Abstract
Hepatic cystic hydatid disease caused by Echinococcus granulosus remains a highly morbid and potentially fatal disease. The main therapeutic options for hydatid cystic disease are pharmacological therapy, surgical therapy, and percutaneous aspiration injection and re-aspiration (PAIR). Surgical expertise is concentrated in centers associated with the global epidemiological distribution of the disease. Surgical therapy is performed with traditional open surgery as well as minimally invasive techniques. The principles of surgery are the extirpation of active cystic contents and components, the intraoperative prevention of content spillage, the detection of biliary leaks, and the treatment of the residual cavity. Even though the potential benefits of a minimally invasive approach to hydatid hepatic disease are well known, this technique has not been widely adopted. It is reserved for carefully selected patients, namely, with uncomplicated hydatid cysts located in the anterior segments of the liver, which lend themselves to easy access with low risks of intraoperative complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Over the past 30 years, minimally invasive techniques in surgery have revolutionized surgical practice. In abdominal surgery, laparoscopy has established itself as the standard of care in the most common surgical disorders, namely, cholecystopathy, appendicitis, and hernia surgery. The adoption of minimally invasive approaches for surgical diseases extended to endocrine surgery, foregut surgery, colorectal surgery, and bariatric surgery. In most of these fields, the learning curve is steeper, and more cases are required to master the techniques.
The increased use of laparoscopy and minimally invasive techniques has been driven by several factors [1]:
-
Smaller incisions, with the benefit of cosmesis and reduced incidence and severity of hernia
-
Less superficial surgical trauma with a decrease in surgical site infections
-
Reduced postoperative pain
-
Enhanced recovery
Minimally invasive surgery introduced new surgical skills. Complex precise dissection is now performed under magnification; attention is given to anatomical structures not previously taken into account in open surgery. Novel instrumentation, such as high-resolution optics, energy devices, and surgical techniques, such as suturing, has led to the adoption of minimally invasive techniques to most of the spectrum of intra-abdominal and gastroenterological surgery.
Laparoscopic Hepatectomy
Laparoscopic hepatic procedures have been added to the armamentarium of liver surgery. However, it has not enjoyed the same adoption rate as other surgical techniques. There are several reasons for this:
-
The anatomical complexity of the liver
-
The proximity of major vascular structures
-
Difficulties in hepatic mobilization, lack of working space
-
The possibility of insufficient oncologic resection
-
Parenchymal transection exposes a risk of hemorrhage and CO2 gas embolization
-
Difficult laparoscopic control of vessels
-
Lack of training among hepatobiliary experts in advanced laparoscopic techniques [1, 2]
It is highly desirable to combine the qualifications of an expert hepatobiliary surgeon and an advanced laparoscopic surgeon to perform minimally invasive hepatic procedures [3].
Additionally, liver procedures comprise a large variety and standardization of the procedure is more difficult, thus prolonging the learning curve [1,2,3,4].
An important point is that a minimally invasive hepatic procedure should achieve the same surgical “quality” as an open procedure with regard to technical aspects and oncological outcomes [2].
Hydatid Cyst
Echinococcus granulosus is a small tapeworm that typically infects carnivores such as dogs and foxes, after consumption of infected intermediate host viscera (sheep and pigs). The parasite enters the small intestine and remains firmly attached to the mucosa. Later, it sheds proglottids which are excreted in the infected animal’s feces. Proglottids (or eggs) are ingested by intermediate hosts (sheep, humans), where they mature into cysts and daughter cysts.
A hydatid cyst usually develops in the liver parenchyma of an intermediate host. Humans are accidental intermediate hosts [5].
Hydatid cysts contain a clear, colorless, odorless hydatid fluid with a large amount of protoscolices (400,000/mm3). This fluid is highly irritating to tissues. The mature cyst consists of three layers:
-
The germinal layer: this is the inner layer, and it surrounds the fluid-filled central hydatid cavity. It is the living component of the parasite. It produces protoscolices, which are released directly into the cyst fluid or an endogenous daughter cyst. Protoscolices are the future taenia heads. The germinal membrane is the source of both the cyst fluid and daughter cysts. Multivesicular hydatid cysts are the result of multiple daughter cysts.
