Abstract
The buccal fat pad is an invaluable source of vascularized soft tissue rich with multi-potent cellules in the maxillofacial region, used mainly for reconstructive purposes. With limitations of using regional soft tissues in the head and neck, a buccal fat pedicle graft provides an excellent alternative in managing a broad range of defects in maxillofacial patients with a history of resective surgery, oro-antral fistulas, trauma, clefts, medication-related osteonecrosis of the jaw (MRONJ), osteoradionecrosis, TMJ disorders, and more recently periimplantitis. Partial removal of the buccal fat is also being extensively used as a key piece in the esthetic surgical treatment plan for patients with buccal fat hypertrophy. In this chapter a brief introduction to the anatomy and physiology of the buccal fat pad will be put forth, and clinical applications of the buccal fat as either a free or pedicled flap will be discussed at length.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Managing soft and hard tissue defects in the oral and maxillofacial region is a far more difficult challenge to the reconstructive surgeon, in comparison to defects in other regions of the body. This is primarily because the esthetic and functional demands in the face are at their highest peak. Also local mucosal, skin, and bony tissues that can be used in this region for reconstruction are scarce [1]. Utilizing free skin, mucosal, and musculoskeletal autogenous grafts and allografts have long been considered for reconstruction of maxillofacial defects, often requiring extensive procedures with additional morbidity [2].
In the last three decades, more attention has been drawn toward using the buccal fat pad (BFP) as a local reservoir of tissue for reconstructing maxillofacial defects [3, 4]. If allowed by the size, location, and type of the defect, BFP is an attractive choice for closure of and covering oral defects, creating a proper bed for osteogenesis and augmenting facial defects when a lack of subdermal tissues exists. The appeal for using BFP in reconstructing oral and facial defects is fueled by its availability, easy access, rich blood supply, and the proven fact that adipose tissues possess multi-potent cells with the potential to transform to adipocytes, osteoblasts, myoblasts, chondroblasts, and epithelial cells [4]. Research shows that “adipose-derived stem cells” (ADSCs) have the potential to express and produce proteins and osteogenic markers such as alkaline phosphatase, collagen type 1, osteopontin, and osteocalcin. These stem cells are also capable of producing a mineral matrix [5, 6].
Conclusively, given its rich blood supply, ease of access, and reservoir of multi-potent cells, BFP is an excellent biologic membrane and a source of stem cells for covering and reconstructing soft tissue and bony defects in the oral and maxillofacial region.
2 Anatomy
The buccal fat pad is a biconcave mass with a substantial role in the form and function of the masticatory muscles. It is encapsulated by a thin membrane and is located in the buccal space. It can be recognized in a 6–8 centimeter fetus. It is comprised of three distinct lobes: the anterior, intermediate, and posterior lobes, each being separately encapsulated. These lobes are attached to adjacent structures by six ligaments: the maxillary, posterior zygomatic, medial and lateral infra-temporal, temporal tendon, and buccinator ligaments.
The maxillary ligament is fibrotic and firm in nature and extends from the anterior lobe to the maxilla. The posterior zygomatic ligament attaches the intermediate ligament to the maxillary process of the zygomatic bone. The medial and lateral infra-temporal ligaments originate from the intermediate lobe and attach this lobe to the infra-temporal crest. The posterior lobe is suspended from the temporal muscle tendon by a ligament of the same name, and finally the buccinator ligament extends from the anterior lobe to the fascia of the buccinator muscle. In addition to the three main lobes described, the BFP mass also contains four processes exclusively originating from the posterior lobe, namely, the buccal, pterygoid, temporal (superficial temporal), and pterygopalatine (deep temporal) processes.
The main trunk of BFP is situated on the anterior edge of the masseter muscle. Anteriorly, it extends to the maxillary vestibule, and it extends medially and posteriorly to the posterior of the maxillary bone. The buccal process is located more superficially in the cheeks and is responsible for the fullness of this segment. The pterygoid process is located deep and medial to the ramus between the ramus and the medial and lateral pterygoid muscles [7, 8].
The buccal branches of the facial nerve are in contact with the anterior lobe of BFP in roughly 75% of the time, and in 26% of these cases, the two extensions of the buccal branch traverse the anterior lobe. The zygomatic branch of the facial nerve, however, is adjacent to the lateral surface of BFP 90% of the time. The relation between the parotid duct and BFP takes three forms: the duct might [1] pass over the buccal extension (42%), [2] traverse deep through the buccal extension (26%), and [3] pass above the buccal extension (32%) [9].
