Abstract
Adrenaline is an important pharmacologic treatment during cardiac arrest (CA) for resuscitation. Recent studies suggest that adrenaline increases the likelihood of return of spontaneous circulation (ROSC) but does not contribute to improving neurological outcomes of CA. The mechanisms have not been elucidated yet. A bimodal increase in mean arterial pressure (MAP) is observed after adrenaline injection in rodent CA models [17]. In this study, we focused on alteration of systemic arterial pressure in conjunction with the measurement of cerebral blood oxygenation (CBO) such as oxyhemoglobin (Oxy-Hb), deoxyhemoglobin (Deoxy-Hb), and tissue oxygenation index (TOI) by near-infrared spectroscopy (NIRS). Male Sprague–Dawley rats were used. We attached NIRS between the nasion and the upper cervical spine. Rats underwent 10-minute asphyxia to induce CA. Then, cardiopulmonary resuscitation (CPR) was started, followed by a 20 μg/kg of bolus adrenaline injection at 30 seconds of CPR. This injection accelerated the first increase in MAP, and ROSC was observed with an abrupt increase in CBO. Interestingly, the second increase in MAP, once it exceeded a certain value, was accompanied by paradoxical decreases of Oxy-Hb and TOI, while Deoxy-Hb increased. Based on this finding, we compared Oxy-Hb, Deoxy-Hb, and TOI at the first MAP ≈ 100 mmHg and the second MAP ≈ 100 mmHg. The average of Oxy-Hb and TOI from the 13 animals significantly decreased at the second increase in MAP over 100 mmHg, while Deoxy-Hb significantly increased. NIRS identified a decrease in Oxy-Hb after ROSC. These findings may be a clue to understanding the mechanism of how and why adrenaline alters the neurological outcomes of CA post-resuscitation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiac arrest
- Adrenaline
- Cerebral blood oxygenation (CBO)
- Near-infrared spectroscopy (NIRS)
- Autoregulation
1 Introduction
The 2015 International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support (ALS) Guidelines [1] recommended the use of intravenous adrenaline during resuscitation after cardiac arrest (CA), while recent studies have revealed that adrenaline increased the likelihood of return of spontaneous circulation (ROSC) but did not contribute to improving the neurological outcomes in CA [2, 3]. The mechanisms have not been elucidated yet. A bimodal increase in mean arterial pressure (MAP) is observed after adrenaline injection in rodent CA models [4]. There are a few studies that evaluated the cerebral blood oxygenation (CBO) by near-infrared spectroscopy (NIRS) in swine, but there is no study focused on the relationship between CBO by NIRS and the bimodal increasing in MAP observed in rodent CA models [5]. In this study, we focused on this alteration of the systemic arterial pressure in conjunction with the measurement of cerebral blood oxygenation (CBO) such as oxyhemoglobin (Oxy-Hb), deoxyhemoglobin (Deoxy-Hb), and tissue oxygenation index (TOI) by near-infrared spectroscopy (NIRS). We also tested the effects of adrenaline on both MAP and CBO in sham animals.
2 Methods
2.1 Animal Preparation
The details of the methods for a rat asphyxia CA model have been described previously [6, 17]. In brief, 12 adult male Sprague–Dawley rats (450–550 g, Charles River Laboratories) were anesthetized with 4% isoflurane (Isothesia, Butler-Schein AHS) and intubated with a 14-gauge plastic catheter (Surflo, Terumo Medical Corporation). Animals were mechanically ventilated. Anesthesia was maintained with isoflurane 2% at a fraction of inspired O2 (FIO2) of 0.3. The left femoral artery was cannulated (sterile polyethylene-50 catheter inserted for 20 mm) for continuous arterial pressure monitoring. A temperature probe was placed in the esophagus for continuous temperature monitoring. The core temperature was maintained at 36.5 ± 1.0 °C during the surgical procedure. The left femoral vein was cannulated with a polyethylene-50 catheter, which was advanced into the inferior vena cava for drug infusion. We attached NIRS (NIRO-200NX, Hamamatsu Photonics, Japan) from the nasion to the upper cervical spine of the rats. The distance between the emission and the detection probes was 3 cm. We examined the mean arterial pressure (MAP), end-tidal carbon dioxide (ETCO2), and Oxy/Deoxy-Hb and TOI. The NIRS device records the oxygen saturation level (TOI) and the changes in concentration of Oxy-Hb and Deoxy-Hb in real time (100 Hz). Data averaged every 20 seconds were used. After instrumentation setup, neuromuscular blockade was achieved by slow intravenous administration of 2 mg/kg of vecuronium bromide (Hospira, USA). Asphyxia was induced in the rats by switching off the ventilator and CA occurred 3–4 minutes after asphyxia started. We defined CA as a MAP below 20 mmHg; CA was completely untreated during 10 minutes of CA. Mechanical ventilation was restarted at an FIO2 of 1.0, and manual cardiopulmonary resuscitation (CPR) was delivered to CA animals. Chest compressions were performed at a rate of 240–300 per minute. At 30 seconds after the beginning of CPR, a 20 μg/kg bolus of adrenaline was given to animals through the venous catheter. Following ROSC, defined as a systolic blood pressure above 60 mmHg, CPR was discontinued. If ROSC did not occur by 5 minutes of CPR, resuscitation was terminated. In order to evaluate the dynamics of CBO in post-CPR, we assessed Oxy-Hb, Deoxy-Hb, and TOI 20 seconds before and after the time when MAP reached 100 mmHg. MAP always increased twice after the injection of adrenaline in post-CPR. Six rats underwent sham surgeries including vecuronium and adrenaline administrations but without asphyxia or CPR. The sham animals had one peak of increased MAP after the injection of adrenaline.
