosomes are tightly packed up to the chromatin fiber and then condensed further yielding the chromosome structure. Two different types of chromatin exist which can be distinguished by their condensation state. Euchromatin is the less condensed active form, which allows gene transcription. Heterochromatin is the highly condensed and transcriptionally inactive form that can either be constitutive and serve as structural element of the chromosomal centro- and telomeres or be facultative and switch to transcriptionally active euchromatin. Covalent modifications of DNA or histones determine whether hetero- or euchromatin is formed. DNA can be reversibly modified by methylation, whereas lysine residues of histones can be methylated, acetylated, ubiquitinated, or sumoylated, and serine residues can be phosphorylated (Kouzarides 2007). Writers and erasers, which attach and remove modifications, modify chromatin in different possible combinations. This results in a histone code in a certain genomic region, which is recognized by readers. Readers induce the restructuring of chromatin into the open euchromatin or the closed heterochromatin form and by this regulate fungal development and secondary metabolism (Pfannenstiel et al. 2018; Keller 2018). In the following paragraphs, we will discuss some writers and erasers in more detail with the focus on consequences of histone or DNA modification on secondary metabolism and development.
Epigenetic control of chromatin dynamics for the coordination of fungal development and secondary metabolism. Secondary metabolite gene clusters are mostly silenced during vegetative growth by negative histone tags (red) resulting in heterochromatin and have to be activated by positive tags (green) to allow transition to euchromatin. Methyltransferases (MT), histone acetyltransferases (HAT), and histone deacetylases complexes (HDAC) add (writer) or remove (eraser) these tags. Histone H3K4Me3 represents a positive (euchromatin) and H3K9Me3 a negative (heterochromatin) example for a hallmark chromatin tag. Fungal gene expression can be induced by methylation of H3K4 (COMPASS), demethylation of H3K9 (VapB, LaeA), methylation of H4R3 (RmtA), and acetylation of H3K9 (SAGA/ADA) resulting in increased transcription supporting fungal growth, development, and the corresponding secondary metabolism
A. DNA and Histone Methylation and Demethylation
Methylation of chromatin can occur at DNA and histones. Cytosine modification of DNA is conserved between plants, mammals, and the fungus N. crassa , but has not been found in all fungi. Histone methylations and demethylations at lysine and arginine residues are present in plants, mammals, and fungi and important for fungal development and the associated secondary metabolism (Liu et al. 2010a; Greer and Shi 2012; Nie et al. 2018; Fig. 8.3). Arginines can be mono- or dimethylated (symmetric or asymmetric) and are less well studied than lysine modifications, which include mono-, di-, or trimethylations. Protein arginine (R) methyltransferases (PRMTs) are divided into four major classes according to the methylation pattern they provide (Bachand 2007; Stopa et al. 2015). Three of nine human PRMTs (PRMT1, PRMT3, PRMT5) are conserved in yeast or filamentous fungi (Bachand 2007). The corresponding A. nidulans proteins RmtA and RmtC possess H4R3Footnote 4 specificity, whereas RmtB can methylate the histones H4, H3, and H2A in vitro and all three methylate additional non-histone substrates that affect transcriptional regulation (Lee and Stallcup 2009; Bauer et al. 2010). RmtA and RmtC can affect mycelial growth, oxidative stress response, development, or secondary metabolism in different Aspergilli, whereas the role and the non-histone substrates of RmtB still remain unclear (Bauer et al. 2010; Satterlee et al. 2016).
Lysine methylation usually occurs at histones 3 and 4. The methyltransferase Set1 (SET domain-containing 1) is part of the eight subunit COMPASS (COMPlex ASsociated with Set1) complex involved as writer in mono-, di-, and trimethylation of histone H3K4 euchromatin marks in fungi and in humans. Whereas dysfunction in humans has been linked to several types of human cancer (Meeks and Shilatifard 2017), the degree of H3K4 methylation in fungi has important implications on development and secondary metabolism. Defects in COMPASS subunits, which significantly reduce H3K4Me3Footnote 5 euchromatin marks, change significantly the secondary metabolite production profile in Aspergillus and Fusarium, and the development of Aspergillus (Palmer et al. 2013a; Studt et al. 2017). COMPASS-dependent methylation of H3K4 is connected to the methylation of H3K79 by the non-Set domain-containing enzyme Dot1 (disruptor of telomeric silencing 1) and dependent on histone H2B ubiquitination (Shilatifard 2012). Dot1 is to date the only non-SET domain-containing enzyme for histone H3K79 methylation and controls, e.g., production of aflatoxin, conidiation, sclerotia formation, and pathogenicity of A. flavus (Liang et al. 2017).
Another epigenetic mark commonly associated with “active” chromatin is the trimethylation of H3K36 performed by the methyltransferase Set2, which is able to mono-, di-, and trimethylate this residue (Wagner and Carpenter 2012). Methylation of H3K36 controls N. crassa development as well as vegetative growth, fungal virulence, and secondary metabolism of F. verticillioides (Adhvaryu et al. 2005; Gu et al. 2017). Ash1 (asymmetric synthesis of HO 1) represents a second histone methyltransferase for H3K36 in N. crassa or F. fujikuroi . N. crassa methyl tags deposited by Set2 mark actively transcribed genes, whereas the H3K36 methylation by Ash1 occurs in inactive genes. F. fujikuroi Ash1 is involved in developmental processes and secondary metabolism (Janevska et al. 2018; Bicocca et al. 2018).
Repressive marks associated with gene silencing and heterochromatic regions include the trimethylation of H3K9 or H3K27, which are enriched in silenced fungal secondary metabolite gene clusters. The H3K27 mark is not used in A. nidulans , but in F. graminearum , where the methyltransferase Kmt6 is responsible for the establishment of the H3K27Me3 and regulates production of many secondary metabolites as well as development (Connolly et al. 2013).
Lysine demethylation is performed by histone demethylases, such as KdmA (lysine (K)-demethylase A) and KdmB of A. nidulans . Both are erasers that remove methyl marks from histone H3. KdmA demethylates H3K36Me3 and has a dual role in transcriptional regulation as co-repressor of primary metabolism genes and activator of secondary metabolite genes. KdmB demethylates H3K4Me3 and promotes transcriptional downregulation as prerequisite for accurate induction of A. nidulans secondary metabolism (Gacek-Matthews et al. 2015, 2016).
One of the first described Aspergillus proteins with a methyltransferase domain is LaeA, a master regulator of secondary metabolism. LaeA, which is part of the velvet complex (see Sect. II.A.2), possesses automethylation activity (Patananan et al. 2013). Additionally, it counteracts H3K9Me3 marks in repressive heterochromatin, activating many secondary metabolite biosynthetic gene clusters (Reyes-Dominguez et al. 2010, Fig. 8.3). However, the exact molecular mechanism of LaeA function is yet unknown. There are nine additional A. nidulans LaeA-like methyltransferases (LlmA-LlmG, LlmI, LlmJ) that share sequence similarity with LaeA. LlmF interacts with VeA in the cytoplasm and reduces its nuclear import, resulting in altered development and secondary metabolism, whereas the interaction of VeA with LaeA takes place in the nucleus (Palmer et al. 2013b, Fig. 8.2). VeA interacts with at least two additional methyltransferases in both cellular compartments, the VeA interacting protein C (VipC) and the VipC-associated protein B (VapB). The nuclear heterodimeric methyltransferases VipC-VapB are, together with the membrane tethering zinc finger domain protein VapA (VipC associated protein A), part of a novel type of epigenetic signal transduction pathway (Sarikaya-Bayram et al. 2014, Fig. 8.2). VapA can exclude VipC-VapB from the nucleus by forming the membrane-bound trimeric VapA-VipC-VapB complex. Release of the VipC-VapB heterodimer from VapA is induced by a yet elusive molecular trigger and leads to its transport from the membrane to the nucleus. VipC-VapB interaction with VeA in the cytoplasm inhibits its nuclear accumulation, resulting in decreased sexual development and corresponding secondary metabolism. Without VeA interaction, VipC-VapB enters the nucleus and activates the light-promoted brlA master regulatory gene of conidiation and thereby the asexual differentiation program, for instance, by decreasing heterochromatin through VapB, which was shown to reduce H3K9Me3 marks (Sarikaya-Bayram et al. 2014; Figs. 8.2 and 8.3).
B. Histone Acetylation and Deacetylation
Histone hyperacetylation at amino groups of lysine residues is, in most cases, associated with euchromatin, whereas deacetylation results in heterochromatin formation (Fig. 8.3). Promoter spreading of histone H3 or H4 acetylation results in higher transcription and production of secondary metabolites of the aflatoxin family in Aspergilli (Roze et al. 2007; Reyes-Dominguez et al. 2010).
Histone acetylation is performed by type A or type B histone acetyltransferases (HATs) as writers. Cytoplasmic type B HATs acetylate newly synthesized histones prior to their assembly into nucleosomes, whereas nuclear type A HATs acetylate nucleosomal histones.
A well-studied group of type A HATs are the Gcn5-related N-acetyltransferases (GNAT). They catalyze the transfer of acetyl groups to lysine residues on the histones H2B, H3, or H4 from acetyl-CoA donors. Founding member of the family is the yeast Gcn5 (general control non-derepressed 5), a transcriptional cofactor associating as HAT with several complexes (e.g. SAGA: Spt-Ada-Gcn5-Acetyltransferase). The A. nidulans SAGA complex acetylates the histone sites H3K9 and H3K14 and regulates the biosynthesis of penicillin, sterigmatocystin, terrequinone, and orsellinic acid (Nützmann et al. 2011). Gcn5 is important for the control of the mycotoxin deoxynivalenol biosynthesis in Fusarium graminearum (Kong et al. 2018). Numerous homologs of S. cerevisiae Gcn5 in filamentous fungi are involved in growth, development, regulation of secondary metabolite production, and pathogenicity.
The MYST (MOZ, Ybf2, Sas2, and Tip60) family represents another type A HAT group with a characteristic zinc finger in the highly conserved MYST domain, which acetylates H2A, H3, and H4 lysine residues. EsaA (essential SAS2-related acetyltransferase A), a MYST HAT of A. nidulans , is an activator of secondary metabolism by acetylating H4K12 in the sterigmatocystin, penicillin, terrequinone, and orsellinic acid gene clusters (Soukup et al. 2012). MYST3 of A. parasiticus is required for aflatoxin production (Roze et al. 2011b).
Histone deacetylation in fungi is performed by erasers such as the classical histone deacetylases (HDACs) of class I (Rpd3/Hos2-type) or class II (HDA1-type), with a zinc ion in their catalytic site, or by non-conventional class III SIR2-type sirtuin HDACs, which require NAD+ as cofactor (Brosch et al. 2008).
Yeast Rpd3 (reduced potassium dependency factor 3) as name-giving class I HDAC regulates transcription by RNA polymerases I and II through chromatin silencing and controls mitosis, meiosis, aging, or macroautophagy (see Sect. V.B). The corresponding A. nidulans RpdA deacetylates H3 and H4 and is essential for growth and conidiation (Tribus et al. 2010). Trichostatin A, an inhibitor of classical HDACs such as RpdA, is a promising anticancer drug (Bauer et al. 2016) and inhibits appressorium formation and decreases pathogenicity of the rice blast fungus Magnaporthe oryzae (Izawa et al. 2009). A. nidulans HosA (corresponding to yeast Hos2 (Hda One similar 2)) is another class I HDAC, which deacetylates H4 and represses orsellinic acid production but has inducing effects on other secondary metabolites by overriding the regulatory effects of other HDACs (Pidroni et al. 2018). The corresponding A. oryzae protein (HdaD/Hos2) is involved in the regulation of growth, asexual development, stress response, and the biosynthesis of the industrially important chelator agent kojic acid (Kawauchi and Iwashita 2014).
