Abstract
Pulmonary embolism (PE) is potentially life threatening and a common cause of morbidity and mortality that can constitute a thoracic emergency. The definitions, diagnosis, and treatment of this disease have evolved considerably over the last several decades warranting a careful review of the current state of its management. Because abundant, high-quality data is lacking for many of the management branch points, correct decision making around PE can be challenging. This chapter focuses on summarizing the state of the field with respect to diagnosis and treatment including relevant clinical studies and consensus opinion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Pulmonary embolism (PE) is a common cause of significant morbidity and mortality. In fact, rates of pulmonary embolism have been estimated to affect greater than 1 out of every 1000 adults annually [1]. The majority of pulmonary embolisms originate from the deep venous system of the pelvis and thighs. As a result, factors associated with deep venous thrombosis (DVT) of the lower extremities increase the risk of PE. Among these factors are prolonged bed rest, congestive heart failure, recent myocardial infarction, malignancy, shock, hypercoagulable states, trauma, and major surgery. Although there has been considerable progress in clinical management and a decreasing incidence of the disease, the severity of PE has been increasing due to simultaneous enhanced survival of patients at greatest risk for PE including advanced malignancy, multisystem severe trauma, and complex major surgery [1, 2]. Although prevention is key to mitigating the disease, appropriate diagnosis when a PE occurs is essential to providing the right treatment to the correct patient without the associated complications of PE treatment in the wrong patient.
Pathophysiology
Acute pulmonary artery obstruction due to embolism or primary thrombosis results in obstructive pathophysiology (Fig. 1). In a minority of patients, pulmonary infarction occurs as small thrombi lodge in distal subsegmental vessels causing pleuritic chest pain and hemoptysis. This is further aggravated by an intense local inflammatory response. As the embolus and subsequent inflammation alters perfusion to otherwise ventilated lung units, dead space physiology occurs. Inflammation also precipitates surfactant dysfunction, atelectasis, and compensatory pulmonary vascular changes where high flow pulmonary perfusion is directed to areas affected by bronchospasm and edema combining to cause functional intrapulmonary shunting. Ultimately, poor V/Q matching occurs and hypoxia can ensue. Patients may develop severe dyspnea, oppressive substernal chest pain, hypotension, and acute cor pulmonale.
Classification
Acute pulmonary embolism represents a spectrum of clinical disease. As a result, pulmonary embolism is categorized into different subgroups that are directly related to the severity of clinical illness. Appropriate categorization is essential to providing appropriate level of care and treatment decisions. Although previous categorizations relied on overall clot burden, the correlation of clot burden and clinical outcome is poorly correlated. As a result, more accurate predictive classifications have been developed that more closely reflect the patient’s underlying physiologic and hemodynamic response [3,4,5].
Currently, the classification systems incorporate validated risk scores, biomarkers, and the patient’s hemodynamic status. Three categories of acute pulmonary embolism have been developed—nonmassive (low-risk), submassive (intermediate-risk), and massive (high-risk) (Table 1). Nonmassive pulmonary embolism is not associated with any hemodynamic instability, and more specifically demonstrates no evidence of right ventricular strain, elevation of troponin, or elevation of B-type natriuretic peptide. Submassive pulmonary embolism is not associated with hemodynamic instability but does demonstrate some evidence of cardiac dysfunction as demonstrated by evidence of right ventricular dysfunction by either CT or ECHO, or has elevated biomarkers including B-type natriuretic peptide and/or troponin. Massive pulmonary embolism is associated with hemodynamic instability and shock that is not attributed to a different cause. Thus, the current consensus classification system divides pulmonary embolism into three categories not based on clot size but based on the level of physiologic or clinical deterioration resulting from the embolus.
Diagnosis
Clinical Decision Rules
Several scoring systems have been developed to aid in the diagnosis of pulmonary embolism. Each scoring system should be applied to individual patients appropriately and with caution since clinical suspicion plays a critical role in the actual diagnosis of patients that have suffered from a pulmonary embolism. Three scoring systems are routinely used, the Pulmonary Embolism Rule-Out Criteria (PERC) has been applied in the outpatient and emergency room setting, while the Wells Score and Geneva Score have been applied to hospitalized patients (Tables 2, 3, and 4).
