Abstract
Clinical investigators have focused on the real-time evaluation of cerebral blood oxygenation (CBO) by near-infrared spectroscopy (NIRS) during cardiopulmonary resuscitation (CPR). A previous study showed that an abrupt increase of oxy-hemoglobin (Hb) level and tissue oxygenation index (TOI) was associated with the timing of return of spontaneous circulation (ROSC). However, it is not clear how TOI alters before and after CPR including a period of cardiac arrest (CA). Therefore, this study aimed to assess CBO with asphyxia CA and its association with CPR to ROSC in rats. Male Sprague-Dawley rats were used. We attached NIRS (NIRO-200NX, Hamamatsu Photonics, Japan) from the nasion to the upper cervical spine in rats. A ten-minute asphyxia was given to induce CA. After CA, mechanical ventilation was restarted, and manual CPR was performed. We examined the mean arterial pressure (MAP), end-tidal carbon dioxide (ETCO2), and Oxy/Deoxy-Hb and TOI. Out of 14 rats, 11 obtained sustained ROSC. After the induction of asphyxia, a rapid drop of TOI was observed, followed by a subsequent increase of Oxy-Hb, Deoxy-Hb, and TOI with CPR. Recent CPR guidelines suggest the use of ETCO2 during CPR since its abrupt increase is a reasonable indicator of ROSC. In this study, abrupt increases in MAP, ETCO2, and TOI were observed at the time of ROSC. TOI can be an alternative to ETCO2 for identifying ROSC after CA, and it also has the capability of monitoring CBO during and after CPR.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiopulmonary resuscitation (CPR)
- Near-infrared spectroscopy (NIRS)
- Return of spontaneous circulation (ROSC)
- Tissue oxygen saturation (StO2)
- Cardiac arrest
1 Introduction
Cardiac arrest (CA) is a major public health issue worldwide. Despite significant improvements to care for CA, mortality remains high [1]. To improve CA survival, recent guidelines on cardiopulmonary resuscitation (CPR) indicated that it may be reasonable to use physiologic parameters when feasible to monitor and optimize CPR quality, guide vasopressor therapy, and detect return of spontaneous circulation (ROSC) (Class IIb) [2]. Clinical investigators have focused on the real-time evaluation of cerebral blood oxygenation (CBO) by near-infrared spectroscopy (NIRS) during CPR [3, 4]. A previous study showed that abrupt increases of oxy-hemoglobin (Hb) level and tissue oxygenation index (TOI) were associated with the timing of ROSC [4]. Although there have been studies supporting the use of NIRS during CPR [3,4,5,6,7], the guidelines do not make any statements regarding tissue oxygen saturation (StO2) or CBO [2] because it is not clear yet how StO2, including TOI, alters before and after CPR that includes a period of CA. Therefore, this study aimed to assess CBO by using a rat asphyxia CA model to investigate its association with CPR to ROSC. To the best of our knowledge, there have been no reports measuring CBO using NIRS in a rat CA model.
2 Methods
The Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research approved the study protocol. The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.1 Animal Surgical Procedure
The details of methods for a rat asphyxia CA model have been described previously [8]. In brief, 34 adult male Sprague-Dawley rats (450–550 g, Charles River Laboratories) were anesthetized with 4% isoflurane (Isosthesia, Butler-Schein AHS) and intubated with a 14-gauge plastic catheter (Surflo, Terumo Medical Corporation). Animals were mechanically ventilated. Anesthesia was maintained with 2% isoflurane at a fraction of inspired O2 (FIO2) of 0.3. The left femoral artery was cannulated (sterile polyethylene-50 catheter inserted for 20 mm) for continuous arterial pressure monitoring. A temperature probe was placed in the esophagus for continuous temperature monitoring. The core temperature was maintained at 36.5 ± 1.0 °C during the surgical procedure. The left femoral vein was cannulated with a polyethylene-50 catheter, which was advanced into the inferior vena cava for drug infusion. We attached NIRS (NIRO-200NX, Hamamatsu Photonics, Japan) from the nasion to the upper cervical spine of the rats. The distance between the emission and the detection probes was 3 cm. We examined the mean arterial pressure (MAP), end-tidal carbon dioxide (ETCO2), Oxy/Deoxy-Hb, and TOI. The NIRS device records the oxygen saturation level (TOI) and the changes in concentration of oxygenated hemoglobin (ΔO2Hb) and deoxygenated hemoglobin (ΔHHb) in real time (100 Hz). Averaged data every 500 msec were used. After instrumentation, neuromuscular blockade was achieved by slow intravenous administration of 2 mg/kg of vecuronium bromide (Hospira, USA). Asphyxia was induced by switching off the ventilator, and CA occurred 3–4 minutes after asphyxia started. We defined CA as a MAP below 20 mmHg; CA was completely untreated for 10 minutes. Mechanical ventilation was restarted at an FIO2 of 1.0, and manual CPR was delivered to CA animals. Chest compressions were performed at a rate of 240–300 per minute. At 30 seconds after the beginning of CPR, a 20 μg/kg bolus of epinephrine was given to animals through the venous catheter. Following ROSC, defined as a systolic blood pressure above 60 mmHg, CPR was discontinued. If ROSC did not occur by 5 minutes of CPR, resuscitation was terminated.