2.2 Statistical Analysis
Data are shown as mean and SD. The Mann–Whitney U test was used for continuous variables. One-way analysis of variance (ANOVA) was used for the group comparison with post hoc analysis of the Tukey test. We also presented the results of our multiple parameters. All statistical analyses were performed with JMP (version 10.1 software; SAS Institute, Cary, NC, USA). P-values less than 0.05 were considered significant.
3 Results
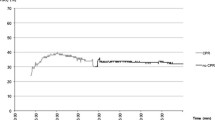
Figure 6.1 shows the trend of MAP, heart rate, TOI, Oxy-Hb, Deoxy-Hb, and EtCO2 in a CA animal and a sham animal. In CA animals, the first peak of increased MAP was observed at ROSC, and it was always followed by the second slow peak of MAP that occurred approximately 10 minutes after ROSC. In CA animals, there was a significant difference in the trend of CBO between the first and the second peak of increased MAP. When animals achieved ROSC and MAP reached 100 mmHg for the first time, Oxy-Hb and TOI increased but Deoxy-Hb decreased. However, when MAP reached 100 mmHg for the second time, Oxy-Hb and TOI decreased but Deoxy-Hb increased. This CBO alteration pattern at the second peak was considered paradoxical compared to that of the first peak (n = 6). In sham animals, the CBO alteration when MAP increased to 100 mmHg was the paradoxical pattern, in which Oxy-Hb and TOI decreased and Deoxy-Hb increased as MAP increased (Fig. 6.2).
The representative changes in mean arterial pressure (MAP), heart rate, end-tidal carbon dioxide (EtCO2), and cerebral blood oxygenation (CBO) including oxyhemoglobin (Oxy-Hb), Deoxy-Hb, and tissue oxygenation index (TOI) in (a) a cardiac arrest rat and (b) a sham rat. Bimodal peaks in MAP were seen in cardiac arrest, while single peak was observed in sham
The dynamic trend’s comparison of cerebral blood oxygenation (CBO) including oxyhemoglobin (Oxy-Hb), Deoxy-Hb, and tissue oxygenation index (TOI) when mean arterial pressure (MAP) reached 100 mmHg after adrenaline injection in sham model (sham100) and at the first time (first 100) and at the second time (second 100) in cardiac arrest model. rSO2, regional cerebral tissue oxygen saturation
4 Discussion
In the present study, we found significantly different patterns of CBO between the first and the second increase in MAP after CPR. The phenomenon of CBO alteration at the second peak of MAP after CPR was similar to that of the effect of adrenaline on sham animals.
It has been accepted that higher Oxy-Hb and rSO2(TOI) suggests improvement in brain function [7, 8]. Because of this, adrenaline is considered damaging to the brain since it paradoxically decreases blood perfusion into the brain. Likewise, after cardiac arrest, adrenaline may decrease brain oxygenation post-CA [5], and it could be harmful even though it is considered necessary for CPR [2, 3].
Adrenaline is referred to as a double-edged sword because it has a beneficial effect on the heart by promoting resuscitation but also has a harmful effect on blood flow to the brain, which in turn is considered to deteriorate neurological outcomes. The mechanisms of altering CBO, the cerebral blood flow (CBF), and the microcirculation have been poorly understood [3, 9, 10]. A previous study indicated that adrenaline might increase and stabilize CBF under isoflurane [11]. Given our findings that Oxy-Hb and TOI decreased after adrenaline administration, adrenaline might affect the microcirculation in a different way from that for CBF. Previous studies indicated that the vasoactive tone might be incompetent after prolonged CA, resulting in reduced cerebrovascular resistance and increased cerebral microcirculation [12, 13]. Therefore, a linear relationship between MAP and CBO is plausible under conditions of severely devastated cerebral autoregulation as was seen at ROSC [14, 15]. The mechanism that explains the second peak of increased MAP is not clear, but as heart rate also indicated, it may originate from a delayed release of endogenous catecholamine and/or an activation of the sympathetic nervous system that changes the microcirculatory autoregulation [4].