PERC
The PERC scoring system (Table 2) was initially described in 2004. The eight PERC criteria are (1) age greater than or equal to 50; (2) heart rate greater than 100/min; (3) pulse oximetry oxygen saturation less than 95%; (4) unilateral leg swelling; (5) hemoptysis; (6) recent surgery or trauma; (7) prior PE or DVT; and (8) exogenous estrogen use. When none of these factors are present, a pulmonary embolism is unlikely with a sensitivity of 97%, specificity of 22%, and a false-negative rate of under 1% [6]. However, if the clinician suspicion is high, then appropriate laboratory and imaging studies should still be performed.
Wells Score
The classic Wells score (Table 3) was initially described in 1998 [7]. Since then, modifications including different cutoffs and corresponding probability levels or alternate weightings of the score items have been proposed, leading to a modified Wells score and a simplified version. The seven Wells criteria are (1) signs of DVT; (2) alternative diagnosis less likely than PE; (3) heart rate greater than 100/min; (f) immobilization for greater than or equal to 3 days/surgery in past 4 weeks; (5) prior DVT or PE; (6) hemoptysis; and (7) active malignancy/palliative situation. In the simplified version, if one or less of these factors is present, then a pulmonary embolism is unlikely.
Geneva Score
The original Geneva score was initially described in 2001. Since then, modifications have been developed to further refine and simplify the scoring in both the Revised Geneva Score and Simplified Geneva Score. Although accurate, it is less dependent on the provider assessment than the Wells score. The nine Geneva criteria are (1) age greater than 65; (2) prior DVT or PE; (3) surgery or fracture within a month; (4) active malignancy; (5) unilateral lower limb pain; (6) hemoptysis; (7) heart rate between 75 and 95/min; (8) heart rate greater than or equal to 95/min; and (9) pain on deep palpation of the lower limb with unilateral edema (Table 4). In the simplified version, if a score of 4 or less is present, then a pulmonary embolism is unlikely [8].
D-Dimer
D-Dimer is a sensitive marker for pulmonary embolism and can exclude PE without need for further testing in patients with low clinic probability. D-dimer levels greater than 500 ng/ml may suggest the presence of PE. However, since D-dimer increases with age, the false-positive rate in older individuals increases. Thus, the threshold for a positive D-dimer should be calculated as the patient’s age multiplied by 10 ng/ml for patients older than 50 years. When used in combination with the Wells score in patients with a low clinical suspicion, further testing can be safely withheld. Although the use of D-dimer is useful in the outpatient setting, it appears less useful in the inpatient setting and patients with either a history of previous venous thromboembolic events, trauma, or malignancy [9].
Diagnostic Imaging
Venous Duplex
The presence of a proximal deep venous thrombosis (DVT) in a patient with a suspected PE is highly predictive of a PE and thus warrants treatment. As a result, Doppler and B-mode ultrasonography may be used to demonstrate the presence of a DVT. However, a negative result does not exclude PE and requires further investigation [10].
Chest Radiography
Chest X-ray is not specific for pulmonary embolism, but is important in ruling out other diseases. Traditional radiographic findings, including Westermark’s sign (focal peripheral hyperlucency) and Hampton’s hump (wedge-shaped consolidation), are neither specific nor sensitive for pulmonary embolism.
Radionucleotide (V/Q) Scan
Historically, V/Q scanning was the most common means of diagnosis of pulmonary embolism, but it is no longer the most sensitive or specific study. The study is based on the presumption that an embolic obstruction of the pulmonary vasculature will produce a perfusion defect without a change in ventilation. However, prior to the rapid emergence of CT, the PIOPED study demonstrated a specificity of only 10% [11]. As a result, the clinical probability plays a critical role in the interpretation of this study.
Pulmonary Angiogram
Although angiography continues to be referred to as the “gold standard” for diagnosis with a sensitivity of 63–100% and a specificity of 55–96%, the increasing accuracy of CT has limited the need for this invasive test. Although this study is limited by the invasive nature, it does allow for potential interventions to be undertaken during the angiography (Fig. 2). As a result, in rare instances in patients with suspected massive PE that may require catheter-directed interventions, this may be considered an option for both diagnosis and therapy.
CT Scan
Computed tomography pulmonary angiography has good diagnostic accuracy for pulmonary embolism and has become widely available (Fig. 3). The test is relatively easy to perform, and as a result has become the major means of diagnostic imaging for pulmonary embolism. However, similar to angiography, it exposes patients to ionizing radiation and contrast media that may be contraindicated in patients with severe renal impairment [12].
