Abstract
Laser interstitial thermal therapy (LITT) offers a minimally invasive approach to the treatment of both regrowth of brain metastases and/or radiation necrosis following radiosurgery (known together as metastatic in-field recurrence). Current indications for LITT include patients who have KPS >70, have good expected survival or systemic therapy options, and are deemed suitable surgical candidates. While LITT treatment was previously offered when patients required initiation of steroids for symptom management, LITT results have been shown to be better when the lesion is smaller and therefore there is a current trend toward using LITT earlier in the course of the progression of these lesions. While surgical access is less invasive, adverse neurological outcomes following LITT are similar to those encountered in a craniotomy.
Post-LITT imaging changes can be variable but generally follow a trend of an initial increase in the size of contrast-enhanced volume followed by a steady decrease over the subsequent 3–6 months. Surprisingly, FLAIR volumes often do not increase but rather can decrease rapidly in some cases and is often associated with the ability to wean steroids. With regard to clinical outcomes, recent multicenter prospective series suggest worthwhile short-term progression-free survival rates for LITT, with better outcomes for patients with radiation necrosis as opposed to tumor regrowth. LITT also resulted in an improvement in neurological outcomes and preservation of KPS in many patients. Pilot data suggest that LITT may serve as an alternative to a craniotomy in these patients and additional benefit may be a result of disruption of the blood-brain barrier by the LITT procedure, thus potentially having implications on post-LITT therapeutic management. Larger prospective studies with longer-term follow-up are needed to further refine the indications for LITT and clarify its role in the cancer survivor.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Laser interstitial thermal therapy
- Laser ablation
- Stereotactic ablation
- Brain metastasis
- Radiation necrosis
- Thermocoagulation
- Metastatic in-field recurrence
- Tumor regrowth
- LAASR
- NeuroBlate
- Visualase
- Neuro-oncology
- Oncology
Introduction
The management of patients with brain metastases has become increasingly complex with advancements in systemic therapies resulting in increased duration of survival in cancer patients. Typically a late-term complication, brain metastases represent the most common brain tumors diagnosed and their presence can significantly impact patients’ overall survival and quality of life (QOL). The treatment of brain metastases has undergone multiple shifts in recent decades. Most significantly, stereotactic radiosurgery (SRS) has evolved to become first-line treatment for many patients.
The cumulative incidence of recurrent tumor or radiation necrosis after SRS is reported to be up to 9.2–14% in patients surviving beyond one year [1]. While these entities have distinct pathophysiologies, when symptomatic, patients present with similar symptoms related to mass effect and edema as seen as progressive enhancement on magnetic resonance imaging (MRI) sometimes causing focal neurological deficits and seizures, among other symptoms.
Radiation necrosis is typically a result of a late irreversible injury to the brain surrounding the tumor after SRS. Risk factors for its development include large lesional volume, higher radiation dose, and adjuvant chemotherapy around the time of SRS [1, 2]. Multiple hypotheses exist in the pathophysiology of radiation necrosis, including endothelial cell damage causing capillary dysfunction and injury to glial cells leading to demyelination and necrosis [2, 3]. Radiation necrosis can occur months to years after SRS treatment and its incidence has been rising with the increased use of immunotherapies. Given that not all radiation necrosis becomes symptomatic, the initial treatment of radiation necrosis is typically conservative. Corticosteroids are used if patients develop neurological symptoms. If lesional regrowth or symptoms are progressive, especially despite corticosteroid therapy, surgical resection is an option to relieve the mass effect caused by radiation necrosis.
In contrast, the recurrent tumor has a more straightforward pathophysiology and often results from incomplete resection or radiation, regrowth of treatment-resistant tumor cells, or invasion of metastatic cells to the previously treated site. The incidence of tumor regrowth is related to the type of primary tumor, the size of the initial target, and the radiation dose. Recurrent metastases can be treated with a surgical resection or additional radiation in the form of SRS, whole brain radiation therapy (WBRT) , or a combination of the two.
In practice, it is often difficult to distinguish between radiation necrosis and tumor regrowth by means of imaging or presentation alone [4]. When progressive and symptomatic, the management of both entities often converges. For these reasons, some authors have proposed the use of the term “metastatic in-field recurrence” to include and sometimes obviate the need to distinguish between radiation necrosis and tumor regrowth. Craniotomy for resection of metastatic in-field recurrence offers excellent local control but may result in prolonged recovery time, worsening neurological deficits, infection, and significant psychiatric implication including depression [5]. Laser interstitial thermal therapy (LITT) has emerged as a minimally invasive treatment option for metastatic in-field recurrence especially in tumors that are difficult to access surgically. This chapter will discuss the current evidence for LITT as a treatment of metastatic in-field recurrence, including patient selection, outcome, imaging changes, and its role in disrupting the blood-brain barrier (BBB).