Aside from racial differences, BFP’s volume is in average 10.2 cubic milliliters (7.8–11.2 cc) in men and 8.9 cubic milliliters (7.2–10.8 cc) in women. When comparing right-side volume with the left side, no significant difference is found, though the volume slightly decreases to roughly 7 cc as the patient ages. Average thickness and weight of the BFP are reported 6 mm (4.8–7.2) and 9.7 g (7.2–12.3), respectively [10].
The BFP is highly vascularized by three different vessels: side branches of the facial artery, internal maxillary artery, and the superficial temporal artery. Most of these vessels enter the posterior lobe and spread and anastomose under BFP’s membrane (capsule) creating a vascular-membranous plexus. In clinical practice, care must be taken not to inadvertently injure the rich blood supply of the posterior lobe. A branch of the posterior alveolar artery enters the intermediate lobe. The venous plexus of the BFP is connected to the cranial circulation by the pterygoid plexus of veins [7, 10] (Fig. 71.1).
3 Physiology
BFP fills in the spaces between the masticatory muscles and around the mimetic muscles. It also fills the deep facial spaces including the masseteric, infra-temporal, and supra-parotid spaces. When the masticatory and mimetic muscles contract, lobes of BFP cushion the muscles by allowing them to slide over adipose tissues. BFP plays an integral role in the suckling mechanism in infants. As children grow and masticatory muscles develop, BFP decreases in size. BFP also helps protect deep neurovascular bundles from external forces and contractional forces of the masticatory muscles. A segment of the anterior lobe compresses the infra-orbital nerve and veins deeper and enters the infra-orbital tube with them. The pterygopalatine extension of the posterior lobe encapsulates the pterygopalatine veins. Likewise, the pterygoid extension cushions the mandibular and lingual neurovascular bundles in the pterygomandibular space [11].
4 Clinical Applications of Buccal Fat Pad
Utilizing the buccal fat pad as a pedicle graft for the purpose of reconstructing oral defects has well been documented and gained vast popularity through the years. Recent studies have also investigated the application of free buccal fat to restore defects of the periodontium. But perhaps the most widespread surgical procedure involving the buccal fat today is the esthetic buccal fat removal also known as bichectomy. A detailed list of the clinical applications of BFP can be shown as follows:
-
1.
Oro-antral and oro-nasal fistula closure.
-
2.
Reconstructing regional pathologic defects and clefts.
-
3.
Reconstructing regional traumatic defects.
-
4.
Covering bone grafts as a biologic membrane.
-
5.
Restoring perforations of the Schneiderian membrane unamenable to other techniques.
-
6.
TMJ reconstruction as interpositional grafts.
-
7.
Free graft for nerve coverage.
-
8.
Esthetic surgery.
-
9.
Managing MRONJ and osteoradionecrosis.
-
10.
Free graft to restore periodontium around teeth and implants.
Before elaborating on the applications listed above, techniques for approaching the BFP will be discussed.
5 Surgical Approach to the Buccal Fat Pad
There are many instances when the buccal fat is inevitably or inadvertently exposed to the surgical site, such as traumatic and pathologic defects and, at times, during orthognathic surgery. Besides from these, the intraoral approach to expose, free, and mobilize the buccal fat pad can be done by one of the two methods explained below:
5.1 When a Mucoperiosteal Flap Is Not Already Elevated
In the maxillary buccal vestibule, a 2–3-cm-long horizontal incision is made distal to the zygomatic buttress extending backward above the maxillary second molar. Care is taken to avoid the Stensen duct and orifice while sharply incising through the oral mucosa and the buccinator muscle. A blunt supra-periosteal dissection through the buccinators and loose fascia below the buccinator muscle allow the BFP to herniate. Placing pressure on the cheek might help to better introduce the fat into the mouth. Once the fat pad is dissected from its surroundings, it can be grasped with a vascular forceps and gently pulled toward the desired site. Repetitive motions of gentle pulling, opening the forceps, and grasping the fat pad at a place closer to its base ensures a safe method to prepare a fat graft pedicle with proper size and intact blood supply and a wide base [12].
