Abstract
Biopolymers or the natural polysaccharides like alginate, chitosan, pectin, cellulose and their derivatives, etc., have been used in recent research studies for a number of significant advantages like their biocompatibility, biodegradability, safety, and cost-effectivity. They have been evaluated in a number of formulation strategies including matrix tablets, microencapsulation, nanoparticulate delivery, targeted drug delivery in various parts of the gastrointestinal tract according to pH or microbial population, etc. Further, they have been extensively utilized in the formation of gels by physical or chemical cross-linking methods for advanced drug delivery. Such biopolymeric gels find applications not only in controlled or targeted drug delivery but also in biomedical fields. Such gel formulations would provide controlled or targeted drug release based on their physicochemical properties including thermal sensitivity, pH sensitivity, analyte sensitivity or presence or absence of microbial population, etc. Recent inventions in this field include the smart gels which produce significant changes in drug delivery with minimum changes in the environment or the in situ gels which remain in the liquid state outside the body and would turn into gel at body temperature, once delivered. Further, modified or grafted biopolymers have been tried out for the formation of stable gels with favorable physicochemical properties for better control on drug delivery. The present chapter would present a review of the potential biopolymeric gels, their preparation, characterization, and most importantly their applications in modern drug delivery taking into account the recent innovations in the area.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Biopolymers can be defined as the polymeric substances produced from natural resources either by chemical synthesis of biological matter or by biosynthesis of living organisms [1,2,3,4]. The main advantage of biopolymer is its capability of degradation when it comes into contact of living microorganisms. This phenomenon has gained popularity in recent years in pharmaceutical, industrial, and medical fields [5]. Further, due to their biocompatible nature, they have created an interesting impact on drug delivery systems [6, 7].
In pharmaceutical field, polymeric gels have been established as promising delivery systems to overcome the challenges of drug delivery. Gels are generally semisolid and homogeneous formulations consist of medicinal dispersion in satisfactory hydrophilic or hydrophobic three-dimensional (3D) network [8, 9]. These formulation have attained popularity due to easy preparation. They also provide close contact between therapeutic element and the site of action followed by controlled delivery of drug in different routes. Generally, gels are classified into two types: (a) hydrogels and (b) organogels. Hydrogels are hydrophilic 3D polymeric network that binds with great volume of water or biological fluids without dissolution of polymer [10, 11]. Immobilization of gelator fibers with organic liquid phase followed by formation of 3D network is known as organogels or oleogels [12].
On the basis of the responsive nature, novel gels are generally distinguished by two different types: stimuli-responsive and non-responsive. The former one swells when exposed to external stimuli, like pH, temperature, magnetic field, ionic strength and the later one swells after engulfing water. Gels that counter more than one environmental stimulus are known as multiresponsive gels [9, 13].
2 Classification of Gels
Due to the versatile characteristics and wide background of applications, novel gels are classified as follows:
2.1 Hydrogels
These are hydrophilic 3D polymer networks, which are closer to the extracellular matrix of cells [9, 14]. From decades in the field of drug delivery system, hydrogels contribute their promising aspects. Due to biocompatibility, pliability, presence of higher volume of water, and broad range of applications, they have gained the popularity in the sphere of drug delivery system from since 50 years [14,15,16,17,18,19,20]. Hydrogels can be synthesized in number of classic chemical processes, which involves one step approaches, such as polymerization and aligned cross-linking of multifunctional monomers. The synthesis also involves various techniques associated with reactive groups of polymer molecules, which result in their cross-linking as well as possible reactions between polymers and cross-linking agents. As the hydrogels are 3D hydrophilic polymer networks, they have capability of swelling and de-swelling in aqueous media and thereby retain high volume of fluid in swollen condition [19, 21]. Hydrogels perform volume transformation markedly in the presence of different physical and chemical factors like light, pressure, magnetic field, sound and pH, ionic strength, molecular species as well as composition of solvent [19, 22].
The first synthetic hydrogel for biological application was established by Wichterle and Lim in 1960 [23]. Later on hydrogels have come into application in different fields, such as agriculture, [24] drug delivery systems, [25, 26] ophthalmic products, [27] dehydration of coal, [28] food additives, [29] pharmaceuticals, [30] biomedical background, [31, 32] tissue engineering, [33] and regenerative medicines, [34] Moreover, hydrogels are also used in separation of biomolecules or cells, [35] wound dressing, [36] as well as biosensor [37].
2.2 Emulgels
Emulgel has now widely emerged as a topical drug delivery system. If adequate efforts are applied in its formulation development with topically effective drugs, it would prove to be a great achievement for derma care and cosmetology. Emulgels are defined as an emulsion of w/o or o/w type, which is converted to gel form followed by addition of gelling agent [38, 39]. Emulsion itself acts as a controlled release system in which drug is encapsulated in an internal phase and thereby moves toward the external phase to the skin, from where it can be absorbed gradually in a controlled manner. Internal phases function as drug reservoir and release the drug to the external phase for a longer period of time. Gel possesses cross-linked network form that catches tiny drug particles and produces controlled release. Owing to its mucoadhesive nature, it extends the period of contact time of medication on the skin. Emulgel acts as dual controlled release system as it possesses the characteristics of both emulsion and gel [39].
There are several marketed emulgels available, which have been widely used as antifungal, topical corticosteroids, anticancer, exfoliating, and anti-inflammatory medications [8, 40].
2.3 Microgels
The term ‘microgel’ was first introduced by Baker by implementing cross-linked polybutadiene latex particles [41]. Basically, microgels are defined as micron-sized gel, which is having cross-linked network structure possessing particle size range higher than 1 micron. Furthermore, microgels are composed of colloidal dispersion of gel and solvent [42]. Due to strong physical forces, microgels have their steady construction [43].
Microgels are widely used in the field of oral and non-oral drug delivery system, [44,45,46] topical drug delivery system [8, 47,48,49,50], etc.
2.4 Nanogels
In recent years, nanogels have emerged as promising hydrophilic formulations for entrapment of guest molecules having the capacity to respond to external stimuli that may be possibly used for multiple applications. Nanogels are 3D polymeric hydrogel-based cross-linked network having nanoscale range with a capacity to carry large volume of water in the absence of aqueous media [8, 51]. Nanogels can be prepared by synthetic, natural polymers and combination of both. By altering the composition of nanogels, their size, shape, amphiphilicity, charge, and softness can be modified [52]. Nanogel triggers drug release at target site. However, owing to their speciality, nanogels offer number of advantages, such as biocompatibility, swelling capability, biodegradability, high drug loading capability, enhanced efficacy over renal excretion [8].
Nanogels have wide number of applications in the field of cancer treatment. As compared to single responsive nanogels, multistimuli-responsive nanogels are very effective for targeted drug delivery in cancer [53, 54]. Moreover, nanogels are used in local anesthesia, [55] Alzheimer’s disease, [56] gene therapy, [57] neurodegenerative diseases, [58] and rheumatic disorders [59].
2.5 In Situ Gel
In situ gel is one of the most elegant drug delivery systems that stimulates establishment of various medical and biomedical controlled delivery systems. In situ gel appears in liquid form at ambient temperature and markedly change based on different factors like: change in pH and alteration of temperature. Moreover, it simultaneously changes its appearance when it comes to the contact of body fluid [60]. In situ gelation is a mechanical process of gel formation at the target site after application [61]. The advantage of in situ gels includes excellent thixotropic characteristics, enhanced contact time of drug, decrease frequency of drug administration, quick absorption and rapid onset of action, low dose strength, minimized systemic and local effects, direct penetration to systemic circulation and central nervous system (CNS) as well as possible administration through rectal, vaginal, oral, ocular, and intraperitoneal routes [62, 63].
In situ gels are used as in antibiotic formulations to overcome the problems associated with eye drops [64], used as an antitussive and anti-angiogenic agent [65].
2.6 Vesicular Gel
Vesicles are small-scale carriers composed of a number of amphiphilic molecules like lipids, surface active agents and block polymers. Vesicles are generally made up of hydrophilic core bordered by bilayer of amphiphiles. They have gained attraction for their exclusive delivery system of drugs, pharmaceuticals, food nutrients, fragrances and dyes in cosmetics and textiles, and so on. Vesicular gel enables topical drug delivery via niosomes, liposomes, ethosomes, novasomes, and other vesicular system. Vesicular system provides innumerable applications of locally acting drugs on dermal delivery as well as transdermal delivery for systemic effect. Hence, non-invasive route has gained popularity over oral route from decades [8].
3 Biopolymeric Gels
These are molecular coils which proceed through coil helix transformations; either produces network or enables the network to establish via lateral helix alliance [66]. Different levels of structural heterogeneity are shown by biopolymer gel networks. At one degree, where polysaccharide network strands are thick (e.g., agarose gel), such network may be considered as a microphase separation. On the other hand, where a network represents itself to be necessarily molecular, large-scale variation of network density repeatedly occurs in both the cases of associative and particulate gels. The gels thus are made heterogeneous over long-scale distance and opaque [66, 67].