Patient Selection
The use of stereotactic laser therapy for the treatment of brain tumors was described as early as 1966 [6]. As discussed in the previous chapters, the availability of MRI for the guidance of stereotaxis and heat delivery has expanded the current interest in and use of LITT. In the early studies, the indications for using LITT for metastatic in-field recurrence were less clear. The cases that were described largely used thermal therapy to treat tumors that had previously exhausted other treatments, were about 2–3 cm in diameter or less, and were deemed accessible by LITT in patients who had good expected survival [7, 8]. Subsequently, the selection criteria have evolved to highlight some of the strengths of LITT, which will be discussed in the following sections. In the most recent LAASR (laser ablation after stereotactic radiosurgery) multicentered study, patients who qualified for LITT were those with metastases from a known primary cancer who previously underwent SRS treatment for the LITT-intended lesion, with KPS score ≥60 and age ≥18 years, and who were deemed to be suitable surgical candidates [5]. In another study by Rao et al., stricter KPS scores of >70 were used as a cutoff [9]. LITT was reported to be used for lesions with volumes ranging from 0.4 to 38.9 cm3 [5, 9,10,11,12].
Aside from the patient’s age and functional status, other indications for LITT can include patients with radiographically regrowing treated brain metastases who need biopsy for diagnosis, or those in whom symptoms related to the regrowth are not controllable with steroids [1]. Patel et al. proposed that LITT should be considered in a progressive lesion that meets any of the following criteria: (1) patient requiring long-term low-dose steroid or (2) the lesion has grown at least 1 cm, grown by 50% in two out of three linear dimensions, and has grown on two consecutive scans [13]. The authors concluded that patients who required higher preoperative steroid dosages were unlikely to benefit from LITT, and a craniotomy should be considered in these cases, if possible, to immediately address the mass effect. The idea here is that the LITT procedure can increase edema and mass effect in the acute phase and the natural course of edema after LITT can sometimes take weeks, if not months, to resolve [14].
In general, therapeutic decision making for radiographic regrowth after SRS follows the principle that tumor regrowth typically requires immediate treatment whereas radiation necrosis can be followed and treated only if progressively symptomatic. However, this last indication is changing with the increasing use of immunotherapy and the need to wean off steroids quickly to enable restarting of cancer treatment. This is also challenging when in the midst of attempting to resolve the underlying pathophysiology, the growth can become exponential, and one can miss the window to treat irrespective of the underlying physiology [4, 15]. When LITT is proposed as a treatment modality, however, authors have failed to achieve consensus on whether or not to perform a biopsy for diagnosis prior to LITT. On one hand, complete ablation of the lesion using LITT has been shown to be effective for both diagnoses and eliminates the need to distinguish between these two pathologies. The concern about the biopsy arises because bleeding in the area can make intraoperative LITT imaging, specifically that related to the Visualase System (Medtronic), more difficult to interpret, thus compromising the treatment [13]. Furthermore, within SRS-treated targets, there can be areas where both radiation necrosis and tumor coexist and sampling errors can occur making the value of biopsy debatable. However, from a cancer management standpoint, it can be critically important to understand if cancer control is being achieved in the brain using SRS. Many patients have multiple brain metastases treated using SRS and the presence or absence of tumor within the biopsy sample likely reflects the pathology of the next regrowing lesion. In the experience of some authors, the presence of any tumor, regardless of the presence of radiation necrosis, should be treated as tumor recurrence [16]. In the age of targeted therapies and immunotherapies, biopsy is an opportunity not only to make a diagnosis but also to determine whether the genetic profile of the tumor is the same in the brain as it is peripherally in the cases of regrowing tumors, thus helping to determine if the systemic therapy being prescribed might be effective in the brain.
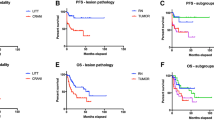
The LAASR study revealed that both survival and local control outcomes after LITT alone are significantly better for patients with radiation necrosis than those with tumor regrowth [5]. Additional analysis of the results was performed based on the completeness of LITT ablation for 21 patients. For patients with radiation necrosis, resolution of the LITT lesion was seen in 100% of the treated lesions with both total and subtotal ablation. This was compared with tumor patients where 75% resolved with total ablation, 25% of lesions partially resolved with total ablation, and 63% of lesions progressed if subtotally ablated. Not only does this translate in the radiation necrosis patients to an overall better prognosis and therefore continued aggressive cancer care, but for the progressive tumor patient, a possibly different discussion regarding goals of care and whether adjuvant radiation after LITT may be needed. In addition, from a technical standpoint, complete ablation was necessary for controlling a regrowing tumor whereas the subtotal ablation of radiation necrosis could still result in local control, thus affecting the goals of the LITT procedure. These authors therefore recommended that a biopsy be performed where possible at the time of LITT as it can guide follow-up care decisions. The decision to perform a biopsy in the setting of lesional regrowth after SRS needs to take the care of the patient holistically into the context relative to the capability of the LITT technology.