Some clinicians prefer to place the sharp incision in a more inferior position adjacent to the maxillary occlusal plane. If correctly made, the incision will be positioned below the parotid duct opening, and the surgeon must be cautious not to penetrate the duct during the procedure. Dissection through the mucosa and blunt dissection of the buccinator muscle are carried out in a similar fashion. This approach is mostly applied for buccal fat removal (bichectomy) procedures and if the surgeon is planning to tunnel the BFP below the buccal mucosa to cover a mandibular defect. If reconstruction of maxillary defects is planned, the buccal vestibular approach is preferred (Fig. 71.2a–d).
5.2 When a Mucoperiosteal Flap Is Already Elevated
In almost all cases involving reconstruction of a maxillary defect, the defect site must be exposed by a mucoperiosteal flap before reconstructive measures are to be applied. In this scenario, a 1.5–2 cm incision on the elevated periosteum distal to the zygomatico-maxillary buttress will allow the surgeon to bluntly dissect through the buccinator fascia and expose the buccal fat. The grasping and pulling of the fat graft toward the desired site is then carried out similarly to the approach explained above [13] (Fig. 71.3).
6 Oro-Antral and Oro-Nasal Closure
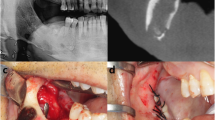
Egyedi was the first to use the buccal fat pad for closing an oro-antral defect in 1977 [14]. Since then, the fistula coverage with a buccal fat pedicle has become the go-to technique for managing fistulas, specifically if the surrounding oral tissues have been scarified by previous unsuccessful surgical attempts [15]. The key to success in this surgery is thorough exposure of the fistula by elevating a mucoperiosteal flap and debriding and refreshing of both the hard and soft tissue edges of the defect. Next, the buccal fat should be approached by making an incision through the periosteum in the apico-distal aspect of the flap adjacent to the second upper molar, and the buccal fat can be freed and mobilized toward the defect site with gentle dissection and pulling. Once we have made sure that an intact bulk of buccal fat is advanced enough to cover the fistula with no tension and has maintained its vascular supply and wide base, the fat pad is fixated to the palatal mucosa with at least one horizontal mattress suture and then single sutures next to the mattress suture to fully stabilize the pedicle graft. Complete coverage of the buccal fat pedicle with the previously elevated mucoperiosteal flap is not mandatory for proper healing, but it is certainly beneficial to get as much coverage as possible. If the BFP pedicle vasculature is preserved, rapid healing of the region will ensue, and epithelialization of the flap can be witnessed 2–3 weeks post-operatively [16] (Fig. 71.4a–f).
7 Reconstructing Regional Pathologic Defects and Clefts
Removing or resecting a pathology at the maxilla, anterior ramus, or buccal mucosa might leave a defect with no possibility of primary closure of the tissues. In more severe cases, a composite defect of the mucosa, muscles, and bone might exist. In these cases the buccal fat pad can be pulled over the defect to seal oro-antral defects, prevent potential nasal air escape, and reduce scar formation [17, 18].
In patients who have received radiotherapy of the head and neck region following surgical resection of a pathology, several factors will complicate defect coverage by BFP. Primarily, the BFP of the ipsilateral site might be partially obliterated as a result of the surgical manipulation and the radiotherapy, making it impossible to advance the fat pedicle to the desired site (Figs. 71.5 and 71.6). The most consistent and accurate way to investigate the existence and the size of the buccal fat pad is probably the MRI [19]. An ultrasonography can also be done and has the advantage of lower cost and availability, yet with inexperienced operators without comprehensive knowledge of the buccal fat anatomy, false-positive results might be reported, and it is inherently unable of differentiating soft tissue types as MRI does. It is worth noting that the resultant scarring of tissues after radiotherapy will make it difficult to dissect through.
Palatal cleft closure is another realm in which a buccal fat pedicle graft can be used. Surgeons who treat patients with a history of previous unsuccessful cleft closure surgery will testify that the scarring of tissues and compromised blood supply of the palatal mucosa around the cleft pose a great challenge to the surgical team. A buccal fat graft pedicle, if managed with finesse, can cover clefts located in the palatal midline. For larger defects or those less amenable to closure, bilateral buccal fat pedicle grafts have been used successfully [20, 21].
In the author’s personal experience, removing the ipsilateral wisdom tooth or any hopeless teeth will allow the pedicled flap to course a direct route toward the defect and be under less tension. Also, if the wisdom tooth is removed or is non-existent, the bone in the tuberosity can be reduced with a rongeur to facilitate advancing the BFP flap.