The biopolymeric gels are widely used in the field of agriculture [68], food, and pharmaceuticals [69]. On the other side, the gels prepared with polymeric blends have wide ranges of applications in pharmaceutical, cosmetics, food, and biotechnological background [8, 70].
Recent research has been focused on natural biopolymers and biopolymer gels, various types of gel formulations due to the immense benefits that could be achieved from such delivery systems targeting several diseases; for local as well as systemic action; for drug delivery and medical devices; for various routes of drug delivery including oral, rectal, vaginal, topical nasal, or buccal. Few of such studies would be highlighted in the next section concerning several biopolymers.
3.1 Guar Gum
Pandey et al. prepared o/w type of emulsion gels using aqueous solutions of guar gum and xantham gum together with sunflower oil for the administration of combinations of probiotics and drugs. The emulgels were characterized by FTIR, fluorescence microscopy, XRD, DSC, evaluated for mechanical properties and disintegration studies. The encapsulated probiotic Lactobacillus plantarum 299v was found to be viable in the emulgel formulation when stored under different conditions of temperature, 4 °C, 20 °C, and −196 °C. Further, when the drug metronidazole was loaded on to the probiotic emulsion gels, sustained release of the drug was produced [71].
An in situ gelling ophthalmic delivery system was developed by Bhowmick et al. [72] using poloxamer-407, xanthan gum, and guar gum in a ratio of 3:7 which was sufficient to convert the poloxamer, used in concentrations less than 18% from sol to gel at temperatures below the body temperature. Further, the gums added helped the formulations to retain the drug for longer duration than the poloxamer alone.
Tetrakis(2-hydroxyethyl)orthosilicate (THEOS) which is a hydrophilic silica precursor was combined with hydroxypropyl guar gum (HPGG) which is a hydrophilic guar gum derivative to obtain a biocompatible sol–gel silica matrix which can be used for the delivery of drugs or biomolecules. The addition of HPGG not only catalyzed the sol–gel transition of THEOS in water yielding a homogeneous gel, with much shorter gelation times but also affected the mechanical strength of the gels. When Vit B12 was encapsulated within the formulation, it produced a sustained release. The release properties were further dependent on the concentration of HPGG, with lower rates of release with higher amounts of the gum derivative [73].
3.2 Xanthan Gum
Anionic gels containing agarose along with carbomer 934 and xanthan gum were applied to produce electric field induced drug delivery that would follow zero-order drug release. The gel content and the strength of the electric current were observed to affect the drug migration under the effect of the applied electric field [74].
A nanoemulgel was designed for the nasal delivery of the antiepileptic drug carbamazepine containing oil/surfactant as oleic acid and labrasol in a ratio of 1:5 and 0.1% xanthan gum as the mucoadhesive agent which was anionic in nature. The prepared formulations were characterized with respect to the size of the droplets, the drug release and drug uptake as well as mucoadhesion properties. Further, in vivo studies were conducted on animal model using albino mice to evaluate the anticonvulsant activity induced by chemical and electrical means after the application of the nasal gel. Drug uptake reached up to 65% within an hour. Thus, an alternative means for the delivery of the drug could be developed which has erractic absorption on oral administration [75].
Chlorhexidine gels containing 10% doxycycline hyclate and xanthan gum were evaluated for use along with scaling and root planning for the treatment of chronic periodontitis. The results showed marked improvements in the patients when the gel was applied in the treatment [76].
Cross-linked gel beads with xanthan gum and sodium alginate entrapping diclofenac sodium as the model drug were developed. The presence of hydrogen bonds between xanthan gum and sodium alginate resulted in difference of physicochemical properties of the beads, like higher drug entrapment efficiency, higher water uptake, and swelling in distilled water pH 6.8 phosphate buffer. However, in increased concentrations of the gum, drug release increased [77].
In a novel study, an aerogel prepared with methoxy pectin and xanthan gum was placed as a coating on medical grade stainless steel and evaluated for orthopedic applications on osteoblast cells derived from human bones. The gelation was produced using ethanol and then dried. Drugs such as indomethacin and diclofenac sodium were added in the gel coatings, and the release was obtained till a period of 24 h. The delivery system was proven to be biocompatible and thus has potential to be used for clinical purposes [78].
Lamotrigine was added into polymeric solutions of gellan and xantham gums, so as to produce in situ gels in the nasal cavity, which would improve the bioavailability of the drug, bypassing the first pass effect, and increase residence time due to higher mucoadhesion. In vitro and ex-vivo studies were conducted for the formulations. The in vitro studies showed immediate drug release with a maximum of 97% within 20 min. The ex-vivo studies showed sustained drug release over a period of 12 h with greater permeability compared to the control batch. The formulations were stable for 45 days when temperature maintained within 4 ± 2º C [79].
Mucoadhesive nasal inserts were prepared with a number of water-soluble polymers like sodium alginate, carrageenan, Carbopol, chitosan, polyvinyl pyrrolidone, hydroxypropyl methylcellulose (HPMC) K15M and E5, sodium carboxy methylcellulose, and xanthan gum. It was observed that the polymers with low molecular weights dissolved and released the drug quickly, whereas the inserts prepared with high molecular weight polymers like xantham gum, HPMC K15, or carbopol decreased the drug release rate [80].
Xanthan gum and locust bean gum were used to produce gels loaded with vesicles composed of non-ionic surfactants and a model drug to evaluate topical drug delivery compared to other marketed topical gel formulations. The developed formulations matched the marketed products with respect to mechanical integrity and strength and produced sustained release but improved permeability through the skin as it was loaded with permeation enhancing vesicles. Further, the formulations were stable for a period of about one year without the addition of any preservatives [81].
HPMC K100 and xanthan gum were used to prepare in situ gels encapsulating loratidine for nasal delivery. The gels had mucoadhesive characteristics. The temperature for the sol-to-gel transformation for the formulations varied between 33.1 ± 0.43 and 34.8 ± 0.82 °C, and the gelling time ranged from 4.0 ± 0.21 to 11.3 ± 0.22 s. The pH of the formulations was favorable such that they produced no mucosal irritation. The drug release was sustained for a period of around 10 h. The gels were stable for about six months under accelerated stability testing. Thus, the prepared formulations had the potential to be used as mucoadhesive gels to improve bioavailability of drugs [82].
Microemulsion formulations of Repaginate, a class II hypoglycemic drug was gelled using xanthan gum for improvement of its bioavailability by improving the residence time and permeation of drugs. Ex-vivo permeation tests were conducted using rat skin for both the microemulsion and microemulsion gel formulations. They showed 12.30 and 10.97 fold increase in permeation across the membrane compared to normal drug suspension. In vivo studies on Sprague Dawley rats confirmed the efficacy of the developed formulations to control glucose level [83].
3.3 Shellac
In situ gels and microparticles were developed using bleached shellac and different solvents like dimethyl sulfoxide (DMSO), N-methyl pyrrolidone (NMP), and 2-pyrrolidone (PYR) encapsulating the model drug doxycline hyclate. The drug and solvent release from the microparticles were slower than that of gels. The drug release rates were minimum when 2-pyrrolidone (PYR) was used as the solvent and was chosen as the solvent which gave the best sustained release from in situ gels with good biodegradation properties [84].
In situ gels comprising of bleached shellac, ethylcellulose and Eudragit RS were developed using N-methyl pyrrolidone as solvent for possible use in periodontitis treatment. The formulations could be easily administered through injection and could inhibit various bacterial species owing to the antibacterial activity of N-methyl pyrrolidone. They could form in situ gels in vitro [85].
Three types of oleogels were formed with hydrophilic polymer like HPMC, with shellac, and with emulsion droplets and were compared with respect to various physicochemical properties. They were characterized with respect to their process of preparation, their mechanical strength and rheological properties and the response of the oleogels to differences in temperature, water content, or shearing force [86].
3.4 Alginate
Beads produced from alginate aerogels are known to have a highly porous structure nanostructure. In a study, dried beads were produced from alginate cryogels, xerogels, or aerogels under different gelation conditions like aqueous or alcoholic solutions of calcium chloride and different drying conditions like freeze drying, supercritical drying, or oven drying. The effect of such gelation or drying methods on the physicochemical properties and stability of the beads were studied. The alginate aerogels were found to be suitable after 3 months of storage at 25 °C and 65% relative humidity [87].
Beads from alginate aerogels with cross-linked iron (III) were developed, and ibuprofen and ascorbic acid were loaded. When the drug release was tested in HCl (pH 2) and phosphate buffers (pH 6.8), the release was found to be faster in phosphate buffer. Further, incorporation of ascorbic acid in some of the formulations increased the drug release as the iron (III) to iron (II) cross-linking was reduced by the acid and led to the erosion of the matrix [88].