The class II Hda1-type HDACs include HdaA of A. nidulans , which represses sterigmatocystin and penicillin gene clusters as well as additional clusters located close to telomeres. HdaA, which can be overridden by class I HDAC HosA, is therefore an antagonist of the positive secondary metabolite gene cluster regulator LaeA (see Sects. II.B.2 and III.A). Analysis of the corresponding proteins of Alternaria alternata or Penicillium expansum further support HDAC-mediated repression of secondary metabolism as conserved function in fungi. One exception is the A. fumigatus gliotoxin cluster, which is activated by HdaA. The protein is additionally required for growth and germination but not for virulence in this fungus (Shwab et al. 2007; Lee et al. 2009).
Class III SIR2-type sirtuins HDACs include SirA, which deacetylates H4K16 in the promotor regions of the sterigmatocystin and penicillin gene clusters and controls together with the sirtuin HstA (homolog of SIR Two A) the formation of these metabolites. Some secondary metabolite genes are repressed, whereas others are activated, which reflects the complex control of histone modifications (Shwab et al. 2007; Shimizu et al. 2012; Itoh et al. 2017). This is further illustrated by the Aspergillus oryzae sirtuin HstD, which controls the expression of the velvet complex member LaeA and thus the kojic acid production and conidiation (Kawauchi et al. 2013).
C. Histone Phosphorylation, Ubiquitination, and Sumoylation
In addition to methylation and acetylation, further post-translational histone modifications comprise phosphorylation, ubiquitination, or sumoylation. These modifications were rather analyzed in yeast than in filamentous fungi. Histone phosphorylations by kinases that transfer the phosphate group from ATP to the hydroxyl group of the modified amino acid take place at serines, threonines, or tyrosines and are reversed by phosphatases. Phosphorylation of H3S10 is crucial for yeast chromosome condensation and cell cycle progression during mitosis and meiosis (Nowak and Corces 2004).
Ubiquitination of histones by ubiquitin ligases is the covalent attachment of the small modifier ubiquitin to lysine residues and is reversed by deubiquitinating enzymes (DUBs, see Sect. IV.B). Methylation of H3K4 is mediated by the ubiquitination of H2BK123 in yeast (Sun and Allis 2002). These concerted histone modifications on distinct histone tails are referred to as trans-tail regulation (Zheng et al. 2010). Similar to ubiquitination, sumoylation is the covalent attachment of the small ubiquitin-like modifier (SUMO) to lysines of proteins through the activity of an E1-E2-E3 enzyme cascade. Sumoylation has been found for all four histones and is associated with transcriptional repression. SUMO, which is required for multicellular fungal development in A. nidulans , most likely blocks the histone modification sites to prevent acetylation or ubiquitination (Nathan et al. 2006; Harting et al. 2013). Co-purification experiments identified SUMO-associated proteins connected to the ubiquitin network involved in A. nidulans histone modification. They include one subunit of the COMPASS complex required for histone methylation as well as subunits of the SAGA complex for histone acetylation (Harting et al. 2013). Like other post-translational modifications, ubiquitin and ubiquitin-like modifications do not only modify histones but also other proteins and thereby can influence their activity, function, localization, or stability and affect fungal secondary metabolism and development (see Sects. III and IV).
D. Epigenetic Tools to Activate Silenced Gene Clusters
The crucial role of histone modifications such as methylation or acetylation in the regulation of secondary metabolism can be utilized in the laboratory for the identification of new secondary metabolites. Epigenetically silenced clusters can be reactivated by changes in chromatin modifications. Deletion of the genes encoding HDACs, which are generally associated with transcriptional repression, can lead to derepression of secondary metabolite gene clusters. Accordingly, penicillin and sterigmatocystin gene clusters of A. nidulans are activated when the HDAC gene hdaA had been deleted (Shwab et al. 2007). These types of experiments have resulted in chemical epigenetics as an emerging new research field (Okada and Seyedsayamdost 2017). The addition of HDAC or methyltransferase inhibitors to fungal cultures alters the epigenetic status of the cells to activate, identify, and study new secondary metabolite gene clusters and their products. The advantage of this method is that the fungus does not have to be genetically modified, and it can therefore be applied to any organism. Examples for such inhibiting chemicals are the HDAC inhibitors valproic acid, trichostatin A (see Sect. III.B) and its synthetic derivative suberoylanilide hydroxamic acid (SAHA), or the DNA methyltransferase inhibitor 5-azacytidine (5-AZA). Successful chemical epigenetics approaches were applied in many different fungi, such as A. alternata and P. expansum (Shwab et al. 2007), Cladosporium cladosporioides and Diatrype disciformis (Williams et al. 2008), A. niger (Fisch et al. 2009; Henrikson et al. 2009), and A. fumigatus (Magotra et al. 2017).
IV. The Role of Ubiquitination and Deubiquitination in Fungal Development and Secondary Metabolism
The importance of post-translational modifications for coordinated fungal development and secondary metabolism is not restricted to histone modifications such as the epigenetic control in connection with transcriptional networks. Target protein modifications by phosphate, ubiquitin, or ubiquitin-like proteins are also essential for the accurate adjustment of the fungal proteome. They alter the physical and chemical properties of a protein in response to external or internal stimuli in order to regulate and control its activity, half-life, transport, and subsequent cellular localization. These processes, which include the highly conserved ubiquitin-26S proteasome pathway (UPP) for protein stability control, are prerequisites for accurate fungal differentiation and physiology.
A. The Ubiquitin Attachment Machinery Influences Fungal Development and Secondary Metabolism on Several Layers
Ubiquitin is the most prominent modifier of the family of ubiquitin-like proteins (UBL), which includes SUMO, the cullin modifier neural precursor cell expressed, developmentally downregulated 8 (Nedd8) or autophagy-related modifiers (Atg8, Atg12; see Sect. V). Ubiquitin consists of 76 amino acids and is encoded as fusion to ribosomal proteins or as head-to-tail fusions of many ubiquitin moieties encompassing two ubiquitin genes in filamentous fungi such as A. nidulans or four in S. cerevisiae (Noventa-Jordão et al. 2000; Lee et al. 2017). These ubiquitin fusion proteins have to be cleaved by deubiquitinating enzymes (DUBs) to create a pool of ubiquitin monomers that can be used for the actual ubiquitination reaction (Grou et al. 2015). Ubiquitin is essential for fungal growth, although single deletion strains can grow but have strong defects in fungal growth, stress response, and development (Leach et al. 2011; Oh et al. 2012).
About 20% of all A. nidulans proteins are ubiquitinated during hyphal growth and are located in the nucleus, whereas in S. cerevisiae the biggest portion is contained in the transmembrane protein fraction (Peng et al. 2003; Chu et al. 2016). Ubiquitin itself contains seven conserved lysine residues (K6, K11, K27, K29, K33, K48, K63), which can be used for ubiquitin chain formation. The attachment of ubiquitin chains linked through a certain lysine residue can alter activity, localization, or stability of the substrate. The K48-linked polyubiquitin chains are the most abundant in yeast and usually mark the modified protein for degradation by the 26S proteasome (Spasser and Brik 2012; Zuin et al. 2014).
The attachment of ubiquitin as well as other ubiquitin-like proteins involves an enzyme cascade consisting of E1 activating, E2 conjugating, and E3 ligase enzymes (Fig. 8.4). An E1 enzyme activates ubiquitin in an ATP-dependent reaction by formation of a thioester bond. The corresponding E1 encoding UBA1 (ubiquitin activating 1) gene of S. cerevisiae is essential for spore formation and vegetative growth (McGrath et al. 1991). The activated ubiquitin molecule is transferred to E2, which can physically interact with E3 ubiquitin ligases. Several yeast genes encoding E2 enzymes as well as the polyubiquitin encoding locus are induced during heat stress or starvation conditions (McGrath et al. 1991; Hiraishi et al. 2006). E3 ubiquitin ligases are classified into the cullin-RING ligase (CRLs) and the HECT (homologous to E6AP carboxyl terminus) ubiquitin ligase families.
Protein degradation pathways and their involvement in growth, development, pathogenicity, and secondary metabolite production. Free ubiquitin is generated from ubiquitin precursors and used to post-translationally label substrates by the concerted action of E1 activating, E2 conjugating, and E3 SCF (SkpA-CulA-Fbox) ligase enzymes. E3 SCF is activated by neddylation and disassembled by deneddylation. Deneddylation is primarily catalyzed by the COP9 signalosome or in addition by DenA. Exchange of the adaptor/receptor complex (SkpA/Fbox) requires binding of the CandA receptor exchange factor. E3 SCF activity results primarily in K48 polyubiquitinated substrates, which are tagged for degradation by the 26S proteasome. Damaged proteasomes, organelles, or aggregated cellular material of misfolded proteins are enclosed by autophagosomes for vacuolar degradation. Fungal proteins that are damaged, misfolded, or only required for a certain timespan are targets for degradation by the 26S proteasome in the nucleus or cytosol. Misfolded ER proteins are recognized by the unfolded protein response (UPR) and the ER-associated degradation (ERAD) pathway and exported to the cytoplasm for proteasomal degradation. Signal transduction pathways (e.g., the target of rapamycin Tor kinase) inhibit the formation of the fungal Atg1 autophagy initiation complex for phagophore assembly site (PAS) in the presence of nutrients. Atg1 is activated by starvation or during development or interaction with other organisms and results in coupling of the ubiquitin-like Atg8 to the lipid phosphatidylethanolamine (PE). Atg8-PE is recruited to PAS, which promotes nucleation and elongation of the phagophore. The phagophore engulfes proteins, protein aggregates or organelles like mitochondria or ribosomes resulting in the double membraned autophagosome. Autophagosomes fuse with the vacuole. The membrane of the resulting autophagic body is degraded by Atg15 lipase and the contents by vacuolar hydrolases. Permeases such as Atg22 can release degraded material back to the cytoplasm for recycling. The budding of vesicles from mitochondria is called mitophagy and the budding from peroxisomes is called pexophagy. Aflatoxin producing secondary metabolite enzymes and their substrates can be transported by cytoplasm-to-vacuole transport (Cvt) vesicles and exported from the vacuole out of the fungal cell by aflatoxisomes
Fungi such as A. nidulans express three cullin proteins (CulA, CulC and CulD) and humans even eight (Marín 2009; von Zeska Kress et al. 2012). CulA, corresponding to human cullin-1, is part of the largest group of CRLs, the SCF (SkpA, CullinA, Fbox) complexes. They are activated by the attachment of Nedd8 to CulA, a process called neddylation. Nedd8 and proteins of the neddylation cascade and the SCF complex are essential for A. nidulans , whereas SUMO-deficient mutants can grow but are impaired in multicellular development and secondary metabolite production (von Zeska Kress et al. 2012; Harting et al. 2013). In S. cerevisiae , it is the other way round, SUMO is essential but not Nedd8 (Liakopoulos et al. 1998).