Technical Aspects
Like gliomas, the locations of metastatic in-field recurrence can vary significantly as can the prior management of the patients. Because of this, some preoperative planning is required to ensure successful access to the target and then complete coverage with the ablation. Given the lack of pre-procedural planning ability within the current software packages, goals to be achieved by the surgery and approach limitations need to be considered prior to surgery. In general, one of the most important factors to be determined is trajectory. Preoperative MRI brain with and without contrast should be reviewed preoperatively. General anesthesia is preferred given the sometimes complex trajectories and target locations. Patients undergo preoperative MRI with fiducials which is then transferred to the stereotactic navigation system. Given that the diameter of the deliverable heat region is typically 2–3 cm in maximum dimension, planning to place the laser fiber along the long axis of the target typically allows for best LITT coverage. Many of the trajectory planning systems allow for a diameter circle to be created around the planned trajectory and this often enables the surgeon to visualize which parts of the target can be ablated with the planned trajectory and what normal brain may be at risk as the cylinder of heating is created. Figure 5.1 demonstrates a typical trajectory used for an occipital regrowing tumor in a patient with metastatic breast cancer (NeuroBlate System; Monteris Medical).
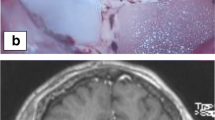
Figure 5.2, however, shows how planning may vary depending upon the goals of the surgery. This example shows a patient with metastatic melanoma who was treated with radiosurgery to a right basal ganglia lesion with SRS followed by the initiation of ipilimumab and nivolumab. Unfortunately, the metastatic focus (medial portion of the enhancing region) did not decrease in size in response to SRS but rather enlarged laterally within the next 3 months. Systemic response to immunotherapy in contrast was excellent.
Due to its deeper location, LITT was felt to be a reasonable option but biopsy was also needed to understand the discrepancy in response to immunotherapy between the intracranial disease and the systemic disease. The NeuroBlate System was also used in this example. Given the difference in radiographic appearance between the medial nodule and the more lateral changes, it was decided to plan trajectory to allow for separate biopsies of the two areas (Fig. 5.2a) rather than along the classical long axis of the lesion (Fig. 5.2b). This was possible in this scenario because the longest length of the target in the AP direction was still less than 3 cm. In addition, given that this target almost abutted the internal capsule, having the structure at risk at the end of the laser (Fig. 5.2a) rather than on the side of the laser (Fig. 5.2b) also allowed for the safest heat delivery. Heat emanates from the sides of the tip of the NeuroBlate laser and forward heat delivery is limited and therefore unlikely to spread out of control. Three specimens were sent for pathology, and a diagnosis of radiation necrosis was made from the lateral specimen compared with residual tumor from the medial portion. The follow-up MRI approximately one month after LITT demonstrated improved perilesional edema and mass effect.
The other major consideration in trajectory planning is the presence of a prior craniotomy. Usually there is scarred dura and/or dural substitute which can increase the insertional hemorrhage rate or cause catheter deviations. Therefore, choosing a trajectory outside of the original surgery can be advantageous. Figure 5.3 demonstrates a trajectory that may be used for a patient who previously underwent a craniotomy.
Lastly, odd target configurations and locations, as well as the need to treat multiple lesions, can pose a challenge largely unique to metastatic cancer patients. Figure 5.4 demonstrates some examples of how LITT can be planned and used for bilateral occipital regrowing tumors (Fig. 5.4a) or unique trajectories across midline (Fig. 5.4b).
Most patients receive 10 mg of dexamethasone and 1 g of levaciteram intraoperatively. A stab incision followed by a twist drill burr hole is made through this system, and the laser introduction bolt is secured to the skull. A biopsy is performed at this point if needed. Following the biopsy, a laser fiber is introduced and a repeat MRI obtained to confirm its position prior to initiation of LITT.
From a technical standpoint, an early report from the Case Comprehensive Cancer Center in patients with gliomas using the NeuroBlate System suggested a possible risk of proximal seeding of tumor along the laser tract, and recommendations were made for heat delivery to start at the shallowest point and advance to the deepest point [17]. Since this initial report, however, no further cases have been reported and this practice has not been instituted in our practice.
Immediately postoperatively, LITT patients are monitored in the neuro-intensive care unit. CT head without contrast is obtained postoperatively to rule out immediate periprocedural complications. Most patients are continued on a steroid taper postoperatively varying from 5 days to 2 weeks depending on steroid dependence preoperatively. Patients are followed up at 2 weeks postoperatively with an MRI and for wound check. They then typically undergo surveillance MRIs at 1.5, 3, and 6 months.