Microparticles with size less than 50 μm were prepared from alginate aerogels cogelled with pectin with low methoxyl pectin and k-carrageen an, dried using supercritical CO2 and loaded with drugs like ketoprofen and quercetin. The release of drugs from the microparticles was relatively improved than from alginate particles due to larger surface area and superporous characteristics of the aerogel. Further, alginate having bioadhesive properties and the formulations may be applied for mucosal delivery with improved absorption [89].
Aerogel microspheres were also developed with polysaccharides like alginate, starch and pectin, loaded with drugs like ketoprofen or benzoic acid and their release characteristics tested in HCl and phosphate buffers. Controlled drug release from alginate aerogel microspheres could be obtained depending on the composition of matrix. Drug release was observed to be based on both diffusion and erosion of alginate matrix [90].
Nanoparticles based on nanogels prepared from alginate and chitosan as biopolymers were developed for corneal delivery for treating glaucoma. Timolol maleate was used as the model drug. The particles gave sustained release for a period of 24 h after a burst release during the first hour in the drug dissolution studies. The size of the particles ranged from 80–100 nm. Promising results were also obtained from the ex-vivo permeation studies conducted through Franz diffusion cell and fluorescent microscopy where it was observed that the drug permeation from the formulations was twice than that from pure drug [91].
Smart nanogels were developed from alginate derivatives linked to iron oxide nanoparticles and loaded with doxorubicin which would have magnetic resonance imaging properties for diagnosis of disease as well as target tumor cells for therapeutic activity as they were loaded with drug. They were relatively safe to normal tissues [92].
Cisplatin-loaded alginate nanogels were developed for targeting macrophages for treating artherosclerosis. Cisplatin served as the therapeutic agent as well as the cross-linking agent for alginate molecules. The formulation showed a pH-dependent drug release with 100% release within 48 h at pH 5, whereas the release was even less than 15% at pH 7.4. The drug uptake was evaluated on macrophage and human cell lines. Further, it was observed that the nanogels were selectively taken up by macrophage cells rather than human cell lines [93].
Alginate along with a thermosensitive polymer poly(N-isopropylacrylamide) was used to develop smart nanogels in situ with cystamine as cross-linker. The developed nanogels gave abrupt swelling upon temperature increase in the environment of the cells from 25 to 37 °C. Due to the increase of temperature, the nanogels could be easily taken up by cancer cells. Further, due to the presence of acidic and reducing conditions in the cells, the drug release from the nanogels was accelerated. Thus, the therapeutic activity of anticancer drugs would be improved to a high extent [94].
In a similar study, gels were prepared by adding poly(N-isopropylacrylamide) or poly(N-isopropylacrylamide-co-acrylic acid) into alginate (AG) emulsion nanodrops and fixed and stabilized with cystamine dihydrochloride. The developed gels showed thermosensitivity, pH sensitivity, and redox sensitivity. The formulations loaded with anticancer drug were taken up by cells and released drug due to thermal changes. Further, the acidic and reducing environments in the cells cause accelerated drug release leading to enhanced toxicity of the drug [95].
Smart nanogels comprising of branched alginate-polyethyleneimine copolymer were used to not only enhance drug delivery but also track the passage of the delivery system through fluorescence spectroscopy. The fluorescence emitted varied with pH γ irradiation. Doxorubin loaded on to the gels was released in a time-dependent manner which was significantly higher in the presence of glutathione and at lower pH than in acidic pH or absence of glutathione. The drug-loaded gels were actively taken up by HeLa cells as confirmed by fluorescence microscopy. Further, the nanogels were much less cytotoxic and hemocompatible [96].
In situ gels using alginate using cystamine as a cross-linker were prepared and loaded with doxorubicin as a model drug which has poor cellular uptake and release characteristics. The nanogels were cytocompatible and had > 95% encapsulation efficiency. The release from the nanogels under reducing conditions of the cells was high. The nanogels were actively taken up by osteosarcoma cell line CAL-72 cells. The cell uptake and death were significantly higher from the developed nanogels compared to free drug [97].
Pressure-sensitive nanogels prepared with alginate cross-linked by modified β-cyclodextrin were developed and utilized for better therapeutic management with 5-fluorouracil which suffers from low cellular uptake. The prepared nanoparticles from the nanogels were cytocompatible with 82% encapsulation efficiency. The in vitro drug release from the formulation was pressure sensitive which indicates that the drug release in vivo would be stimulated by intravascular pressure. They were actively taken up in colon cell lines HT-29 cells. Significantly higher amounts of drug were taken up by the cells and resulted in cell death compared to free drug [98].
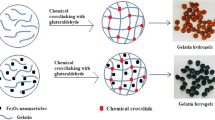
Aldehyde-alginate was cross-linked with gelatin by inverse mini-emulsion technique to develop nanogels in the presence of borax due to Schiff’s base reaction. Spherical particles of the nano range were obtained which were found to be cytocompatible and hemocompatible and thus non-toxic to the body. Such formulations can be thus used targeted drug delivery with fewer side effects [99].
Curcumin-loaded alginate aldehyde-gelatin nanogels were developed and characterized to improve the bioavailability of the medicament. The nanogels had a high encapsulation efficiency > 82% and gave a controlled release of drug over a period of 48 h. Nanogels were found to be cytocompatible and were actively taken by MCF-7 cells which were confirmed by confocal laser scanning microscopy [100].
Biocompatible alginate nanogels have been used as suitable carriers for protein or peptide drugs. However, low encapsulation efficiency and higher release in alkaline pH have been observed due to larger pore size of the nanogel structure. In this study, a novel on-chip method has been devised where the pore size of the gel could be modulated by synthesis of the gels using microfluidic platforms with cross-junction microchannels. For fluid flow ratio of 0.2–2, the size of the nanogels ranged from 68 to 138 nm. Increase in the flow ratio increased the size and decreased the compactness of the formulations. Therefore, the porosity of the gels could be controlled effectively by this method to modulate drug release under different conditions [101].
Nanosized liposomes were used to prepare nanosized cross-linked alginate gels for various therapeutic applications. Alginate was entrapped within the core of nanoscale liposomes and then placed in a calcium chloride containing buffer solution with elevated temperatures which resulted in the absorption of calcium ions by the liposomes to cross-link the alginate in the core. Thereafter, the lipid layer was removed by surfactants and nanoscale alginate gels of size range 120–200 nm resulted. In a similar way, different size of nanogels can be prepared using different sizes of liposome template [102].
3.5 Chitosan
Chitosan-based gels were applied on second degree burns in rabbits to study the wound healing rates. Chitosan has well-known wound healing properties. It was observed that compared to the control group there was better wound healing including healed epidermis and normal melanocyte production [103].
A biodegradable, safe, and biocompatible biomaterial with bioadhesive properties which would stop bleeding and help to bind tissues was developed by reacting chitosan with oxidized dextran. A biocompatible gel was produced as a result which was non-toxic and showed good adhesive properties on tissues. The gel did not swell in phosphate buffer. The in vivo efficacy of the produced gel was demonstrated in a rabbit model with liver injury. The gel can also be used for drug delivery [104].
Myricetin was loaded on to chitosan nanogels with a particle size of 100–300 nm. Fickian drug release was accompanied with swelling and erosion in acidic medium. Further, the bioavailability of the drug was found to improve when administered in rats via the oral route. The formulation showed no cytotoxicity in MTT assay [105].
Chitosan gels with mucoadhesive properties encapsulating antifungal agents like econazole and miconazole nitrates were developed and evaluated for possible vaginal use. Various grades of chitosan with differing molecular mass and viscosity were used. Several evaluation studies were conducted on the developed gels to determine their mechanical strength, rheological properties, and syringe ability, in vivo retention in vagina in rats, mucoadhesive properties, release studies, and studies to demonstrate anticandidal effect. After evaluating all the formulations, the gels prepared with medium-molecular weight chitosan were optimized as it showed suitable mucoadhesive and release properties and effective vaginal retention times [106].
Film-forming gels have the specific advantage of protecting the skin and to provide continuous release at the target site. Thus, a chitosan-based film-forming gel encapsulating the drug ketoprofen was developed with various skin permeation enhancers and evaluated for various physicochemical characteristics. Skin permeation study was conducted in Franz diffusion cell using excised rat skin. In vivo studies in rheumatoid arthritis-induced animal model were also conducted. The gel was found to form the film well in the skin permeation study. Oleic acid was found to act as a useful skin permeation enhancer compared to others. In the in vivo study, significant anti-inflammatory and analgesic activities were reported [107].
Such film-forming chitosan gels have also been produced containing tyrothricin and evaluated in various wound types and burns. Once solidified, the chitosan layer protected the wound and healed it gradually. The wound size was measured periodically after induction and results compared with negative control where no drug was applied and positive control where sodium fusidate ointment and marketed tyrothricin gel were applied. The developed gel formulation demonstrated significantly better results than negative and positive controls for various types of wounds, which may be due to better occlusion properties of chitosan [108].