-
The laminated layer: this supports the germinal layer externally. It is acellular, always separable from the pericyst, with a thickness of 1–2 mm. The layer is permeable to water, K+, Cl-, Ca++, and urea. It protects the cysts from bacteria, enzymes, and bile.
-
The adventitial layer: this has an abundant blood supply and does not have a clear cleavage plane between itself and the surrounding normal host tissue. A fibrous capsule that develops from the host tissue as an inflammatory reaction is the ectocyst or pericyst. With time the adventitial tissue may partially or totally calcify [5, 7].
If rupture of the cyst occurs , secondary hydatidosis results from implantation of protoscolices and daughter cysts on the surrounding viscera [5, 7, 8].
Cyst morphology and cyst integrity are instrumental to the surgical approach of hydatid disease. All aspects of surgical technique are based on knowledge of these specific anatomical details.
Cyst Complications
-
Compression. Hydatid cysts may cause compression toward Glisson’s capsule, compression of the bile ducts with obstructive jaundice, and compression of the hepatic veins resulting in Budd-Chiari syndrome. Presinusoidal portal hypertension can occur; sinistral portal hypertension is a result of splenic vein compression.
-
Cyst infection. Hydatid cysts can become infected as a result of a bacteremic episode or communication with bile ducts. Clinical presentation is similar to that of a pyogenic liver abscess.
-
Rupture into the biliary tract. This is the most common complication of hepatic hydatid cysts and leads to cystobiliary communication. Such communications can be major or minor. Major cystobiliary communication can lead to obstructive jaundice, cholangitis, or both.
-
Rupture into the bronchial tree. This may occur with hepatic hydatid cysts located in segments IVa, VII, and VIII of the liver. Bronchio-biliary fistula is the main clinical presentation.
-
Rupture into the peritoneum. This may occur as a result of trauma or spontaneously. Multiple cysts usually develop. Patients with intraperitoneal rupture may be asymptomatic or have acute abdominal pain, nausea and vomiting, and, rarely, anaphylactic reactions.
-
Rupture into organs or cavities [8].
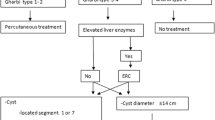
The WHO Ultrasound (US) Classification System
Ultrasound is the first-line imaging technique for hepatic hydatid disease. The WHO classification system [6] classifies hydatid cysts into six types:
-
1.
CL (cystic lesion) type: This is a well-circumscribed liquid image with a clearly defined wall that may be difficult to differentiate from a simple biliary cyst. These cysts are active.
-
2.
CE1: A concentric, hyperechoic halo is present around the cyst, which may contain free-floating hyperechoic foci called hydatid sand. These cysts are active.
-
3.
CE2: Multivesicular daughter cysts present. A honeycomb or rosette image is characteristic. These cysts are active.
-
4.
CE3: There is partial or total detachment of the laminated layer with floating and undulating hyperechoic membranes showing the dual wall. Water-lily sign. These cysts are transitional.
-
5.
CE4: Cystic and solid components are present. Daughter cysts are absent. The cysts are inactive.
-
6.
CE5: Cysts with matrix or amorphous mass, solid or semisolid appearance, calcification in wall, complete or incomplete. The cysts are inactive.
Indications for Surgery
-
Large CE2–CE3 cysts with multiple daughter cysts.
-
Single liver cysts located superficially, as these may rupture spontaneously.
-
Infected cysts.
-
Cystobiliary communication.
-
Cysts exerting pressure on adjacent organs.
-
Surgery is contraindicated in patients with inactive asymptomatic cysts, cysts which are difficult to access surgically, and very small cysts [8].
Surgical Objectives
The principal objectives of surgery are removal of the entire parasite, removal of the residual cavity, and identification and treatment of biliary leak. The main operative procedures offered are conservative surgery and radical surgery. Conservative surgery includes cystectomy or cyst unroofing, whereas radical surgery includes total pericystectomy, total cystectomy, and hepatectomy. The major pitfalls of hepatic hydatid surgery are uncontrolled spillage of active cystic contents, insufficient extirpation of cystic contents and components, bile leakage, and poor access secondary to location [8, 9].