Once a palatal defect induced by a pathology or cleft is closed with the BFP, the drawbacks of covering the palate with a surgical stent outweigh its advantages. The author believes placing the stent might cause pressure on the vasculature of the pedicled flap and deprives the patient and the clinician to keep the surgical site clean and free of debris. Placing a naso-gastric or an oro-gastric tube for the first week after surgery should be considered, specially if the surgeon is uncertain about the level of patient’s compliance in keeping a liquid-only diet for the first post-operative 2 weeks and soft diet for at least the next 6 weeks (Fig. 71.7).
8 Reconstructing Midfacial Traumatic Defects
Managing trauma to the zygomatico-maxillary complex is aimed at creating facial symmetry and restoring normal facial prominence and contour. Yet even with the best attempts, incomplete reduction, atrophy of superficial and deep fat compartments, and formation of scars can lead to asymmetries and depressions in the facial contour. To tackle this problem, a buccal fat pedicle graft can be mobilized and moved underneath the depressed area requiring augmentation. The scarred or atrophied site should be prepared by undermining subperiosteally or supra-periosteally. A supra-periosteal dissection below the depression is technically more difficult due to scar formation but keeps the periosteum fixed on the bone and allows the surgeon to fixate the buccal fat to the periosteum with sutures. A subperiosteal undermining of the defect is more straightforward, but the fat graft should either be fixated by transcutaneous sutures, or with sutures to the unelevated periosteum located more cephalic to the defect. In the authors’ experience, regardless of the plane of undermining used, advancing the buccal fat pedicle and suturing it to a more cephalic periosteum yields better long-term results, as the effect of gravity will ultimately cause a slight collapse in the position of the graft [22].
9 Covering Bone Grafts as a Biologic Membrane
Alloplastic and allograft membranes have been widely used in guided tissue regeneration. Also, platelet-rich fibrin (PRF) and leukocyte-platelet-rich fibrin (LPRF) which are processed from the patient’s own blood sample have shown to not only successfully function as a barrier membrane but also boost healing properties of the soft tissue in the region they are applied at. The buccal fat pedicle graft can be mobilized to cover sites augmented by bone grafts. Hassani and Khojasteh showed in their paper published in 2009 that the BFP pedicle can successfully replace bioresorbable collagen membranes in maxillary and sinus floor bone grafts. This is highly recommended when the oral mucosal coverage lacks a proper seal due to a perforation or when tension might still exist on the tissue edges after suturing and the risk for bone graft exposure is increased [23] . The morbidity of approaching the buccal fat via the already existing mucoperiosteal flap is negligible, and in the author’s experience, no apparent asymmetry in the facial fullness and contour ensues. Still, we believe approaching the buccal fat should be reserved for cases where an apparent benefit in doing so exists, such as a simultaneous need for sinus roof repair, perforation in the oral mucosa, or conditions necessitating boosting the healing potential of tissues, like a history of previous unsuccessful surgeries (Fig. 71.8).
10 Restoring Perforations of the Schneiderian Membrane
If the location or the size of the membrane perforation makes it unamenable to repair with collagen membranes or suturing, perforations with a diameter larger than 10 mm or between 5 and 10 mm that are adjacent to the medial or nasal sinus wall, the clinician will only be left with a few choices. One option is to discontinue the treatment and postpone it to 6–9 months later. Another option will be using a “Loma Linda pouch” technique, in which a large resorbable membrane is inserted in the sinus, covering all the walls and the Schneiderian membrane while portions of it extend outside the lateral window osteotomy and can be fixated via tag screws. This membrane will act as a pouch in which graft material can be inserted [24]. If the perforation is too large or when the lateral sinus wall has been severely traumatized, not even the Loma Linda pouch technique will be able to isolate the graft material from the sinus and repair the sinus roof. In these cases the last resort will be using the pedicled buccal fat graft.
The buccal fat pad will be approached in a similar fashion already discussed earlier in this chapter and gently grasped from its wide base and pulled while reserving its capsule and maintaining its blood supply until it can easily rest inside the sinus with no tension. Next, two holes are made in the palatal sinus wall by a fissure bur (702) approximately 1 cm above the sinus floor. Care is taken not to disrupt the greater palatine bundle while drilling with the bur. Then, a Vicryl 4–0 suture with an 18-gauge needle is introduced to the sinus cavity through the hole prepared on the palatal side and grasped with a forceps from the lateral window osteotomy. The needle then takes at least two bites of the fat graft and then exits the sinus through the other previously drilled hole in the palate. Finally, a knot is created to pull the BFP and create a new roof for the sinus [25] (Fig. 71.9).