Photocytotoxic agents like Toluidine blue O (TBO) have been in use to produce toxic effects on tumor cells. Thus, TBO was incorporated in 4% chitosan gels containing Tween 80 as a permeation enhancer for management of oral cancer. The formulations were characterized with respect to physicochemical properties like mucoadhesion, viscosity, etc., and in vitro release or mucosal retention and in vivo penetration studies through mucous layer. The 4% chitosan gels with 5% Tween 80 and 1% TBO had good mucoadhesion properties. The release of TBO was sustained by Tween 80 and allowed greater mucosal retention times. The formulation also improved tumor cell death by apoptosis [109].
Nanoemulsions were prepared containing Labrafac PG + Triacetin as the oil phase, Tween 80 and polyethylene glycol (PEG 400) as surfactant and co-surfactant, respectively, and characterized with respect to physicochemical properties, ex-vivo skin permeation and deposition studies. The optimized emulsion was then incorporated into 1–3% chitosan gel for preparing nanoemulsion gels. Curcumin was used as the model drug and entrapped within both the nanoemulsion and the nanoemulsion gel formulations, and both the formulations were compared with respect to wound healing characteristics. The permeation of curcumin was higher in case of the nanoemulsion, whereas the retention of curcumin on skin was significantly higher in case of the gel formulation [110].
Nanofibers have been found to be beneficial for accelerated wound healing as they can induce hemostasis, induce cell growth, and absorb the exudates from wound. Chitosan nanofibers have been prepared for the above purpose with their surface modified with arginine and electrostatically interacted with sodium alginate under different pH conditions with an average diameter of 100–150 nm as observed by scanning electron microscope. The release of arginine from the nanofibers took place at a sustained rate. The formulation was viscous and could spread quite well and therefore easily applied in the wound area. The wound healing properties observed were significantly improved in in vivo rat model when compared to control groups [111].
Chitosan gel-containing Lysostaphin was developed and evaluated against multidrug resistant Staphylococcus aureus. The antibacterial activity of the developed gel formulation was evaluated using agar well diffusion method and ex-vivo porcine skin model. Both the studies showed significant reductions in bacterial count [112].
Hydrogel beads of succinyl chitosan were prepared and stabilized with glycopolymeric network and loaded with anticancer drug doxorubicin. The drug release was sustained for more than 15 days with zero-order release profile [113].
Moxifloxacin was loaded on to niosomes and entrapped within chitosan gel for topical drug delivery. The formulations were evaluated for various physicochemical characteristics, and activity on the pathogens like Pseudomonas aeruginosa and Staphylococcus aureus was studied by agar well diffusion methods. The optimized formulation demonstrated a drug entrapment efficiency of 73%, and sustained drug release of 47% in 8 h. The gel formulations exhibited improved sustained release characteristics compared to niosomal formulations. The niosomes showed greater activity toward P. aeruginosa, while the noisome containing gel formulations showed greater activity toward S. aureus [114].
Acrylic-based nanocapsules with cationic and anionic characteristics were loaded onto chitosan gels for vaginal delivery which is a challenging route. Nile red was used as the model drug which is lipophilic in nature. The formulations were characterized with respect to pH and viscosity and evaluated in porcine vaginal mucosa. The formulations were found to be retained for prolonged periods of time and penetrate the vaginal membrane better. The gels were found to be sufficiently viscous and had an acidic pH of about 4.5 in both the formulations containing cationic and anionic nanocapsules. The mucoadhesion on the vaginal mucosa was found to be better than control without any nanocapsules. The penetration of gels with cationic nanocapsules was better than anionic ones [115].
Biocompatible chitosan gel along with cationic protamine sulfate, an arginine-rich protein was used for controlled delivery of DNA. Protamine has been known to be beneficial for effective cell penetration and localization in the nucleus. The formulations were characterized with respect to DNA entrapment efficiency, release, release kinetics, and swelling studies. The hydrophilic nature of the gel particles was assayed by Rose Bengal partition assay method. Further, the formulations were found to be non-hemolytic and therefore can be used in devices that stay in contact with blood [116].
3.6 Inulin
Inulin plasma clearance data were determined and compared for inulin solution given through i.v and i.m routes and an inulin/poloxamer gel formulation administered through i.m route. It was found that the clearance values of inulin in case of the gel formulations were significantly reduced compared to inulin solutions given through i.v and i.m routes [117].
3.7 Cellulose
Cellulose gels were produced with tetramethylguanidine as the cross-linking agent in ratios of 1:1, 1:2, and 1:3 which gave stable formulations. Such gels would be beneficial for drug delivery [118].
Aerogels were prepared with cellulose and polyethoxydisiloxane resulting in polymer networks, and they were characterized using two different methods like molecular diffusion and pressure difference induced forced flow. The latter method resulted in significantly reduced impregnation times. The specific surface area of the gel network was evaluated using nitrogen adsorption analysis method and was found to increase by threefold compared to organic–inorganic composites [119].
A mucoadhesive cellulose polymer gel formulation encapsulating the antifungal agent miconazole was prepared and characterized for drug delivery in the buccal cavity. The gel was cross-linked with triethanolamine and evaluated with respect to ex-vivo permeation study. The texture of the formulations was found to be consistent over a period of 90 days. Greater diameters of zones of inhibitions of the antifungal gel were observed when compared to marketed formulations. Thus, stable formulations with improved residence times in the oral mucosa were developed [120].
Sodium carboxymethylcellulose was used along with microcrystalline cellulose to formulate a mucoadhesive nanogel formulation. Few nanofillers were also incorporated in the nanogels like MMT-clay or porous starch for a stable structure. The formulations were able to achieve better controlled release compared to other nanogels [121].
One of the recent promising methods for gastric emptying tests is the alternate current biosusceptometry (ACB). It is sensitive, easy to perform, non-invasive, and economic. In a study, formulations with magnetic properties such as Mn-Zn ferrite nanoparticles, nanoparticles modified with dextrose, and cellulose gel-containing ferrite nanoparticles were developed to function as tracers in gastric emptying tests. When evaluated in rats, the ferrite nanoparticles were pH-sensitive along the length of the gastrointestinal tract, while nanoparticles modified with dextrose were found suitable for rapid gastric emptying tests. The cellulose gel formulation on the other hand was found to be sufficiently bioadhesive and retained in stomach and easily detected by ACB analysis [122].
Ibuprofen was loaded onto oxidized cellulose gels containing nanofibrils and compared with marketed products for topical delivery. The gels were evaluated using silicone membrane and pig skin by in vitro tests and in human volunteers by in vivo tests. The permeation of the drug from the gels was similar to that of the marketed products as observed from the in vitro or in vivo tests [123].
In a study based on preparation of cellulose aerogels, zinc chloride tetrahydrate was used which was later removed from the gel by using solvents such as alcohol, acetone, water, or isopropanol. It was further observed that aerogels washed with acetone had a significant increase in surface specific area compared to those washed with water [124].
Novel topical cellulose-based gel formulations were developed in order to minimize the loss of viscosity after sterilization in autoclave. Initially, viscous gels were produced with methylcellulose and hypromellose entrapping a protein molecule; however, they lost their viscosity after autoclaving. When edetate disodium was added into the formulations, the loss of viscosity was minimized in the presence of 0–100 ppm of hydrogen peroxide. However, when methionine was added, the loss of viscosity could be completely prevented in the presence of 0–50 ppm of hydrogen peroxide [125].
Aqueous NaIO4 was used to partially oxidize cellulose gel and then reacted with polyallylamine for formation of Schiff’s base. Three different gel formulations were prepared by the method with a content of amino groups such as 0.35, 0.59, and 0.96 mmol/g cellulose, and protein retentions were evaluated and compared with DEAE-cellulose gel-containing amino groups in the ratio of 1.07 mmol/g cellulose. It was observed that the protein retention was much higher in case of the developed gel formulations. Further pairs of proteins such as human and bovine serum albumins which have similar isoelectric points and molecular weights could be effectively separated using the developed gels, which may be due to the high density of polyallylamine [126].
In a novel study, cellulose polymer was modified with different concentrations of HMDI and this modified polymer was then added to castor oil to form oleogels due to the reaction of the polymer with the ricinoleic fatty acid chain hydroxyl groups. When characterized, the oleogels were found to possess suitable viscosity and thermal-resistant properties [127].
Lysine-based surfactants were added to ethyl (hydroxyethyl) cellulose gels which reduced their cytotoxicty which was tested on HeLa cells. The polymer interacted with the surfactant molecules to form mixed micelles due to which they became more biocompatible. The biocompatibility of the gels was improved with the most hydrophilic or longest chain surfactant molecule [128].