MRI
Magnetic resonance imaging angiography offers a noninvasive examination of the pulmonary vasculature without the need for ionic contrast. However, the accuracy of MRI in establishing the diagnosis of PE is poor, with nearly 19% of cases being inconclusive [12].
V/Q Single-Photon Emission Computed Tomography
Ventilation/perfusion single-photon emission computed tomography is an emerging technique that results in considerably less radiation exposure than CT angio with PE protocol (CTPE)and avoids the need for intravenous contrast. The diagnostic accuracy of PE in terms of sensitivity and specificity is similar to CTPE. However, the precise role of this modality has not been sufficiently validated for use in routine clinical practice.
Cardiac/Hemodynamic Evaluation
Troponin
Circulating troponin aids in evaluation of evidence of cardiac ischemia. Circulating levels of both troponin I and troponin T are associated with early and late morbidity and mortality following an acute PE. Elevation of troponin I of a level of greater than 0.1 ng/ml implies evidence cardiac ischemia and is consistent with a submassive PE [13].
Natriuretic Peptides
Elevated natriuretic peptides are associated with increased early morbidity and mortality following an acute pulmonary embolism. Both brain natriuretic peptide (BNP) and N-terminal pro-BNP if elevated have been associated with a 9.51- and 5.74-fold increased risk of early mortality, respectively. A BNP value of greater than 100 pg/ml or NT-proBNP greater than 900 pg/ml is suggestive of a submassive pulmonary embolism [14].
Electrocardiogram
Obtaining an EKG helps in differentiating between acute myocardial infarction, pericarditis, and pulmonary embolism. Signs of acute right ventricular overload due to acute pulmonary artery obstruction include sinus tachycardia, rightward shift of the axis, right bundle branch block, and an SIQIIITIII pattern. However, lack of these finding does not rule out a less significant pulmonary embolism.
CT
Dilation of the right ventricle determined by CT is associated with increase in hospital and mortality within 3 months. Although the determination of right ventricular dilation by CT varies, a right ventricle diameter divided by left ventricle diameter of greater than 0.9 is the typical value demonstrating right ventricular dysfunction and suggestive of a submassive PE. The degree of clot burden, however, as demonstrated by CT has not been shown to be prognostic for early or late-term outcome.
ECHO
Transesophageal echocardiography (TEE) usually reveals indirect evidence of pulmonary embolism with acute dilation of the right ventricle and elevated pulmonary artery pressures. Transthoracic echocardiography and TEE can identify clot in the main PA and proximal right PA, but the proximal left PA is often obscured. The primary benefit of TEE is to rule out segmental wall motion defects that might suggest acute infarction, tamponade, and to define whether there is clot proximal enough to warrant an attempt at catheter-directed or surgical removal. Evidence of right ventricular dysfunction by echocardiography following an acute PE is associated with a 2.53-fold increase in early mortality [15].
Prognostication
One of the reasons diagnosing PE into specific classifications is for prognosis. As VTE and PE began to be more associated with hospitalized patients, a formal strategy to predict and prevent development of PE was launched and Various Risk Assessment Models (RAMs) have been developed to quantitatively predict risk. The Padua Prediction Score and IMPROVE RAM were the first widely used models that were valid in medical patients [16, 17]. However, a more encompassing tool emerged that was also valid among surgical populations—The CAPRINI RAM. This is currently the most robust and used prediction model having been validated in 250,000 patients and 100 clinical trials. The model works via a series of short scoring surveys for both the patient and the healthcare provider. It is dynamic in that it can be used for re-evaluation over the clinical course of a patient’s illness and provide new scores and updated treatment options [18].
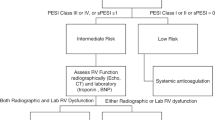
The pulmonary embolism severity index (PESI) was developed as a clinical prediction tool for 30-day mortality [19]. The PESI consists of 11 clinical variables that can be assessed at the time of diagnosis (Table 5). The PESI does not rely on any imaging or laboratory studies, but several studies have demonstrated increased predictive value with incorporation of laboratory values, specifically troponin and BNP. The benefit of this prediction model is to aid in initial management of patients and to determine which patients are best treated solely with anticoagulation and potential limited hospitalization. This index has been demonstrated to be reliable and reproducible for prognostication of patients with acute PE.