Complications and Postoperative Management
Adverse outcomes following LITT for metastatic in-field recurrence have varied depending on the definitions used in the studies. In the LAASR study, adverse outcomes were defined as any undesirable medical occurrence regardless of its association with the use of the device itself [5]. The most common side effects were headache, nausea/vomiting, cardiopulmonary events including pneumonia, urinary tract infection, and complications from the progression of systemic cancer. When considering only LITT-related neurological complications, 12% of the patients had adverse outcomes including weakness, paralysis, and neglect. In 80% of these patients, LITT was performed adjacent to the motor, sensory, or speech areas. Asymptomatic intracerebral hemorrhage (ICH) occurred in 2% of the patients and seizures in 17%. The rates of complications overall did not differ between the tumor regrowth group and the radiation necrosis group in this study.
Overall, ICH was reported in 2–13% of cases in the published studies [5, 9, 11]. The Chaunzwa et al. series of 30 patients reported a 13% incidence of ICH, occurring during the LITT portion rather than the biopsy portion of the case, but very few resulted in worsening of preoperative symptoms. Rao et al. reported an asymptomatic ICH in one patient (7%) in their series of 15 patients. One out of 23 patients who had left thalamic metastasis in the Ali et al. series developed hydrocephalus requiring a temporary ventricular drainage after LITT, and one patient developed malignant cerebral edema requiring an emergency hemicraniectomy [10]. See Table 5.1 for a listing of the complications.
It can be concluded that while the scalp and bony access to the target is less invasive compared to a craniotomy, the risks associated with LITT are similarly dependent on several variables including lesion location, pre-ablation edema, and size of the pre-ablation targets. Postoperatively, LITT patients therefore require observation in a setting equivalent to a neuro-intensive care unit. Time to recovery after an uncomplicated procedure and anesthesia, however, is still relatively short compared to a standard craniotomy, with the median length of hospital stay after LITT being 1–2 days [4, 5, 9, 11]. See section later in this chapter, Outcomes of LITT for Metastatic In-field Recurrence, for discussion of postoperative steroids management.
Imaging Changes after LITT
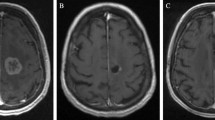
Radiographic changes after LITT can be variable but generally follow a trend of an initial increase in the size of the contrast-enhanced volume followed by a steady decrease. In the initial series published by Carpentier et al., the thermal ablation zone showed postoperative expansion of the necrotic area followed by a decrease in size [7]. Interestingly, the authors noted that the FLAIR volume did not increase postoperatively. These results are comparable in the subsequent larger series. Rao et al. reported that in the majority of targets treated (12 out of 14), the immediate postoperative volume had an average increase to 2.78 times the preoperative volume [9]. Thereafter, some treated areas continued to increase in size up to 2–4 weeks, followed by a gradual decrease in size. The majority of these treated areas returned to their preoperative sizes by 16 weeks. Chaunzwa et al. found that at 6 weeks, the contrast-enhanced volume showed a median increase in the volume of up to 34%, but this was associated with a median reduction in FLAIR volume of 36% [11]. At 3 months, the contrast-enhanced volume largely returned to their preoperative baseline, but the FLAIR volume continued to decrease to 74% of the baseline volume. At 6 months, the contrast-enhanced volume showed a decline in size compared to the preoperative volume, with an overall median reduction of 34%. The median FLAIR reduction was 77% at this time point. Beechar et al. reported similar response, with the median post-contrasted volume increase at 3 months, followed by a decrease at 6–9 months post-LITT [18]. Similarly, the FLAIR volumes at 6 months demonstrated significant reduction compared to pre-treatment volumes. Figure 5.5 showcases an example of imaging changes after LITT.
Contrast-enhanced T1W imaging in a patient with metastatic melanoma showed no significant change in the contrast-enhancing lesion size on preoperative imaging (a) and postoperative imaging at two weeks (b), but a significant reduction in associated FLAIR volumes between preoperative imaging (c) and postoperative imaging (d)
Compared to these series, Smith et al. reported a similar increase in volume followed by a decrease, but the trend observed in this study appeared to be on a longer time course [12]. Most notably, an increase in the lesional volume was observed in the majority of patients all the way up to their 6-month follow-up, and the volume reduction only began to be observed in the majority of the patients at 12 months. One explanation here may be that this study included patients with both primary and secondary brain tumors failing radiosurgery as opposed to metastatic in-field recurrence alone. The degree and timing of FLAIR signal resolution has also not been well studied or well stratified by pre-LITT lesional sizes. Whereas no significant associations were found with these factors in the LAASR study [5], Beechar et al. found that the smaller preoperative volumes respond better radiographically than those with larger volumes [18]. The authors postulated that this may be because of residual tumor cells that may be left unablated in patients with larger tumor volumes.