Preoperative Evaluation
Preoperative evaluation for hydatic disease includes standard tests such as an electrocardiogram, complete blood count and electrolyte panel, and renal function tests. Of great importance is recent high-quality cross-sectional imaging. This is done to exclude hidden or occult pelvic, retroperitoneal, and chest hydatid cysts. A hepatic triphasic CT is performed to evaluate major vascular structures. Finally, MR cholangiography should be performed for central cysts located close to the hilum, to evaluate for possible biliary fistula [8, 9].
Perioperative Benzimidazoles
Perioperative adjunctive drug therapy with benzimidazoles (albendazole, mebendazole) is administered to prevent secondary seeding of the peritoneal cavity in case of intraoperative cyst rupture. Usually, either albendazole or mebendazole is given 4 days prior to surgery. Mebendazole is continued for 3 months postoperatively, whereas albendazole is continued for 1 month postoperatively [5, 7,8,9, 15].
Operative Strategy During Cystectomy or Cyst Unroofing for Hydatid Disease
Cystectomy is the most commonly performed surgical procedure for hepatic hydatidosis. It consists of puncture and cyst aspiration, injection of a scolicidal agent, hydatidectomy (removal of hydatid sand, daughter cysts, laminated and germinal layers), and unroofing. The main risk during hydatid surgery is twofold: intraoperative spillage and missing a biliary leak [8, 15, 20].
One of the important adjunctive intraoperative measures taken in hydatic surgery is the placement of scolicidal-soaked gauzes around the hepatic surface at the cyst location. The most common scolicidal agent used is 20% hypertonic saline and povidone-iodine [8, 9, 15].
Below is a step-by-step brief outline of cystectomy for hepatic hydatid disease [8, 9, 15, 17].
-
1.
Cyst puncture. This is performed using a large-gauge needle. A three-way stopcock is used ideally. Aspiration is performed as much as possible. The color of the aspiration fluid is carefully noted for pus or bile.
-
2.
Scolicidal solution Injection. If the fluid is clear, 20% hypertonic saline is injected into the cyst. The ideal contact time is 6 minutes [21]. The goal of this step is to sterilize the cyst contents thus preventing recurrence.
-
3.
Cyst aspiration. Usually the laminated membrane will collapse into the cystic cavity at this stage.
-
4.
Cyst incision. This is done in order to inspect cyst contents initially and to remove them subsequently. The typical contents are clear fluid, hydatid sand, and daughter cysts. After drainage of the fluid, the laminated membrane collapses into the cavity and the cyst contents including daughter cysts can be evacuated. The laminated membrane is removed by forceps. It is important to inspect the cavity for bile staining. Scolicidal injection should never be done in this case, as this poses a risk for sclerosing cholangitis [22].
-
5.
Removal of cyst roof.
-
6.
Management of residual cavity.
As mentioned above, careful inspection of the cyst contents is performed for detection of biliary leakage. This can be done with direct inspection of the cyst cavity, placement of a dry gauze in the cavity with inspection upon removal, methylene blue testing, and intraoperative cholangiography. Obvious small biliary leaks can be sutured, whereas large ones may necessitate endoscopic drainage (ERCP), T-tube drainage, or Roux-en-Y hepaticojejunostomy [8, 9, 15, 17].
The residual cavity is packed with omentum adequately mobilized from the transverse colon. With large cavities, a drain is placed along with the omentoplasty [8, 9, 15, 17].
Radical Operations
Pericystectomy
This technique involves creating a surgical plane outside the pericyst layer. This will prevent inadvertent opening of the cyst during surgery, and an en bloc resection of the lesion is thus achieved. Of note, a clear anatomic plane does not exist; adjunctive tools such as the ultrasonic aspirator or other energy devices may be used to facilitate parenchymal transection as in hepatic resection. If the hydatid cyst is in proximity of major hepatic veins, the venal cava, or the liver hilum, pericystectomy should be avoided [8, 20].