Management
The principle behind PE treatment is to reduce or remove clot burden in order to prevent hemodynamically significant strain on the heart and improve pulmonary gas exchange. The treatment options for PE occupy a spectrum of increasingly invasive options from medical and systemic treatments, to more invasive and targeted approaches up to and including circulatory assist treatments.
Medical Therapy
Anticoagulation
Early therapeutic anticoagulation has been demonstrated conclusively to reduce recurrence and improve mortality in acute PE [20]. For this reason, in patients with relatively low bleeding risk, systemic anticoagulation should be empirically started in all patients with high clinical suspicion of PE even before diagnosis is confirmed. And, systemic anticoagulation should remain a foundation of treatment even when additional interventional treatments are considered [21]. The particular choice of anticoagulant used depends on clinical factors such as hepatic and renal function, but in general when more advanced treatments are sought for intermediate- and high-risk PE, the flexibility of unfractionated heparin drip is the preferred choice for interventional radiology and surgical teams. For low-risk PEs, and for outpatient management of clinically stable intermediate- and high-risk PEs after advanced treatment, the Direct Oral Anticoagulants (DOACs) are now a first-line treatment along with low-molecular-weight heparin [5, 22]. Duration of treatment in PE mirrors that of DVT and accounts for provoking events and risk factors for bleeding. Specifically, patients with a first episode of low-risk or intermediate-risk PE should receive anticoagulation for at least 3 months. If there were transient provoking factors (i.e. major surgery, immobility >3 days, hormone therapy that was subsequently stopped) within the past 3 months, the duration should not be extended. If, there are sustained provoking factors, anticoagulation therapy may be increased up to 6–12 months on a case-by-case basis, although there continues to be no high-level evidence for specific duration recommendations longer than 6 months [23]. Additionally, patients that do not have evidence of provoking factors should undergo workup for hypercoagulable state that may require lifelong therapy.
Thrombolysis
Systemic thrombolysis is given with the intent of clot dissolution, restoration of pulmonary perfusion and subsequent V/Q matching, and relief of right ventricular afterload. It has been shown that decreased right ventrical (RV) afterload and treating with systemic thrombolytics compared to heparin alone improve outcomes such as hemodynamic collapse when administered in intermediate- and high-risk PE [24, 25]. However, significant complications at prohibitively high rates in the form of intracranial hemorrhage have been conclusively demonstrated, and now systemic thrombolytics are cautiously administered to a narrow group of patients [26]. This group may include patients in cardiac arrest from known PE, patients with right heart thrombus, and patients with high-risk PE and low risk of bleeding. In fact, for patients with massive PE and no contraindications, systemic thrombolysis is considered first-line therapy based mainly on meta-analysis data that found a reduction in recurrent PE or death from 19.0% without treatment to 9.4% with thrombolytic [27]. There is currently not significant high-quality data regarding use of systemic thrombolytics in submassive PE specifically with regard to which outcomes would improve or how that should tip the risk/benefit analysis given the known high complication rates [28].
Inhaled Nitric Oxide
The intended use of inhaled nitric oxide is to harness its property of selective vasodilation only to pulmonary vasculature adjacent to airways participating in inhalation of the gas, thereby reducing RV afterload and strain without causing systemic hypotension. There have been several studies including a randomized control trial demonstrating improved hemodynamics and reduced RV strain; however, the data has not yet been conclusive enough to drive use of nitric oxide into current guidelines [29, 30].
Catheter-Directed Therapy
Catheter-Directed Thrombolysis
Thrombolysis with a continuous infusion of low-dose thrombolytic via a catheter in the pulmonary artery attempts to achieve the advantages of systemic thrombolysis without the distant bleeding risks. There have been a few prominent prospective trials assessing the benefits of this technology, and they have demonstrated better RV-to-left ventrical (LV) ratio reduction compared to heparin alone as well as improved hemodynamics without significant incidence of catastrophic bleeding events [31, 32]. These studies were done in patients with predominantly high-risk PE and underscore the relative safety and efficacy of the technology. However, there is still not good data about which intermediate-risk PE patients would benefit nor do these studies address the challenges of expertise availability and rapid mobilization of high resource interventions compared to lower-resource intravenous systemic thrombolytics. For now, the indicated population appears to be high-risk PE patients with relative contraindications to systemic thrombolysis and intermediate-risk PE patients with signs of impending clinical deterioration [33].