Overall, these results cautioned against interpreting LITT failure as an increase in the lesional volume alone. As these studies would suggest, capturing imaging changes at an early time point could lead to an inaccurate interpretation that the treatment has failed. Treatment response may be more accurately represented by a trend in the lesional volumes and the FLAIR volumes over time.
Outcomes of LITT for Metastatic In-Field Recurrence
Local Control and Overall Survival
In 2008, Carpentier et al. described their group’s initial experience with real-time MRI-guided LITT for metastatic in-field recurrence [7]. Their series included four subjects who were previously treated with chemotherapy or radiotherapy (SRS or WBRT) who were not candidates for craniotomy. Following LITT treatment using a prototype Visualase System, the authors reported no tumor recurrence within the thermal ablation zone. In patients whose treatment was partial, peripheral recurrence was observed, making the rate of local control approximately 50% overall at 90 days after LITT. In 2013, Torres-Reveron et al. described the use of LITT in six patients who had metastatic in-field recurrence after gamma knife SRS [8]. This was the first series for which all patients with metastatic in-field recurrence underwent a biopsy at the time of the procedure, and pathology was consistent with radiation necrosis in all cases. One patient died of systemic progression, and local control was seen in 80% of the remainder of the patients at 3 months. Rao et al. series reported a similar local control rate of 75.8% at a median follow-up time of 6 months [9]. However, pathology was not available in this series.
In 2016, Ali et al. reported their results in 26 brain metastases and observed local control in 65% of their patients over a median follow-up duration of 4.7 months (range 2.1–26.5 months) [10]. Interestingly, <80% ablation was achieved in patients who were later noted to have a progression of the disease. In patients who underwent postoperative adjuvant SRS for consolidation of <80% ablation with LITT, 100% control rate was obtained, leading to a suggestion that hypofractionated SRS may enhance the efficacy of LITT. Pathology was also not available in this series. During the same year, Smith et al. described single-institution long-term outcomes for 25 patients with biopsy-proven radiation necrosis [12]. The primary targets were metastasis in seven cases. In these patients, mean survival from LITT was 19.2 months, and progression-free survival was 11.4 months.
In the following years, larger multicentered series were added to the body of experiences of LITT as a treatment of metastatic in-field recurrence. Chaunzwa et al. reported an overall survival rate of 52.3% at 6 months [11]. Pathology reports were available in 80% of the cases and radiation necrosis made up 79% of the cases versus tumor regrowth in 21%. Most recently published was the multicentered LAASR study [5]. Here, all patients underwent a biopsy at the time of the surgery, with an approximately equal distribution between radiation necrosis and recurrent tumor (45.2% and 47.6%, respectively). Progression-free survival was 74% and overall survival was 72% at 6.5 months. Progression-free survival was significantly different at 3 months between the radiation necrosis group and the recurrent tumor group (100% vs 54%, respectively), and trended toward significance at 6 months (90.9% vs 62%, respectively). Of note, patients who had progression of disease after LITT had a lower preoperative baseline KPS score than those without progression (70 vs 90). Table 5.2 summarizes the outcomes of these series.
Heterogeneity may exist in the rate of local control and survival between these series for multiple reasons. In the early series, partial treatment was elected in some cases for safety reasons [19], as was seen in the Carpentier et al. series. Smith et al.’s reported local control rate of 14.3% was a stark difference compared to other series, whose local control rates ranged from 65% to 92.9% [5, 8,9,10,11]. It is worth mentioning that Smith’s series median follow-up time is longer than most other series (12.1 months vs 3–6 months), and that their progression-free survival was impressive at 11.4 months. As was discussed in the prior section, lesions treated by LITT tend to initially undergo an increase in the volume before a decrease. As a result, one may argue that capturing these volumes at earlier time points could overestimate the rate of LITT failure, and that the local control rate may be better defined as a trend in volume reduction over multiple time points. Unfortunately, the failure of LITT was not uniformly defined. For example, while one study defined local control as the absence of regrowth on MRI associated with increase in FLAIR and no recurrence of symptoms [11], another study defined local control as a decrease in size of the ablated targets, or <25% enlargement in volume compared to volume 24 hours after the procedures, and absence of new enhancement progressing over two MRIs [9]. In many studies, however, definitions were not provided [8, 12].
While their reported local control rate may be different, Smith et al.’s progression-free survival of 11.4 months was not inconsistent with the LAASR data in the patient group with radiation necrosis. This raises the question of whether survival outcomes may be affected by the pathology at hand. Unfortunately, pathology reports were not uniformly available in all series, and a biopsy may not always be feasible due to the differences in the LITT procedure set-up available at each institution. As discussed previously, the LAASR data showed significant difference in progression-free survival between the tumor regrowth and the radiation necrosis cohorts at 3 months and rates of progression-free survival remained higher for the radiation necrosis group at 6 months compared to the tumor regrowth group. Thus, from a cancer-control perspective, biopsy at the time of LITT is recommended as long as it does not compromise the ability to perform LITT therapy, since the lower local control rate achieved by LITT in the tumor regrowth group may be curbed by considering postoperative radiation or systemic therapy [5].