Liver Resection
Liver resection is indicated for infection with E. multilocularis [20]. In cases of E. granulosus, resection is considered in cases of peripherally sited cysts such as the left lateral segment, in cases of pedunculated cysts, as well as in cases of hepatic atrophy secondary to biliary obstruction. When a major bile leak occurs during hepatic hydatid surgery, liver resection may be performed if endoscopic or surgical biliary drainage or diversion is not deemed effective [20].
The Laparoscopic Approach
The adoption of minimally invasive techniques in hepatic surgery is unquestionable. Surgery for hepatic hydatidosis has been no exception to this trend. However, widespread adoption of minimally invasive techniques for this specific indication has not been widespread. It has not replaced standard time-tested open approaches even with conservative surgical options such as cystectomy. Strict selection criteria based on location and size of the lesion, experience in minimally invasive surgery, and available instrumentation are extremely important for consideration of a laparoscopic approach to hydatidosis. Overall, minimally invasive approaches are reserved for uncomplicated cysts in the anterior liver segments [1, 8].
Significant pitfalls for laparoscopy are as follows:
-
Limited space for instrumentation.
-
Complexity in controlling spillage after cyst puncture.
-
Difficulty in aspirating degenerated thick cystic contents.
-
Pneumoperitoneum can increase risk of hydatid fluid contamination and has been reported as a risk factor for spillage and recurrence [16].
-
Difficulty in controlling hemorrhage.
In centers with extensive experience in treating hepatic hydatidosis, exclusion criteria for minimally invasive surgery are intraparenchymal cyst location, the presence of more than 3 cysts, and a thick, calcified cyst wall. Hydatid cysts in proximity to major vessels and relapsed cysts are also contraindications to the minimally invasive approach.
Both conservative and radical procedures have been utilized in minimally invasive hepatic hydatid surgery [8, 15, 20].
Positioning and Access
The “French” position, supine with legs spread apart, surgeon between the legs and assistants on either side, is frequently employed. Trocar sites vary according to location and size of cyst. Usually a supraumbilical port is placed for the optics; a 30-degree laparoscope is frequently used. On average, four trocars are placed in total. A pneumoperitoneum at 12 mm pressure is established. With laparoscopic cystectomy, the same principals are followed as with open surgery. Gauzes soaked with scolicidal fluid (20% hypertonic saline or povidone-iodine) are carefully placed around the hydatid cyst. The cyst is punctured, aspirated, and filled with the scolicidal fluid, and re-aspirated after 10 min. A cystotomy is performed with electrocautery. The laparoscope can be introduced in to the cyst cavity and enhanced magnification and high optic resolution can be useful to explore for remnant daughter cysts, laminated membranes, and bile leakage. Laparoscopic sutures or clips are used to ligate biliary-cyst communications. Omentoplasty is employed for the residual cavity and drains can be placed [8, 9].
Special laparoscopic techniques and instruments have been used in minimally invasive hydatid surgery. Most of these are a result of surgical enterprise, using relatively cheap components to achieve a cost-effective solution to a problem endemic in specific geographical areas. A perforator-grinder-aspirator has been used to access and evacuate hydatid cysts [13, 14]. A specialized trocar (umbrella-shaped) enables suspension of the cyst wall against the abdominal wall for facilitated evacuation of cyst contents [19]. Bickel and Eitan described the use of a transparent large cannula with a beveled tip for safe aspiration of hydatid cyst contents [12]. Hemmati described a laparoscopic system for access and evacuation of hydatid cysts using a Maryland laparoscopic grasper/dissector, standard laparoscopic trocar, and a modified endotracheal tube [17]. All these techniques are designed to achieve access and evacuation of hydatid cysts with minimal spillage and risk of contamination and recurrence. Perihepatic irrigation creating a pool of scolicidal solution has been recommended by some experts [15, 18].