The active SCF complex requires at one side of the post-translational neddylated cullin scaffold a small RING protein RbxA, which binds to E2-ubiquitin, and at the other side the target substrate, which is connected to the cullin by the general SkpA (suppressor of kinetochore protein mutant A) adaptor. SkpA binds the specific substrate receptors, the Fbox proteins , which differ considerably to interact with different substrates, but share a characteristic N-terminal 40–50 amino acids F-box motif as SkpA interaction domain. The genome of A. nidulans encompasses approximately 70 different Fbox proteins and the recruited target proteins are often primed by phosphorylation prior to ubiquitination (Draht et al. 2007). Fungal adaptation to changing environmental conditions requires the rapid exchange of Fbox proteins at the SCF complexes in order to label and degrade different substrates, what requires the removal of Nedd8 from CulA (Flick and Kaiser 2013). Several Fbox proteins are involved in fungal development. Fbox protein GrrA (glucose repression-resistant A) is needed for the induction of meiosis and thus for the development of mature ascospores in A. nidulans (Krappmann et al. 2006). The corresponding orthologs of the fungal pathogens Cryptococcus neoformans F-box protein 1 (Fbp1) and Gibberella zeae FBP1 are also involved in sexual reproduction and virulence (Han et al. 2007; Liu et al. 2011). A. nidulans Fbx15 is required for asexual and sexual and Fbx23 for light-dependent development (von Zeska Kress et al. 2012). The homologous Fbx15 of the human pathogen A. fumigatus represses the formation of the secondary metabolite gliotoxin and provides a general stress response and virulence to the fungus (Jöhnk et al. 2016).
The disruption of the UPP leads to an imbalanced protein degradation of, e.g., secondary metabolite cluster-specific or global transcription factors. This can be used for the identification of novel interesting secondary metabolites (Gerke et al. 2012; Zheng et al. 2017). For instance, the deletion of csnE (COP9 signalosome E), encoding the catalytically active subunit of the COP9 signalosome, interferes with the deneddylation of cullins, which mediate the ubiquitination of proteins (Beckmann et al. 2015; see Sect. IV.B). In A. nidulans, this deneddylation defect leads to the accumulation of 100 metabolites and resulted in the identification of the antimicrobial 2,4-dihydroxy-3-methyl-6-(2-oxopropyl)benzaldehyde (DHMBA) (Nahlik et al. 2010; Gerke et al. 2012).
B. Controlled Removal of Ubiquitin Family Proteins from Substrates Is Important for Fungal Growth and Development
The attachment of ubiquitin and UBLs such as Nedd8 or SUMO to proteins is a reversible process. Two desumoylating enzymes UlpA and UlpB were characterized in A. nidulans , which are required for asexual conidiospore production and the formation of mature sexual fruiting bodies (Harting et al. 2013).
The removal of Nedd8 (deneddylation) from SCF complexes leads to a conformational change of the cullin protein (Duda et al. 2008) and is performed by deneddylases such as the COP9 signalosome (constitutive photomorphogenesis 9, CSN) (Beckmann et al. 2015). In most fungi, plants or in humans, it is a highly conserved complex consisting of eight CSN subunits including an intrinsic JAMM motif with Nedd8 isopeptidase activity in one subunit. Neurospora crassa and S. pombe possess seven- and six-subunit CSNs, respectively, and in S. cerevisiae there is only the conserved deneddylase subunit as part of an alternative CSN complex. Dysfunction of CSN results in lethality in plants or mammals but not in fungi, which makes them an attractive model organism to study CSN complex assembly and the function of the different subunits (Braus et al. 2010). CSN recognizes substrate free CRLs (Lingaraju et al. 2014) and physically interacts within the nucleus with a second deneddylase Den1/A. This interaction is found in A. nidulans as well as in human cells (Christmann et al. 2013; Schinke et al. 2016). Fungal CSN is composed of an inactive seven-subunit pre-complex, which incorporates the deneddylase subunit in a final step, resulting in a catalytically active complex (Beckmann et al. 2015). In N. crassa , CSN mutant strains result in stabilization of the circadian clock regulator FRQ, defects in light control (see Sect. II.A.2), and impaired asexual development (Wang et al. 2010). Loss of any of the eight CSN subunits of A. nidulans leads to impaired light control, a block in sexual development and a brownish colony with more than a hundred accumulated secondary metabolites including orsellinic acid derivatives (Busch et al. 2003, 2007; Nahlik et al. 2010).
COP9-mediated deneddylation of CulA deactivates E3 SCF ubiquitin ligases and results in disassembly. Cand1 (cullin associated Nedd8 dissociated 1) acts as Fbox receptor exchange factor by blocking the neddylation site of CulA. Multiple cycles of disassembly and assembly ensure the appropriate exchange of different Fbox proteins to degrade different target proteins in different environments or during development (Choo et al. 2011; Wu et al. 2013). Cand1/A is encoded as single protein in higher eukaryotes and fungi like N. crassa , Verticillium dahliae , or the opportunistic pathogen C. albicans , whereas the A. nidulans gene has been split into two separate genes, which encoded proteins are interacting (Helmstaedt et al. 2011; Sela et al. 2012). Deletion of one or both cand genes in A. nidulans leads to impaired conidiospore and fruiting body formation and to a similarly altered secondary metabolism with a brownish colony color as for the defective CSN (Helmstaedt et al. 2011; Nahlik et al. 2010). The deneddylation of cullins, the dissociation of the SkpA adaptor/Fbox receptor complex, the binding of the Cand protein, and the following reactivation of the SCF complex are highly dynamic processes that require tight regulation (Liu et al. 2018).
Deubiquitinases (DUBs) are required for the processing of ubiquitin precursors and for the removal of ubiquitin molecules from target proteins in order to generate the free ubiquitin pool for E1 ubiquitin-activating enzymes (Fig. 8.4). DUBs are classified into seven different families (Komander et al. 2009; Rehman et al. 2016). (1) The JAMM domain metalloprotease DUBs, such as the Rpn11 (regulatory particle non-ATPase 11) of the proteasomal lid,Footnote 6 contain an MPN+ domain and are similar to the deneddylase subunit of CSN (Meister et al. 2016). All other DUBs are cysteine proteases: (2) ubiquitin C-terminal hydrolases (UCH); (3) Machado-Joseph domain (Josephin-domain) containing proteases (MJD), which are missing in some yeasts (Hutchins et al. 2013); (4) ovarian tumor proteases (OTU); (5) ubiquitin-specific proteases (USP); (6) the motif interacting with Ub-containing novel DUB family (MINDY) (Rehman et al. 2016); and (7) the zinc finger with UFM1-specific peptidase domain protein/C6orf113/ZUP1 (ZUFSP) family (Haahr et al. 2018).
The DUB distribution to the different families is quite conserved between fungi and humans with most members in the ubiquitin-specific proteases family with 17 USPs in S. cerevisiae and more than 50 in humans (Hutchins et al. 2013). Any single deletion of the genes for the 17 USP DUBs of S. cerevisiae is viable with only partially moderate growth defects (Amerik et al. 2000). The ubiquitin-specific protease Doa4 (degradation of Alpha 4) is associated to the 26S proteasome and recycles ubiquitin chains from proteins that were targeted for degradation to the 26S proteasome or from membrane proteins that are targeted to the vacuole (Swaminathan et al. 1999). The ubiquitin-specific protease Ubp14 hydrolyzes rather free polyubiquitin chains, which are not degraded by the 26S proteasome as its unfolding would require too much energy (Amerik et al. 2000). The C. neoformans ubiquitin-specific protease Ubp5 is required for virulence and controls melanin and capsule formation (Fang et al. 2012).
V. Protein Degradation Pathways
Clearance of misfolded, damaged , or no longer needed proteins and organelles directs time controlled developmental transitions, multicellular development, pathogenicity, and secondary metabolism in filamentous fungi and leads to recycling of building bricks for the synthesis of new proteins and organelles. Protein degradation is mediated through different pathways (Fig. 8.4) including the nuclear and cytoplasmic ubiquitin-26S proteasome pathway (UPP) and autophagy pathways, which target defective cellular compartments and 26S proteasomes for degradation in the fungal vacuole. Misfolded proteins of the endoplasmic reticulum (ER) activate the unfolded protein response and the ER associated degradation (ERAD) pathway, which finally results in degradation of these proteins in 26S proteasomes of the cytoplasm. The plant pathogenic fungus Ustilago maydis requires the unfolded protein response of the ER for pathogenicity (Heimel et al. 2013; Hampel et al. 2016).
A. Protein Degradation by the 26S Proteasome
The conserved 2.5 MDa 26S proteasome is one of the major degradation machineries in eukaryotes and has approximately half the size of a ribosome. This multi-protease complex consists of the 20S barrel-like core particle and on one or both sides associated 19S regulatory particles consisting of lid and base subcomplexes (Tomko and Hochstrasser 2013). The lid receives ubiquitinated substrates and the base, containing six subunits with ATPase activity, unfolds substrates by ATP hydrolysis, and translocates them into the barrel of the core particle, while the JAMM domain metalloprotease DUB Rpn11 cleaves the ubiquitin chains (Lander et al. 2012; de la Peña et al. 2018). The core particle contains four rings. Each ring is built of seven subunits providing gate and catalytic activity with different trypsin-, chymotrypsin-, or caspase-like peptidases. These degrade substrates processively, resulting in recyclable amino acids (Budenholzer et al. 2017).
26S proteasomal-mediated degradation is initiated by the recognition of K48-polyubiquitinated substrates by Rpn1, Rpn10, or Rpn13, which possess ubiquitin-binding domains (Finley 2009). After capturing these substrates, ubiquitin tags are released by the intrinsic lid Rpn11 deubiquitinase, which is embedded in a similar protein subunit architecture as the intrinsic deneddylase of the COP9 signalosome (Meister et al. 2016; see Sect. IV.A). The eight CSN subunits correspond to eight Rpn (Rpn3, 5–9, 11, 12) subunits of the lid of the regulatory particle. The lid contains as ninth additional subunit the versatile small multifunctional and intrinsically disordered Sem1/Dss1 (suppressor of exocyst mutations 1; deletion of split hand/split foot 1), and a ninth COP9 signalosome subunit (CSN acidic protein) is also present in metazoa and some plants and fungi (Barth et al. 2016).
Sem1 stabilizes the assembly of 26S proteasomes by recruiting the receptors Rpn13 and Rpn10 as well as the tethering factor Ecm29 (extracellular mutant 29) to support complex formation. The A. nidulans sem1 deletion strain is delayed in conidiophore development, has less conidiospores, and produces immature fruiting bodies without ascospores. Sem1 of A. nidulans links fungal stress response to development and controls secondary metabolism similar to CSN or CandA (Kolog Gulko et al. 2018; see Sect. IV.A).
A link between the 26S proteasome of filamentous fungi and development and control of secondary metabolism is further corroborated by the application of proteasome inhibitors such as bortezomib. Proteasome inhibitors delay germination, appressoria formation, and host infection processes of the rice blast fungus M. oryzae (Wang et al. 2011; Oh et al. 2012). Inhibition of the proteasome resulted in the identification of several new compounds in Pleosporales or in the plant pathogen Pestalotiopsis sydowiana (VanderMolen et al. 2014; Xia et al. 2016). Although the targets of the 26S proteasome, which coordinate fungal development and secondary metabolism in these specific cases, are still unknown, it seems likely that they include regulatory proteins with a limited half-life that interconnect different cellular pathways.
