Abstract
The transplantation of spermatogonial stem cells (SSCs) to the testis has the potential to become a fertility restoration strategy in patients at risk for germ cell loss due to gonadotoxic therapies. Initial transplantation of SSCs was performed by injecting a single cell suspension in the seminiferous tubules of the testis. Later on, testicular tissue transplantation seemed to be easier and more efficient. Full spermatogenesis can be obtained in transplanted testicular tissue in many species, including nonhuman primates. Recently, the birth of a monkey after intracytoplasmic injection with spermatozoa isolated from ectopic testicular tissue transplants in the monkey was achieved.
Cryopreservation of testicular biopsies to boys facing gonadotoxic treatments is already offered by several fertility centers, but transplantation of these stored biopsies to a patient has not yet been performed. It will be a matter of time before patients will inform about the restoration strategies with their cryopreserved testicular biopsies. Next to a clinical fertility preservation strategy, the transplantation of SSCs can be useful for the preservation of endangered livestock or the creation of transgenic animals.
This book chapter will provide an overview of the current achievements in the transplantation of SSCs in animal models and factors to be considered for a human clinical application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
The transplantation of spermatogonial stem cells (SSCs) represents a potential approach to restore fertility in patients who are at risk of germ cell loss but do not have the option to cryopreserve a semen sample to preserve their fertility. SSCs are the founder cells of spermatogenesis and are thus indispensable for the production of sperm cells (90 million per day in the human). The function of SSCs is to maintain the stem cell population (self-renewal) in the testis on the one hand and to generate enough progenitor cells (differentiation) which will further differentiate in mature spermatozoa on the other hand [1]. To succeed in these functions, SSCs are positioned in a tightly regulated stem cell niche.
The interaction between Sertoli cells and the SSCs is of high importance for the regulation of spermatogenesis. The number of Sertoli cells also determines the number of SSCs in the testis and is thus responsible for the sperm output. They are connected to each other and to the germ cells by junctions allowing close communication between both cell types [2]. The Sertoli cells are assisted in their function to regulate the tight balance between SSC renewal and SSC differentiation by other somatic cells, including the Leydig cells, the peritubular myoid cells, other interstitial cell types and the vasculature [3]. Leydig cells support spermatogenesis by the synthesis of androgens which regulate spermatogenesis by interaction with androgen receptors present on the Sertoli cells and peritubular myoid cells. Increasing evidence shows that also the peritubular myoid cells play a role in the regulation of spermatogenesis. They provide structural support by forming the basement membrane together with the extracellular matrix and fibroblasts and stimulate attachment of Sertoli cells and SSCs to the basement membrane. Their myoid characteristics assist in the transport of mature spermatozoa in the seminiferous tubules toward the epididymis [4]. The close interaction between these different cell types ensures the tight regulation of spermatogenesis within the SSC niche (Fig. 1). It is obvious that any damage to this organ will eventually result in infertility.
A lot of the knowledge that is gathered today about the organization of spermatogenesis, SSC renewal, and the testicular niche has been obtained by transplanting SSCs to a germ cell-depleted testis. The transplantation of a testicular cell suspension resulted in the restoration of spermatogenesis in an infertile mouse testis [5, 6]. These very first reports in 1994 paved the way to a new field of research: fertility preservation. The cryopreservation and transplantation of SSCs could become a means to preserve and restore fertility in patients facing SSC loss due to gonadotoxic treatments. Initially, testicular cell suspensions were transplanted, soon followed by the transplantation of testicular tissue [7]. Now, 25 years and many research papers later, a clinical application of this technique is within reach. However, although cryopreservation of testicular tissue is already offered worldwide, the transplantation procedure remains experimental, and the first clinical transplantation is still awaited [8].
This chapter provides an overview of the achievements and insights gathered by the transplantation of SSCs and its possible applications.
The Transplantation of Spermatogonial Stem Cells in Animal Models
SSC transplantation was originally developed in 1994 by Brinster and Avarbock in a mouse model. Isolated mouse testicular cells could recolonize the testis from an infertile mouse and differentiate into fully matured sperm with fertilization capacity as proven by the birth of viable offspring [5, 6]. This was the first proof of concept that genetic information could be transmitted to the next generation via SSCs. The identification of donor-derived cells and offspring could be realized by the transplantation of labeled donor cells. The first reported detection system was based on the transplantation of ZFLacZ donor cells that stain blue after staining with X-gal [5]. The second system and also the most common one involved donor cells from transgenic animals that express green-fluorescent protein (GFP) in all cells under control of the β-actin promoter [9]. Next to their use in the development of fertility preservation strategies, these models could also add valuable information to the knowledge of the fundamental aspects of SSCs and spermatogenesis or be used in the preservation of endangered animal species and the generation of transgenic animals. Moreover, in the search for the ultimate SSC population in the testis, SSC transplantation was, and still is, the only way to prove stem cell activity.
Alternatively, instead of isolating and transplanting SSCs which have to be followed by SSCs finding their way to the niche (homing), SSCs could be transplanted within their own niche (testicular tissue grafting). The first reports showing restoration of spermatogenesis in testicular tissue grafted to immunosuppressed mice date from 2002 [7, 10, 11].
During the past 25 years, transplantation of SSCs (either as cell suspension or tissue) has been investigated thoroughly.
Xenotransplantations
After the success of restoring spermatogenesis by the transplantation of SSCs in a mouse model, the efficiency of interspecies transplantations was evaluated. Mature spermatozoa could be isolated from the mouse epididymis after SSC transplantation with donor cells from adult rat and hamster. The presence of donor-derived spermatogenesis was confirmed by colorimetric staining (β-galactosidase/X-gal) [12] and/or by the identification of morphologically different spermatozoa in the mouse epididymis [13]. Although testicular cell suspensions including germ cells and somatic cells were transplanted to the mouse recipient testis, only SSCs were able to home to an empty niche and colonize the seminiferous tubules, while other cells were phagocytized by Sertoli cells [14]. This implies that the somatic environment from the recipient mouse was able to support rat and hamster spermatogenesis. However, the efficiency of the microenvironment in recipient seminiferous tubules to support spermatogenesis from transplanted SSCs seems to decrease with increasing phylogenetic distance between donor and host. After transplantation of testicular cell suspensions from immature rabbits and pigs, colonization and limited spermatogonial expansion were noticed, but differentiation did not proceed to meiosis. Colonization, albeit with a low efficiency, was observed when testicular cells from dog, bull, and horse were transplanted to the mouse testis, but no differentiation was observed [15, 16]. This indicates that the interactions between Sertoli cells and germ cells are only partially conserved between different species.
The problem of increased phylogenetic difference between niche and stem cell can be circumvented by transplanting testicular tissue. This gives the advantage of transplanting SSCs within their own niche and avoids the homing step which might result in low colonization efficiency [17]. Initially, grafting of immature testicular tissue was performed under the back skin of immunodeficient mice. Ectopic transplantation of immature tissue from hamster [18], pig, goat [7], cat [19], dog [20], horse [21], bovine [22], and rhesus macaques [7, 23] resulted in the production of complete spermatogenesis in the xenografts. The functionality of the ectopically produced sperm was confirmed by embryonic development with sperm isolated from mouse, goat [7], pig [24], and rhesus macaques [23]. Spermatozoa isolated from porcine xenografts could successfully activate embryonic development in porcine oocytes and support development up to the blastocyst stage [24]. Convincing evidence for the functionality of the spermatozoa generated in xenografts came with the birth of live offspring in pigs and monkeys [25, 26]. Initially, spermatozoa were retrieved from fresh xenografts, but the same has been performed with spermatozoa isolated from xenografts that had been vitrified before transplantation [27]. The sexual maturation and fertility of piglets born from spermatozoa isolated from cryopreserved xenografts was evaluated. Male offspring showed normal testosterone concentration and testicular histology and were able to impregnate sows after natural mating resulting in the birth of piglets with normal litter size and birth weight. Female first-generation offspring gave birth to healthy pups after artificial insemination with sperm from conventional male pigs [28]. Sperm cells were also collected from monkey xenografts and were either directly used for ICSI or cryopreserved for later use. Transfer of in vitro fertilized oocytes resulted in the birth of seven monkeys [25].
Although successful sperm production in ectopic xenografts was reported for many species, the establishment of full spermatogenesis could not be achieved in human xenografts. The first reports about the xenografting of human tissue only showed limited survival of spermatogonia [10, 29, 30]. In these initial reports, adult testicular tissue was grafted, but this mostly resulted in degenerated and hyalinized tubules. In analogy to what was observed in hamster tissue, this limited success was attributed to the fact that adult tissue was used. The presence of ongoing spermatogenesis seems to have a negative influence on graft survival [10]. Transplantation of testicular tissue from immature human tissue (10 and 11 years) resulted in a better-preserved integrity of the seminiferous tubules, but with only limited survival of spermatogonia 4 and 9 months after transplantation [31]. Similar results were observed when newborn marmoset tissue was transplanted to the back skin of mice [10]. The weight of the seminal vesicles collected after grafting indicated poor androgen production in human and marmoset grafts, while seminal vesicle weight in castrated mice receiving immature mouse or hamster tissue was recovered to values in control mice [10, 30]. The capacity of marmoset and human ectopic tissue grafts to respond to mouse gonadotrophins and initiate testosterone production was lower compared to other species. Meiotic arrest was also observed in rat xenografts, which is exceptional since incomplete spermatogenesis is mostly observed in xenografts from larger species. Spermatogenesis in rat xenografts did not proceed beyond pachytene spermatocytes [32, 33]. Seminal vesicle weight in transplanted mice was restored to a size similar than intact control male mice. Luteinizing hormone (LH) levels were also similar to intact controls, while follicle-stimulating hormone (FSH) levels remained elevated compared to non-castrated mice. Expression of FSH receptor was lacking and could be responsible for the elevated FSH concentrations. Dysregulated expression of Sertoli cell transcripts was also observed, indicating that altered protein expression in the somatic compartment could have played a role in the spermatogenic arrest in rat xenografts [33]. This shows that the functional establishment of spermatogenesis in xenografts is species dependent.