Cellulose sulfate vaginal gel formulation was tested in a clinical study on 1398 women of sub-Saharan Africa in order to test their efficacy to prevent HIV infection. The studies indicated that the cellulose sulfate was not able to prevent HIV infection in the tested women and was in no way better than placebo [129].
3.8 Pectin
In a novel study, the efficacy of preactivated thiolated pectin for buccal delivery of lidocaine was evaluated in a gel formulation and compared with other pectin and thiolated pectin formulations with respect to drug release and other physicochemical properties like viscosity, swelling, and mucoadhesive properties. Thiolated pectin was cross-linked resulting in gel formation due to the presence of thiol groups and did not require any excipient for the cross-linking reaction, whereas pectin could not do so. Viscosity of the gels formed with thiolated pectin was increased by 92 times and that of preactivated thiolated pectin increased by 4958 times than pectin gels. Gels swelled in water but would not dissolve for several hours. Further, mucoadhesive properties improved significantly. Higher retention of drug in the buccal mucosa and sustained release properties of the novel gel indicated that the gel formulation would be promising for buccal delivery [130].
Beads were prepared from calcium pectin-silica gel using the ionotropic gelation method entrapping mesalazine for controlled release in colon. Increasing the amount of sodium silicate led to higher gel strength and a decrease in swelling of the beads. Further, when the reaction time with calcium chloride was increased up to 1 h, sustained release properties were improved [131].
Pectin-calcium gels have the property of adhesion on gram positive bacterial cells like Bacillus subtilis. Pectins obtained from various sources were used in the study. The adhesion was attributed to the high molecular mass, length of carbohydrate chain, and low degree of methyl esterification [132].
A pulsatile capsule was developed for colon-targeted delivery. The capsule was impermeable and filled with an immediate release tablet containing the drug 5-aminosalicylic acid and had a plug at the capsule opening which was prepared with high-methoxy pectin and lactose or low-methoxy pectin and hydroxypropylmethylcellulose. In vitro release profile showed rapid release after a lag time and adding pectinase or rat cecal contents to the medium shortened the lag time for drug release. In the in vivo studies, it was observed that the plasma concentration for the drug could be detected after a lag time of 6 h which showed that it would be suitable for colon-targeted drug delivery [133].
In a novel study, spherical aerogel particles were produced via the jet cutting method using chitosan, amidated pectin, and sodium alginate. Gels were produced by diffusion method in calcium chloride solution and by internal setting method using calcium carbonate as the cross-linking agent; citric and acetic acids were used for adjusting pH. The aerogel particles thus produced had a size range of 400–1500 μm and a specific area of 500m2/g [134].
In a study, ketoprofen-loaded tablet formulations were prepared by adding pectin/dextrin mixtures to microparticles of zinc pectinate for controlled drug delivery in the colon. The formulations were characterized and optimized using factorial design. It was observed that the release was sustained when the microparticles were added in the formulations. The in vitro release studies were conducted by mimicking the pH and gastric retention times throughout the gastrointestinal tract. Lag times ranging from 4.125 to 4.85 h were observed, whereas the time taken for 50% drug release was in the range of 7.45–8.70 h. The formulations were able to achieve controlled release and were better by 5.28–37.82 folds than calcium pectinate beads [135].
Pectin gel had been used for adsorption and removal of methylene blue, but the adsorption rate was low. Therefore, particles of pectin microgel were prepared and used for the adsorption of methylene blue. It was found that the microgel particles had a high adsorption rate and therefore would be beneficial for methylene blue removal from samples [136].
Aerogel microspheres were prepared with biopolymers like alginate and pectin and cross-linked with calcium ion and dried by lyophilization. It was observed that on increasing the pectin amount the porosity and solubility of the microspheres increased and on increasing the alginate concentration, the microspheres were stiffer. The microspheres were able to achieve controlled release of the encapsulated agent proanthocyanidins and those with higher pectin contents showed higher antioxidant activity [137].
Modified pectins were obtained from callus cultures and when cross-linked with higher concentrations of calcium chloride resulted in gels with higher degree of rhamnogalacturonan I branching of pectin. Such a cross-linked pectin structure could achieve retarded release of encapsulated prednisolone in simulated gastric and intestinal media, whereas a rapid release pattern was observed in simulated colonic medium from which it may be concluded that the resulting gel would be suitable for colonic delivery [138].
Gels with double network were prepared with sugar beet pectin and isolates from soy protein by laccase catalysis and application of heat. It was observed that the gels had improved water holding capacities and mechanical strength, and therefore, such gels could be effectively used for delivery of various components [139].
Floating emulsion gel beads were prepared by emulsion gelation methods with pectin and wax, where the wax added in mixtures of pectin and olive oil was melted, homogenized, added to the cross-linking solution of calcium chloride and the formed beads after washing were dried. Metronidazole was used as the model drug and loaded onto the beads. When the amount of oil used was sufficient, the beads floated on the simulated gastric fluid. Various types of waxes were used in the study. When water-soluble waxes were used, the drug release increased, but when water insoluble waxes were used, the drug release was reduced significantly. However, the incorporation of waxes in the formulation resulted in sustained drug release from the gel beads, while they floated for prolonged periods of time [140].
4 Conclusion
From the above chapter, it was observed that there are innumerable natural polymers with significant benefits which are used for preparation of different biopolymeric gel formulations entrapping active agents and used for drug delivery or other biological applications. Such biopolymeric gel formulations have immense opportunities for further research for targeting different disease models. Further research on discovery and development of new biopolymers is also the call of the day. However, a further research should be focused on proper in vivo studies, clinical, and toxicological study to ensure the safety and efficacy of biopolymeric formulations. Overall, it can be concluded that biopolymers hold significant potential with respect to targeting new disease models, development of novel formulations, and medical device and ensure safety and efficacy for the benefit of the common man.
References
Smith, A.M., Moxon, S., Morris, G.A.: Biopolymers as wound healing materials. Wound Heal. Biomater. 2, 261–287 (2016). https://doi.org/10.1016/B978-1-78242-456-7.00013-1
Mohiuddin, M., Kumar, B., Haque, S.: Biopolymer composites in photovoltaics and photodetectors. Biopolym. Compos. Electron., 459–486 (2017). https://doi.org/10.1016/B978-0-12-809261-3.00017-6
Numata, K., Kaplan, D.L.: Biologically derived scaffolds. In: David, F. (ed.) Advanced Wound Repair Therapies. Woodhead Publishing Series in Biomaterials, pp. 524–551 (2011)