B. Degradation by Autophagy
High protein turnover results in damaged fungal proteasomes, which are degraded in yeasts and presumably also in filamentous fungi by self-eating autophagy (Waite et al. 2016; Hoeller and Dikic 2016). This is, besides the proteasomal degradation, the second major eukaryotic degradation system. It is a highly organized membrane-trafficking pathway, specialized for long-lived proteins as well as quality control for large and heterogeneous cellular material including protein aggregates or organelles. Malfunction of this conserved process is, e.g., associated to neurodegenerative diseases in humans as well as impairment of accurate fungal secondary metabolism and differentiation. Autophagy provides nutrients during stress conditions, starvation, or transition phases of developmental programs (Voigt and Pöggeler 2013; Popova et al. 2018, Fig. 8.4). Fungi produce secondary metabolites, which directly affect autophagy such as the beneficial rasfonin from Talaromyces , which reduces different cancer cells by inducing autophagy (Xiao et al. 2014; Sun et al. 2016).
The targeted engulfment of cellular material by membrane invaginations of the vacuole is termed microautophagy. Macroautophagy corresponds to the sequestration of bulk cellular material such as damaged organelles or protein aggregates by de novo formed phagophore, e.g., during yeast starvation (Li et al. 2012; Reggiori et al. 2012). Cargo proteins are engulfed during this process by the double membrane phagophore, which closes to form the autophagosome. This compartment fuses with the fungal vacuole at its outer membrane, while the inner autophagic body is digested by the hydrolytic vacuolar milieu. Autophagy can be either non-selective or selective. Selective autophagy for the degradation of different cellular organelles, e.g., the pexophagy for defective peroxisomes in Sordaria macrospora , requires specific cargo receptors (Werner et al. 2019).
Instead of degradation, selective autophagy pathways can also be used for hydrolytic enzyme transportation to the vacuole as the yeast cytoplasm-to-vacuole transport (Cvt) pathway (Lynch-Day and Klionsky 2010). Fungal secondary metabolism takes place in different cellular compartments such as the cytoplasm, the peroxisomes, vesicles, or vacuoles. The transport of enzymes and metabolic compounds or precursors is therefore often mediated through autophagic pathways. For instance, aflatoxin production requires a complex interplay of different autophagic processes. Vesicles bud from mitochondria and peroxisomes, which deliver precursors and enzymes to Cvt vesicles containing other enzymes of the biosynthetic pathway to form the aflatoxisomes. Aflatoxisomes mediate the biosynthesis by the transport of active enzymes as well as compartmentalization by storing different aflatoxin intermediates and the aflatoxin export to the cell exterior (Chanda et al. 2009; Roze et al. 2011a).
Autophagy-associated atg genes were first identified in the unicellular fungus S. cerevisiae . Until 2016, 42 yeast atg genes were identified including 18 core genes for autophagosome formation, which are mostly conserved in filamentous fungi (Wen and Klionsky 2016; Parzych et al. 2018).
In the initiation phase, starvation-induced autophagy requires an active Atg1 Ser/Thr kinase complex, which is inhibited under non-starvation conditions by the active Tor (target of rapamycin) kinase complex, sensing nitrogen sources, and the cAMP-dependent protein kinase A complex, sensing glucose (Budovskaya et al. 2004; González and Hall 2017). In the following nucleation phase, Atg1 complex kinase activity results in the assembly of several other Atg proteins and the phosphatidylinositol kinase Vps34, which action recruits transmembrane protein Atg9-containing vesicles for the transport to the phagophore assembly site (He et al. 2008; Stjepanovic et al. 2017). Phagophore expansion as the third phase includes two ubiquitin-like conjugation systems (Atg8, Atg12), which cooperate to transfer Atg8 to the lipid phosphatidylethanolamine (PE). Conjugation of Atg8 to Atg8-PE requires a similar mechanism of E1 activating, E2 conjugating, and E3 ligase cascade as previously described for other ubiquitin-like proteins (Liu et al. 2010b; see Sect. IV.A). After the fusion of the outer phagosomal membrane with the vacuolar membrane, vacuolar lipases such as Atg15 degrade the membrane of the released autophagic body. The permease Atg22 exports the degradation products into the cytoplasm for recycling (Sugimoto et al. 2011; Ramya and Rajasekharan 2016; Fig. 8.4).
Numerous fungal atg genes are essential for growth or are required for development, pathogenicity, or the formation of secondary metabolites. An A. fumigatus atg1 deletion strain is impaired in vegetative growth in the presence of different nitrogen sources and produces less asexual spores, but is not affected in pathogenicity (Richie et al. 2007; Richie and Askew 2008). A Penicillium chrysogenum atg1 mutant strain shows increased penicillin production due to an increased number of peroxisomes as a result of impaired pexophagy (Bartoszewska et al. 2011). Penicillin production takes place in the cytosol and in peroxisomes (Meijer et al. 2010). The relocation of AcvA, the first enzyme of the penicillin biosynthesis (δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine synthetase), from the cytosol to the peroxisomes significantly increased the penicillin production of A. nidulans by more than three-fold (Herr and Fischer 2014).
The genes for the ubiquitin-like Atg12 from different yeasts are required for ascosporogenesis under nitrogen starvation, and the corresponding gene of N. crassa supports efficient fruiting body formation (Mukaiyama et al. 2010; Chinnici et al. 2014). In contrast, a M. oryzae atg12-deficient strain can undergo sexual development but is impaired in host infection. Whereas the atg genes participating in selective autophagy are dispensable, 22 non-selective M. oryzae atg genes are required for pathogenicity (Kershaw and Talbot 2009). This includes non-selective autophagy mutants, which are unable to transport the cellular content from the conidiospore into the appressoria, impairing leaf penetration.
Defects in the UBL encoding gene atg8 as well as in several other atg genes result in impaired vegetative growth and a block in sexual development in S. macrospora (Nolting et al. 2009; Voigt and Pöggeler 2013). Both autophagy UBLs are involved in aging of hyphae, which change in their thickness from young thin to old thick hyphae. Deletion of atg8 for the UBL or of atg10 encoding the E2 conjugating enzyme for the Atg12 UBL shortened this aging process in A. niger (Nitsche et al. 2013). Aging of hyphae by autophagy might be due to degradation of organelles in older hyphae, which provides nutrients and building bricks for tip growth (Shoji et al. 2010). The degradation by mitophagy of damaged mitochondria increases presumably also fungal life span, because it prevents the accumulation of ROS or proapoptotic factors (Tyler and Johnson 2018).
Autophagy ensures survival during starvation, mediates nutrient supply for ascospore formation, and transports hydrolytic enzymes into the vacuoles in yeast. Autophagy protects cells against toxic metabolites or macromolecules and damaged organelles by their degradation and export. In ascomycetes, autophagy is required for development under starvation and nutrient-rich conditions. Furthermore, pathogenicity and secondary metabolism rely on autophagy.
VI. Conclusion
The burden of infectious diseases caused by bacteria and fungi, which are resistant to the usual antibiotics or antimycotics such as azoles, is growing (Cassini et al. 2019; Lestrade et al. 2019). Filamentous fungi form a vast reservoir of yet unknown beneficial or toxic secondary metabolites, which are only produced as responses to environmental biotic or abiotic stimuli. A more comprehensive picture of the complex and subtle control of transcriptional networks, which are nested within each other, are more and more emerging. They interconnect distinct fungal developmental programs with the production of specific secondary metabolites. This includes the genetic networks of the fungal velvet domain proteins and their sub-networks, which link transcriptional to epigenetic control of gene expression, but also protein degradation machineries like the 26S proteasome and the autophagy membrane trafficking pathway. They control maintenance or changes of fungal proteomes as well as cellular localization and transport of proteins as additional levels of coordinating secondary metabolite production in response to environmental parameters or during development, aging, or pathogenesis. Manipulation of gene expression including chemical epigenetics as well as genetic or chemical reprogramming of fungal protein degradation are promising approaches to find new biologically active secondary metabolites acting as antibiotics or as antifungal drugs.
Notes
- 1.
Ascus = skin bag.
- 2.
Virulence = severity of a disease caused by a pathogen.
- 3.
Pathogenicity = ability of a pathogen to cause a disease.
- 4.
H3R4 = histone H4 modified at arginine residue 3 (R3).
- 5.
H3K4Me3 = histone H3 trimethylated (Me3) at lysine residue 4 (K4).
- 6.
lid = removable or hinged cover of a container, here: the cover of the proteasome core.