Based on these hypothetical mechanisms and the findings in the present study, we hypothesized that the administration of adrenaline would affect the cerebral microcirculation and CBO (Fig. 6.3), which may depend on the status of cerebral autoregulation. CA is a time-sensitive disorder so autoregulation in the cerebral microcirculation is heavily impacted by the time after resuscitation [16]. As the cerebral autoregulation recovers, the effect of adrenaline on the cerebral microcirculation alters, and it in turn changes the effect on CBO, which may have a harmful effect on the neurological outcomes.
The current study includes several limitations. First, we did not show a correlation between the CBO by NIRS and the CBF. It is necessary to monitor CBF, but due to technical difficulties in small animal models, further work will be required to better define the relationship of CBO with CBF using larger animal models. Second, the next study needs to examine the effect of exogenous and endogenous adrenaline, which may include additive effects after CA.
5 Conclusion
NIRS identified a decrease in Oxy-Hb after ROSC. This phenomenon might be a clue to help understand the mechanism of how and why adrenaline interferes with the improvement of neurological outcomes in post-CA.
References
Link MS, Berkow LC, Kudenchuk PJ et al (2015) Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132:S444–S464
Hagihara A, Hasegawa M, Abe T et al (2012) Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 307:1161–1168
Perkins GD, Ji C, Deakin CD, Quinn T et al (2018) A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med 379:711–721
Neumar RW, Bircher NG, Sim KM et al (1995) Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 29:249–263
Ristagno G, Tang W, Huang L et al (2009) Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med 37:1408–1415
Shinozaki K, Becker LB, Saeki K et al (2018) Dissociated oxygen consumption and carbon dioxide production in the post-cardiac arrest rat: a novel metabolic phenotype. J Am Heart Assoc 7(13):e007721. https://doi.org/10.1161/jaha.117.007721
Hallacoglu B, Sassaroli A, Fantini S et al (2011) Cerebral perfusion and oxygenation are impaired by folate deficiency in rat: absolute measurements with noninvasive near-infrared spectroscopy. J Cereb Blood Flow Metab 31:1482–1492
Nosrati R, Lin S, Ramadeen A et al (2017) Cerebral hemodynamics and metabolism during cardiac arrest and cardiopulmonary resuscitation using hyperspectral near infrared spectroscopy. Circ J 81:879–887
Sigal AP, Sandel KM, Buckler DG et al (2019) Impact of adrenaline dose and timing on out-of-hospital cardiac arrest survival and neurological outcomes. Resuscitation 139:182–188
Gough CJR, Nolan JP (2018) The role of adrenaline in cardiopulmonary resuscitation. Crit Care 22:139
Myburgh JA, Upton RN, Grant C et al (2002) The cerebrovascular effects of adrenaline, noradrenaline and dopamine infusions under propofol and isoflurane anaesthesia in sheep. Anaesth Intensive Care 30:725–733
Lee SK, Vaagenes P, Safar P et al (1989) Effect of cardiac arrest time on cortical cerebral blood flow during subsequent standard external cardiopulmonary resuscitation in rabbits. Resuscitation 17:105–117
van den Brule JMD, van der Hoeven JG, Hoedemaekers CWE (2018) Cerebral perfusion and cerebral autoregulation after cardiac arrest. Biomed Res Int 2018:4143636
Nishizawa H, Kudoh I (1996) Cerebral autoregulation is impaired in patients resuscitated after cardiac arrest. Acta Anaesthesiol Scand 40:1149–1153
Tsuji M, Saul JP, du Plessis A et al (2000) Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106:625–632
Weisfeldt ML, Becker LB (2002) Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA 288:3035–3038
Okuma Y, Shinozaki K, Yagi T et al (2019) Combination of cardiac and thoracic pump theories in rodent cardiopulmonary resuscitation: a new method of three-side chest compression. Intensive Care Med Exp 7(1)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Okuma, Y. et al. (2021). Effect of Adrenaline on Cerebral Blood Oxygenation Measured by NIRS in a Rat Asphyxia Cardiac Arrest Model. In: Nemoto, E.M., Harrison, E.M., Pias, S.C., Bragin, D.E., Harrison, D.K., LaManna, J.C. (eds) Oxygen Transport to Tissue XLII. Advances in Experimental Medicine and Biology, vol 1269. Springer, Cham. https://doi.org/10.1007/978-3-030-48238-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-48238-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48236-7
Online ISBN: 978-3-030-48238-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)