2.2 Statistical Analysis
Data are expressed as means ± standard deviation for continuous variables. Levels of TOI, ΔO2Hb, and ΔHHb were compared using repeated measures analysis of variance. P values ≤0.05 were considered to be statistically significant. All analyses were performed using the SPSS software (version 24.0, J SPSS).
3 Results
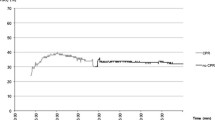
We monitored CBO by NIRS in 14 rats in CA. Of these, ROSC was observed in 11 rats. After the induction of asphyxia, TOI and ΔO2Hb rapidly dropped, whereas ΔHHb increased (Fig. 49.1). The values of TOI and ΔO2Hb were significantly lower during CA than at the start of asphyxia, whereas ΔHHb was significantly higher (TOI: 66.0 ± 5.4, at the start of asphyxia vs. 34.2 ± 10.4 during CA, p < 0.001). Towards the end of the 10 minutes of CA period, the level of TOI remained constantly low; ΔO2Hb kept decreasing; ΔHHb increased at 1–2 minutes after a start of asphyxia and gradually decreased thereafter (Fig. 49.1). This phenomenon was observed until about 8 minutes after starting asphyxia. Figure 49.2 shows the changes in TOI, ETCO2, MAP, ΔO2Hb, and ΔHHb after starting CPR in a typical case. During CPR, TOI, ΔO2Hb, and ΔHHb increased in synchrony with chest compressions. An abrupt increase in MAP, ETCO2, and TOI was observed at the time of ROSC (TOI: 36.9 ± 6.8 during CPR vs. 45.9 ± 4.6 at 30 seconds after ROSC, p < 0.001). In addition, ΔO2Hb increased, whereas ΔHHb decreased after ROSC. On the other hand, TOI did not increase in rats without ROSC (n = 3).
(a) Tissue oxygenation index (TOI) and (b) the changes in Oxy-hemoglobin (ΔO2Hb) concentration and Deoxy-Hb (ΔHHb) concentration during cardiac arrest (CA), before and after cardiopulmonary resuscitation (CPR). The number of rats in asphyxia, at 1-minutes after asphyxia, at 2 minutes after asphyxia, during CA was 14. The number of rats during CPR, at 30-seconds after ROSC, and at 1-minutes after ROSC was 11. Asphyxia indicates the time when starting asphyxia (baseline). During CA indicates at 5 minutes after the induction of asphyxia. During CPR indicates before return of spontaneous circulation (ROSC). The asterisk (*) indicates statistical significance (p < 0.05)
The changes in tissue oxygenation index (TOI), mean arterial pressure (MAP), end-tidal carbon dioxide (ETCO2), Oxy-hemoglobin (ΔO2Hb), and Deoxy-Hb (ΔHHb) during cardiac arrest (CA) and resuscitation in a typical case. The asterisk symbol indicates the period of the interruption of chest compression due to the administration of epinephrine. ROSC, return of spontaneous circulation; CPR, cardiopulmonary resuscitation
4 Discussion
This study showed that TOI rapidly dropped after induction of asphyxia in a rat CA model. We observed a synchrony of CBO waveforms with chest compression followed by an abrupt increase of TOI at the time of ROSC. Our major finding was the observation of CBO by NIRS during CA, before and after resuscitation in rats. NIRO-200NX has two measurement methods running independently of each other: a modified Beer-Lambert (MBL) method and a spatially resolved spectroscopy (SRS) method. Some studies reported that the MBL method may detect mainly shallow layers, and the SRS method may be able to detect deeper layers of the brain [9, 10]. Kakihana et al. showed that excessive exogenous and endogenous epinephrine caused contraction of the scalp blood vessels, and the blood flow and oxygenation of the scalp decreased, because the systolic blood pressure had risen up to 200 mmHg at ROSC in their experiments [10]. Our results were consistent with their findings, and the systolic blood pressure increased to approximately 200 mmHg (Fig. 49.2). Thus, it is plausible that CBO of rats can be measured by NIRO-200NX, which has features of both MBL and SRS.