In the author’s experience, this technique can also be used to simultaneously repair an oro-antral fistula or whenever there is limited soft tissue for primary closure of the oral mucosa. This can be achieved by preparing a bi-lobular BFP flap. One lobule is used as discussed to repair the sinus roof, and the other covers the lateral window osteotomy or remains exposed to the oral cavity. Theoretically, another advantage of repairing the sinus perforation in comparison to the Loma Linda pouch technique is that the BFP flap has its own blood supply and the combination of the sinus floor blood supply from below and the BFP flap from above can lead to better maturing and ossification of the graft material.
11 TMJ Reconstruction as Interpositional Grafts
Surgical treatment for inter-capsular TMJ ankylosis includes gap arthroplasty, interpositional arthroplasty (IA), reconstruction of the articulation with grafts, and total joint replacement. Interpositional arthroplasty is favored over gap arthroplasty since it is involved with lower rates of re-ankylosis and the technique’s potential for reconstructing or maintaining the ramus height [26].
Materials such as temporal fascia (most common), the masseter muscle, full-thickness skin, autologous costochondral cartilage, and synthetic PTFE have been used as interpositional grafts in IA. The buccal fat is also considered a choice not just because it is relatively easy to harvest through the same surgical access to the glenoid fossa and its surrounding structures, but also since due to its multi-potent cellular content and ample blood supply it is less likely to go through fibrosis and has even showed cartilaginous changes in reports [27, 28].
12 Free Graft for Nerve Coverage
The infra-alveolar branch of the mandibular division of the trigeminal nerve is safely situated in the mandibular bone, and the other branches of the mandibular nerve course through lingual and buccal soft tissue. There are three situations in which these nerve branches become susceptible to damage. First, these nerves might be severed during flap elevation and dental implantation and require exploration through the surrounding tissues for neurorrhaphy. Second, extensive benign pathologies of the mandible might require partial or total resection of the mandible while preserving the infra-alveolar nerve. Last but not least, the infra-alveolar nerve will be released from its bony canal in nerve lateralization procedures for implantation in posterior mandible with severe atrophy. In all these situations, it will be beneficial to provide coverage for the denuded nerve. Without this additional coverage from external sources, nerve repair might be compromised, and sensory changes might ensue. Bioabsorbable alloplastic material has been used with controversial and less than ideal results around nerve trunks. Alloplastic material such as Gore-Tex and more recently NeuraGen have been used as nerve-guiding conduits when primary anastomosis of the nerve has not been accomplished by sutures with some success [29]. A reliable nerve coverage which can provide a gliding path for denuded nerves is autogenous soft tissue grafts, including free or pedicle buccal fat grafts. Providing nerve coverage by fat pedicles is routine practice in other regions of the body, and it can be carried out using the buccal fat pad in the orofacial regions as well [30].
13 Esthetic Surgery
Removing hypertrophied or herniating buccal fat in patients with increased fullness of the anterior buccal and sub-malar area with round faces can help create a more feminine and angulated appearance of the face. Based on the concept of the “triangle of beauty,” a face with prominent malar bones which narrows down inferiorly toward a semi-pointed chin is considered more attractive and youthful. As a result of aging, this inverted triangle flips vertically as the collapse of the facial fat compartments and skin laxity in the lower third of the face, with fat atrophy in the temporal, infra-orbital and malar regions changes the fullness of different facial segments [31].
Removing excess buccal fat or “buccal fat partial lipectomy” is also referred to as “bichectomy” in honor of Marie François Xavier Bichat, who was the first to describe the buccal fat pads as adipose compartments in 1902. This procedure can help hollow out the facial segment between the malar prominence and the mandibular border, giving these boney ridges a more augmented appearance. The fullness of this region is mostly caused by a hypertrophied buccal extension of the posterior lobe of the buccal fat pad.