Jaiswal, L., Shankar, S., Rhima, J.W.: Applications of nanotechnology in food microbiology. Methods Microbiol. 46, 43–60 (2019). https://doi.org/10.1016/bs.mim.2019.03.002
Vroman, I., Tighzert, L.: Biodegrad. Polym. Mater. 2, 307–344 (2009). https://doi.org/10.3390/ma2020307
Gracia, M.C.: Drug delivery systems based on non immunogenic biopolymers. In: Parambath, A. (ed.) Engineering of Biomaterials for Drug Delivery Systems, pp. 317–344 (2018). https://doi.org/10.1016/B978-0-08-101750-0.00012-X
Jacob, J., Haponiuk, J.T., Thomas, S., Gopi, S.: Biopolymer based nanomaterials in drug delivery: a review. Mater. Today Chem. 9, 43–55 (2018). https://doi.org/10.1016/j.mtchem.2018.05.002
Paul, S.D., Sharma, H., Jeswani, G., Jha, A.K.: Novel gels: implications for drug delivery. In: Nanostructures for Drug Delivery, pp. 379–412 (2017). https://doi.org/10.1016/B978-0-323-46143-6.00012-9
Aggarwal, G., Nagpal, M.: Pharmaceutical polymer gels in drug delivery. In: Polymer Gels, pp. 249–284 (2018). https://doi.org/10.1007/978-981-10-6080-9_10
Saka, R., Sathe, P., Khan, W.: Brain local delivery strategy. In: Gao, H., Gao, X. (eds.) Brain Targeted Drug Delivery System, pp. 241–286 (2019). https://doi.org/10.1016/B978-0-12-814001-7.00011-1
Peppas, N.A., Buresa, P., Leobandunga, W., Ichikawa, H.: Hydrogels in pharmaceutical formulations. Eur. J. Pharm Biopharm. 50, 27–46 (2000). https://doi.org/10.1016/S0939-6411(00)00090-4
Vintiloiu, A., Leroux, J.C.: Organogels and their use in drug delivery- a review. J. Control Release 125, 179–192 (2008). https://doi.org/10.1016/j.jconrel.2007.09.014
Sasaki, Y., Akiyoshi, K.: Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem. Rec. 10, 366–376 (2010). https://doi.org/10.1002/tcr.201000008
Bayat, M., Nasri, S.: Injectable microgel-hydrogel composites “plum pudding gels”: new system for prolonged drug delivery. In: Nanomaterials for Drug Delivery and Therapy, pp. 343–372 (2019). https://doi.org/10.1016/B978-0-12-816505-8.00001-1
Calo, E., Khutoryanskiy, V.V.: Biomedical applications of hydrogels: a review of patents and commercial products. Eur. Polym. J. 65, 252–267 (2015). https://doi.org/10.1016/j.eurpolymj.2014.11.024
Mauney, J.R., Cannon, G.M., Michael, L., Lovett, M.L., Gong, E.D., Vizio, D.D., Gomez, P., III., Kaplan, D.L., Adam, R.M., Estrada, C.S., Jr.: Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials 32, 808–818 (2011). https://doi.org/10.1016/j.biomaterials.2010.09.051
Buchholz, F.L., Peppas, N.A.: Superabsorbent polymers-science and technology. In: Buchholz, F.L., Peppas, N.A. (eds.) Polymer International, vol. 39, pp. 1–148 (1994)
Peppas, L.B., Harland, R.S.: Absorbent polymer technology. In: Peppas, L.B., Harland, R.S. (eds.) Studies in Polymer Science, vol. 8, 1st edn., pp. 3–272 (1991)
Ahmed, E.M.: Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 6, 105–121 (2015). https://doi.org/10.1016/j.jare.2013.07.006
Li, Y., Huang, G., Zhang, X., Li, B., Chen, Y., Lu, T., Lu, T.J., Xu, F.: Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 23, 1–13 (2012). https://doi.org/10.1002/adfm.201201708
Shin, J., Braun, P.V., Lee, W.: Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sens. Act. B Chem. 150(1), 183–190 (2010). https://doi.org/10.1016/j.snb.2010.07.018
Burkert, S., Schmidta, T., Gohsb, U., Dorschnerc, H., Arndt, K.F.: Cross-linking of poly(N-vinyl pyrrolidone) films by electron beam irradiation. Rad. Phys. Chem. 76, 1324–1328 (2007). https://doi.org/10.1016/j.radphyschem.2007.02.024
Wichterle, O., Lim, D.: Hydrophilic gels for biological use. Nature 185, 117–118 (1960)
Saxena, A.K., Graz, M.D.: Synthetic biodegradable hydrogel (PleuraSeal) sealant for sealing of lung tissue after thoracoscopic resection. J. Thor. Cardio. Surg. 139, 496–497 (2010). https://doi.org/10.1016/j.jtcvs.2008.11.003
Li, J., Mooney, D.J.: Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 1–31 (2016)
Hamidi, M., Azadi, A., Rafiei, P.: Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 60(15), 1638–1649 (2008). https://doi.org/10.1016/j.addr.2008.08.002
Narayanaswamy, R., Torchilin, V.P.: Hydrogels and their applications in targeted drug delivery. Molecules 24, 1–21 (2019). https://doi.org/10.3390/molecules24030603
Park, J.H., Kim, D.: Preparation and characterization of water-Swellable natural rubbers. J. App. Polym. Sci. 80, 115–121 (2001). https://doi.org/10.1002/1097-4628(20010404)80:1<115::AID-APP1079>3.0.CO;2-K
Chen, X., Martin, B.D., Neubauer, T.K., Linhardt, R.J., Dordick, J.S., Rethwisch, D.G.: Enzymatic and chemoenzymatic approaches to synthesis of sugar-based polymer and hydrogels. Carbohydr. Polym. 28, 15–21 (1995). https://doi.org/10.1016/0144-8617(95)00082-8
Kashyap, K., Kumar, N., Ravi Kumar, M.N.V.: Hydrogels for pharmaceutical and biomedical applications. Critic. Rev. Ther. Drug Carrier Sys. 22, 107–150 (2005). https://doi.org/https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v22.i2.10
Kaihara, S., Matsumura, S., Fisher, J.P.: Synthesis and characterization of cyclic acetal based degradable hydrogels. Eur. J. Pharm. Biopharm. 68, 67–73 (2008). https://doi.org/10.1016/j.ejpb.2007.05.019
Stamatialis, D.F., Papenburg, B.J., Girones, M., Saiful, S., Bettahalli, S.N.M., Schmitmeier, S., Wessling, M.: Medical applications of membranes: drug delivery, artificial organs and tissue engineering. J. Mem. Sci. 308(1–2), 1–34 (2008). https://doi.org/10.1016/j.memsci.2007.09.059
Zhang, X., Zhang, W., Yang, M.: Application of hydrogels in cartilage tissue engineering. Curr. Stem Cell Res. Ther. 13, 497–516 (2018). https://doi.org/10.2174/1574888X12666171017160323
Slaughter, B.V., Khurshid, S.S., Fisher, O.Z., Khademhosseini, A., Peppas, N.A.: Hydrogels in regenerative medicine. Adv. Mater. 21, 3307–3329 (2009). https://doi.org/10.1002/adma.200802106
Wang, F., Li, Z., Khan, M., Tamama, K., Kuppusamy, P., Wagner, W.R., Sen, C.K., Guan, J.: Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers. Acta Biomaterialia 6, 1978–1991 (2010). https://doi.org/10.1016/j.actbio.2009.12.011
Sikareepaisan, P., Ruktanonchai, U., Supaphol, P.: Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr. Polym. 83, 1457–1469 (2011). https://doi.org/10.1016/j.carbpol.2010.09.048
Krsko, P., McCann, T.E., Thach, T.T., Laabs, T.L., Geller, H.M.: Length-scale mediated adhesion and directed growth of neural cells by surface-patterned poly(ethylene glycol) hydrogels. Biomaterials 30, 721–729 (2009). https://doi.org/10.1016/j.biomaterials.2008.10.011
Mohamed, M.I.: Optimization of chlorphenes in emulgel formulation. AAPS J. 6, 1–7 (2004). https://doi.org/10.1208/aapsj060326
Ajazuddin, Alexander, A., Khichariya, A., Gupta, S., Patel, R.J., Giri, T.K., Tripathi, D.K.: Recent expansions in an emergent novel drug delivery technology. Emulgel. J. Controll. Rel. 171, 122–132 (2013). https://doi.org/10.1016/j.jconrel.2013.06.030
Sah, S.K., Badola, A., Nayak, B.K., Nayak, B.K.: Emulgel: magnifying the application of topical drug delivery. Ind. J. Pharm. Biol. Res. 5, 25–33 (2017). https://doi.org/10.30750/ijpbr.5.1.4
Baker, W.O.: Microgel, a new macromolecule. Relation to sol and gel as structural elements of synthetic rubber. Rubber Chem. Technol. 22(4), 511–520 (1949). https://doi.org/10.5254/1.3543023
Thorne, J.B., Vine, J.G., Snowden, M.J.: Microgel applications and commercial considerations. Colloid Polym. Sci. 289, 625–646 (2011). https://doi.org/10.1007/s00396-010-2369-5
Forsling, M.: Cells, gels and the engines of life. In: Pollack, G.H., pp. 320. Ebner and Sons Publishers (2001). ISBN 0 9626895 2 1. Exper. Physiol. 87(3), 401–401 (2002). https://doi.org/10.1113/eph8702381
Chowdary, K.P.R., Rao, Y.S.: Mucoadhesive microspheres for controlled drug delivery. Biol. Pharm. Bull. 27, 1717–1724 (2004). https://doi.org/10.1248/bpb.27.1717