References
Adams TH, Wieser JK, Yu JH (1998) Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev 62:35–54
Adhvaryu KK, Morris SA, Strahl BD, Selker EU (2005) Methylation of histone H3 lysine 36 is required for normal development in Neurospora crassa. Eukaryot Cell 4:1455–1464
Ahmed YL, Gerke J, Park H-S, Bayram Ö, Neumann P, Ni M, Dickmanns A, Kim SC, Yu J-H, Braus GH, Ficner R (2013) The velvet family of fungal regulators contains a DNA-binding domain structurally similar to NF-κB. PLoS Biol 11:e1001750
Alberti F, Foster GD, Bailey AM (2017) Natural products from filamentous fungi and production by heterologous expression. Appl Microbiol Biotechnol 101:493–500
Amerik AY, Li S, Hochstrasser M (2000) Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae. Biol Chem 381:981–992
Andersen MR, Nielsen JB, Klitgaard A, Petersen LM, Zachariasen M, Hansen TJ, Blicher LH, Gotfredsen CH, Larsen TO, Nielsen KF, Mortensen UH (2013) Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc Natl Acad Sci USA 110:E99–E107
Bachand F (2007) Protein arginine methyltransferases: from unicellular eukaryotes to humans. Eukaryot Cell 6:889–898
Bahn Y-S, Xue C, Idnurm A, Rutherford JC, Heitman J, Cardenas ME (2007) Sensing the environment: lessons from fungi. Nat Rev Microbiol 5:57–69
Barth E, Hübler R, Baniahmad A, Marz M (2016) The evolution of COP9 signalosome in unicellular and multicellular organisms. Genome Biol Evol 8:1279–1289
Bartoszewska M, Kiel JAKW, Bovenberg RAL, Veenhuis M, van der Klei IJ (2011) Autophagy deficiency promotes beta-lactam production in Penicillium chrysogenum. Appl Environ Microbiol 77:1413–1422
Bauer I, Graessle S, Loidl P, Hohenstein K, Brosch G (2010) Novel insights into the functional role of three protein arginine methyltransferases in Aspergillus nidulans. Fungal Genet Biol 47:551–561
Bauer I, Varadarajan D, Pidroni A, Gross S, Vergeiner S, Faber B, Hermann M, Tribus M, Brosch G, Graessle S, Turgeon EBG (2016) A class 1 histone deacetylase with potential as an antifungal target. MBio 7:e00831–e00816
Bayram Ö, Braus GH (2012) Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev 36:1–24
Bayram O, Biesemann C, Krappmann S, Galland P, Braus GH (2008a) More than a repair enzyme: Aspergillus nidulans photolyase-like CryA is a regulator of sexual development. Mol Biol Cell 19:3254–3262
Bayram Ö, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu J, Braus GH (2008b) VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504–1506
Bayram Ö, Krappmann S, Seiler S, Vogt N, Braus GH (2008c) Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet Biol 45:127–138
Bayram Ö, Braus GH, Fischer R, Rodriguez-Romero J (2010) Spotlight on Aspergillus nidulans photosensory systems. Fungal Genet Biol 47:900–908
Bayram Ö, Feussner K, Dumkow M, Herrfurth C, Feussner I, Braus GH (2016) Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet Biol 87:30–53
Becker K, Ziemons S, Lentz K, Freitag M, Kück U (2016) Genome-wide chromatin immunoprecipitation sequencing analysis of the Penicillium chrysogenum velvet protein PcVelA identifies methyltransferase PcLlmA as a novel downstream regulator of fungal development. mSphere 1:e00149–e00116
Beckmann EA, Köhler AM, Meister C, Christmann M, Draht OW, Rakebrandt N, Valerius O, Braus GH (2015) Integration of the catalytic subunit activates deneddylase activity in vivo as final step in fungal COP9 signalosome assembly. Mol Microbiol 97:110–124
Bicocca VT, Ormsby T, Adhvaryu KK, Honda S, Selker EU (2018) ASH1-catalyzed H3K36 methylation drives gene repression and marks H3K27me2/3-competent chromatin. Elife 7:e41497
Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R (2005) The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15:1833–1838
Bode HB, Bethe B, Höfs R, Zeeck A (2002) Big effects from small changes: possible ways to explore nature’s chemical diversity. ChemBioChem 3:619–627
Bok JW, Keller NP (2016) Insight into fungal secondary metabolism from ten years of LaeA research. In: Hoffmeister D (ed) Biochemistry and molecular biology: the Mycota (A comprehensive treatise on fungi as experimental systems for basic and applied research), vol III. Springer International Publishing, Cham, pp 21–29
Braus GH, Irniger S, Bayram Ö (2010) Fungal development and the COP9 signalosome. Curr Opin Microbiol 13:672–676
Brodhun F, Feussner I (2011) Oxylipins in fungi. FEBS J 278:1047–1063
Brosch G, Loidl P, Graessle S (2008) Histone modifications and chromatin dynamics: a focus on filamentous fungi. FEMS Microbiol Rev 32:409–439
Brown NA, Schrevens S, van Dijck P, Goldman GH (2018) Fungal G-protein-coupled receptors: mediators of pathogenesis and targets for disease control. Nat Microbiol 3:402–414
Budenholzer L, Cheng CL, Li Y, Hochstrasser M (2017) Proteasome structure and assembly. J Mol Biol 429:3500–3524
Budovskaya Y, Stephan J, Reggiori F, Klionsky DJ, Herman PK (2004) The Ras/PKA signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J Biol Chem 279:20663
Busch S, Eckert SE, Krappmann S, Braus GH (2003) The COP9 signalosome is an essential regulator of development in the filamentous fungus Aspergillus nidulans. Mol Microbiol 49:717–730
Busch S, Schwier EU, Nahlik K, Bayram Ö, Helmstaedt K, Draht OW, Krappmann S, Valerius O, Lipscomb WN, Braus GH (2007) An eight-subunit COP9 signalosome with an intact JAMM motif is required for fungal fruit body formation. Proc Natl Acad Sci USA 104:8089–8094
Cassini A, Diaz Högberg L, Plachouras D, Quattrocchi A, Hoxha A, Skov Simonsen G (2019) Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19:56–66
Castrillo M, García-Martínez J, Avalos J (2013) Light-dependent functions of the Fusarium fujikuroi CryD DASH cryptochrome in development and secondary metabolism. Appl Environ Microbiol 79:2777–2788
Cetz-Chel JE, Balcázar-López E, Esquivel-Naranjo EU, Herrera-Estrella A (2016) The Trichoderma atroviride putative transcription factor Blu7 controls light responsiveness and tolerance. BMC Genomics 17:327
Chanda A, Roze L, Kang S, Artymovich KA, Hicks GR, Raikhel N, Calvo AM, Linz JE (2009) A key role for vesicles in fungal secondary metabolism. Proc Natl Acad Sci USA 106:19533–19538
Chiang YM, Oakley CE, Ahuja M, Entwistle R, Schultz A, Chang S-L, Sung CT, Wang CCC, Oakley BR (2013) An efficient system for heterologous expression of secondary metabolite genes in Aspergillus nidulans. J Am Chem Soc 135:7720–7731
Chinnici JL, Fu C, Caccamise LM, Arnold JW, Free SJ (2014) Neurospora crassa female development requires the PACC and other signal transduction pathways, transcription factors, chromatin remodeling, cell-to-cell fusion, and autophagy. PLoS One 9:e110603
Choo YY, Boh BK, Lou JJW, Eng J, Leck YC, Anders B, Smith PG, Hagen T (2011) Characterization of the role of COP9 signalosome in regulating cullin E3 ubiquitin ligase activity. Mol Biol Cell 22:4706–4715
Christmann M, Schmaler T, Gordon C, Huang X, Bayram Ö, Schinke J, Stumpf S, Dubiel W, Braus GH (2013) Control of multicellular development by the physically interacting deneddylases DEN1/DenA and COP9 signalosome. PLoS Genet 9:e1003275
Chu X-L, Feng M-G, Ying S-H (2016) Qualitative ubiquitome unveils the potential significances of protein lysine ubiquitination in hyphal growth of Aspergillus nidulans. Curr Genet 62:191–201
Connolly LR, Smith KM, Freitag M (2013) The Fusarium graminearum histone H3 K27 methyltransferase KMT6 regulates development and expression of secondary metabolite gene clusters. PLoS Genet 9:e1003916
de la Peña AH, Goodall EA, Gates SN, Lander GC, Martin A (2018) Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation. Science 362:eaav0725
de Vries RP, Riley R, Wiebenga A, Aguilar-Osorio G, Amillis S, Uchima CA, Anderluh G, Asadollahi M, Askin M, Barry K, Battaglia E, Bayram Ö, Benocci T, Braus-Stromeyer SA, Caldana C, Cánovas D, Cerqueira GC, Chen F, Chen W, Choi C, Clum A, dos Santos RAC, de Damásio ARL, Diallinas G, Emri T, Fekete E, Flipphi M, Freyberg S, Gallo A, Gournas C, Habgood R, Hainaut M, Harispe ML, Henrissat B, Hildén KS, Hope R, Hossain A, Karabika E, Karaffa L, Karányi Z, Kraševec N, Kuo A, Kusch H, LaButti K, Lagendijk EL, Lapidus A, Levasseur A, Lindquist E, Lipzen A, Logrieco AF, MacCabe A, Mäkelä MR, Malavazi I, Melin P, Meyer V, Mielnichuk N, Miskei M, Molnár ÁP, Mulé G, Ngan CY, Orejas M, Orosz E, Ouedraogo JP, Overkamp KM, Park H-S, Perrone G, Piumi F, Punt PJ, Ram AFJ, Ramón A, Rauscher S, Record E, Riaño-Pachón DM, Robert V, Röhrig J, Ruller R, Salamov A, Salih NS, Samson RA, Sándor E, Sanguinetti M, Schütze T, Sepčić K, Shelest E, Sherlock G, Sophianopoulou V, Squina FM, Sun H, Susca A, Todd RB, Tsang A, Unkles SE, van de Wiele N, van Rossen-Uffink D, de Oliveira JVC, Vesth TC, Visser J, Yu J-H, Zhou M, Andersen MR, Archer DB, Baker SE, Benoit I, Brakhage AA, Braus GH, Fischer R, Frisvad JC, Goldman GH, Houbraken J, Oakley B, Pócsi I, Scazzocchio C, Seiboth B, vanKuyk PA, Wortman J, Dyer PS, Grigoriev IV (2017) Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol 18:28
Draht O, Busch S, Hofmann K, Braus-Stromeyer S, Helmstaedt K, Goldman G, Braus G (2007) Amino acid supply of Aspergillus. In: Osmani SA, Goldman GH (eds) The Aspergilli: genomics, medical aspects, biotechnology, and research methods. CRC Press/Taylor & Francis Group, Boca Raton, FL, pp 143–175
Du L, Sánchez C, Shen B (2001) Hybrid peptide–polyketide natural products: biosynthesis and prospects toward engineering novel molecules. Metab Eng 3:78–95
Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA (2008) Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134:995–1006
Estrada AF, Avalos J (2008) The white collar protein WcoA of Fusarium fujikuroi is not essential for photocarotenogenesis, but is involved in the regulation of secondary metabolism and conidiation. Fungal Genet Biol 45:705–718
Fang W, Price MS, Toffaletti DL, Tenor J, Betancourt-Quiroz M, Price JL, Pan W, Liao W, Perfect JR (2012) Pleiotropic effects of deubiquitinating enzyme Ubp5 on growth and pathogenesis of Cryptococcus neoformans. PLoS One 7:38326
Finley D (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78:477–513
Fisch KM, Gillaspy AF, Gipson M, Henrikson JC, Hoover AR, Jackson L, Najar FZ, Wägele H, Cichewicz RH (2009) Biosynthetic pathway transcription in Aspergillus niger. J Ind Microbiol Biotechnol 36:1199–1213
Fischer R, Aguirre J, Herrera-Estrella A, Corrochano LM (2016) The complexity of fungal vision. Microbiol Spectr 4:1–22
Flick K, Kaiser P (2013) Set them free: F-box protein exchange by Cand1. Cell Res 23:870–871
Gacek-Matthews A, Noble LM, Gruber C, Berger H, Sulyok M, Marcos AT, Strauss J, Andrianopoulos A (2015) KdmA, a histone H3 demethylase with bipartite function, differentially regulates primary and secondary metabolism in Aspergillus nidulans. Mol Microbiol 96:839–860