Like any other surgical procedure, the primary step is to make a proper diagnosis. Bichectomy should not be recommended for everyone. A distinction needs to be made among patients with round faces due to excessive buccal fat compartments and masseter hypertrophy. The former are appropriate candidates for buccal fat lipectomy, whereas the latter might initially benefit from Botox injections of 35 to 50 IU near the mandibular angle in the bulk of the masseter muscle. Examination with ultrasound by an experienced radiologist might reveal hypertrophied buccal fat pads. A more reliable para-clinical adjunct is the MRI. MRI T1 images can effectively distinguish the buccal fat from its surrounding soft tissues. In patients with round faces and buccal fat hypertrophy, ultrasound and MRI examinations will show voluminous buccal fat compartments with larger than the mean volume corresponding to the patient’s age groups, already alluded to earlier in this chapter.
Buccal fat lipectomy is either done as a stand-alone procedure in an out-patient setting with local anesthesia or can be carried out on a patient under general anesthesia for another surgical procedure such as rhinoplasty or orthognathic surgery, etc. During the face lift, bichectomy can be done via an extraoral approach, but aside from that, approaching the buccal fat pad is safely done intraorally. An incision 1.5–2 cm long is made on the buccal mucosa either close to the maxillary buccal vestibule or the maxillary occlusal plane at a point near the buccal surface of the first maxillary molar extending posteriorly, parallel to the path of the Stensen duct, making sure the duct and its orifice are preserved. Once the oral mucosa and the buccinator muscle are cut through by a surgical knife or electrocautery, the loose fascia of the buccinator muscle is bluntly dissected, and the buccal fat is herniated to the oral mucosa. Mobilizing the bulk of the buccal extension of BFP is done by gently grasping and pulling out the fat with a vascular forceps while trying to keep its capsule intact. The forceps should only grasp the fat pad toward its wide base. Once adequate volume of BFP is pulled toward the oral cavity, the excess fat can be grasped by a curved hemostat and cut by connecting the hemostat to electrocautery. Care must be taken to provide complete hemostasis before the wound is closed with resorbable sutures. The patient should be prescribed with painkillers and antibiotics and advised to apply ice on the buccal skin to prevent edema [32].
The most common complications reported are edema and hematoma in cases with poor hemostasis, yet the procedure is considered to be safe if the steps are followed as suggested [33].
In the scarce literature available, significantly devoid of clinical trials, there is controversy pertaining to the safe amount of buccal fat that can be excised without risking over-hollowing of the face specially in the long term. There are authors who believe this is not much of a concern as long as only fat which is easily pulled toward the oral cavity is excised. Others, however, believe the amount of fat removed should be limited to the excess volume already evaluated pre-operatively via ultrasound or MRI studies. Sezgin B et al. reported that excising a mean volume of 2.74 ± 0.69 mL in patients presented in their study was effective, posed little risks, and resulted in high post-operative patient satisfaction [34].
14 Managing MRONJ and Osteoradionecrosis
Cases of medication-related osteonecrosis of the jaw (MRONJ) have become more widespread as prescription of certain drugs for cancer and osteoporosis (e.g., bisphosphonates, denosumab, and antiangiogenic agents) has become more common. A thorough description of these conditions and their treatment protocols are beyond the scope of this chapter. In many cases, a surgical debridement is not necessary and even contraindicated. Yet in cases of MRONJ and osteoradionecrosis for which surgery is indicated, the surgical technique should focus on not just removing necrotic and unsalvageable bone, but also enhancing the healing potential of the remaining vital bone and the surrounding soft tissue. In a study by Merigo et al., a combined approach is recommended; piezosurgery is suggested for removing gross necrotic bone tissue, and Er:YAG laser (2940 nm) is then used to vaporize necrotic hard tissue until reaching bleeding bone; Merigo also recommends applying platelet-rich plasma (PRP) and diode laser (808 nm) to stimulate hard and soft tissue healing of the surgical site [35].
The buccal fat pad pedicle can be freed and advanced to cover defects in both maxilla and the mandible and significantly boost the healing potential of the tissues it is covering owing to its vascular supply and cellularity. The literature is filled with an abundance of reports on the efficacy of using BFP pedicle grafts in treatment of these defects [36] (Fig. 71.10).
15 Free Graft to Restore Periodontium around Teeth and Implants
While injecting free fat is routine practice in esthetic procedures, using a free fat graft with no pedicle for vascular supply is still a new concept for maxillofacial reconstruction.