Quality of Life (QOL) and Neurological Outcome
Both radiation necrosis and tumor regrowth can present similarly with neurologic deficits from mass effect. In patients with metastatic in-field recurrence, LITT was reported to improve neurological symptoms in approximately 27.3–71.4% of cases [5, 8, 9, 11]. These symptoms included motor deficits, speech difficulties, and ambulatory status, among others. The median time to symptom resolution was reported to be 2 weeks in the series by Chaunzwa et al. [11].
Overall, the functional outcomes following a LITT surgery for metastatic in-field recurrence have focused on stabilization of the KPS score. Untreated, both radiation necrosis and tumor regrowth have been seen to cause progressive decline in KPS due to neurological impairment. This decline often results in cessation of systemic cancer therapy and transition of the patient to hospice care regardless of lesional pathology. Currently, limited data are available to compare the functional outcomes of LITT to other treatment modalities. However, early results suggest that a successful LITT procedure may preserve the KPS score, improve quality of life, and preserve cognition in many cases. In several series, preservation or improvement of the KPS score was reported in 43.3–75% of the patients, with the median follow-up time ranging from 3 to 6.5 months (Table 5.3). Similar to the other treatment modalities, these numbers may be affected by the patients’ baseline KPS scores. For example, in the series by Chaunzwa et al., KPS score preservation was much more likely for those with a preoperative KPS score of 70 or higher (59%), compared to 100% of those with a preoperative KPS score of 60 who all deteriorated and died after LITT [11]. Similarly, the LAASR study had a median baseline KPS score of 85 and reported a stable to improved KPS score in 60% of their patients at 6 months post-LITT [5].
Early results have also reported no significant impact of LITT on cognition, as measured by the pre- and postoperative Hopkins Verbal Learning Test-Revised (HVLT-R) scores and Mini-Mental State Examination (MMSE) scores [5]. Furthermore, although a decline in Social Well-Being scores and Emotional Well-Being scores overall has been reported [5], Smith et al. found that LITT results in statistically significant improvement of overall mental health and vitality at 12 months, as measured by the Short-Form Health Survey (SF-36) [12].
In practice, functional outcomes and mental health effects in patients with metastatic in-field recurrence may be influenced by multiple factors other than LITT treatment alone. These factors can include baseline functional status and mental health, duration, type and success of systemic cancer therapy, and the presence of disease progression elsewhere. Larger series with a longer follow-up time are needed to fully understand the long-term effects of LITT on patient’s mental health, functional status, and quality of life.
Steroid Dependence
Steroids are an effective tool to treat symptomatic perilesional edema but are often associated with significant adverse effects when used chronically. These adverse effects include weight gain, hypertension, difficult-to-control diabetes, impaired wound healing, GI ulceration, osteoporosis, and infection. In addition, it is thought that immunosuppression facilitates the progression of cancer [20]. Therefore, the inability to wean steroids is one of the most robust indications today for the use of LITT [11]. Most studies reported that following LITT, the majority of patients are able to wean off steroids within one to two months [8,9,10,11, 21], with the percentages ranging from 66.7% of patients within one month [21] to 100% within two months [8]. Chaunzwa et al. cited detailed information on preoperative and postoperative steroid usage and reported that 73.3% of their patients were able to stop steroids, with the median time to cessation of 4.5 weeks [11]. Both lesional volume and the corresponding FLAIR volume were recorded in this study post-LITT. Their results suggested that although the lesional volume as measured by contrast-enhanced images may initially increase at 6 weeks, a FLAIR reduction of as much as 36% was seen at that time, with the trend in FLAIR reduction continuing at 6 months follow-up. A larger reduction in FLAIR volume was found to be associated with an increased ability to stop steroids. In a study by Hernandez et al., 25% of patients with preoperative steroid use were continued on steroids indefinitely, whereas only approximately 13.5% with no preoperative steroid use had to be continued on steroids post-LITT [4]. The authors concluded that LITT should be offered prior to metastatic in-field recurrence becoming symptomatic, as patients with preoperative steroids use tended to remain dependent on steroids postoperatively and were more likely to experience post-LITT complications.
Interestingly, only 31% of patients in the LAASR study were able to stop or reduce steroids by their 3-month follow-up [5]. Although the authors did not offer an explanation to this finding, it is worth mentioning that 42.9% of the patients in this series were dependent on steroids use at baseline, compared to 26.7% as reported by Rao et al. [9], or 33% as reported by Chaunzwa et al. [11]. Moreover, the average pre-LITT volume in the LAASR study was larger at 6.4 cm3 compared to 3.7 cm3 reported by Rao et al., which may explain the smaller percentage of patients being able to stop steroids. The ability to wean steroids was not statistically significantly different between the radiation necrosis group and the tumor regrowth group [5]. Patel et al. proposed that patients who required high-dose steroids preoperatively may not benefit as much from LITT [13]. The effect of preoperative steroid dosages on neurological outcomes was not investigated.