Alternative to the ectopic location, donor testicular tissue can also be transplanted to an orthotopic location, i.e., the scrotum or the most natural place for testicular tissue, the testis itself. Human testicular tissue was transplanted to the scrotum after castration of the recipient mouse. Short-term evaluation (3 weeks after grafting immature testicular tissue from cryptorchid boys) showed a well-preserved integrity of the tubular structures, no fibrosis, and survival of spermatogonia, albeit with a significant loss of germ cells. The number of spermatogonia decreased from 0.55 spermatogonia per tubule in fresh tissue to only 0.08 after the freezing and grafting procedure [34]. Long-term evaluation (6 months after grafting) confirmed the survival of human spermatogonia in scrotal grafts from prepubertal boys. Although sperm cell-like cells were identified in the grafted tissue, these cells did not express meiotic or postmeiotic markers [35].
Intratesticular grafting was first reported by Shinohara et al. Testicular tissue from mouse and rabbit was transplanted under the tunica of the testis from immune-deficient mice. Spermatogenesis was restored, and sperm cells isolated from the grafted piece were used to fertilize oocytes, which resulted in the birth of healthy offspring. Murine sperm cells could be identified by the expression of GFP since a GFP+ donor was used, while rabbit sperm cells were identified based on their unique sperm head [11]. Full spermatogenesis has also been reported in intratesticular xenotransplants from marmoset [36], but complete spermatogenesis was not achieved in testicular xenografts from pre- and peripubertal boys [37,38,39]. An overview of the testicular tissue xenograft achievements in different species is presented in Fig. 2.
Allogeneic Transplantations
The feasibility of restoring spermatogenesis by the transplantation of SSCs to the testis has been evaluated in many species other than the mouse. Successful transplantation of SSCs and regeneration of spermatogenesis was reported in goat, pig, dog, sheep, bovine, and monkey [40,41,42,43,44,45]. The first successful transfer of germ cells into large species was reported by the group of Schlatt [46].
Allogeneic transplantations might have to deal with immunological response due to a different genetic background between donor cells and recipient. Xeno- or allogeneic transplantations in rodents are performed in immunosuppressed animals, but this is not always feasible in larger species. In rodents, allogeneic pups were born by natural mating when recipient mice were treated with immunosuppressive factors [47]. Although spermatogenesis was observed in the testes, no sperm cells were observed in the epididymis of untreated rodents. However, successful generation of sperm cells in the ejaculate could be achieved after transplantation of allogeneic germ cells without any immunosuppression of the recipients indicating that a certain level of immune tolerance is present in the testis [41,42,43]. Even the transplantation between unrelated immunocompetent animals of different sheep breeds resulted in the production of donor-derived spermatozoa. The functionality of these spermatozoa was proven by the birth of live progeny after artificial insemination [44].
To guarantee empty niches for the SSCs to home to, recipients need to be depleted of endogenous germ cells. In analogy to the rodent model, this was achieved by treating the recipients with chemotherapeutic drugs [42] or local irradiation of the testes [43, 44]. Alternatively, transplantation was also performed to prepubertal animals which lack spermatogenesis [41]. However, spermatogonia are still present in these animals and might thus prevent efficient homing of transplanted SSCs. Optimal preparation of the recipients can improve the efficiency of the transplantation procedure and result in an increased proportion of donor spermatozoa in the ejaculate [44].
Identification of donor-derived spermatozoa and thus proof of successful SSC transplantation are hampered by the presence of endogenous spermatogenesis. To confirm the presence of donor-derived spermatogenesis, donor cells need to be distinguished from recipient cells. One option is to label the cells with a fluorescent dye which enables detection of donor spermatogonia after colonization. However, the fluorescent marker appeared to be diluted by cell divisions and thus does not permit the identification of fluorescent sperm cells [41]. A recessively inherited condition resulting in immotile and anatomically abnormal sperm was used to prove the success of SSC transplantation in pigs. Affected boars were transplanted with donor cells from young normal crossbred boars. Motile sperm was observed in the ejaculate between 13 and 59 weeks after SSC transplantation [42]. Genotyping epididymal sperm cells could also be a way to differentiate between donor and recipient sperm cells by the use of microsatellite markers [43, 44].
In some species, testicular tissue has been transplanted under the back skin. Sperm cells produced in ectopic murine allografts showed full capability of fertilization resulting in the birth of live offspring which were proven to be fertile as well as indicating the lack of major damage to the germ cell lineage. Although functional sperm cells were produced in these ectopic grafts, premature sloughing and enlargement of the luminal space were noticed already at week 4 after transplantation, probably as a result of the accumulation of fluid [48]. Ectopic testicular tissue grafting has been explored in rhesus monkey and marmoset, but as a model in nonhuman primate species, for a clinical application, grafting was mainly performed autologously and will thus be discussed in the next paragraph.
Autologous Transplantations
In allo- or xenogeneic transplants, problems with immune tolerance due to the different genetic background from donor and recipient have to be taken into account. In a clinical application, this problem does not arise since SSCs will be preserved during therapy with the aim of performing an autologous transplantation.
Autologous transplantation has been performed in the bovine. Injection of bovine SSCs resulted in the presence of spermatozoa in 15% of the seminiferous tubules, while only spermatogonia were present in 45% of the tubules in the non-transplanted control testis [45].
To investigate the potential of SSC transplantation in a human clinical application, the restoration of spermatogenesis after injection of SSCs was evaluated in nonhuman primates. A cell suspension generated from a hemi-orchidectomized cynomolgus monkey was injected via the rete testis, guided by ultrasonography, in the contralateral testis of the same monkey. Four weeks after transplantation, differentiated spermatogonia were present in the testis [46]. Similar experiments were performed in a cynomolgus monkey made sterile by X-ray irradiation. However, spontaneous recovery of spermatogenesis could be observed in the saline-injected testes, making it hard to form conclusions about the presence of donor-derived spermatogenesis, although a higher testicular weight was reported for the SSC-injected testis [49]. Proof that spermatogenesis originated from transplanted donor cells was generated in busulfan-treated macaques. The presence of donor sperm was evaluated by single nucleotide polymorphisms which enable the differentiation between donor and recipient sperm. Complete spermatogenesis was observed 15–63 weeks after transplantation. This study showed that restoration of spermatogenesis is possible from SSCs which were transplanted to a chemotherapy-treated testis [40]. As testis biology, endocrine regulation, and immune function are similar between rhesus macaques and human, this is a reliable model for a human application.
The proof that spermatogenesis originated from the autologously transplanted donor cells and not from endogenous spermatogenesis is however difficult. Initially, BrdU labeling of the donor cells was used but seemed not efficient for long-term identification of donor cells after transplantation [46, 49]. The use of lentiviral vectors to induce GFP expression in the donor cells was efficient for detection of donor signal by polymerase chain reaction (PCR). However, differentiation between donor-derived and endogenous sperm cells was difficult since lentiviral fluorescence could not be distinguished from autofluorescence [40]. Stable transduction of SSCs can now be achieved as shown by the lentiviral transduction of goat SSCs with the green fluorescent protein. These transfected cells were able to colonize the seminiferous tubules of recipient mice [50].
Autologous grafting has been performed with testicular tissue from rhesus monkey [51, 52]. In 2012, complete spermatogenesis was only achieved in the orthotopic grafts, while ectopic grafts showed meiotic arrest [51]. However, in a recent report, the feasibility to achieve spermatogenesis in ectopic rhesus monkey grafts was shown. Four testicular tissue pieces were individually attached by sutures under the back skin or the scrotal skin. Grafts were collected 8–12 months after grafting, and individual grafts were found to be fused into one single large mass with a fivefold increase in weight. Complete spermatogenesis was present in both the ectopic and orthotopic grafts. Sperm cells were isolated and used for ICSI which resulted in the birth of a healthy monkey girl, Grady [52].
The generation of full spermatogenesis seemed more difficult to achieve in autologous grafts from marmoset. Full spermatogenesis was obtained but only in testicular tissue that was grafted to the scrotum, while spermatocytes were the most advanced germ cell type present in ectopic grafts [53, 54].
Although intratesticular tissue grafting was highly efficient in xenotransplantation studies, autologous transplantations to the testicular parenchyma have not been performed so far (Fig. 3).
Applications of SSC Transplantation in Animals
Production of Transgenic Animals
The production of transgenic animals has practical applications in several fields. Transgenic mice have been produced for years, especially for their use in biomedical research. However, there is also an increasing interest and need for the production of transgenic livestock animals. The term livestock refers to domesticated animals which are kept in an agricultural setting for their use of animal products such as milk, beef, eggs, fur, wool, etc. They have their use in the production of biopharmaceutical products or agricultural products but could also serve as a biomedical model for human diseases [55]. The first genetically modified livestock animals were produced by pronuclear injection, which includes the injection of DNA in the pronucleus of a fertilized oocyte. Somatic cell nuclear transfer (referred to as cloning) is another technique to generate large transgenic animals. However, both techniques are technically challenging, costly, time-consuming, and inefficient. The SSC is unique in its ability to transmit genes to the subsequent generations, which makes it an ideal target for genetic manipulations. The transplantation of genetically modified germ cells can decrease the time to produce transgenic spermatozoa compared to somatic cell nuclear transfer. It also eliminates the labor-intensive outcross breeding to produce non-mosaic germline mutants. A single genetically modified SSC can result in the production of a large number of sperm cells to generate transgenic progeny. Moreover, spermatogenesis is a natural selection mechanism to eliminate spermatozoa with undesired mutations [56]. Goat SSCs were successfully transduced with eGFP by lentiviral transduction. These transduced cells were able to colonize the testis when transplanted to germ cell-depleted recipient mice [50].