Dental implants are now an integral part of routine dental treatment, and managing complications such as periimplantitis and thread exposure will be, and for some clinicians already is, a common challenge. Application of free buccal fat pad in regenerative treatments for periimplantitis is relatively new compared to more conventional techniques such as guided tissue regeneration and gingival grafts and is mostly publicized by the work of Kablan et al.
In 2018, inspired by the success of using free buccal fat in bone augmentation, Kablan reported several cases where he used free buccal fat around exposed implant threads, debrided mechanically by curettes, to gain new soft tissue attachments [37]. Kablan describes free buccal fat graft (FBFG) to be a viable choice for regenerative periimplantitis treatment with excellent healing by fibrosis around implant surfaces and low morbidity of graft harvest for the patient. Free buccal fat graft has also been successfully used for treating gingival recessions, where the exposed root surfaces are covered with free buccal fat and covered with a coronally advanced mucoperiosteal flap [37, 38].
Nevertheless, there is still a great lack of evidence in the literature pertaining to the use of free buccal fat grafts and their long-term success in comparison to buccal fat pedicle grafts, and this will be an exciting realm for research for years to come.
References
Raghoebar GM, Meijer GJ, Smeele LE. Reconstruction of defects in the oral and maxillofacial region. A review of the various options for treatment. Ned Tijdschr Tandheelkd. 2007;114(1):47–53.
Cummins DM, Kim B, Kaleem A, Zaid W. Pedicle orientation in free-flap microvascular maxillofacial reconstruction. J Oral Maxillofac Surg. 2017;75(4):875.e1–4.
Rapidis AD, Alexandridis CA, Eleftheriadis E, Angelopoulos AP. The use of the buccal fat pad for reconstruction of oral defects: review of the literature and report of 15 cases. J Oral Maxillofac Surg. 2000;58(2):158–63.
Komatsu S, Ikemura K, Kimata Y. Pedicled buccal fat pad for the augmentation of facial depression deformity, a case report. Medicine. 2017;96(30):e7599.
Ogawa R, Mizuno H, Watanabe A, Migita M, Shimada T, Hyakusoku H. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun. 2004;313(4):871–7.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Stuzin JM, Wagstrom L, Kawamoto HK, Baker TJ, Wolfe SA. The anatomy and clinical applications of the buccal fat pad. Plast Reconstr Surg. 1990;85(1):29–37.
Zhang H-M, Yan Y-P, Qi K-M, Wang J-Q, Liu Z-F. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109(7):2509–18.
Hwang K, Cho HJ, Battuvshin D, Chung IH, Hwang SH. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16(4):658–60.
Loukas M, Kapos T, Louis RG, Wartman C, Jones A, Hallner B. Gross anatomical, CT and MRI analyses of the buccal fat pad with special emphasis on volumetric variations. Surg Radiol Anat. 2006;28(3):254–60.
Alkan A, Dolanmaz D, Uzun E, Erdem E. The reconstruction of oral defects with buccal fat pad. Swiss Med Wkly. 2003;133(33–34):465–70.
Matarasso A. Managing the buccal fat pad. Aesthet Surg J. 2006;26(3):330–6.
Stajčić Z. The buccal fat pad in the closure of oro-antral communications: a study of 56 cases. J Cranio-Maxillofac Surg. 1992;20(5):193–7.
Egyedi P. Utilization of the buccal fat pad for closure of oro-antral and/or oro-nasal communications. J Maxillofac Surg. 1977;5:241–4.
Adeyemo W, Ogunlewe M, Ladeinde A, James O. Closure of oro-antral fistula with pedicled buccal fat pad. A case report and review of literature. Afr J Oral Health. 2004;1:1.
Candamourty R, Kumar M, Sankar K. Double-layered closure of oroantral fistula using buccal fat pad and buccal advancement flap. J Nat Sci Biol Med. 2012;3(2):203–5.
Tideman H, Bosanquet A, Scott J. Use of the buccal fat pad as a pedicled graft. J Oral Maxillofac Surg. 1986;44(6):435–44.
Martín-Granizo R, Naval L, Costas A, Goizueta C, Rodriguez F, Monje F, et al. Use of buccal fat pad to repair intraoral defects: review of 30 cases. Br J Oral Maxillofac Surg. 1997;35(2):81–4.
Tarallo M, et al. Clinical significance of the buccal fat pad: how to determine the correct surgical indications based on preoperative analysis. Int Surg J. 2018;5(4):1192–4.