Berberoglu et al. [15] described laparoscopic cystectomy with gasless laparoscopy. Theoretical advantages of this approach are the amelioration of dissemination/contamination as a result of positive pressure exerted by the CO2 gas; the use of high-volume suction and irrigation systems without loss of pneumoperitoneum; as well as avoidance of hemodynamic and metabolic changes attributable to pneumoperitoneum. In addition, CO2 embolization can be avoided.
Review of Literature
Minimally invasive technique for treatment of hydatic hepatic cystic disease was first described by Katkhouda et al. [10]. In the ensuing decades, a significant body of literature has accumulated regarding minimally invasive techniques for hepatic hydatid disease. Tuxun et al. [11] reviewed the world literature on laparoscopic treatment of hepatic cystic hydatid disease. The study included 914 patients. The patient age range was 3–70 years; the cyst diameter range was 3–18 cm. The majority (84%) were 5–10 cm in diameter. There were 466 males and 368 female patients. The location of the hepatic cysts were as follows: in 643 patients, right lobe; 295 patients, left lobe and 32 patients, both lobes. The procedures performed were:
-
Cystectomy: 75.16%
-
Partial pericystectomy: 14.77%
-
Pericystectomy: 5.84%
-
Left lobectomy: 1.09%
-
Left lateral segmentectomy: 0.98%
Pneumoperitoneum was used in 89.17% of cases and gasless laparoscopy in 5.58%.
The conversion rate to an open procedure observed in this review was 4.92% overall. Reasons for conversion included adhesions, bleeding, poor exposure, inappropriate staging, inability to identify cyst laparoscopically, imminent risk of rupture and uncontrolled spillage, and inadequate evacuation. The operative time ranged from 50 to 144 min. This decreased as experience accumulated. The average length of hospital stay was 1–8 days. Overall mortality was 0.22%. The range of morbidity in the report included within this review ranged from 0% to 53%. Overall morbidity was 15%. The incidence of bile leak was 6.24%. Treatment for bile leak included endo-biliary stenting and sphincterotomy. Abscess was formed in 2.2% of patients. Anaphylactic shock occurred in three patients in this review. Complications reported in the review included port-site infection, incisional hernia, subphrenic abscess, small bowel perforation, fever of unknown origin, subcutaneous hematoma, pleural effusion, empyema, pneumonia, and drug-induced fever. Recurrence of hydatid disease was 1.09% overall. The authors concluded that minimally invasive approaches are safe in selected patients, comparable to open surgery with acceptable morbidity and mortality. Conservative and radical surgery is feasible laparoscopically [11]. Finally, few cases of robotic-assisted surgery have been reported. Goja et al. [20] described robotic-assisted cysto-pericystectomy. Reported advantages of this approach include three-dimensional visualization, tremor reduction, motion scaling, and additional degrees of freedom allowing for improved dissection, parenchymal transection, intracorporeal suturing, and hemostatic control [20].
Conclusion
Minimally invasive techniques have been added to the armamentarium of surgeons treating patients with hepatic hydatid cystic disease. These should be employed in carefully selected patients. High-quality cross-sectional imaging is important for patient selection. In general, minimally invasive approaches are reserved for uncomplicated cysts in the anterior liver segments. Special instrumentation for access and cyst aspiration may be necessary. All the important steps taken in open surgery should be reproduced in laparoscopic hepatic hydatic cases. This includes field protection of cyst area with scolicidal-soaked gauze, meticulous care to prevent spillage during puncture and aspiration, careful inspection of the cyst cavity for remnant daughter cysts and bile leakage, and making an effort to perform omentoplasty in the remaining cavity. Careful use and selection of scolicidal solutions may prevent complications such as sclerosing cholangitis and chemical peritonitis. Laproscopic hydatid surgery requires expertise in both the treatment of hydatid disease and advanced laparoscopic techniques. Conversion to open surgery should not be considered a failure. There should be a low threshold for conversion when exposure is inadequate or deemed unsafe, access to the cyst itself is unsatisfactory, cyst rupture and uncontrolled spillage are imminent or apparent, or daughter cysts cannot be removed. Inability to control a bile leak is an indication for conversion. Of note, no randomized controlled trials have been performed to compare the various therapeutic options for hepatic cystic hydatidosis.