Ectopic grafting could be an even better approach to generate transgenic animals. While the phylogenetic distance between donor and recipient is of more importance when SSC transplantation is performed, full spermatogenesis was achieved in ectopic xenografts for many species. The isolation of transgenic spermatozoa from these grafts is also easier since they can be retrieved from the subcutaneous grafts on the back side of a nude mouse. The isolation of transgenic spermatozoa after SSC transplantation will be more difficult since they have to be distinguished from endogenous spermatozoa. In a study where testicular tissue from a transgenic monkey was grafted, the birth of a donor-derived monkey was reported after ICSI using spermatozoa isolated from the ectopic xenografts. The presence of the transgene in the offspring was confirmed by RT-PCR and Western blot [25].
Preservation of Endangered Species
Due to human activities, such as habitat destruction, overhunting/fishing, and climate change, the extinction of several animal and plant species is occurring at a much higher rate than predicted [57]. Cryopreservation and subsequent SSC transplantation can be useful in the preservation of valuable or endangered species. Techniques involving the preservation of SSCs have several advantages over the preservation of mature spermatozoa. The cryopreservation of SSCs enables the preservation of the entire genetic potential of an individual species, since genetic recombination will occur in differentiated germ cells when transplanted. SSCs can be harvested from sexually immature males which is critical for species subject to high neonatal or juvenile mortality [58]. This can also be useful in the preservation of valuable species that undergo early castration (e.g., horses) but display superior traits later in life [59].
Fertility Preservation in Patients at Risk for Germ Cell Loss
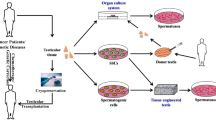
A direct clinical application relies in the preservation of fertility in patients who are at risk of germ cell loss. The incidence of childhood cancers is continuously increasing. Luckily, mortality rates are at the same time decreasing [60]. In most industrialized countries, cancer is the most frequent non-accidental cause of death. The overall 5-year survival rate in children and adolescents diagnosed with cancer ranges from 72% up to 78% in Europe and the United States, with an individual difference between different types of cancer. The long-term survival rate of some cancer types can even be as high as 99% [61]. Unfortunately, the malignant cells are not the only cells that are targeted by chemo- and radiotherapy. The rapidly dividing cells in the testis are also destroyed by aggressive cancer treatments. This side effect from cancer treatment only becomes apparent many years later, when these childhood cancer survivors have a child wish. For many years, fertility of adult cancer patients can be preserved by the cryopreservation of sperm [62]. However, in prepubertal and young adolescent patients, spermatogenesis has not started yet making sperm collection impossible. The preservation and transplantation of SSCs provide hope for these patients. By isolating and storing the SSCs during therapy, the fertility potential of these patients could be safeguarded (Fig. 4).
Indications for Fertility Preservation
The inability to reproduce can have a major impact on the psychological aspects of life quality. The cryostorage of SSCs can offer hope to patients for having children after surviving childhood cancer. However, next to cancer patients, other patient groups might also benefit from SSC banking.
Gonadotoxic Treatments
The cancers diagnosed during childhood are of different nature than those in adults, and their occurrence is age dependent. While young children are mostly affected by tumors of embryonal origin such as neuroblastomas, the incidence of leukemia, central nervous tumors, and lymphomas (non-Hodgkin and Hodgkin) is higher in older children. The treatment regimens for these kind of pediatric cancers often consist of chemotherapy with multiple agents [63]. Initially, it was thought that impact of childhood gonadotoxic treatments would be less severe compared to those given to adults since active spermatogenesis is lacking. However, germ cell proliferation and maturation of the somatic cells occur during childhood. From data in the marmoset testis, it is hypothesized that the transition toward the mature status of the testis occurs much earlier than at puberty [64]. The impact on fertility depends on the survival of two morphologically distinguishable stem cell populations present in the testis. The Adark spermatogonia are considered to be the true testicular stem cells and function as testicular reserve. Their proliferation rate is low under normal circumstances but increases after a cytotoxic insult. The Apale spermatogonia have a higher proliferation rate and serve as progenitor cells for the differentiating germ cells [65]. If only the Apale spermatogonia are destroyed, restoration of fertility is possible due to replenishment of the progenitor pool by the Adark spermatogonia. However, complete depletion of both stem cell populations will indefinitely lead to permanent infertility. Next to the survival of the germ cells, the damage to the somatic environment can have a high impact on the restoration of spermatogenesis [66]. Not only the kind of drug but also the dose, the duration of the treatment, and the age of the patient are influencing factors [63].
Treatment regimens can be subdivided in low-risk and high-risk treatments. Alkylating agents like cyclophosphamide, cisplatin, and busulfan are classified as high-risk treatments. These treatments have a high risk of both destroying the total stem cell population and affecting the somatic compartment reducing the chances of spontaneous recovery of spermatogenesis dramatically. Studies on the effect of these treatments on prepubertal testes are scarce. In a large cohort of childhood lymphoma survivors, a damaging effect on testicular function was observed in 42% of the patients. The damaging effects were mainly dependent on the cumulative doses and less related to the age or pubertal status [67, 68]. A significantly lower number of SSCs was observed in testicular biopsies from prepubertal boys having already received alkylating agents before testicular tissue banking, while no difference with control biopsies was observed when non-alkylating agents were received before banking [69].
Next to chemotherapy, irradiation can also induce damage to the germinal epithelium. Impaired spermatogenesis is already described after doses as low as 0.1 Gy, and irreversible gonadal damage occurs at 4 Gy. The damage not only depends on the dose, but also the fractionation of radiotherapy [70]. The long-term effects of irradiation before adulthood were studied in the rhesus monkey. Different doses ranging from 4.0 to 8.5 Gy were administered to monkeys which did not have reached full maturity yet. The long-term effects on testis development were studied 3–8 years after irradiation. Repopulation of the testes by SSCs was observed in most monkeys but did not reach full recovery even in monkeys receiving the lowest dose. Complete sterility was observed in 13% of the irradiated monkeys and occurred in monkeys receiving the highest doses of 8.0 and 8.5 Gy and in monkeys that received a fractionated dose of 6.0 Gy. A decrease in the Sertoli cell number was also reported. Since the number of Sertoli cells determines the number of germ cells, this also negatively impacts fertility [71].
Treatment regimens for nonmalignant conditions might also involve gonadotoxic treatments. Patients with hematological disorders like sickle cell disease or thalassemia often need hematopoietic stem cell transplantation requiring preconditioning treatments involving total body irradiation (10–13 Gy) to deplete the blood stem cell line [66, 72]. These patients also have a high risk of becoming infertile.
Pediatric oncologists/hematologists should inform patients about the risks of infertility and their options for fertility preservation. Since detailed knowledge about the risks of different treatments on infertility is lacking, there is a need to closely follow up fertility potential of these childhood cancer survivors.
Genetic Disorders
Azoospermia, defined as the absence of sperm in the ejaculate, is diagnosed in 10–15% of all infertile men [73]. Next to the idiopathic cases (40%) where the etiology remains unknown, genetic causes can be found in 20–25% of them [74]. Diagnosis only occurs at adult age leaving no option for these men to take precautionary measures to preserve their fertility. However, in about 14% of azoospermic patients, Klinefelter syndrome (KS) is diagnosed [74]. KS patients, having one or more extra copies of the X-chromosome, present with azoospermia resulting from massive germ cell loss which was thought to accelerate from puberty onward [75]. As KS patients are mainly diagnosed at adulthood, initial fertility preservation strategies focused on sperm cryopreservation. Sperm cell retrieval by testicular sperm extraction is feasible in about 40% of the patients [76, 77]. Since, now, more and more KS patients are diagnosed before adulthood [78], the idea was put forward to preserve germ cells already around adolescence (spermatozoa) or before puberty (SSCs). However, collecting spermatozoa at adolescent age did not prove to be beneficial [79,80,81,82]. Moreover, testicular biopsies from adolescent patients that were preserved for testicular tissue banking only showed a very low number of SSCs [83, 84]. Even in testicular biopsies that were cryopreserved at prepubertal age, SSC numbers were extremely low [85]. Therefore, testicular tissue banking is considered to have no additional benefit for fertility preservation in KS patients.
Cryopreservation of Spermatogonial Stem Cells
For testicular tissue transplantation to succeed, the testicular biopsy needs to be stored without affecting its functionality. Cryopreservation makes use of very low temperatures to preserve structurally intact living cells and tissues and thus enables the long-term storage of biological samples outside the body. The biological effects that occur when cooling below 0 °C are dominated by the freezing of water, which involves 80% of the tissue mass. Freezing converts liquid water into ice, resulting in the concentration of dissolved solutes in the remaining liquid phase and the precipitation of the solutes exceeding their solubility limit [86]. The formation of intracellular ice crystals must be avoided to assure cell survival during freezing and thawing procedures.
Cryopreservation damage can be controlled and diminished by the addition of cryoprotective agents (CPAs). Their cryoprotective action involves a decrease in the concentration of solutes and an increase in membrane stability during the dehydration and rehydration phases. Two classes of CPAs exist, permeating CPAs (e.g., dimethyl sulfoxide [DMSO], glycerol, formamide, and propanediol) and non-permeating CPAs including sugars (e.g., sucrose, trehalose, dextran, lactose, and d-mannitol) and high molecular weight compounds (e.g., polyethylene glycol and hydroxyethyl starch) [87]. The addition of CPAs to the freezing medium has enabled the storage of biomaterials at low temperature without losing functionality. However, the balance between CPA toxicity and their ability to protect cells from freezing damage needs to be carefully considered.