Our institutional experiences are in line with those published by Hernandez et al. In our experience, offering LITT early before patients become dependent on steroid and while the targets and the surrounding FLAIR are small best facilitates the ability to wean off steroids post-LITT. In addition, obtaining an early post-LITT MRI within 2 weeks has also facilitated decision-making regarding the length of steroid taper. In some patients, a significant visible decrease in the amount of perilesional edema was seen by 2 weeks post-LITT and anecdotally in these patients, even if immunotherapy is re-initiated, these patients seemed to be able to remain off steroids without recurrence of their symptoms and eventual resolution of the LITT lesion on imaging.
LITT as an Alternative to Craniotomy
In the early years, LITT was initially proposed for deep targets where a craniotomy may incur excess morbidity. However, LITT is now increasingly performed for easy-to-access targets due to it being perceived as minimally invasive. In our experience, patients are much more likely to agree to LITT than a craniotomy when offered the option of both choices, even with the knowledge that a craniotomy may be needed as a salvage therapy should LITT fail. Only one single-institution retrospective study has been published comparing LITT to craniotomy for the management of metastatic in-field recurrence. This series included a total of 75 patients: 41 (55%) treated with craniotomy and 34 (45%) treated with LITT. No significant difference was found between the two surgical options in the ability to wean off steroids, the ability to initiate or resume postoperative immunotherapy, progression-free survival (PFS), or overall survival (OS) [22]. Given the retrospective nature of this study, the overall mean volume treated by craniotomy was larger than that treated by LITT (8.1 cm3 vs 4.1 cm3). Craniotomy was therefore found to result in a higher rate of relief of preoperative symptoms. To control for the volume difference between the two groups, 14 patients with lesions >3 cm diameter were excluded in the subanalysis. Overall survival and local control were even more significantly associated with the pathology of the lesion rather than the type of procedure, with greater PFS and OS reported in the radiation necrosis group compared to the recurrent tumor group. A larger randomized prospective study of more directly comparable targets is needed in order to validate these results.
Disruption of Blood-Brain Barrier after LITT
Other than the direct effect of laser heat on the lesion itself, early results by Leuthardt et al. have demonstrated a potentially useful unintended effect of LITT in disrupting blood-brain barrier (BBB) in glioma patients [23]. In this study, pharmacokinetic parameters and brain-specific enolase (BSE) were measured following a LITT procedure. The authors found that a forward volume transfer constant reflecting capillary permeability and peritumoral BBB disruption peaked immediately after LITT and was persistently elevated for another 4 weeks. Serum BSE, on the other hand, demonstrated a steady rise after LITT, peaked by 2–3 weeks, and remained elevated for up to 6 weeks. The authors concluded that there is a prolonged window after LITT during which BBB is reversibly disrupted. Reversible BBB disruption may conceivably play a role in enhancing the effectiveness of a therapeutic agent after LITT for many patients with metastatic in-field recurrence. A study is now ongoing looking to see if a similar effect might be found after LITT for metastatic in-field recurrence.
Conclusion and Future Development
In summary, multiple retrospective and prospective series have demonstrated that LITT offers a safe and efficacious treatment modality for patients with metastatic in-field recurrence. The indications for LITT continue to expand and highlight some of the strengths of LITT, including accessibility to deep-seated targets, minimally invasive access, stabilization of good KPS scores, ability to wean off steroids, and favorable cognitive, functional, and survival outcomes. Better outcomes are obtained after LITT if lesions are treated when they are smaller in size thus allowing for more complete ablation of the lesion. Larger prospective series with longer follow-up periods comparing LITT to other treatment modalities are needed to clarify the role of LITT in an armamentarium of options available for treating patients with metastatic in-field recurrence. Futures studies might investigate steroid use and its correlation to imaging changes, local changes in tumor and brain microenvironment after LITT, and the relationship of these changes to post-LITT therapies. In addition, techniques by which to ensure total ablation of larger lesions also need to be developed.