Gacek-Matthews A, Berger H, Sasaki T, Wittstein K, Gruber C, Lewis ZA, Strauss J (2016) KdmB, a jumonji histone H3 demethylase, regulates genome-wide H3K4 trimethylation and is required for normal induction of secondary metabolism in Aspergillus nidulans. PLoS Genet 12:e1006222
García-Esquivel M, Esquivel-Naranjo EU, Hernández-Oñate MA, Ibarra-Laclette E, Herrera-Estrella A (2016) The Trichoderma atroviride cryptochrome/photolyase genes regulate the expression of blr1-independent genes both in red and blue light. Fungal Biol 120:500–512
García-Martínez J, Brunk M, Avalos J, Terpitz U (2015) The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination. Sci Rep 5:7798
Gerke J, Braus GH (2014) Manipulation of fungal development as source of novel secondary metabolites for biotechnology. Appl Microbiol Biotechnol 98:8443–8455
Gerke J, Bayram Ö, Feussner K, Landesfeind M, Shelest E, Feussner I, Braus GH (2012) Breaking the silence: protein stabilization uncovers silenced biosynthetic gene clusters in the fungus Aspergillus nidulans. Appl Environ Microbiol 78:8234–8244
Gessler NN, Aver AA, Belozerskaya TA (2007) Reactive oxygen species in regulation of fungal development. Biochemist 72:1091–1109
González A, Hall MN (2017) Nutrient sensing and TOR signaling in yeast and mammals. EMBO J 36:397–408
Greer EL, Shi Y (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13:343–357
Gressler M, Hortschansky P, Geib E, Brock M (2015) A new high-performance heterologous fungal expression system based on regulatory elements from the Aspergillus terreus terrein gene cluster. Front Microbiol 6:184
Grimaldi B, Coiro P, Filetici P, Berge E, Dobosy JR, Freitag M, Selker EU, Ballario P (2006) The Neurospora crassa white collar-1 dependent blue light response requires acetylation of histone H3 lysine 14 by NGF-1. Mol Biol Cell 17:4576–4583
Grou CP, Pinto MP, Mendes AV, Domingues P, Azevedo JE (2015) The de novo synthesis of ubiquitin: identification of deubiquitinases acting on ubiquitin precursors. Sci Rep 5:12836
Gu Q, Wang Z, Sun X, Ji T, Huang H, Yang Y, Zhang H, Tahir HAS, Wu L, Wu H, Gao X (2017) FvSet2 regulates fungal growth, pathogenicity, and secondary metabolism in Fusarium verticillioides. Fungal Genet Biol 107:24–30
Haahr P, Borgermann N, Guo X, Typas D, Achuthankutty D, Hoffmann S, Shearer R, Sixma TK, Mailand N (2018) ZUFSP deubiquitylates K63-linked polyubiquitin chains to promote genome stability. Mol Cell 70:165–174.e6
Haas H (2014) Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat Prod Rep 31:1266–1276
Hampel M, Jakobi M, Schmitz L, Meyer U, Finkernagel F, Doehlemann G, Heimel K (2016) Unfolded protein response (UPR) regulator Cib1 controls expression of genes encoding secreted virulence factors in Ustilago maydis. PLoS One 11:e0153861
Han Y, Kim M, Lee S, Yun S, Lee Y (2007) A novel F-box protein involved in sexual development and pathogenesis in Gibberella zeae. Mol Microbiol 63:768–779
Harting R, Bayram Ö, Laubinger K, Valerius O, Braus GH (2013) Interplay of the fungal sumoylation network for control of multicellular development. Mol Microbiol 90:1125–1145
He C, Baba M, Cao Y, Klionsky DJ (2008) Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol Biol Cell 19:5506–5516
Hedtke M, Rauscher S, Röhrig J, Rodríguez-Romero J, Yu Z, Fischer R (2015) Light-dependent gene activation in Aspergillus nidulans is strictly dependent on phytochrome and involves the interplay of phytochrome and white collar-regulated histone H3 acetylation. Mol Microbiol 97:733–745
Heimel K, Freitag J, Hampel M, Ast J, Bolker M, Kamper J (2013) Crosstalk between the unfolded protein response and pathways that regulate pathogenic development in Ustilago maydis. Plant Cell 25:4262–4277
Helmstaedt K, Schwier EU, Christmann M, Nahlik K, Tansey WP (2011) Recruitment of the inhibitor Cand1 to the cullin substrate adaptor site mediates interaction to the neddylation site. Mol Biol Cell 22:153–164
Henrikson JC, Hoover AR, Joyner PM, Cichewicz RH (2009) A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org Biomol Chem 7:435–438
Herr A, Fischer R (2014) Improvement of Aspergillus nidulans penicillin production by targeting AcvA to peroxisomes. Metab Eng 25:131–139
Hiraishi H, Mochizuki M, Takagi H (2006) Enhancement of stress tolerance in Saccharomyces cerevisiae by overexpression of ubiquitin ligase Rsp5 and ubiquitin-conjugating enzymes. Biosci Biotechnol Biochem 70:2762–2765
Hoeller D, Dikic I (2016) How the proteasome is degraded. Proc Natl Acad Sci USA 113:13266–13268
Holighaus G, Rohlfs M (2018) Volatile and non-volatile fungal oxylipins in fungus-invertebrate interactions. Fungal Ecol 38:28–36
Hutchins AP, Liu S, Diez D, Miranda-Saavedra D (2013) The repertoires of ubiquitinating and deubiquitinating enzymes in eukaryotic genomes. Mol Biol Evol 30:1172–1187
Idnurm A, Verma S, Corrochano LM (2010) A glimpse into the basis of vision in the kingdom Mycota. Fungal Genet Biol 47:881–892
Inglis DO, Binkley J, Skrzypek MS, Arnaud MB, Cerqueira GC, Shah P, Wymore F, Wortman JR, Sherlock G (2013) Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol 13:91
Itoh E, Shigemoto R, Oinuma K-I, Shimizu M, Masuo S, Takaya N (2017) Sirtuin A regulates secondary metabolite production by Aspergillus nidulans. J Gen Appl Microbiol 63:228–235
Izawa M, Takekawa O, Arie T, Teraoka T, Yoshida M, Kimura M, Kamakura T (2009) Inhibition of histone deacetylase causes reduction of appressorium formation in the rice blast fungus Magnaporthe oryzae. J Gen Appl Microbiol 55:489–498
Janevska S, Baumann L, Sieber CMK, Münsterkötter M, Ulrich J, Kämper J, Güldener U, Tudzynski B (2018) Elucidation of the two H3K36me3 histone methyltransferases Set2 and Ash1 in Fusarium fujikuroi unravels their different chromosomal targets and a major impact of Ash1 on genome stability. Genetics 208:153–171
Jöhnk B, Bayram Ö̈, Abelmann A, Heinekamp T, Mattern DJ, Brakhage AA, Jacobsen ID, Valerius O, Braus GH (2016) SCF ubiquitin ligase F-box protein Fbx15 controls nuclear co-repressor localization, stress response and virulence of the human pathogen Aspergillus fumigatus. PLoS Pathog 12:1–10
Kawauchi M, Iwashita K (2014) Functional analysis of histone deacetylase and its role in stress response, drug resistance and solid-state cultivation in Aspergillus oryzae. J Biosci Bioeng 118:172–176
Kawauchi M, Nishiura M, Iwashita K (2013) Fungal-specific sirtuin HstD coordinates secondary metabolism and development through control of LaeA. Eukaryot Cell 12:1087–1096
Keller NP (2018) Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol 17:167–180
Keller NP, Turner G, Bennett JW (2005) Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol 3:937–947
Kershaw MJ, Talbot NJ (2009) Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc Natl Acad Sci USA 106:15967–15972
Kolog Gulko M, Heinrich G, Gross C, Popova B, Valerius O, Neumann P, Ficner R, Braus GH (2018) Sem1 links proteasome stability and specificity to multicellular development. PLoS Genet 14:e1007141
Komander D, Clague MJ, Urbé S (2009) Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 10:550–563
Kong X, van Diepeningen AD, van der Lee TAJ, Waalwijk C, Xu J, Xu J, Zhang H, Chen W, Feng J (2018) The Fusarium graminearum histone acetyltransferases are important for morphogenesis, DON biosynthesis, and pathogenicity. Front Microbiol 9:654
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Krappmann S, Jung N, Medic B, Busch S, Prade RA, Braus GH (2006) The Aspergillus nidulans F-box protein GrrA links SCF activity to meiosis. Mol Microbiol 61:76–88
Kumar D, Barad S, Chen Y, Luo X, Tannous J, Dubey A, Glam Matana N, Tian S, Li B, Keller N, Prusky D (2016) LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose. Mol Plant Pathol 18:1150–1163
Künzler M (2018) How fungi defend themselves against microbial competitors and animal predators. PLoS Pathog 14:e1007184
Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A (2012) Complete subunit architecture of the proteasome regulatory particle. Nature 482:186–191
Lara-Ortíz T, Riveros-Rosas H, Aguirre J (2003) Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol 50:1241–1255
Leach MD, Stead DA, Argo E, Maccallum DM, Brown AJP (2011) Molecular and proteomic analyses highlight the importance of ubiquitination for the stress resistance, metabolic adaptation, morphogenetic regulation and virulence of Candida albicans. Mol Microbiol 79:1574–1593
Lee Y-H, Stallcup MR (2009) Minireview: protein arginine methylation of nonhistone proteins in transcriptional regulation. Mol Endocrinol 23:425–433
Lee I, Oh J-H, Keats Shwab E, Dagenais TRT, Andes D, Keller NP (2009) HdaA, a class 2 histone deacetylase of Aspergillus fumigatus, affects germination and secondary metabolite production. Fungal Genet Biol 46:782–790
Lee S, Tumolo JM, Ehlinger AC, Jernigan KK, Qualls-Histed SJ, Hsu P-C, Mcdonald H, Chazin WJ, Macgurn JA (2017) Ubiquitin turnover and endocytic trafficking in yeast are regulated by Ser57 phosphorylation of ubiquitin. Elife 6:e29176
Lestrade PP, Bentvelsen RG, Schauwvlieghe AFAD, Schalekamp S, van der Velden WJFM, Kuiper EJ, van Paassen J, van der Hoven B, van der Lee HA, Melchers WJG, de Haan AF, van der Hoeven HL, Rijnders BJA, van der Beek MT, Verweij PE (2019) Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clin Infect Dis 68:1463–1471
Li W, Li J, Bao J (2012) Microautophagy: lesser-known self-eating. Cell Mol Life Sci 69:1125–1136
Liakopoulos D, Doenges G, Matuschewski K, Jentsch S (1998) A novel protein modification pathway related to the ubiquitin system. EMBO J 17:2208–2214
Liang L, Liu Y, Yang K, Lin G, Xu Z, Lan H, Wang X, Wang S (2017) The putative histone methyltransferase DOT1 regulates aflatoxin and pathogenicity attributes in Aspergillus flavus. Toxins 9:232
Lim FY, Won TH, Raffa N, Baccile JA, Wisecaver J, Rokas A, Schroeder FC, Keller NP (2018) Fungal isocyanide synthases and xanthocillin biosynthesis in Aspergillus fumigatus. MBio 9:e00785–e00718
Lind AL, Wisecaver JH, Smith TD, Feng X, Calvo AM, Rokas A (2015) Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus Aspergillus. PLoS Genet 11:e1005096
Lind AL, Smith TD, Saterlee T, Calvo AM, Rokas A (2016) Regulation of secondary metabolism by the velvet complex is temperature-responsive in Aspergillus. G3 6:4023–4033
Lingaraju GM, Bunker RD, Cavadini S, Hess D, Hassiepen U, Renatus M, Fischer ES, Thomä NH (2014) Crystal structure of the human COP9 signalosome. Nature 512:161–165
Liu C, Lu F, Cui X, Cao X (2010a) Histone methylation in higher plants. Annu Rev Plant Biol 61:395–420