Alpar, H.O., Somavarapu, S., Atuah, K.N., Bramwell, V.W.: Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 57, 411–430 (2005). https://doi.org/10.1016/j.addr.2004.09.004
Peppas, N.A., Huang, Y.: Nanoscale technology of mucoadhesive interactions. Adv. Drug Deliv. Rev. 56(11), 1675–1687 (2004). https://doi.org/10.1016/j.addr.2004.03.001
Lu, S., Liu, M., Ni, B.: Degradable, injectable poly(N-isopropylacrylamide)-based hydrogels with low gelation concentrations for protein delivery application. Chem. Eng. J. 173, 241–250 (2011). https://doi.org/10.1016/j.cej.2011.07.052
Sahiner, N., Ozay, O., Aktas, N.: 4-Vinylpyridine-based smart nanoparticles with N-isopropylacrylamide, 2-hydroxyethyl methacrylate, acrylic acid, and methacrylic acid for potential biomedical applications. Curr. Nanosci. 7, 453–462 (2011). https://doi.org/10.2174/157341311795542507
Cirillo, G., Iemma, F., Puoci, F., Parisi, O.I., Curcio, M., Spizzirri, U.G., Picci, N.: Imprinted hydrophilic nanospheres as drug delivery systems for 5-fluorouracil sustained release. J. Drug Target 17, 72–77 (2009). https://doi.org/10.1080/10611860802455813
Gomez, C., Benito, M., Katime, I., Teijon, J.M., Blanco, M.D.: In vitro transdermal and biological evaluation of ALA-loaded poly(N-isopropylacrylamide) and poly(N-isopropylacrylamideco-acrylic acid) microgels for photodynamic therapy. J. Microencapsul. 29, 626–635 (2012). https://doi.org/10.3109/02652048.2012.676091
Soni, K.S., Desale, S.S., Bronich, T.K.: Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Controll. Rel., 1–14 (2015). https://doi.org/10.1016/j.jconrel.2015.11.009
Rolland, J.P., Maynor, B.W., Euliss, L.E., Exner, A.E., Denison, G.M., DeSimone, J.M.: Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 127(28), 10096–10100 (2005). https://doi.org/10.1021/ja051977c
Morimoto, N., Qiu, X.P., Winnik, F.M., Akiyoshi, K.: Dual stimuli-responsive nanogels by self-assembly of polysaccharides lightly Grafted with thiol-terminated poly(N-isopropylacrylamide) chains. Macromolecules 41, 5985–5987 (2008). https://doi.org/10.1021/ma801332x
Rijcken, C.J., Soga, O., Hennink, W.E., van Nostrum, C.F.: Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: an attractive tool for drug delivery. J. Control Rel. 120(3), 131–148 (2007). https://doi.org/10.1016/j.jconrel.2007.03.023
Yin, Q.Q., Wu, L., Gou, M.L., Qian, Z.Y., Zhang, W.S., Liu, J.: Long-lasting infiltration anaesthesia by lidocaine-loaded biodegradable nanoparticles in hydrogel in rats. Acta Anaesthesiol. Scand. 53(9), 1207–1213 (2009). https://doi.org/10.1111/j.1399-6576.2009.02030.x
Ikeda, K., Okada, T., Sawada, S., Akiyoshi, K., Matsuzaki, K.: Inhibition of the formation of amyloid beta-protein fibrils using biocompatible nanogels as artificial chaperones. FEBS Lett. 580(28–29), 6587–6595 (2006). https://doi.org/10.1016/j.febslet.2006.11.009
Lee, J.I., Kim, H.S., Yoo, H.S.: DNA nanogels composed of chitosan and Pluronic with thermo-sensitive and photo-crosslinking properties. Int. J. Pharm. 373(1–2), 93–99 (2009). https://doi.org/10.1016/j.ijpharm.2009.01.016
Vinogradov, S.V., Bronich, T.K., Kabanov, A.V.: Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 54(1), 135–147 (2002). https://doi.org/10.1016/s0169-409x(01)00245-9
Schmitt, F., Lagopoulos, L., Käuper, P., Rossi, N., Busso, N., Barge, J., Wagnières, G., Laue, C., Wandrey, C., Juillerat-Jeanneret, L.: Chitosan-based nanogels for selective delivery of photosensitizers to macrophages and improved retention in and therapy of articular joints. J. Control Rel. 144(2), 242–250 (2010). https://doi.org/10.1016/j.jconrel.2010.02.008
Miyazaki, S., Aoyama, H., Kawasaki, N., Kubo, W., Attwood, D.: In situ-gelling gellan formulations as vehicles for oral drug delivery. J. Control Rel. 60(2–3), 287–295 (1999). https://doi.org/10.1016/s0168-3659(99)00084-x
Bajpai, V.: In situ gel nasal drug delivery system-a review. Int. J. Pharm. Sci. 4(3), 577–580 (2014)
Cho, E., Gwak, H., Chun, I.: Formulation and evaluation of ondansetron nasal delivery systems. Int. J. Pharm. 349(1–2), 101–107 (2008). https://doi.org/10.1016/j.ijpharm.2007.07.028
Hosny, E.A., Elkheshen, S.A., Saleh, S.I.: Buccoadhesive tablets for insulin delivery: in-vitro and in-vivo studies. Boll. Chim. Farm. 141(3), 210–217 (2002)
Makwana, S.B., Patel, V.A., Parmar, S.J.: Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma. Sci. 6, 1–6 (2015). https://doi.org/10.1016/j.rinphs.2015.06.001
Miyazaki, S., Suzuki, S., Kawasaki, N., Endo, K., Takahashi, A., Attwood, D.: In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int. J. Pharm. 229, 29–36 (2001). https://doi.org/10.1016/s0378-5173(01)00825-0
Clark, A.H.: Biopolymer gels. Curr. Opin. Colloid Int. Sci. 1, 712–717 (1996). https://doi.org/10.1016/S1359-0294(96)80072-0
Stading, M., Langton, M., Hermansson, A.M.: Small and large deformation studies of protein gels. J. Rheol. 39, 1445–1450 (1995). https://doi.org/10.1122/1.550646
Mishra, S., Thombare, N., Ali, M., Swami, S.: Applications of biopolymeric gels in agricultural sector. In: Vijay Kumar, T., Manju Kumari, T., Stefan Ioan, V. (eds.) Polymer Gels. Springer Publicaton, pp. 185–228 (2018)
Silva, K.C.G., Sato, A.C.K.: Biopolymer gels containing fructooligosaccharides. Food Res. Int. 101, 88–95 (2017). https://doi.org/10.1016/j.foodres.2017.08.042
Buruiana, L.I., Ioan, S.: Polymer gel composites for bio applications. In: Vijay Kumar, T., Manju Kumari, T., Stefan Ioan, V. (eds.) Polymer Gels. Springer Publicaton, pp. 111–123 (2018)
Pandey, S., Senthilguru, K., Uvanesh, K., Sagiri, S.S., Behera, B., Babu, N., Bhattacharyya, M.K., Banerjee, P.K.: Natural gum modified emulsion gel as single carrier for the oral delivery of probiotic-drug combination. Int. J. Biol. Macromol. 92, 504–514 (2016). https://doi.org/10.1016/j.ijbiomac.2016.07.053
Bhowmik, M., Kumari, P., Sarkar, G., Bain, M.K., Bhowmick, B., Mollick, M.M., Mondal, D., Maity D., Rana, D., Bhattacharjee, D., Chattopadhyay, D.: Effect of xanthan gum and guar gum on in situ gelling ophthalmic drug delivery system based on poloxamer-407. Int. J. Biol. Macromol. 62, 117–123 (2013). https://doi.org/10.1016/j.ijbiomac.2013.08.024
Wang, G.H., Zhang, L.M.: Manipulating formation and drug-release behavior of new sol-gel silica matrix by hydroxypropyl guar gum. J. Phys. Chem. B 111(36), 10665–10670 (2007). https://doi.org/10.1021/jp070370a
Hsu, C.S., Block, L.H.: Anionic gels as vehicles for electrically-modulated drug delivery. I. Solvent and drug transport phenomena. Pharm. Res. 13(12), 1865–1870 (1996). https://doi.org/10.1023/a:1016045427545
Samia, O., Hanan, R., el Kamal, T.: Carbamazepine mucoadhesive nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv. 19(1), 58–67 (2012). https://doi.org/10.3109/10717544.2011.644349
Gupta, R., Pandit, N., Aggarwal, S., Verma, A.: Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J. Contemp. Dent. Pract. 9(7), 25–32 (2008) (PMID: 18997913)
Pongjanyakul, T., Puttipipatkhachorn, S.: Xanthan-alginate composite gel beads: molecular interaction and in vitro characterization. Int. J. Pharm. 331(1), 61–71 (2007). https://doi.org/10.1016/j.ijpharm.2006.09.011
Horvat, G., Xhanari, K., Finšgar, M., Gradišnik, L., Maver, U., Knez, Ž., Novak, Z.: Novel ethanol-induced pectin-xanthan aerogel coatings for orthopedic applications. Carbohydr. Polym. 166, 365–376 (2017). https://doi.org/10.1016/j.carbpol.2017.03.008
Paul, A., Fathima, K.M., Nair, S.C.: Intra nasal in situ gelling system of lamotrigine using ion activated mucoadhesive polymer. Open Med. Chem. J. 11, 222–244 (2017). https://doi.org/10.2174/1874104501711010222
Bertram, U., Bodmeier, R.: In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form. Eur. J. Pharm. Sci. 27(1), 62–71 (2006). https://doi.org/10.1016/j.ejps.2005.08.005