The cryopreservation of SSCs can be performed by either freezing the whole testicular tissue or freezing cell suspensions. Generally, freezing whole testicular tissue is more challenging since different cell types are involved, each cell type with its own characteristics and requirements. Tissue freezing is also hampered by the fact that rapid cooling and warming rates are not easy to achieve. Usually, cooling rates are not uniform across the tissue piece: the temperature in the center of the sample will change more slowly compared to the temperature at the surface of the tissue [88]. Although it is technically easier to cryopreserve cell suspensions, the viability and function of the SSCs can also be affected by the enzymatic dissociation. Besides that, freezing cell suspensions limits the options for transplantation after thawing [89].
Testicular Tissue Freezing
Freezing testicular tissue implies that not only the functionality of SSCs is maintained but also the integrity and functionality of the testicular stem cell niche. Testicular tissue freezing was already reported in the 1990s with the aim of cryopreserving testicular biopsies to avoid repetitive surgery in patients with azoospermia. Glycerol was used as CPA since this was the worldwide choice for the cryopreservation of spermatozoa and these protocols mainly aimed to recover the most mature stages of spermatogenesis [90, 91]. However, the use of glycerol did not seem to be beneficial for the storage of testicular tissue aimed at preservation of SSCs and testicular integrity. The same was observed when 1,2-propanediol was used. In contrast, DMSO was able to maintain testicular integrity and functional activity of Leydig cells as was evaluated by testosterone production in vitro [92]. A controlled slow-freezing (CSF) protocol using a programmable freezer was used to cryopreserve testicular tissue from immature nonhuman primates [93]. Frozen-thawed primate tissue retained the capacity to initiate spermatogenesis when xenografted in an adult mouse recipient, and, recently, sperm isolated from autologous grafts resulted in the birth of a healthy baby monkey [52]. Also, human spermatogonia survived in xenografted testicular tissue after CSF (with DMSO and sucrose as CPAs) [34, 35].
The previously mentioned protocol involves the use of an expensive freezing device and is rather time-consuming. Therefore, a simpler and more time-efficient protocol was developed, and, when evaluated in a mouse model, this proved to result in similar preservation of cellular and tubular integrity compared to CSF. In this single-step slow-freezing (SSF) protocol, vials are put in an isopropyl alcohol container for 24 h at −80 °C before plunging in liquid nitrogen [94]. This protocol was successfully used to cryopreserve immature human testicular tissue with survival of spermatogonia, maintenance of germ cell proliferation, and integrity of the seminiferous epithelium and the interstitial compartment [95]. Human immature SSCs were able to initiate differentiation after SSF in intratesticular tissue xenografts [37,38,39].
An alternative method to CSF and SSF is vitrification, which is an ultrarapid freezing method avoiding the formation of ice crystals. Vitrification might thus be beneficial for cell viability and preservation of tissue integrity. Vitrification of mouse prepubertal testicular tissue seemed efficient in preserving the tubular and cellular integrity [94, 96] and did not influence the graft survival and spermatogenic recovery in mouse intratesticular grafts [94]. Successful vitrification of testicular tissue has been reported in pig [27], cat [97], primate [98], and human [99, 100]. The birth of live offspring after vitrification of pig testicular tissue and ectopic xenografting proves that functional integrity is maintained [27].
Cell Suspension Freezing
The cryopreservation of testicular cell suspensions is studied less intensively and mostly applies to human adult cell suspensions [101,102,103]. Most fertility centers offering testicular tissue banking as a fertility preservation strategy to immature boys perform testicular tissue freezing with slow-freezing protocols. However, there might also be a need to store testicular cell suspensions when the stored testicular tissue is enzymatically digested for decontamination of malignant cells (see later) and subsequent SSC transplantation. During the safety testing procedure, the cell suspension might need to be cryopreserved again until the results of the decontamination procedure are available. When decontamination of the cell suspension is successful, the cell suspension can be thawed again to proceed to the SSC transplantation.
Optimization of a cryopreservation protocol was performed for immature mouse testicular cell suspensions. The initial protocol to cryopreserve a testicular cell suspension was an uncontrolled slow-freezing protocol with DMSO as CPA [104]. The use of a controlled protocol with the same CPA improved the recovery of viable cells after thawing. The use of EG as a CPA in the controlled protocol yielded similar recovery rates. However, the reinitiation of spermatogenesis after SSC transplantation showed slightly better results with the DMSO protocol. Reinitiation of spermatogenesis from frozen-thawed cell suspension was limited compared to restoration of spermatogenesis after the injection of fresh cells. This indicates that the number of viable SSCs in these frozen-thawed suspensions might be very low and thus that cryopreservation protocols need further improvement [105]. The use of a vitrification protocol did not increase the recovery of viable cells compared to the controlled slow-freezing protocol. The storage in vials instead of straws and the addition of an anti-apoptotic factor (z-VAD[Oe]-FMK) were beneficial for cell survival and could restore spermatogenesis after SSC transplantation [106].
The Transplantation of Spermatogonial Stem Cells in a Clinical Situation
After 25 years of research involving the restoration of spermatogenesis by reintroducing SSCs to an infertile recipient, the clinical application of this technique is still awaited. Although many fertility centers already offer cryopreservation of prepubertal tissue before undergoing gonadotoxic therapies [8, 107], the transplantation of SSCs did not yet occur in a human clinical setting. The reported acceptance rate for cryopreservation of a testicular biopsy in boys diagnosed with cancer is 60% or higher as concluded from surveys in the United States and Europe [108,109,110]. Sooner or later, the wish for autotransplantation of the stored SSCs will be expressed in one of the centers offering testicular tissue banking. Several factors need to be put into consideration in the decision on how SSCs should be transplanted to the patient.
Tissue or Cells?
Since SSC cryopreservation is mostly offered as testicular tissue banking, both SSC transplantation and testicular tissue grafting are possible. Testicular tissue grafting offers the possibility to preserve the interaction between stem cells and their niche in the original state.
When SSC transplantation will be performed, SSCs have to pass the blood-testis barrier to colonize an empty niche. The efficiency of SSCs to successfully colonize an infertile mouse testis was reported to be only 12% [17]. In the adult testis, SSCs are very rare and barely constitute 0.01–0.03% of all cells [111]. While their proportion is higher in the prepubertal testis, merely small biopsies are available.
Although injection of single cells to the mouse testis was mostly performed via the efferent duct, translation of this technique to larger testes seemed difficult and inefficient. Injection via the rete testis, guided with ultrasound, seemed more promising [46, 112, 113]. The advantage of using ultrasound-guided rete testis injection is that this technique does not require surgery since the injection needle can be inserted through the scrotal skin [40]. Different injection sites were evaluated by injection of contrast liquid in human cadaver testes under ultrasound guidance and subsequent evaluation of the injected testes by micro-CT scan and histology. Infusions of the testicular parenchyma were not observed after injection through the efferent duct or through the head of the epididymis. Blind injections in the seminiferous tubules resulted in limited infusions, while clear filling of the testicular parenchyma was observed by rete testis infusions [112]. The feasibility of injecting a testicular cell suspension by the same injection method was evaluated by the injection of mouse GFP+ testicular cells which were labeled with 99m technetium for subsequent evaluation with single-photon-emission computerized tomography imaging [113]. Injection of the cells in the previous experiments was performed by hydrostatic pressure injection. However, high variation and leakage in the interstitial space were observed, which could be reduced by using an infusion pump [114].
The low colonization efficiency and the need for a challenging injection method suggest that testicular tissue transplantation might be easier to perform in a human application. When compared in a mouse model, intratesticular tissue grafting resulted in a higher testis weight after transplantation and a higher graft recovery rate. In addition, SSC transplantation might fail, and, on top of that, not all successfully transplanted testes showed donor-derived spermatogenesis [9]. However, for patients who were diagnosed with blood cancer or a metastatic cancer, the autologous transplantation of cryopreserved testicular tissue involves a major risk in reintroducing malignant cells. Leukemic infiltrates in the testis of patients diagnosed with acute lymphocytic leukemia can be found in 21% of prepubertal patients [115]. This drawback implicates that SSC transplantation might be the only option for these patients. In contrast to testicular tissue, the removal of malignant cells from a cell suspension is theoretically possible. The efficiency in removing malignant cells from the testicular cell suspension needs to be high since it was reported that transplantation of as few as 20 leukemic cells can result in the transmission of leukemia in rats [116]. Decontamination strategies have been investigated by several research groups. Elimination of malignant cells by positive selection of germ cells and/or negative selection of cancer cells by magnetic-activated cell sorting (MACS) techniques did not seem to be sufficient to deplete all malignant cells since transmission of leukemia to the recipients occurred after transplantation of this MACS-decontaminated cell suspension [117,118,119]. Negative selection using two markers specific for malignant cells sorted by fluorescence-activated cell sorting (FACS) seemed to be sufficient to eliminate the risk of transmission of leukemic cells for at least 8 weeks after SSC transplantation [120]. However, these promising findings were not confirmed in another study where a combination of MACS and FACS was performed to selectively enrich for SSCs and decontaminate by negatively sorting out malignant cells. Analysis of the sorted fraction still showed the presence of malignant cells (0.4%) which was proven to be sufficient to induce transmission after transplantation in mice [117]. Total elimination of malignant cells is hampered by the similarities between antigens expressed on the membrane of malignant cells and SSCs. In addition, malignant cells can attach nonspecifically to germ cells which means that positive selection of germ cells still includes a risk of contamination. A combination of both negative and positive selection in two repeated FACS cycles did not induce leukemia for 120 days in transplanted mice [118]. The use of singlet discrimination to avoid the inappropriate sorting of cell clumps, together with positive and negative selection further improved the sorting efficiency. However, purity checks of the sorted fractions by FACS still detected remnant contamination [121]. Decontamination of human testicular cell suspensions was achieved using one surface marker for positive selection of SSCs and two markers for negative selection of leukemic cells. It was however shown that different leukemic cell lines required different sets of markers [122]. Immune-phenotyping analysis of the malignant cells will thus be necessary to select the most effective markers for negative selection. Further improvement of the sorting technique in combination with individually determined marker selection will be necessary. Furthermore, depletion of malignant cells from a testicular cell suspension in a clinical application will have to be proven by PCR, since this is the only way to sensitively detect the smallest remnants of contaminating cells.