Catheter-Directed Embolectomy
Percutaneous embolectomy is an approach without any significant supportive data which may be an option for patients who have a contraindication or failure of thrombolysis, do not have a cardiothoracic surgeon available for surgical embolectomy, and do have interventionalists and expertise to offer catheter-directed embolectomy. As there are potentially a sizeable number of patients with intermediate- and high-risk PE who may fit this scenario, additional research is warranted. Nevertheless, when patients with contraindication to thrombolysis do not have the option of surgical embolectomy, it is reasonable to consider this approach [33].
Surgery
The indication for surgical pulmonary embolectomy is a patient with intermediate- to high-risk PE who has an absolute contraindication to thrombolysis or is failing thrombolysis. A majority of consensus guidelines support this role for surgical embolectomy even in the presence of pre-operative thrombolysis [4, 5, 21]. Observational studies have found that while mortality used to be high for this surgery, it is beginning to come down into the single-digit percent range. And while there has never been a randomized control trial comparing systemic thrombolysis to surgical embolectomy, both have shown improved RV function and hemodynamics while thrombolysis is associated with more bleeding [34, 35]. Given the challenging decision making around endovascular and surgical treatment in these critically ill patients, it is important to rapidly involve a structured, expert-led, multi-disciplinary Pulmonary Embolism Response Team (PERT) to make these decisions (PERT flow diagram) which have been shown to improve outcomes [36] (Fig. 4).
Extracorporeal Life Support (ECLS)
ECLS if available should be performed in high-risk PE and cardiogenic shock with cardiac arrest as a lifesaving procedure. Cardiopulmonary support with venoarterial extracorporeal membrane oxygenation (ECMO) should be considered in patients that have failed catheter or systemic thrombolytics, or who are deteriorating so rapidly that cardiopulmonary arrest is imminent. These patients usually demonstrate severe and worsening shock despite therapy directed at reducing clot burden and use of vasopressor agents. There is no published randomized controlled data comparing ECMO to other treatments of high-risk PE; however, numerous observational reports have indicated favorable outcomes [37]. And, among all ECMO indications, the treatment of PE remains one of ECMO’s most successful uses [38]. The general duration of ECMO in these patients is typically 4–6 days and can be used in combination with systemic thrombolysis, catheter-directed therapy, or surgical embolectomy providing cardiopulmonary support while the clot burden resolves. Despite this, mortality remains high in patients treated with ECLS in the range of 40–60%.
Prevention
The need for thromboprophylaxis is based upon the high incidence of venous thromboembolism in high-risk patients. The apparent need for adequate prophylaxis may be further magnified within the critically ill patient because of limited cardiopulmonary reserve. Although intensive, non-invasive surveillance may be an alternative to prophylaxis, tests such as duplex ultrasonography have only moderate sensitivity in asymptomatic high-risk patients [10]. Furthermore, patients with primary pelvic thrombosis leading to PE will not be identified by this approach.
Optimal prophylaxis for high-risk medical and surgical patients has been defined by randomized studies with appropriate guidelines developed. However, appropriate prophylaxis has been underutilized, and thus successful minimization of this disease has not been achieved. Most patients with low and moderate risk of VTE would benefit from either unfractionated heparin, low-molecular-weight heparin, or intermittent pneumatic compression devices. However, patients at high risk for VTE should be treated with low-molecular-weight heparin if possible [39]. The use of low-molecular-weight heparin in this patient population reduces the risk of VTE by greater than 25% in comparison to unfractionated heparin. However, this reduction in the rate of VTE has only led to modest benefit in high-risk trauma patients with a rate between 4 and 28% [40]. As a result, despite limited data, some experts continue to advocate prophylactic IVC filter placement. Although placement may reduce in the incidence of PE, the rate of DVT is significantly increased in this patient population.
Conclusion
The definition and treatment of pulmonary embolism, especially massive PE, are coming into focus with new studies and clearer guidelines. However, there continues to be a general paucity of high-quality evidence for deciding among treatment modalities especially in the area of submassive PEs. As this knowledge gap fills in, it will be important to continue leaning on the expertise of institutional PERTs to guide clinical decision making in higher-risk PEs.