Recent CPR guidelines suggest a use of ETCO2 during CPR since its abrupt increase is a reasonable indicator of ROSC [2]. However, the guidelines recommended that ETCO2 should not be used in isolation and should not be used in non-intubated patients [2]. In the current study, abrupt increases in MAP, ETCO2, and TOI were observed at the time of ROSC. In clinical settings, it is often difficult to monitor ETCO2 for non-intubated patients and to continuously monitor MAP in CA patients. NIRS, an optical and noninvasive technique, can be used as an alternative to detect ROSC [4] since NIRS does not require intubation for measuring CBO.
Previous experimental porcine CA studies showed that StO2 decreased during CA [10, 11]. Two studies reported that the StO2 decrease was rapid, like that observed in the present study [10, 12], but another study reported that it was gradual [11]. One reason for the differences in reports may be the cause of CA, and the capacity of oxygen contents may differ between species. The latter study reported that they induced CA by ventricular fibrillation, while we used an asphyxia CA model.
Some clinical studies reported that initial StO2 can be applied to patients as a predictor of ROSC [5,6,7]. However, we could not describe the correlation between the initial StO2 and ROSC. In a clinical setting, we cannot employ NIRS at the time of sudden cardiac arrest; therefore, no baseline measure of NIRS is available, but we can usually begin the measurement of CBO by NIRS after CPR is initiated. In future studies, it will be necessary to evaluate the association between the value of TOI and the value at the beginning of CPR and to detect ROSC in human beings.
Since our instrument is intended for use in humans and the scull of rats is much smaller than humans, the values of the TOI and O2Hb and HHb may not be quantitatively correlated with the data from humans. However, comparing the trend in rats is considered legitimate because of its internal standardization.
5 Conclusions
In this study, abrupt increases in MAP, ETCO2, and TOI were observed at the time of ROSC. On the basis of these findings, TOI can be an alternative to ETCO2 to detect ROSC after CA and has the potential to monitor CBO during and after CPR.
References
Geocadin RG, Callaway CW, Fink EL et al (2019) Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation 140(9):e517–e542
Link MS, Berkow LC, Kudenchuk PJ et al (2015) Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132(18 Suppl 2):S444–S464
Koyama Y, Wada T, Lohman BD et al (2013) A new method to detect cerebral blood flow waveform in synchrony with chest compression by near-infrared spectroscopy during CPR. Am J Emerg Med 31(10):1504–1508
Yagi T, Nagao K, Kawamorita T et al (2016) Detection of ROSC in patients with cardiac arrest during chest compression using NIRS: a pilot study. Adv Exp Med Biol 876:151–157
Tsukuda J, Fujitani S, Morisawa K et al (2019) Near-infrared spectroscopy monitoring during out-of-hospital cardiac arrest: can the initial cerebral tissue oxygenation index predict ROSC? Emerg Med J 36(1):33–38
Nishiyama K, Ito N, Orita T et al (2015) Characteristics of regional cerebral oxygen saturation levels in patients with out-of-hospital cardiac arrest with or without return of spontaneous circulation: a prospective observational multicentre study. Resuscitation 96:16–22
Takegawa R, Shiozaki T, Ogawa Y et al (2019) Usefulness of cerebral rSO2 monitoring during CPR to predict the probability of return of spontaneous circulation. Resuscitation 139:201–207
Shinozaki K, Becker LB, Saeki K et al (2018) Dissociated oxygen consumption and carbon dioxide production in the post-cardiac arrest rat: a novel metabolic phenotype. J Am Heart Assoc 7(13):e007721
Al-Rawi PG, Smielewski P, Kirkpatrick PJ (2001) Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke 32(11):2492–2500
Kakihana Y, Kamikokuryo C, Furubeppu H et al (2018) Monitoring of brain oxygenation during and after cardiopulmonary resuscitation: a prospective porcine study. Adv Exp Med Biol 1072:83–87
Boucek T, Mlcek M, Krupickova P et al (2018) Brain perfusion evaluated by regional tissue oxygenation as a possible quality indicator of ongoing cardiopulmonary resuscitation. An experimental porcine cardiac arrest study. Perfusion 33(1_suppl):65–70
Putzer G, Braun P, Strapazzon G et al (2016) Monitoring of brain oxygenation during hypothermic CPR – a prospective porcine study. Resuscitation 104:1–5
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yagi, T. et al. (2021). Assessment of Cerebral Blood Oxygenation by Near-Infrared Spectroscopy before and after Resuscitation in a Rat Asphyxia Cardiac Arrest Model. In: Nemoto, E.M., Harrison, E.M., Pias, S.C., Bragin, D.E., Harrison, D.K., LaManna, J.C. (eds) Oxygen Transport to Tissue XLII. Advances in Experimental Medicine and Biology, vol 1269. Springer, Cham. https://doi.org/10.1007/978-3-030-48238-1_49
Download citation
DOI: https://doi.org/10.1007/978-3-030-48238-1_49
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48236-7
Online ISBN: 978-3-030-48238-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)