Khiabani K, Keyhan SO, Ghanean S. Repair of wide cleft palate by bilateral buccal fat pad: a preliminary study. Regen Reconstr Restor. 2016;1(1):15–9.
Levi B, Kasten SJ, Buchman SR. Utilization of the buccal fat pad flap for congenital cleft palate repair. Plast Reconstr Surg. 2009;123(3):1018–21.
Khiabani K, Keyhan SO, Varedi P, Hemmat S, Razmdideh R, Hoseini E. Buccal fat pad lifting: an alternative open technique for malar augmentation. J Oral Maxillofac Surg. 2014;72(2):403.e1–403.e15.
Hassani A, Khojasteh A, Alikhasi M, Vaziri H. Measurement of volume changes of sinus floor augmentation covered with buccal fat pad: a case series study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):369–74.
Proussaefs P, Lozada J. The "Loma Linda pouch": a technique for repairing the perforated sinus membrane. Int J Periodontics Restorative Dent. 2003;23(6):593–7.
Hassani A, Khojasteh A, Alikhasi M, Repair of the perforated sinus membrane with buccal fat pad during sinus augmentation. J Oral Implantol. 2008;34:320.
De Roo N, Van Doorne L, Troch A, Vermeersch H, Brusselaers N. Quantifying the outcome of surgical treatment of temporomandibular joint ankylosis: a systematic review and meta-analysis. J Cranio-Maxillofac Surg. 2016;44(1):6–15.
Singh V, Dhingra R, Sharma B, Bhagol A, Kumar P. Retrospective analysis of use of buccal fat pad as an Interpositional graft in temporomandibular joint Ankylosis: preliminary study. J Oral Maxillofac Surg. 2011;69(10):2530–6.
Gaba S, Sharma RK, Rattan V, Khandelwal N. The long-term fate of pedicled buccal pad fat used for interpositional arthroplasty in TMJ ankylosis. J Plast Reconstr Aesthet Surg. 2012;65(11):1468–73.
Farole A, Jamal BT. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: a case series. J Oral Maxillofac Surg. 2008;66(10):2058–62.
Tollestrup T, Berg C, Netscher D. Management of distal traumatic median nerve painful neuromas and of recurrent carpal tunnel syndrome: hypothenar fat pad flap. J Hand Surg Am. 2010;35(6):1010–4.
Moura L, Spin J, Spin R. Buccal fat pad removal to improve facial aesthetics: an established technique? Med Oral Patol Oral Cir Bucal. 2018;23(4):e478–84.
De Luccas S. Bichectomy: achieving aesthetic, functional and psychological results with a simple intraoral surgical procedure. EC Dental Sci. 2017;13(3):116–7.
Klüppel L, Marcos RB, Shimizu IA, da Silva MAD, da Silva RD. Complications associated with the bichectomy surgery. Revista Gaúcha de Odontologia. 2018;66(3):278–84.
Sezgin B, Tatar S, Boge M, Ozmen S, Yavuzer R. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39(6):585–92.
Merigo E, Cella L, Oppici A, Cristina Arbasi M, Clini F, Fontana M, Fornaini C. Combined approach to treat medication-related osteonecrosis of the jaws. J Lasers Med Sci. 2018;9(2):92–100.
Rotaru H, Kim M-K, Kim S-G, Park Y-W. Pedicled buccal fat pad flap as a reliable surgical strategy for the treatment of medication-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2015;73(3):437–42.
Kablan F. The use of buccal fat pad free graft in regenerative treatment of peri-implantitis: a new and predictable technique. Ann Maxillofac Surg. 2015;5:179–84.
Kablan FK. The reliability of free buccal fat graft for treatment of severe gingival recessions at mandibular and maxillary exposed roots. Ann Maxillofac Surg. 2018;8:281–6.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mohammadi, A., Hassani, A., Fazlisalehi, O. (2021). Use of Buccal Fat Pad in Facial Cosmetic Surgery. In: Keyhan, S.O., Fattahi, T., Bagheri, S.C., Bohluli, B., Amirzade-Iranaq, M.H. (eds) Integrated Procedures in Facial Cosmetic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-46993-1_71
Download citation
DOI: https://doi.org/10.1007/978-3-030-46993-1_71
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46992-4
Online ISBN: 978-3-030-46993-1
eBook Packages: MedicineMedicine (R0)