Although FACS seemed to be depleting single cell suspensions, a major concern associated with this is the loss of cells after sorting [118, 121]. The combination of this low cell recovery, with the low numbers of SSCs in testicular biopsies and the low colonization efficiency makes the expansion of SSCs indispensable for clinical application of SSC transplantation. Strategies to expand SSCs in vitro have been reported in rodents. However, translation of these culture methods to SSCs from larger animals did not give the expected results [123,124,125]. Recently, in vitro proliferation of porcine SSCs has been reported with preservation of their colonization capacity when xenotransplanted to the mouse testis [126]. It has been estimated that a 1300-fold increase in SSCs is necessary to achieve sufficient colonization in a clinical human application [127]. In vitro culture conditions for the propagation of human SSCs have been reported for adult men [127] and prepubertal boys [128, 129]. The identity of these cultured cells was confirmed by the expression of SSC-specific markers shown by RT-PCR and immunohistochemistry. However, functional assays to prove SSC capacity of long-term cultured human cells are lacking since xenotransplantation of human SSCs is not efficient.
Transplantation Site
Obviously, when SSCs are transplanted as single cell suspension, the only possible option is to transplant them to the seminiferous tubules so they can colonize accessible niches. Healthy offspring has been produced after natural mating of transplanted males with females [130]. This would offer infertile patients the chance to impregnate their partner by natural conception.
The transplantation of testicular tissue has however been performed to different locations, such as the back skin (ectopic), the scrotum, and the testis (orthotopic). In most xenograft studies, testicular tissue was transplanted to the back skin of nude mice. In the model most closely related to the human, full spermatogenesis was achieved in ectopic grafts from rhesus monkeys. Full spermatogenesis could be observed in up to 15% of tubules in ectopic xenografts [23]. However, the same outcome could not be reached in the marmoset. In this species, the site of transplantation seemed to have a major effect on the outcome of the graft. The efficiency of inducing spermatogenesis in marmoset grafts was limited to spermatogonial survival and differentiation up to spermatocyte level [10]. Since androgens were not produced by the marmoset graft, it was hypothesized that failure of testosterone production caused the premeiotic arrest in these grafts. This hypothesis was supported by a deletion in exon 10 of the luteinizing hormone (LH) receptor gene, which makes this species sensitive to chorionic gonadotrophin (CG) instead of LH which is produced in the mouse recipient [131]. However, neither exogenous stimulation of the recipient with human CG nor the co-transplantation of hamster immature tissue to provide a high local testosterone level could overcome the meiotic blockage in the marmoset graft [10, 132]. When marmoset tissue was grafted to a suitable hormonal environment by performing autologous transplantation of immature marmoset testis pieces to the back skin of the same animal, induction of spermatogenesis was achieved but not beyond meiosis. The serum testosterone levels in these transplanted animals only showed a marginal increase which could explain the meiotic arrest. Next to this, the transplantation site was covered with fur resulting in an increased temperature [54]. This hyperthermia probably was the main reason for the failure of obtaining full spermatogenesis in ectopic grafts since the production of spermatozoa could be observed in autologous scrotal grafts in castrated marmosets [53]. Recently, full spermatogenesis was achieved in intratesticular xenografts without altering the hormonal milieu by castration or any exogenous gonadotrophin stimulation [36].
In a study in which cryopreserved rhesus monkey testicular tissue was grafted, the scrotal grafts showed the presence of full spermatogenesis, while pachytene spermatocytes were the most advanced cell type in autologous ectopic grafts. However, it should be mentioned that, in this study, testicular tissue from marmosets in which spermatogenesis was already initiated at the time of cryopreservation was used [51]. Indeed, no difference was observed between scrotal and back skin grafts when immature rhesus monkey testicular tissue was autologously grafted. Complete spermatogenesis was observed in more than 70% of the seminiferous tubules, regardless of the transplantation site. However, graft size was found to be larger in the scrotal grafts compared to the ectopic grafts [52]. The discrepancy between both studies could rely in the age of the donor or the cryopreservation protocol which was slightly different.
Adult human tissue grafted to the scrotal area also resulted in better integrity of the seminiferous tubules compared to ectopic grafts which were almost totally degenerated and fibrotic [34]. Although tubular integrity in ectopic human xenografts was also improved when immature human tissue was used, spermatogonial survival seemed to be better in scrotal or intratesticular grafts [31, 35, 37].
Hormonal Environment
In xenotransplantations to the mouse, the endogenous gonadotrophins were sufficient to initiate spermatogenesis and to ensure its continuation in bovine grafts [22]. Elevated levels of LH and FSH were guaranteed by the castration of the host before transplantation [23]. The high FSH levels stimulate Sertoli cell proliferation and maturation, while LH triggers Leydig cells to mature and produce testosterone as evidenced by an increased weight of the seminal vesicles and increased levels of serum testosterone [7, 48]. Testosterone production by the ectopic graft will then exert negative feedback on gonadotrophin release in the castrated host resulting in decreasing LH and FSH levels [23, 48]. Increased weight of the seminal vesicles at the time of graft collection in castrated mice shows that testosterone production occurs in the grafted tissue. However, seminal vesicle weight, which is indicative for the production of bioactive testosterone, in grafted recipients is higher compared to castrated mice but still lower than in intact male mice. The production of less bioactive testosterone was attributed to the lower responsiveness of donor Leydig cells to murine gonadotrophins. In ectopic xenografts from horses, full spermatogenesis was shown, but could only be achieved in a very limited number of tubules. In most seminiferous tubules, progression up to meiosis was achieved. Treatment with exogenous human CG (LH-like) and pregnant mare’s serum gonadotropin (FSH-like) resulted in improved germ cell differentiation in horse xenografts. This improvement was translated in the fact that elongated spermatids were observed in the grafts from an immature donor, while pachytene spermatocytes were the most advanced cell type in the nontreated controls. No improvement in the percentage of tubules with differentiated cells was noticed. Exogenous treatment with gonadotrophins did also not result in increased seminal vesicle weight [21]. The effect of administration of exogenous gonadotrophins to mice in which prepubertal human tissue was grafted to the testis was also evaluated but did not result in postmeiotic progression of spermatogenesis in these grafts. Meiotic activity was observed in the treated grafts as well as in the nontreated controls [38]. However, exogenous administration of gonadotropins resulted in complete spermatogenesis in rhesus monkey xenografts, while untreated grafts showed no germ cell differentiation. Sertoli cell maturation was observed in both treated and untreated grafts [133].
Postmeiotic progression of spermatogenesis is dependent on testosterone [134], and a low testosterone concentration in the grafts might thus explain the low efficiency of obtaining fully matured spermatozoa in xenografts. In ectopic grafts, testosterone production is solely coming from donor Leydig cells, which need to be stimulated by the mouse gonadotrophins. Since castrated mice were the prevalent model used for xenograft experiments, at the moment of transplantation, the ectopic xenografts are placed in an environment with a low testosterone concentration. This corresponds with the natural environment for neonatal and immature tissue. The high levels of FSH in castrated mice were thought to induce maturation of the somatic cells in the grafted tissue. The responsiveness of donor Leydig cells to the mouse gonadotrophins seems to be higher in immature or young tissue compared to adult tissue as observed by an increased seminal vesicle weight in mice receiving immature or young donor tissue grafts [20]. However, new insights came from a recent study which evaluated the effect of the host environment on the development of marmoset grafts. Grafts transplanted to non-castrated male mice achieved better tubular survival, seminiferous epithelial arrangement, and progression of spermatogenesis compared to female mice and castrated mice [135].
The Effect of Donor Age on the Maturation of Testicular Grafts
To understand the effect of the donor age is important to define the patient’s age range for which the technique could be offered in order to successfully restore spermatogenesis after autotransplantation. At least in xenograft experiments, when transplantation was performed to the back skin, a large variation in the success of achieving full spermatogenesis was noticed. Successful production of spermatozoa in ectopic xenografts has been achieved in many species, but the time needed to collect mature spermatozoa differed between species. In the pig and primate xenografts, spermatozoa production was observed at an earlier tissue age compared to age-matched controls [7, 23], while delayed spermatozoa production was noticed in the cat, horse, and bull [21, 22, 136]. The impact of donor age was evaluated in feline and canine xenografts. Initial acceleration of meiosis was observed in xenografts when young donor tissue was used, but mature spermatozoa were only recovered after the time point they normally appear in the feline testis. Ectopic tissue grafts with tissue from young adults did not develop but showed degeneration, while tissue from pubertal animals in which meiotic germ cells were already present at the time of grafting showed a lower recovery and less efficient production of mature spermatozoa compared to the prepubertal donor grafts [19]. Immature testicular tissue showed the highest potential to induce full spermatogenesis in the grafts. The same findings were observed in canine xenografts [20]. More extensive tubular damage was also observed in scrotal and intratesticular grafts from peripubertal human donors compared to grafts from prepubertal boys [37]. In general, it seems that the presence of spermatogenesis at the time of grafting increases the risk of tubular degeneration in the graft, and this risk increases with the level of spermatogenesis that is present in the donor tissue at the time of grafting [19, 20]. Adult testicular tissue has a low chance in surviving graft periods and mostly results in sclerotic grafts. This is reported in different species including mouse, hamster [10], horse [21], and human [29, 30] and can be explained by the fact that spermatogenesis demands high amounts of oxygen. Mature tissue is therefore more vulnerable to periods of ischemia, while immature tissue is more effective in restoring blood supply after grafting [10]. Also, seminal vesicle weight was higher in mice carrying grafts from immature or young donors compared to mice receiving adult donor tissue. This suggests that immature and young donor tissue is more responsive to the mouse gonadotrophins resulting in a higher production of testosterone in the graft, which in turn stimulates postmeiotic development [20].