Liu T-B, Liu X-H, Lu J-P, Zhang L, Min H, Lin F-C (2010b) The cysteine protease MoAtg4 interacts with MoAtg8 and is required for differentiation and pathogenesis in Magnaporthe oryzae. Autophagy 6:74–85
Liu T, Wang Y, Stukes S, Chen Q, Casadevall A, Xue C (2011) The F-Box protein Fbp1 regulates sexual reproduction and virulence in Cryptococcus neoformans. Eukaryot Cell 10:791–802
Liu X, Reitsma JM, Mamrosh JL, Zhang Y, Straube R, Deshaies RJ (2018) Cand1-mediated adaptive exchange mechanism enables variation in F-box protein expression. Mol Cell 69:773–786.e6
Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, Tollot M, Zuccaro A, Reissmann S, Kahmann R (2015) Fungal effectors and plant susceptibility. Annu Rev Plant Biol 66:513–545
López-Díaz C, Rahjoo V, Sulyok M, Ghionna V, Martín-Vicente A, Capilla J, Di Pietro A, López-Berges MS (2018) Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol Plant Pathol 19:440–453
Lynch-Day MA, Klionsky DJ (2010) The Cvt pathway as a model for selective autophagy. FEBS Lett 584:1359–1366
Macheleidt J, Mattern DJ, Fischer J, Netzker T, Weber J, Schroeckh V, Valiante V, Brakhage AA (2016) Regulation and role of fungal secondary metabolites. Annu Rev Genet 50:371–392
Magotra A, Kumar M, Kushwaha M, Awasthi P, Raina C, Gupta AP, Shah BA, Gandhi SG, Chaubey A (2017) Epigenetic modifier induced enhancement of fumiquinazoline C production in Aspergillus fumigatus (GA-L7): an endophytic fungus from Grewia asiatica L. AMB Express 7:43
Malzahn E, Ciprianidis S, Káldi K, Schafmeier T, Brunner M (2010) Photoadaptation in Neurospora by competitive interaction of activating and inhibitory LOV domains. Cell 142:762–772
Marín I (2009) Diversification of the cullin family. BMC Evol Biol 11:1–11
Matsuda Y, Abe I (2016) Biosynthesis of fungal meroterpenoids. Nat Prod Rep 33:26–53
McGrath JP, Jentsch S, Varshavsky A (1991) Uba1: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J 10:227–236
Meeks JJ, Shilatifard A (2017) Multiple roles for the MLL/COMPASS family in the epigenetic regulation of gene expression and in cancer. Annu Rev Cancer Biol 1:425–446
Meijer WH, Gidijala L, Fekken S, Kiel JAKW, van den Berg MA, Lascaris R, Bovenberg RAL, van der Klei IJ (2010) Peroxisomes are required for efficient penicillin biosynthesis in Penicillium chrysogenum. Appl Environ Microbiol 76:5702–5709
Meister C, Gulko MK, Köhler AM, Braus GH (2016) The devil is in the details: comparison between COP9 signalosome (CSN) and the LID of the 26S proteasome. Curr Genet 62:129–136
Meyer V, Andersen MR, Brakhage AA, Braus GH, Caddick MX, Cairns TC, de Vries RP, Haarmann T, Hansen K, Hertz-Fowler C, Krappmann S, Mortensen UH, Peñalva MA, Ram AFJ, Head RM (2016) Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: a white paper. Fungal Biol Biotechnol 3:6
Mukaiyama H, Nakase M, Nakamura T, Kakinuma Y, Takegawa K (2010) Autophagy in the fission yeast Schizosaccharomyces pombe. FEBS Lett 584:1327–1334
Nahlik K, Dumkow M, Bayram Ö, Helmstaedt K, Busch S, Valerius O, Gerke J, Hoppert M, Schwier E, Opitz L, Westermann M, Grond S, Feussner K, Goebel C, Kaever A, Meinicke P, Feussner I, Braus GH (2010) The COP9 signalosome mediates transcriptional and metabolic response to hormones, oxidative stress protection and cell wall rearrangement during fungal development. Mol Microbiol 78:964–979
Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, Whelan KA, Krsmanovic M, Lane WS, Meluh PB, Johnson ES, Berger SL (2006) Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev 20:966–976
Netzker T, Fischer J, Weber J, Mattern DJ, König CC, Valiante V, Schroeckh V, Brakhage AA (2015) Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front Microbiol 6:299
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Ni M, Yu JH (2007) A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2:e970
Nie X, Li B, Wang S (2018) Epigenetic and posttranslational modifications in regulating the biology of Aspergillus species. In: Gadd GM, Sariaslani S (eds) Advances in applied microbiology. Academic Press, London, pp 191–226
Nitsche BM, Burggraaf-van Welzen A-M, Lamers G, Meyer V, Ram AFJ (2013) Autophagy promotes survival in aging submerged cultures of the filamentous fungus Aspergillus niger. Appl Microbiol Biotechnol 97:8205–8218
Nocedal I, Mancera E, Johnson AD (2017) Gene regulatory network plasticity predates a switch in function of a conserved transcription regulator. Elife 6:e23250
Nolting N, Bernhards Y, Pöggeler S (2009) SmATG7 is required for viability in the homothallic ascomycete Sordaria macrospora. Fungal Genet Biol 46:531–542
Noventa-Jordão MA, Mendes do Nascimento A, Goldman MHS, Terenzi HF, Goldman GH (2000) Molecular characterization of ubiquitin genes from Aspergillus nidulans: mRNA expression on different stress and growth conditions. Biochim Biophys Acta Mol Cell Res 1490:237–244
Nowak SJ, Corces VG (2004) Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet 20:214–220
Nsa IY, Karunarathna N, Liu X, Huang H, Boetteger B, Bell-Pedersen D (2015) A novel cryptochrome-dependent oscillator in Neurospora crassa. Genetics 199:233–245
Nützmann H-W, Reyes-Dominguez Y, Scherlach K, Schroeckh V, Horn F, Gacek A, Schümann J, Hertweck C, Strauss J, Brakhage AA (2011) Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc Natl Acad Sci USA 108:14282–14287
Oakley CE, Ahuja M, Sun W, Entwistle R, Akashi T, Yaegashi J, Guo C, Cerqueira GC, Wortman JR, Clay C, Wang C, Chiang Y, Oakley BR (2016) Discovery of McrA, a master regulator of Aspergillus secondary metabolism. Mol Microbiol 103:347–365
Oh Y, Franck WL, Han SO, Shows A, Gokce E, Muddiman DC, Dean RA (2012) Polyubiquitin is required for growth, development and pathogenicity in the rice blast fungus Magnaporthe oryzae. PLoS One 7:e42868
Okada BK, Seyedsayamdost MR (2017) Antibiotic dialogues: induction of silent biosynthetic gene clusters by exogenous small molecules. FEMS Microbiol Rev 41:19–33
Palmer JM, Bok JW, Lee S, Dagenais TRT, Andes DR, Kontoyiannis DP, Keller NP (2013a) Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 1:e4
Palmer JM, Theisen JM, Duran RM, Grayburn WS, Calvo AM, Keller NP (2013b) Secondary metabolism and development is mediated by LlmF control of VeA subcellular localization in Aspergillus nidulans. PLoS Genet 9:e1003193
Park HS, Yu JH (2012) Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15:669–677
Parzych KR, Ariosa A, Mari M, Klionsky DJ (2018) A newly characterized vacuolar serine carboxypeptidase, Atg42/Ybr139w, is required for normal vacuole function and the terminal steps of autophagy in the yeast Saccharomyces cerevisiae. Mol Biol Cell 29:1089–1099
Patananan AN, Palmer JM, Garvey GS, Keller NP, Clarke SG (2013) A novel automethylation reaction in the Aspergillus nidulans LaeA protein generates S-methylmethionine. J Biol Chem 288:14032–14045
Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP (2003) A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 21:921–926
Pfannenstiel BT, Greco C, Sukowaty AT, Keller NP (2018) The epigenetic reader SntB regulates secondary metabolism, development and global histone modifications in Aspergillus flavus. Fungal Genet Biol 120:9–18
Pidroni A, Faber B, Brosch G, Bauer I, Graessle S (2018) A class 1 histone deacetylase as major regulator of secondary metabolite production in Aspergillus nidulans. Front Microbiol 9:2212
Pöggeler S, Nowrousian M, Teichert I, Beier A, Kück U (2018) Fruiting-body development in ascomycetes. In: Anke T, Schüffler A (eds) The Mycota XV. Physiology and genetics, 2nd edn. Springer International Publishing, Cham, pp 1–56
Popova B, Kleinknecht A, Arendarski P, Mischke J, Wang D, Braus GH (2018) Sumoylation protects against β-synuclein toxicity in yeast. Front Mol Neurosci 11:94
Purschwitz J, Müller S, Kastner C, Schöser M, Haas H, Espeso EA, Atoui A, Calvo AM, Fischer R (2008) Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr Biol 18:255–259
Ramya V, Rajasekharan R (2016) ATG15 encodes a phospholipase and is transcriptionally regulated by YAP1 in Saccharomyces cerevisiae. FEBS Lett 590:3155–3167
Rauscher S, Pacher S, Hedtke M, Kniemeyer O, Fischer R (2016) A phosphorylation code of the Aspergillus nidulans global regulator VelvetA (VeA) determines specific functions. Mol Microbiol 99:909–924
Reggiori F, Komatsu M, Finley K, Simonsen A (2012) Autophagy: more than a nonselective pathway. Int J Cell Biol 2012:1–18
Rehman SAA, Kristariyanto YA, Choi S, Labib K, Hofmann K, Kulathu Y, Arif S, Rehman A, Kristariyanto YA, Choi S, Nkosi PJ, Weidlich S (2016) MINDY-1 is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol Cell 63:146–155
Ren H, Wang B, Zhao H (2017) Breaking the silence: new strategies for discovering novel natural products. Curr Opin Biotechnol 48:21–27
Reyes-Dominguez Y, Bok JW, Berger H, Shwab EK, Basheer A, Gallmetzer A, Scazzocchio C, Keller N, Strauss J (2010) Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol Microbiol 76:1376–1386
Richie DL, Askew DS (2008) Autophagy: a role in metal ion homeostasis? Autophagy 4:115–117
Richie DL, Fuller KK, Fortwendel J, Miley MD, McCarthy JW, Feldmesser M, Rhodes JC, Askew DS (2007) Unexpected link between metal ion deficiency and autophagy in Aspergillus fumigatus. Eukaryot Cell 6:2437–2447
Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U, Mouriño-Pérez RR, Takeshita N, Fischer R (2018) Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol Mol Biol Rev 82:e00068–e00017
Rodriguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R (2010) Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64:585–610
Rodríguez-Urra AB, Jiménez C, Nieto MI, Rodríguez J, Hayashi H, Ugalde U (2012) Signaling the induction of sporulation involves the interaction of two secondary metabolites in Aspergillus nidulans. ACS Chem Biol 7:599–606
Rokas A, Wisecaver JH, Lind AL (2018) The birth, evolution and death of metabolic gene clusters in fungi. Nat Rev Microbiol 16:731–744
Roze LV, Arthur AE, Hong S-Y, Chanda A, Linz JE (2007) The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol Microbiol 66:713–726
Roze LV, Chanda A, Linz JE (2011a) Compartmentalization and molecular traffic in secondary metabolism: a new understanding of established cellular processes. Fungal Genet Biol 48:35–48
Roze LV, Koptina AV, Laivenieks M, Beaudry RM, Jones DA, Kanarsky AV, Linz JE, Roze LV, Linz JE, Koptina AV, Kanarsky AV, Laivenieks M, Beaudry RM, Jones DA (2011b) Willow volatiles influence growth, development, and secondary metabolism in Aspergillus parasiticus. Appl Microbiol Biotechnol 92:359–370
Ruger-Herreros C, Rodríguez-Romero J, Fernández-Barranco R, Olmedo M, Fischer R, Corrochano LM, Canovas D (2011) Regulation of conidiation by light in Aspergillus nidulans. Genetics 188:809–822