References
Silverstein MD, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. JAMA Intern Med. 1998;158(6):585–93.
Tagalakis V, et al. Incidence of and Mortality from Venous Thromboembolism in a Real-world Population: The Q-VTE Study Cohort. Am J Med. 2013;126(9):832.e13–21.
Sharifi M, et al. Pulseless electrical activity in pulmonary embolism treated with thrombolysis (from the “PEAPETT” study). Am J Emerg Med. 2016;34(10):1963–7.
Jaff MR, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–830.
Kearon C, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52.
Singh B, et al. Diagnostic accuracy of pulmonary embolism rule-out criteria: a systematic review and meta-analysis. Ann Emerg Med. 2012;59(6):517–520.e4.
Wells PS, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129(12):997–1005.
Klok FA, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168(19):2131–6.
Nagel SN, et al. Age-dependent diagnostic accuracy of clinical scoring systems and D-dimer levels in the diagnosis of pulmonary embolism with computed tomography pulmonary angiography (CTPA). Eur Radiol. 2019;29:4563–71.
Cipolle MD, et al. The role of surveillance duplex scanning in preventing venous thromboembolism in trauma patients. J Trauma Acute Care Surg. 2002;52(3):453–62.
PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263(20):2753.
Stein PD, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Radiology. 2007;242(1):15–21.
Moores L, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2010;8(3):517–22.
Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178(4):425–30.
Sanchez O, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569–77.
Barbar S, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–7.
Spyropoulos AC, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–14.
Cronin M, et al. Completion of the updated Caprini risk assessment model (2013 version). Clin Appl Thromb Hemost. 2019;25:1076029619838052.
Jiménez D, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with Acute Symptomatic Pulmonary Embolism Simplified Pulmonary Embolism Severity Index. JAMA Intern Med. 2010;170(15):1383–9.
Robertson L, Jones LE. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. 2017;(2):CD001100.
Konstantinides SV, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) endorsed by the European Respiratory Society (ERS). Eur Heart J. 2014;35(43):3033–73.
Groetzinger LM, et al. Apixaban or rivaroxaban versus warfarin for treatment of submassive pulmonary embolism after catheter-directed thrombolysis. Clin Appl Thromb Hemost. 2018;24(6):908–13.
Kearon C, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2):e419S–96S.
Konstantinides S, et al. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998;82(8):966–70.
Goldhaber SZ, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341(8844):507–11.
Meyer G, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–11.
Wan S, et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744–9.
Chodakowski JD, Courtney DM. Pulmonary embolism critical care update: prognosis, treatment, and research gaps. Curr Opin Crit Care. 2018;24(6):540–6.
Kline JA, et al. Randomized trial of inhaled nitric oxide to treat acute pulmonary embolism: the iNOPE trial. Am Heart J. 2017;186:100–10.
Summerfield DT, et al. Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: a case series. Respir Care. 2012;57(3):444–8.
Piazza G, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. J Am Coll Cardiol Intv. 2015;8(10):1382–92.
Kuo WT, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148(3):667–73.
Rivera-Lebron B, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037.
Aymard T, et al. Massive pulmonary embolism: surgical embolectomy versus thrombolytic therapy—should surgical indications be revisited? Eur J Cardiothorac Surg. 2012;43(1):90–4.
Azari A, et al. Surgical embolectomy versus thrombolytic therapy in the management of acute massive pulmonary embolism: short and long-term prognosis. Heart Lung. 2015;44(4):335–9.
Rosovsky R, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis. 2019;47(1):31–40.
Pasrija C, et al. Triage and optimization: a new paradigm in the treatment of massive pulmonary embolism. J Thorac Cardiovasc Surg. 2018;156(2):672–81.
Carroll BJ, et al. Clinical features and outcomes in adults with cardiogenic shock supported by extracorporeal membrane oxygenation. Am J Cardiol. 2015;116(10):1624–30.
Geerts WH, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133(6):381S–453S.
Geerts WH, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Miranda, D., Cuschieri, J. (2021). Pulmonary Embolism. In: Galante, J.M., Coimbra, R. (eds) Thoracic Surgery for the Acute Care Surgeon. Hot Topics in Acute Care Surgery and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-030-48493-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-48493-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48492-7
Online ISBN: 978-3-030-48493-4
eBook Packages: MedicineMedicine (R0)