Although there is a higher risk of tubular disintegration, donor tissue isolated at the onset of puberty shows the best potential to support the development of complete spermatogenesis. This is probably because the somatic environment and germ cells are at that point ready to start spermatogenesis. Nine months after transplantation, human peripubertal testicular tissue grafted to the mouse testis displayed better spermatogonial survival and showed spermatocytes entering meiosis, which was not the case in grafts from younger patients [37]. However, in a follow-up study, meiotic cells could also be observed in xenografts from prepubertal patients (2.5–12.5 years), but postmeiotic differentiation was not achieved [38].
Depending on the species, in vivo maturation of testicular tissue takes a few days (rodents), several months (pigs, sheep, goats), few years (horse, monkey, bovine), or, in case of the human, 9–15 years. This has of course important implications on the future clinical application. The maturation of the transplanted tissue cannot occur at the normal pace, since this could be more than 10 years if cryopreservation was performed during the neonatal period. Such a long incubation time will not only be negatively received by patients with a child wish but might also be too long for efficient graft survival. For testicular tissue that was cryopreserved at a young age and is transplanted during adulthood, accelerated maturation will thus be required for efficient restoration of fertility.
In animal models, accelerated maturation was achieved with [21, 133] as well as without [7, 23] exogenous stimulation of gonadotrophins. In human intratesticular xenografts, maturity was not achieved since Sertoli cells still showed expression of anti-Mullerian hormone. The lack of full maturation is probably the explanation why full spermatogenesis has not yet been achieved in human prepubertal tissue grafts. In marmoset grafts, intratesticular grafting overcame the meiotic blockage which was observed in ectopic grafts, but this was not the case for human xenografts. Although human prepubertal tissue was grafted to the testis for a long period (12 months), no postmeiotic germ cells were achieved in these grafts [37,38,39]. The marmoset is the most accepted preclinical model for human testicular development, but, in the marmoset, testicular maturation is reached by 15–18 months [64], while it takes more than 10 years in the human. Unfortunately, this period cannot be covered in the life span of a mouse.
Delivery of Growth Factors
The first days after the transplantation of testicular tissue are crucial for further graft development. During this time, a connection between the transplant and the host needs to be established in order to reduce the ischemic period and provide the transplant of oxygen, nutrients, and factors supporting proliferation and differentiation of germ cells and maturation of somatic cells. A substantial loss of germ cells has been reported shortly after testicular tissue grafting [34, 37, 51]. Germ cell fate evaluated in bovine xenografts indicates that an initial loss of germ cells is one of the reasons for the low efficiency of generating full spermatogenesis in bovine xenografts. To limit this initial germ cell loss, it is important that blood supply to the transplant is established quickly and efficiently. Graft and germ cell survival may thus be increased by the stimulation of neovascularization. A higher number of blood vessels were detected in functional grafts compared to nonfunctional grafts, which highlight the importance of angiogenesis for the survival, development, and generation of spermatogenesis in grafted tissue [137]. It was shown that the connection of the capillary system between donor and host is established by a combination of outgrowing small capillaries from the graft and large blood vessels coming from the host [32].
With the aim to improve vascularization, the effect of treating grafts with vascular endothelium growth factor (VEGF) was evaluated. VEGF is an important regulator of blood vessel formation. During embryogenesis, it stimulates vasculogenesis and angiogenesis, but it is also known as a key mediator in neovascularization in cancer and other diseases [138]. In bovine xenografts, treatment with VEGF increased the number of seminiferous tubules with elongating spermatids. However, an increase in the number of blood vessels or microvascular density was not observed in these grafts [137]. Instead, the increased number of seminiferous tubules with complete spermatogenesis could be attributed to an increased survival of spermatogonia as a result of the production of the anti-apoptotic factor B-cell lymphoma 2 [139]. Although there was no effect on vascularity in these bovine xenografts, an increase in vascular surface and vessel density could be observed in human prepubertal xenografts. This resulted in a better tubular integrity and spermatogonial survival but did not stimulate postmeiotic differentiation [39]. VEGF treatment of the testicular transplants was either achieved by a direct and single injection at the transplantation site [137] or by treating the testicular tissue with VEGF in vitro for 5 days prior to grafting [39, 139]. Instead of a single injection or incubation of the testicular tissue before transplantation, testicular tissue transplants could benefit from a more prolonged delivery of VEGF to stimulate the connection with the vascular system of the recipient. However, systemic delivery of VEGF can result in uncontrolled effects at distant sites making it unsafe for use in a clinical context. Tissue engineering might be helpful to provide a sustained and controlled delivery of molecules. This involves the encapsulation in a three-dimensional environment, synthetically composed or derived from biological matrices to imitate the extracellular matrix [140]. A local and prolonged delivery of biomolecules might be achieved by the incorporation of testicular tissue transplants in biocompatible hydrogels, together with biomolecules which can be encapsulated in chitosan/dextran sulfate nanoparticles. The requirements for being biocompatible implicate that the used biomaterials should interact with the biological environment of the recipient without inducing immune responses and allow full integration with the recipient. On top of that, products resulting from the biodegradation of the scaffold should be cleared from the body without interfering with other organs [140]. The use of supportive matrices to optimize testicular tissue grafting was evaluated in mouse autografts. Testicular tissue was encapsulated in two different hydrogels, alginate and fibrin. Encapsulation in alginate was superior to fibrin encapsulation and resulted in a higher vascular density and improved the recovery rate of scrotal grafts 5 days after grafting. Although the beneficial results observed few days after transplantation were not maintained at 21 days after transplantation, the additional value of tissue engineering to improve graft survival and differentiation should be explored further [141].
Fertility After Testicular Tissue Transplantation
Although natural conception might be possible when SSC transplantation is performed, this has never been proven for intratesticular grafting. It might be difficult for the grafted tissue to make a connection with the rete testis and allow spermatozoa produced in the grafted tissue to reach the epididymis and leave the testis at the time of ejaculation. Therefore, testicular spermatozoa will probably have to be collected through testicular sperm extraction. The most suitable time point to collect spermatozoa from the grafted tissue in a human application will need to be determined. It is not known how long it will take before mature spermatozoa are produced in the testis, and this might be dependent on the donor’s age at the time of cryopreservation.
Spermatozoa isolated from ectopic porcine xenografts showed a lower fertilization rate compared to testicular, epididymal, and ejaculated spermatozoa. Ectopically produced spermatozoa do not undergo the final steps of maturation (which normally take place in the epididymis) but could be comparable to testicular sperm. However, in boars, a lower fertilization rate was observed for ectopically produced spermatozoa compared to testicular sperm from control boars. This lower fertilization rate could be caused by senescence of spermatozoa in the grafts. In vivo, a constant clearance of the produced spermatozoa from the seminiferous tubules is assured. However, this is not the case in grafts [24]. In theory, when testicular tissue is grafted to the testis, open seminiferous tubules in the grafted tissue could connect with existent seminiferous tubules. Although this could allow clearance of senescent spermatozoa from the grafted tissue, the number of sperm cells produced by the graft will probably be too low for natural conception. To avoid this problem, spermatozoa should be isolated from the graft as soon as possible.
Conclusion
Both SSC transplantation and testicular tissue transplantation seem to be promising strategies to preserve fertility in patients facing germ cell loss. Restoration of spermatogenesis from human spermatogonia has not yet been achieved in a xenotransplantation model. However, autologous transplantation of SSCs in nonhuman primates is able to restore fertility in the recipients. Also, spermatozoa produced in transplanted testicular tissue in nonhuman primates was able to fertilize an oocyte which resulted in the birth of healthy baby monkey. These promising results are reassuring for the translation of this techniques to the clinic. Careful evaluation of the efficiency and safety of these procedures will be necessary. Testicular tissue transplantation is believed to be the most efficient and easy method but holds too many risks when there is risk for contamination with malignant cells in the stored testicular tissue. Thorough evaluation of minimal residual disease will have to be implemented before transplantation to the patient can be performed. SSC transplantation might be an alternative on the condition that total elimination of malignant cells can be guaranteed.
Definitions
Spermatogonial stem cell
Stem cell located at the basement membrane of the seminiferous tubules within the testis. Responsible for renewal of the stem cell pool in the testis and the founder cell of spermatogenesis.
Spermatogonial stem cell transplantation
The transplantation of spermatogonial stem cells to the testis by injecting a testicular cell suspension through the efferent duct.
Testicular tissue grafting
The transplantation of testicular tissue. The testicular tissue is inserted under the tunica albuginea, which is sutured after insertion of the testicular piece.
Practical Clinical Tips
Translation of the spermatogonial stem cell transplantation and testicular tissue transplantation to a clinical application needs to be performed. Testicular tissue freezing is already performed in several centers. All centers offering cryopreservation of testicular tissue use the slow-freezing protocol. Several factors need to be put into consideration in the decision on how SSCs should be transplanted to the patient before translation to the clinic:
-
Should we transplant tissue or cells?
-
If testicular tissue is transplanted, where should we transplant?
-
Should we provide external support to the hormonal environment?
-
Should we deliver growth factors to stimulate graft survival?
Clinical Cases
Clinical cases have not yet been performed.
Key Readings
-
Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30(11):2463–75.
-
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–26.
-
Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363(6433):1314–9.
-
Baert Y, Van Saen D, Haentjens P, In’t Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28(7):1816–26.
Take Home Messages
-
Restoration of spermatogenesis in infertile animals is possible by the transplantation of spermatogonial stem cells or testicular tissue.
-
Healthy offspring have been born after transplantation of spermatogonial stem cells (cell suspension or tissue) in large animal species.