Sakai K, Kinoshita H, Nihira T (2012) Heterologous expression system in Aspergillus oryzae for fungal biosynthetic gene clusters of secondary metabolites. Appl Microbiol Biotechnol 93:2011–2022
Sarikaya-Bayram Ö, Bayram Ö, Feussner K, Kim JH, Kim HS, Kaever A, Feussner I, Chae KS, Han DM, Han KH, Braus GH (2014) Membrane-bound methyltransferase complex VapA-VipC-VapB guides epigenetic control of fungal development. Dev Cell 29:406–420
Sarikaya-Bayram Ö, Palmer JM, Keller N, Braus GH, Bayram Ö (2015) One Juliet and four Romeos: VeA and its methyltransferases. Front Microbiol 6:1
Satterlee T, Cary JW, Calvo AM (2016) RmtA, a putative arginine methyltransferase, regulates secondary metabolism and development in Aspergillus flavus. PLoS One 11:e0155575
Schardl CL, Panaccione DG, Tudzynski P (2006) Ergot alkaloids-biology and molecular biology. In: Cordell GA (ed) The alkaloids.chemistry and biology, vol 63, 1st edn. Academic Press, Cambridge, pp 45–86
Scharf DH, Brakhage AA, Mukherjee PK (2016) Gliotoxin—bane or boon? Environ Microbiol 18:1096–1109
Schinke J, Gulko MK, Christmann M, Valerius O, Stumpf K, Stirz M, Braus GH (2016) The DenA/DEN1 interacting phosphatase DipA controls septa positioning and phosphorylation-dependent stability of cytoplasmatic DenA/DEN1 during fungal development. PLoS Genet 12:e1005949
Schmidt-Dannert C (2014) Biosynthesis of terpenoid natural products in fungi. In: Schrader JBJ (ed) Biotechnology of isoprenoids, Advances in biochemical engineering/biotechnology, vol 148. Springer, Cham, pp 19–61
Sela N, Atir-lande A, Kornitzer D (2012) Neddylation and CAND1 independently stimulate SCF ubiquitin ligase activity in Candida albicans. Eukaryot Cell 11:42–52
Shelest E (2017) Transcription factors in fungi: TFome dynamics, three major families, and dual-specificity TFs. Front Genet 8:53
Shilatifard A (2012) The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu Rev Biochem 81:65–95
Shimizu K, Hicks JK, Huang T, Keller NP (2003) Pka, Ras and RGS protein interactions regulate activity of AflR, a Zn(II)2Cys6 transcription factor in Aspergillus nidulans. Genetics 1104:1095–1104
Shimizu M, Masuo S, Fujita T, Doi Y, Kamimura Y, Takaya N (2012) Hydrolase controls cellular NAD, sirtuin, and secondary metabolites. Mol Cell Biol 32:3743–3755
Shlezinger N, Irmer H, Dhingra S, Beattie SR, Cramer RA, Braus GH, Sharon A, Hohl TM (2017) Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death. Science 357:1037–1041
Shoji J, Kikuma T, Arioka M, Kitamoto K (2010) Macroautophagy-mediated degradation of whole nuclei in the filamentous fungus Aspergillus oryzae. PLoS One 5:e15650
Shwab EK, Bok JW, Tribus M, Galehr J, Graessle S, Keller NP (2007) Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot Cell 6:1656–1664
Soukup AA, Chiang Y-M, Woo Bok J, Reyes-Dominguez Y, Oakley BR, Wang CCC, Strauss J, Keller NP (2012) Overexpression of the Aspergillus nidulans histone 4 acetyltransferase EsaA increases activation of secondary metabolite production. Mol Microbiol 86:314–330
Spasser L, Brik A (2012) Chemistry and biology of the ubiquitin signal. Angew Chem 51:6840–6862
Spatafora JW, Aime MC, Grigoriev IV, Martin F, Stajich JE, Blackwell M (2017) The fungal tree of life: from molecular systematics to genome-scale phylogenies. Microbiol Spectr 5:5
Stergiopoulos I, Collemare J, Mehrabi R, de Wit PJGM (2013) Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol Rev 37:67–93
Stjepanovic G, Baskaran S, Lin MG, Hurley JH (2017) Vps34 kinase domain dynamics regulate the autophagic PI 3-kinase complex. Mol Cell 67:528–534
Stopa N, Krebs JE, Shechter D (2015) The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci 72:2041–2059
Strauss J, Reyes-Dominguez Y (2011) Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet Biol 48:62–69
Studt L, Janevska S, Arndt B, Boedi S, Sulyok M, Humpf HU, Tudzynski B, Strauss J (2017) Lack of the COMPASS component Ccl1 reduces H3K4 trimethylation levels and affects transcription of secondary metabolite genes in two plant-pathogenic Fusarium species. Front Microbiol 7:2144
Sugimoto N, Iwaki T, Chardwiriyapreecha S, Shimazu M, Kawano M, Sekito T, Takegawa K, Kakinuma Y (2011) Atg22p, a vacuolar membrane protein involved in the amino acid compartmentalization of Schizosaccharomyces pombe. Biosci Biotechnol Biochem 75:385–387
Sun ZW, Allis CD (2002) Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418:104–108
Sun H, Wang W, Che Y, Jiang X (2016) Fungal secondary metabolites rasfonin induces autophagy, apoptosis and necroptosis in renal cancer cell line. Mycology 7:81–87
Swaminathan S, Amerik AY, Hochstrasser M (1999) The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol Biol Cell 10:2583–2594
Tan KK (1974) Blue-light inhibition of sporulation in Botrytis cinerea. J Gen Microbiol 82:191–200
Thieme KG, Gerke J, Sasse C, Valerius O, Thieme S, Karimi R, Heinrich AK, Finkernagel F, Smith K, Bode HB, Freitag M, Ram AFJ, Braus GH (2018) Velvet domain protein VosA represses the zinc cluster transcription factor SclB regulatory network for Aspergillus nidulans asexual development, oxidative stress response and secondary metabolism. PLoS Genet 14:e1007511
Toledo AV, Franco MEE, Yanil Lopez SM, Troncozo MI, Saparrat MCN, Balatti PA (2017) Melanins in fungi: types, localization and putative biological roles. Physiol Mol Plant Pathol 99:2–6
Tomko RJ, Hochstrasser M (2013) Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem 82:415–445
Tribus M, Bauer I, Galehr J, Rieser G, Trojer P, Brosch G, Loidl P, Haas H, Graessle S (2010) A novel motif in fungal class 1 histone deacetylases is essential for growth and development of Aspergillus. Mol Biol Cell 21:345–353
Tsitsigiannis DI, Zarnowski R, Keller NP (2004) The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J Biol Chem 279:11344–11353
Tyler JK, Johnson JE (2018) The role of autophagy in the regulation of yeast life span. Ann N Y Acad Sci 1418:31–43
van der Lee TAJ, Medema MH (2016) Computational strategies for genome-based natural product discovery and engineering in fungi. Fungal Genet Biol 89:29–36
VanderMolen KM, Darveaux BA, Chen W-L, Swanson SM, Pearce CJ, Oberlies NH (2014) Epigenetic manipulation of a filamentous fungus by the proteasome-inhibitor bortezomib induces the production of an additional secondary metabolite. RSC Adv 4:18329–18335
Voigt O, Pöggeler S (2013) Autophagy genes Smatg8 and Smatg4 are required for fruiting-body development, vegetative growth and ascospore germination in the filamentous ascomycete Sordaria macrospora. Autophagy 9:33–49
von Zeska Kress MR, Harting R, Bayram Ö, Christmann M, Irmer H, Valerius O, Schinke J, Goldman GH, Braus GH (2012) The COP9 signalosome counteracts the accumulation of cullin SCF ubiquitin E3 RING ligases during fungal development. Mol Microbiol 83:1162–1177
Wagner EJ, Carpenter PB (2012) Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol 13:115–126
Waite KA, De-La Mota-Peynado A, Vontz G, Roelofs J (2016) Starvation induces proteasome autophagy with different pathways for core and regulatory particles. J Biol Chem 291:3239–3253
Wang J, Hu Q, Chen H, Zhou Z, Li W, Wang Y, Li S, He Q (2010) Role of individual subunits of the Neurospora crassa CSN complex in regulation of deneddylation and stability of cullin proteins. PLoS Genet 6:12–16
Wang Y, Kim S-G, Wu J, Yu S, Kang K-Y, Kim S-T (2011) Proteasome inhibitors affect appressorium formation and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Plant Pathol J 27:225–231
Wang Z, Wang J, Li N, Li J, Trail F, Dunlap JC, Townsend JP (2018) Light sensing by opsins and fungal ecology: NOP-1 modulates entry into sexual reproduction in response to environmental cues. Mol Ecol 27:216–232
Wen X, Klionsky DJ (2016) An overview of macroautophagy in yeast. J Mol Biol 428:1681–1699
Werner A, Herzog B, Voigt O, Valerius O, Braus GH, Pöggeler S (2019) NBR1 is involved in selective pexophagy in filamentous ascomycetes and can be functionally replaced by a tagged version of its human homolog. Autophagy 15:78–97
Wiemann P, Brown DW, Kleigrewe K, Bok JW, Keller NP, Humpf HU, Tudzynski B (2010) FfVel1 and Fflae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol Microbiol 77:972–994
Williams RB, Henrikson JC, Hoover AR, Lee AE, Cichewicz RH (2008) Epigenetic remodeling of the fungal secondary metabolome. Org Biomol Chem 6:1895–1897
Wolf JC, Mirocha CJ (1977) Control of sexual reproduction in Gibberella zeae (Fusarium roseum “Graminearum”). Appl Environ Microbiol 33:546–550
Wu S, Zhu W, Nhan T, Toth JI, Petroski MD, Wolf DA (2013) CAND1 controls in vivo dynamics of the cullin 1-RING ubiquitin ligase repertoire. Nat Commun 4:1642
Wu C, Yang F, Smith KM, Peterson M, Dekhang R, Zhang Y, Zucker J, Bredeweg EL, Mallappa C, Zhou X, Lyubetskaya A, Townsend JP, Galagan JE, Freitag M, Dunlap JC, Bell-Pedersen D, Sachs MS (2014) Genome-wide characterization of light-regulated genes in Neurospora crassa. G3 4:1731–1745
Xia X, Kim S, Liu C, Shim S, Xia X, Kim S, Liu C, Shim SH (2016) Secondary metabolites produced by an endophytic fungus Pestalotiopsis sydowiana and their 20S proteasome inhibitory activities. Molecules 21:944
Xiao Z, Li L, Li Y, Zhou W, Cheng J, Liu F, Zheng P, Zhang Y, Che Y (2014) Rasfonin, a novel 2-pyrone derivative, induces ras-mutated Panc-1 pancreatic tumor cell death in nude mice. Cell Death Dis 5:e1241
Xu W, Gavia DJ, Tang Y (2014) Biosynthesis of fungal indole alkaloids. Nat Prod Rep 31:1474–1487
Yu Z, Fischer R (2019) Light sensing and responses in fungi. Nat Rev Microbiol 17:25–36
Zheng S, Wyrick JJ, Reese JC (2010) Novel trans-tail regulation of H2B ubiquitylation and H3K4 methylation by the N terminus of histone H2A. Mol Cell Biol 30:3635–3645
Zheng Y, Ma K, Lyu H, Huang Y, Liu H, Liu L, Che Y, Liu X, Zou H, Yin W-B (2017) Genetic manipulation of the COP9 signalosome subunit PfCsnE leads to the discovery of pestaloficins in Pestalotiopsis fici. Org Lett 19:4700–4703
Zuin A, Isasa M, Crosas B (2014) Ubiquitin signaling: extreme conservation as a source of diversity. Cells 3:690–701
Acknowledgments
We thank Fruzsina Bakti and Christoph Sasse for critical reading of the manuscript. Funding of this research was provided by the Deutsche Forschungsgemeinschaft (DFG) and the SFB860.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gerke, J., Köhler, A.M., Meister, C., Thieme, K.G., Amoedo, H., Braus, G.H. (2020). Coordination of Fungal Secondary Metabolism and Development. In: Benz, J.P., Schipper, K. (eds) Genetics and Biotechnology. The Mycota, vol 2. Springer, Cham. https://doi.org/10.1007/978-3-030-49924-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-49924-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49923-5
Online ISBN: 978-3-030-49924-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)