References
Liu R. Laparoscopic anatomical hepatectomy. In: Liu R, editor. Laparoscopic liver resection. Theory and techniques. Gordecht: Springer; 2017.
Cherqui D, Lin CW, Kluger M. Minimally invasive techniques in hepatic resection. In: Jarnagin W, editor. Blumgart’s surgery of the liver, biliary tract and pancreas. Philadelphia: Elsevier; 2017. p. 1597–612.
Martin RCG, Scoggins CR, McMasters KM. Laparoscopic hepatic lobectomy: advantages of a minimally invasive approach. J Am Coll Surg. 2010;210:627–36.
Komatsu SO, Goumard C, et al. Development process and technical aspects of laparoscopic hepatectomy: learning curve based on 15 years of experience. J Am Coll Surg. 2017;224:841–50.
Pakala T, Molina M, Wu GY. Hepatic echinococcal cysts: a review. J Clin Transl Hepatol. 2016;4:39–46.
WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. 2003;85:253–61.
Bhutani N, Kajal P. Hepatic echinococcosis: a review. Ann Med Surg (Lond). 2018;36:99–105.
Pascal G, Azoulay D, Belghiti J, Laurent A. Hydatid disease of the liver. In: Jarnagin W, editor. Blumgart’s surgery of the liver, biliary tract and pancreas. Philadelphia: Elsevier; 2017. p. 1597–612.
Ertem M, Karahasanoglu T, Yavuz N, et al. Laparoscopically treated liver hydatid cysts. Arch Surg. 2002;137:1170–3.
Katkhouda N, Fabiani P, Benizri E, et al. Laser resection of a liver hydatid cyst under videolaparoscopy. Br J Surg. 1992;79:560–1.
Tuxun T, Zhang J, Zhao J, et al. World review of laparoscopic treatment of liver cystic echinococcosis-914 patients. Int J Infect Dis. 2014;24:43–50.
Bickel A, Eitan A. The use of a large, transparent cannula, with a beveled tip, for safe laparoscopic management of hydatid cysts of the liver. Surg Endosc. 1995;9:1304–5.
Saglam A. Laparoscopic treatment of liver hydatid cysts. Surg Laparosc Endosc. 1996;6:16–21.
Avtan LA. A new ‘perforator-grinder-aspirator apparatus (PGAA)’ for the minimal access surgery of cystic hydatidosis. Hepato-Gastroenterology. 2005;52:339–42.
Berberoglu M, Taner S, Dilek ON, et al. Gasless vs gaseous laparoscopy in the treatment of hepatic hydatid disease. Surg Endosc. 1999;13:1195–8.
Jerraya H, Khalfallah M, Osman SB, et al. Predictive factors of recurrence after surgical treatment for liver hydatid cyst. Surg Endosc. 2015;29:86–93.
Hemmati SH. How to build a simple and safe hydatid evacuation system. JSLS. 2014;18:e2014.00314.
Palacios-Ruiz JA, et al. Hypertonic saline in hydatid disease. World J Surg. 2002;1398.
Seven R, Berber E, Mercan S, et al. Laparoscopic treatment of hepatic hydatid cysts. Surgery. 2000;128:36–40.
Goya S, Saha SK, Yadav SK, et al. Surgical approaches to hepatic hydatidosis ranging from partial cystectomy to liver transplantation. Ann Hepatobiliary Pancreat Surg. 2018;22:208–15.
Besim H, et al. Scolicidal agents in hydatid cyst surgery. HBP Surg. 1998;10:347–51.
Belghiti J, et al. Caustic sclerosing cholangitis. A complication of the surgical treatment of hydatid disease of the liver. Arch Surg. 1986;121:1162–5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Spanos, C.P. (2020). Is There a Role for Minimally Invasive Surgery in the Management of Hydatid Liver Disease?. In: Tsoulfas, G., Hoballah, J., Velmahos, G., Ho, YH. (eds) The Surgical Management of Parasitic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-47948-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-47948-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47947-3
Online ISBN: 978-3-030-47948-0
eBook Packages: MedicineMedicine (R0)