-
Testicular tissue transplantation and spermatogonial stem cell transplantation are promising strategies to restore fertility in patients at risk of germ cell loss during puberty.
-
Cryopreservation of testicular tissue is already offered in several fertility centers.
-
Autotransplantation in a clinical setting has not yet been performed.
References
Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17.
Griswold MD. 50 years of spermatogenesis: sertoli cells and their interactions with germ cells. Biol Reprod. 2018;99(1):87–100.
Caires K, Broady J, McLean D. Maintaining the male germline: regulation of spermatogonial stem cells. J Endocrinol. 2010;205(2):133–45.
Zhou R, Wu J, Liu B, Jiang Y, Chen W, Li J, et al. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol Life Sci. 2019;76(14):2681–95.
Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11303–7.
Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11298–302.
Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418(6899):778–81.
Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30(11):2463–75.
Van Saen D, Goossens E, De Block G, Tournaye H. Regeneration of spermatogenesis by grafting testicular tissue or injecting testicular cells into the testes of sterile mice: a comparative study. Fertil Steril. 2009;91(5 Suppl):2264–72.
Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124(3):339–46.
Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17(12):3039–45.
Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis in mouse testis. Nature. 1996;381(6581):418–21.
Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60(2):515–21.
Parreira G, Ogawa T, Avarbock M, Franca L, Brinster RL, Russel L. Development of germ cell transplants in mice. Biol Reprod. 1998;59(6):1360–70.
Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61(5):1331–9.
Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;57(3):270–9.
Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod. 2003;69(2):701–7.
Ehmcke J, Gassei K, Schlatt S. Ectopic testicular xenografts from newborn hamsters (Phodopus sungorus) show better spermatogenic activity in aged compared with young recipients. J Exp Zool A Ecol Genet Physiol. 2008;309(5):278–87.
Kim Y, Selvaraj V, Pukazhenthi B, Travis AJ. Effect of donor age on success of spermatogenesis in feline testis xenografts. Reprod Fertil Dev. 2007;19(7):869–76.
Abrishami M, Abbasi S, Honaramooz A. The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice. Theriogenology. 2010;73(4):512–22.
Rathi R, Honaramooz A, Zeng W, Turner R, Dobrinski I. Germ cell development in equine testis tissue xenografted into mice. Reproduction. 2006;131(6):1091–8.
Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Spermatogenesis and germ cell transgene expression in xenografted bovine testicular tissue. Biol Reprod. 2004;71(2):494–501.
Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70(5):1500–3.
Honaramooz A, Cui XS, Kim NH, Dobrinski I. Porcine embryos produced after intracytoplasmic sperm injection using xenogeneic pig sperm from neonatal testis tissue grafted in mice. Reprod Fertil Dev. 2008;20(7):802–7.
Liu Z, Nie YH, Zhang CC, Cai YJ, Wang Y, Lu HP, et al. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 2016;26:139–42.
Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, et al. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction. 2010;139(2):331–5.
Kaneko H, Kikuchi K, Nakai M, Somfai T, Noguchi J, Tanihara F, et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. 2013;8(7):e70989.
Kaneko H, Kikuchi K, Tanihara F, Noguchi J, Nakai M, Ito J, et al. Normal reproductive development of pigs produced using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. Theriogenology. 2014;82(2):325–31.
Geens M, De Block G, Goossens E, Frederickx V, Van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum Reprod. 2006;21(2):390–6.
Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rubben H, Dhir R, et al. Limited survival of adult human testicular tissue as ectopic xenograft. Hum Reprod. 2006;21(2):384–9.
Goossens E, Geens M, De Block G, Tournaye H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil Steril. 2008;90(5):2019–22.
Schlatt S, Westernstroer B, Gassei K, Ehmcke J. Donor-host involvement in immature rat testis xenografting into nude mouse hosts. Biol Reprod. 2010;82(5):888–95.
Goel S, Minami N. Altered hormonal milieu and dysregulated protein expression can cause spermatogenic arrest in ectopic xenografted immature rat testis. Sci Rep. 2019;9(1):4036.
Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod. 2007;22(6):1603–11.
Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23(11):2402–14.
Ntemou E, Kadam P, Van Saen D, Wistuba J, Mitchell RT, Schlatt S, et al. Complete spermatogenesis in intratesticular testis tissue xenotransplants from immature non-human primate. Hum Reprod. 2019;34(3):403–13.
Van Saen D, Goossens E, Bourgain C, Ferster A, Tournaye H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum Reprod. 2011;26(2):282–93.
Van Saen D, Goossens E, Haentjens P, Baert Y, Tournaye H. Exogenous administration of recombinant human FSH does not improve germ cell survival in human prepubertal xenografts. Reprod BioMed Online. 2013;26(3):286–98.
Ntemou E, Kadam P, Van Laere S, Van Saen D, Vicini E, Goossens E. Effect of recombinant human vascular endothelial growth factor on testis tissue xenotransplants from prepubertal boys: a three-case study. Reprod BioMed Online. 2019;39(1):119–33.
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–26.
Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64(4):422–8.
Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41(2):124–8.
Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136(6):823–31.
Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81(5):898–905.
Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126(6):765–74.
Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, Nieschlag E. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod. 1999;14(1):144–50.
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Honjo T, et al. Allogeneic offspring produced by male germ line stem cell transplantation into infertile mouse testis. Biol Reprod. 2003;68(1):167–73.
Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68(6):2331–5.
Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17(1):55–62.
Abbasi H, Hosseini SM, Hajian M, Nasiri Z, Bahadorani M, Tahmoorespur M, et al. Lentiviral vector-mediated transduction of goat undifferentiated spermatogonia. Anim Reprod Sci. 2015;163:10–7.
Jahnukainen K, Ehmcke J, Nurmio M, Schlatt S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012;72(20):5174–8.
Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363(6433):1314–9.
Luetjens CM, Stukenborg JB, Nieschlag E, Simoni M, Wistuba J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology. 2008;149(4):1736–47.
Wistuba J, Luetjens CM, Wesselmann R, Nieschlag E, Simoni M, Schlatt S. Meiosis in autologous ectopic transplants of immature testicular tissue grafted to Callithrix jacchus. Biol Reprod. 2006;74(4):706–13.
Long C. Transgenic livestock for agriculture and biomedical applications. BMC Proc. 2014;8:O29.
Savvulidi F, Ptacek M, Savvulidi Vargova K, Stadnik L. Manipulation of spermatogonial stem cells in livestock species. J Anim Sci Biotechnol. 2019;10:46.
Comizzoli P, Holt WV. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol Reprod. 2019;101(3):514–25.
Dobrinski I, Travis AJ. Germ cell transplantation for the propagation of companion animals, non-domestic and endangered species. Reprod Fertil Dev. 2007;19(6):732–9.
Honaramooz A, Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int. 2010;2011:657860.
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the nation on the status of cancer, Part I: National cancer statistics. Cancer. 2018;124(13):2785–800.
Desandes E. Survival from adolescent cancer. Cancer Treat Rev. 2007;33(7):609–15.
Sharma V. Sperm storage for cancer patients in the UK: a review of current practice. Hum Reprod. 2011;26(11):2935–43.
Allen CM, Lopes F, Mitchell R, Spears N. How does chemotherapy treatment damage the prepubertal testis? Reproduction. 2018;156:R209.
Kelnar CJ, McKinnell C, Walker M, Morris KD, Wallace WH, Saunders PT, et al. Testicular changes during infantile ‘quiescence’ in the marmoset and their gonadotrophin dependence: a model for investigating susceptibility of the prepubertal human testis to cancer therapy? Hum Reprod. 2002;17(5):1367–78.
Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12(3):275–82.
Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. 2011;25(2):287–302.
Servitzoglou M, De Vathaire F, Oberlin O, Patte C, Thomas-Teinturier C. Dose-effect relationship of alkylating agents on testicular function in male survivors of childhood lymphoma. Pediatr Hematol Oncol. 2015;32(8):613–23.
Stukenborg JB, Jahnukainen K, Hutka M, Mitchell RT. Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocr Connect. 2018;7(2):R69–r87.
Stukenborg JB, Alves-Lopes JP, Kurek M, Albalushi H, Reda A, Keros V, et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum Reprod. 2018;33(9):1677–83.
Okada K, Fujisawa M. Recovery of spermatogenesis following cancer treatment with cytotoxic chemotherapy and radiotherapy. World J Mens Health. 2019;37(2):166–74.
de Rooij DG, van de Kant HJ, Dol R, Wagemaker G, van Buul PP, van Duijn-Goedhart A, et al. Long-term effects of irradiation before adulthood on reproductive function in the male rhesus monkey. Biol Reprod. 2002;66(2):486–94.
Slavin S, Aker M, Shapira MY, Panigrahi S, Gabriel C, Or R. Non-myeloablative stem cell transplantation for the treatment of cancer and life-threatening non-malignant disorders; past accomplishments and future goals. Transfus Apher Sci. 2002;27(2):159–66.
Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo). 2013;68(Suppl 1):15–26.
Krausz C, Cioppi F, Riera-Escamilla A. Testing for genetic contributions to infertility: potential clinical impact. Expert Rev Mol Diagn. 2018;18(4):331–46.
Aksglaede L, Wikstrom AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2006;12(1):39–48.
Vloeberghs V, Verheyen G, Santos-Ribeiro S, Staessen C, Verpoest W, Gies I, et al. Is genetic fatherhood within reach for all azoospermic Klinefelter men? PLoS One. 2018;13(7):e0200300.
Corona G, Pizzocaro A, Lanfranco F, Garolla A, Pelliccione F, Vignozzi L, et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2017;23(3):265–75.
Herlihy AS, Halliday JL, Cock ML, McLachlan RI. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med J Aust. 2011;194(1):24–8.
Wikstrom AM, Raivio T, Hadziselimovic F, Wikstrom S, Tuuri T, Dunkel L. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89(5):2263–70.