Abbreviations
- BBB:
-
blood-brain barrier
- BSE:
-
brain-specific enolase
- HVLT-R:
-
Hopkins Verbal Learning Test-Revised
- ICH:
-
intracerebral hemorrhage
- KPS:
-
Karnofsky Performance Score
- LAASR:
-
Laser Ablation After Stereotactic Radiosurgery study [5]
- LITT:
-
laser interstitial thermal therapy
- MMSE:
-
Mini-Mental State Examination
- MRI:
-
magnetic resonance imaging
- N/A:
-
not available
- PFS:
-
progression-free survival
- POD:
-
progression of disease
- QOL:
-
quality of life
- RN:
-
radiation necrosis
- SF-36:
-
Short-Form Health Survey
- SRS:
-
stereotactic radiosurgery
- TR:
-
tumor regrowth
- WBRT:
-
whole brain radiation therapy
References
Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–86. https://doi.org/10.3171/2014.10.JNS141610.
Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015;55(Suppl 1):50–9.
Furuse M, Nonoguchi N, Kawabata S, Miyatake S, Kuroiwa T. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol. 2015;48(4):183–90.
Hernandez RN, Carminucci A, Patel P, Hargreaves EL, Danish SF. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following Stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery. 2019;85(1):84–90. https://doi.org/10.1093/neuros/nyy220.
Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–11. https://doi.org/10.3171/2017.11.JNS171273.
Rosomoff HL, Carroll F. Reaction of neoplasm and brain to laser. Arch Neurol. 1966;14(2):143–8.
Carpentier A, McNichols RJ, Stafford RJ, Itzcovitz J, Guichard JP, Reizine D, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 Suppl 1):ONS21–9. https://doi.org/10.1227/01.neu.0000335007.07381.df.
Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neuro-Oncol. 2013;113(3):495–503. https://doi.org/10.1007/s11060-013-1142-2.
Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014;74(6):658–67. https://doi.org/10.1227/NEU.0000000000000332.
Ali MA, Carroll KT, Rennert RC, Hamelin T, Chang L, Lemkuil BP, et al. Stereotactic laser ablation as treatment for brain metastases that recur after stereotactic radiosurgery: a multiinstitutional experience. Neurosurg Focus. 2016;41(4):E11.
Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63. https://doi.org/10.1093/neuros/nyx142.
Smith CJ, Myers CS, Chapple KM, Smith KA. Long-term follow-up of 25 cases of biopsy-proven radiation necrosis or post-radiation treatment effect treated with magnetic resonance-guided laser interstitial thermal therapy. Neurosurgery. 2016;79(Suppl 1):S59–72.
Patel PD, Patel NV, Davidson C, Danish SF. The role of MRgLITT in overcoming the challenges in managing infield recurrence after radiation for brain metastasis. Neurosurgery. 2016;79(Suppl 1):S40–58.
Patel NV, Jethwa PR, Barrese JC, Hargreaves EL, Danish SF. Volumetric trends associated with MRI-guided laser-induced thermal therapy (LITT) for intracranial tumors. Lasers Surg Med. 2013;45(6):362–9. https://doi.org/10.1002/lsm.22151.
Patel PD, Hargreaves EL, Danish AF, Weiner J, Danish SF. Volumetric trends of progressive in-field recurrences after stereotactic radiosurgery of metastatic intracranial tumors. J Radiosurg SBRT. 2018;5(4):293–304.
Nath SK, Sheridan AD, Rauch PJ, Yu JB, Minja FJ, Vortmeyer AO, et al. Significance of histology in determining management of lesions regrowing after radiosurgery. J Neuro-Oncol. 2014;117(2):303–10. https://doi.org/10.1007/s11060-014-1389-2.
Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016;125(4):853–60.
Beechar VB, Prabhu SS, Bastos D, Weinberg JS, Stafford RJ, Fuentes D, et al. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J Neuro-Oncol. 2018;137(1):57–65. https://doi.org/10.1007/s11060-017-2694-3.
Carpentier A, McNichols RJ, Stafford RJ, Guichard JP, Reizine D, Delaloge S, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–50. https://doi.org/10.1002/lsm.21138.
Stewart T, Tsai SC, Grayson H, Henderson R, Opelz G. Incidence of de-novo breast cancer in women chronically immunosuppressed after organ transplantation. Lancet. 1995;346(8978):796–8.
Rammo R, Asmaro K, Schultz L, Scarpace L, Siddiqui S, Walbert T. The safety of magnetic resonance imaging-guided laser interstitial thermal therapy for cerebral radiation necrosis. J Neuro-Oncol. 2018;138(3):609–17. https://doi.org/10.1007/s11060-018-2828-2.
Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neuro-Oncol. 2019;142(2):309–17. https://doi.org/10.1007/s11060-019-03097-z.
Leuthardt EC, Duan C, Kim MJ, Campian JL, Kim AH, Miller-Thomas MM, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613. https://doi.org/10.1371/journal.pone.0148613.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sujijantarat, N., Danish, S.F., Chiang, V.L. (2020). LITT for Metastatic In-Field Recurrence. In: Chiang, V., Danish, S., Gross, R. (eds) Laser Interstitial Thermal Therapy in Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-030-48047-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-48047-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48046-2
Online ISBN: 978-3-030-48047-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)