Gies I, De Schepper J, Van Saen D, Anckaert E, Goossens E, Tournaye H. Failure of a combined clinical- and hormonal-based strategy to detect early spermatogenesis and retrieve spermatogonial stem cells in 47,XXY boys by single testicular biopsy. Hum Reprod. 2012;27(4):998–1004.
Van Saen D, Gies I, De Schepper J, Tournaye H, Goossens E. Can pubertal boys with Klinefelter syndrome benefit from spermatogonial stem cell banking? Hum Reprod. 2012;27(2):323–30.
Nahata L, Yu RN, Paltiel HJ, Chow JS, Logvinenko T, Rosoklija I, et al. Sperm retrieval in adolescents and young adults with klinefelter syndrome: a prospective, pilot study. J Pediatr. 2016;170:260-5.e1–2.
Van Saen D, Pino Sanchez J, Ferster A, van der Werff ten Bosch J, Tournaye H, Goossens E. Is the protein expression window during testicular development affected in patients at risk for stem cell loss? Hum Reprod. 2015;30(12):2859–70.
Wikstrom AM, Hoei-Hansen CE, Dunkel L, De Meyts ER. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: evidence for degeneration of germ cells at the onset of meiosis. J Clin Endocrinol Metab. 2007;92(2):714–9.
Van Saen D, Vloeberghs V, Gies I, Mateizel I, Sermon K, De Schepper J, et al. When does germ cell loss and fibrosis occur in patients with Klinefelter syndrome? Hum Reprod. 2018;33(6):1009–22.
Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2007;368:39–57.
Elliott GD, Wang S, Fuller BJ. Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74–91.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17(3):243–56.
Kanbar M, de Michele F, Wyns C. Cryostorage of testicular tissue and retransplantation of spermatogonial stem cells in the infertile male. Best Pract Res Clin Endocrinol Metab. 2019;33(1):103–15.
Hovatta O, Foudila T, Siegberg R, Johansson K, von Smitten K, Reima I. Pregnancy resulting from intracytoplasmic injection of spermatozoa from a frozen-thawed testicular biopsy specimen. Hum Reprod. 1996;11(11):2472–3.
Crabbe E, Verheyen G, Tournaye H, Van Steirteghem A. Freezing of testicular tissue as a minced suspension preserves sperm quality better than whole-biopsy freezing when glycerol is used as cryoprotectant. Int J Androl. 1999;22(1):43–8.
Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20(6):1676–87.
Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod. 2007;22(4):1060–7.
Baert Y, Goossens E, van Saen D, Ning L, in’t Veld P, Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. 2012;97(5):1152-7.e1–2.
Baert Y, Van Saen D, Haentjens P, In’t Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28(7):1816–26.
Curaba M, Verleysen M, Amorim CA, Dolmans MM, Van Langendonckt A, Hovatta O, et al. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril. 2011;95(4):1229–34.e1.
Lima DBC, Silva T, Aquino-Cortez A, Leiva-Revilla J, Silva L. Vitrification of testicular tissue from prepubertal cats in cryotubes using different cryoprotectant associations. Theriogenology. 2018;110:110–5.
Poels J, Van Langendonckt A, Dehoux JP, Donnez J, Wyns C. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology. 2012;77(5):1008–13.
Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28(3):578–89.
Curaba M, Poels J, van Langendonckt A, Donnez J, Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril. 2011;95(6):2123.e9–12.
Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril. 2001;75(2):269–74.
Pacchiarotti J, Ramos T, Howerton K, Greilach S, Zaragoza K, Olmstead M, et al. Developing a clinical-grade cryopreservation protocol for human testicular tissue and cells. Biomed Res Int. 2013;2013:930962.
Unni S, Kasiviswanathan S, D’Souza S, Khavale S, Mukherjee S, Patwardhan S, et al. Efficient cryopreservation of testicular tissue: effect of age, sample state, and concentration of cryoprotectant. Fertil Steril. 2012;97(1):200–8.e1.
Avarbock MR, Brinster CJ, Brinster RL. Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat Med. 1996;2(6):693–6.
Frederickx V, Michiels A, Goossens E, De Block G, Van Steirteghem AC, Tournaye H. Recovery, survival and functional evaluation by transplantation of frozen-thawed mouse germ cells. Hum Reprod. 2004;19(4):948–53.
Onofre J, Faes K, Kadam P, Vicini E, van Pelt AMM, Goossens E. What is the best protocol to cryopreserve immature mouse testicular cell suspensions? Reprod BioMed Online. 2018;37(1):6–17.
Valli-Pulaski H, Peters KA, Gassei K, Steimer SR, Sukhwani M, Hermann BP, et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum Reprod. 2019;34(6):966–77.
van den Berg H, Repping S, van der Veen F. Parental desire and acceptability of spermatogonial stem cell cryopreservation in boys with cancer. Hum Reprod. 2007;22(2):594–7.
Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, et al. Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer. 2014;61(9):1673–8.
Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, et al. Fertility preservation in the male pediatric population: factors influencing the decision of parents and children. Hum Reprod. 2015;30(9):2022–30.
Helsel AR, Yang QE, Oatley MJ, Lord T, Sablitzky F, Oatley JM. ID4 levels dictate the stem cell state in mouse spermatogonia. Development. 2017;144(4):624–34.
Ning L, Meng J, Goossens E, Lahoutte T, Marichal M, Tournaye H. In search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation: infusion of contrast dyes in isolated cadaveric human testes. Fertil Steril. 2012;98(6):1443–8.e1.
Faes K, Tournaye H, Goethals L, Lahoutte T, Hoorens A, Goossens E. Testicular cell transplantation into the human testes. Fertil Steril. 2013;100(4):981–8.
Faes K, Lahoutte T, Hoorens A, Tournaye H, Goossens E. In search of an improved injection technique for the clinical application of spermatogonial stem cell transplantation. Reprod BioMed Online. 2017;34(3):291–7.
Kim TH, Hargreaves HK, Chan WC, Brynes RK, Alvarado C, Woodard J, et al. Sequential testicular biopsies in childhood acute lymphocytic leukemia. Cancer. 1986;57(5):1038–41.
Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61(2):706–10.
Geens M, Van de Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod. 2007;22(3):733–42.
Hou M, Andersson M, Zheng C, Sundblad A, Soder O, Jahnukainen K. Decontamination of leukemic cells and enrichment of germ cells from testicular samples from rats with Roser’s T-cell leukemia by flow cytometric sorting. Reproduction. 2007;134(6):767–79.
Hou M, Andersson M, Zheng C, Sundblad A, Söder O, Jahnukainen K. Immunomagnetic separation of normal rat testicular cells from Roser’s T-cell leukaemia cells is ineffective. Int J Androl. 2009;32(1):66–73.
Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, et al. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest. 2005;115(7):1855–61.
Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Hum Reprod. 2011;26(12):3222–31.
Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, et al. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest. 2013;123(4):1833–43.
Zheng Y, Tian X, Zhang Y, Qin J, An J, Zeng W. In vitro propagation of male germline stem cells from piglets. J Assist Reprod Genet. 2013;30(7):945–52.
Sahare M, Kim SM, Otomo A, Komatsu K, Minami N, Yamada M, et al. Factors supporting long-term culture of bovine male germ cells. Reprod Fertil Dev. 2016;28(12):2039–50.
Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136(5):543–57.
Zhang P, Chen X, Zheng Y, Zhu J, Qin Y, Lv Y, et al. Long-term propagation of porcine undifferentiated spermatogonia. Stem Cells Dev. 2017;26(15):1121–31.
Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302(19):2127–34.
Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305(23):2416–8.
Dong L, Kristensen SG, Hildorf S, Gul M, Clasen-Linde E, Fedder J, et al. Propagation of spermatogonial stem cell-like cells from infant boys. Front Physiol. 2019;10:1155.
Goossens E, De Rycke M, Haentjens P, Tournaye H. DNA methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod. 2009;24(9):2255–63.
Gromoll J, Wistuba J, Terwort N, Godmann M, Muller T, Simoni M. A new subclass of the luteinizing hormone/chorionic gonadotropin receptor lacking exon 10 messenger RNA in the New World monkey (Platyrrhini) lineage. Biol Reprod. 2003;69(1):75–80.
Wistuba J, Mundry M, Luetjens CM, Schlatt S. Cografting of hamster (Phodopus sungorus) and marmoset (Callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol Reprod. 2004;71(6):2087–91.
Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149(10):5288–96.
Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–20.
Sharma S, Sandhowe-Klaverkamp R, Schlatt S. Differentiation of testis xenografts in the prepubertal marmoset depends on the sex and status of the mouse host. Front Endocrinol. 2018;9:467.
Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25(6):926–30.
Schmidt JA, de Avila JM, McLean DJ. Effect of vascular endothelial growth factor and testis tissue culture on spermatogenesis in bovine ectopic testis tissue xenografts. Biol Reprod. 2006;75(2):167–75.
Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6(2):209.
Caires KC, de Avila J, McLean DJ. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction. 2009;138(4):667–77.
Del Vento F, Vermeulen M, de Michele F, Giudice MG, Poels J, des Rieux A, et al. Tissue engineering to improve immature testicular tissue and cell transplantation outcomes: one step closer to fertility restoration for prepubertal boys exposed to gonadotoxic treatments. Int J Mol Sci. 2018;19(1):286.
Poels J, Abou-Ghannam G, Decamps A, Leyman M, Rieux A, Wyns C. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J Control Release. 2016;234:79–89.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Van Saen, D., Goossens, E. (2022). Testicular Tissue Transplantation. In: Grynberg, M., Patrizio, P. (eds) Female and Male Fertility Preservation. Springer, Cham. https://doi.org/10.1007/978-3-030-47767-7_41
Download citation
DOI: https://doi.org/10.1007/978-3-030-47767-7_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47766-0
Online ISBN: 978-3-030-47767-7
eBook Packages: MedicineMedicine (R0)