Coviello, T., Trotta, A.M., Marianecci, C., Carafa, M., Di Marzio, L., Rinaldi, F., Di Meo, C., Alhaique, F., Matricardi, P.: Gel-embedded niosomes: preparation, characterization and release studies of a new system for topical drug delivery. Colloids Surf. B Biointerf. 125, 291–299 (2015). https://doi.org/10.1016/j.colsurfb.2014.10.060
Sherafudeen, S.P., Vasantha, P.V.: Development and evaluation of in situ nasal gel formulations of loratadine. Res. Pharm. Sci. 10(6), 466–476 (2015)
Shinde, U.A., Modani, S.H., Singh, K.H.: Design and development of repaglinide microemulsion gel for transdermal delivery. AAPS Pharm. Sci. Tech. 19(1), 315–325 (2018). https://doi.org/10.1208/s12249-017-0811-4
Phaechamud, T., Senarat, S., Puyathorn, N., Praphanwittaya, P.: Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and-microparticle. Int. J. Biol. Macromol. 135, 1261–1272 (2019). https://doi.org/10.1016/j.ijbiomac.2018.11.098
Srichan, T., Phaechamud, T.: Designing solvent exchange-induced in situ forming gel from aqueous insoluble polymers as matrix base for periodontitis treatment. AAPS Pharm. Sci. Tech. 18(1), 194–201 (2017). https://doi.org/10.1208/s12249-016-0507-1
Patel, A.R., Dewettinck, K.: Comparative evaluation of structured oil systems: shellac oleogel, HPMC oleogel, and HIPE gel. Eur. J. Lipid. Sci. Technol. 117(11), 1772–1781 (2015). https://doi.org/10.1002/ejlt.201400553
Rodríguez-Dorado, R., López-Iglesias, C., García-González, C.A., Auriemma, G., Aquino, R.P., Del Gaudio, P.: Design of aerogels, cryogels and xerogels of alginate: effect of molecular weight, gelation conditions and drying method on particles’ micromeritics. Molecules 24(6), 1049 (2019). https://doi.org/10.3390/molecules24061049
Veres, P., Sebők, D., Dékány, I., Gurikov, P., Smirnova, I., Fábián, I., Kalmár, J.: A redox strategy to tailor the release properties of Fe(III)-alginate aerogels for oral drug delivery. Carbohydr. Polym. 188, 159–167 (2018). https://doi.org/10.1016/j.carbpol.2018.01.098
Gonçalves, V.S., Gurikov, P., Poejo, J., Matias, A.A., Heinrich, S., Duarte, C.M., Smirnova, I.: Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 107, 160–170 (2016). https://doi.org/10.1016/j.ejpb.2016.07.003
García-González, C.A., Jin, M., Gerth, J., Alvarez-Lorenzo, C., Smirnova, I.: Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 117, 797–806 (2015). https://doi.org/10.1016/j.carbpol.2014.10.045
Ilka, R., Mohseni, M., Kianirad, M., Naseripour, M., Ashtari, K., Mehravi, B.: Nanogel-based natural polymers as smart carriers for the controlled delivery of timolol maleate through the cornea for glaucoma. Int. J. Biol. Macromol. 109, 955–962 (2018). https://doi.org/10.1016/j.ijbiomac.2017.11.090
Peng, N., Ding, X., Wang, Z., Cheng, Y., Gong, Z., Xu, X., Gao, X., Cai, Q., Huang, S., Liu, Y.: Novel dual responsive alginate-based magnetic nanogels for onco-theranostics. Carbohydr. Polym. 204, 32–41 (2019). https://doi.org/10.1016/j.carbpol.2018.09.084
Hong, S.H., Li, Y., Eom, J.B., Choi, Y.: Responsive alginate-cisplatin nanogels for selective imaging and combined chemo/radio therapy of proliferating macrophages. Quant. Imag. Med. Surg. 8(8), 733–742 (2018). https://doi.org/10.21037/qims.2018.09.01
Xu, X., Wang, X., Luo, W., Qian, Q., Li, Q., Han, B., Li, Y.: Triple cell-responsive nanogels for delivery of drug into cancer cells. Colloids Surf. B Biointerf. 163, 362–368 (2018). https://doi.org/10.1016/j.colsurfb.2017.12.047
Ji, P., Zhou, B., Zhan, Y., Wang, Y., Zhang, Y., Li, Y., He, P.: Multistimulative nanogels with enhanced thermosensitivity for intracellular therapeutic delivery. ACS Appl. Mater, Interf. 9(45), 39143–39151 (2017). https://doi.org/10.1021/acsami.7b08209
Wu, S.Y., Debele, T.A., Kao, Y.C., Tsai, H.C.: Synthesis and characterization of dual-sensitive fluorescent nanogels for enhancing drug delivery and tracking intracellular drug delivery. Int. J. Mol. Sci. 18(5), 1090 (2017). https://doi.org/10.3390/ijms18051090
Maciel, D., Figueira, P., Xiao, S., Hu, D., Shi, X., Rodrigues, J., Tomás, H., Li, Y.: Redox-responsive alginate nanogels with enhanced anticancer cytotoxicity. Biomacromol 14(9), 3140–3146 (2013). https://doi.org/10.1021/bm400768m
Hosseinifar, T., Sheybani, S., Abdouss, M., Hassani Najafabadi, S.A., Shafiee, A.M.: Pressure responsive nanogel base on alginate-cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J. Biomed. Mater. Res. A 106(2), 349–359 (2018). https://doi.org/10.1002/jbm.a.36242
Sarika, P.R., Anil Kumar, P.R., Raj, D.K., James, N.R.: Nanogels based on alginic aldehyde and gelatin by inverse miniemulsion technique: synthesis and characterization. Carbohydr. Polym. 119, 118–125 (2015). https://doi.org/10.1016/j.carbpol.2014.11.037
Sarika, P.R., James, N.R., Anil Kumar, P.R., Raj, D.K.: Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde-gelatin nanogels. Mater. Sci. Eng. C Mater. Biol. Appl. 68, 251–257 (2016). https://doi.org/10.1016/j.msec.2016.05.046
Bazban-Shotorbani, S., Dashtimoghadam, E., Karkhaneh, A., Hasani-Sadrabadi, M.M., Jacob, K.I.: Microfluidic directed synthesis of alginate nanogels with tunable pore size for efficient protein delivery. Langmuir 32(19), 4996–5003 (2016). https://doi.org/10.1021/acs.langmuir.5b04645
Hong, J.S., Vreeland, W.N., Lacerda, S.H., Locascio, L.E., Gaitan, M., Raghavan, S.R.: Liposome-templated supramolecular assembly of responsive alginate nanogels. Langmuir 24(8), 4092–4096 (2008). https://doi.org/10.1021/la7031219
Honardar, S., Kordestani, S.S., Daliri, M., NayebHabib, F.: The effect of chitosan-based gel on second degree burn wounds. J. Wound Care 25(8), 488–494 (2016). https://doi.org/10.12968/jowc.2016.25.8.488
Balakrishnan, B., Soman, D., Payanam, U., Laurent, A., Labarre, D., Jayakrishnan, A.: A novel injectable tissue adhesive based on oxidized dextran and chitosan. Acta Biomater. 53, 343–354 (2017). https://doi.org/10.1016/j.actbio.2017.01.065
Yao, Y., Xia, M., Wang, H., Li, G., Shen, H., Ji, G., Meng, Q., Xie, Y.: Preparation and evaluation of chitosan-based nanogels/gels for oral delivery of myricetin. Eur. J. Pharm. Sci. 25(91), 144–153 (2016). https://doi.org/10.1016/j.ejps.2016.06.014
Senyiğit, Z.A., Karavana, S.Y., Eraç, B., Gürsel, O., Limoncu, M.H., Baloğlu, E.: Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 64(2), 139–156 (2014). https://doi.org/10.2478/acph-2014-0013
Oh, D.W., Kang, J.H., Lee, H.J., Han, S.D., Kang, M.H., Kwon, Y.H., Jun, J.H., Kim, D.W., Rhee, Y.S., Kim, J.Y., Park, E.S., Park, C.W.: Formulation and in vitro/in vivo evaluation of chitosan-based film forming gel containing ketoprofen. Drug Deliv. 24(1), 1056–1066 (2017). https://doi.org/10.1080/10717544.2017.1346001
Kim, J.Y., Jun, J.H., Kim, S.J., Hwang, K.M., Choi, S.R., Han, S.D., Son, M.W., Park, E.S.: Wound healing efficacy of a chitosan-based film-forming gel containing tyrothricin in various rat wound models. Arch. Pharm. Res. 38(2), 229–238 (2015). https://doi.org/10.1007/s12272-014-0368-7
Garcia, M., de Paula Freitas, C., Graciano, T.B., Coutinho, T.S., Cressoni, C.B., de Lima Pereira, S.A., Shimano, M.M.: Chitosan-based mucoadhesive gel for oral mucosal toluidine blue O delivery: The influence of a non-ionic surfactant. Photodiagnosis Photodyn. Ther. 20, 48–54 (2017). https://doi.org/10.1016/j.pdpdt.2017.08.009
Thomas, L., Zakir, F., Mirza, M.A., Anwer, M.K., Ahmad, F.J., Iqbal, Z.: Development of curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol. Macromol. 101, 569–579 (2017). https://doi.org/10.1016/j.ijbiomac.2017.03.066
Hoseinpour Najar, M., Minaiyan, M., Taheri, A.: Preparation and in vivo evaluation of a novel gel-based wound dressing using arginine-alginate surface-modified chitosan nanofibers. J. Biomater. Appl. 32(6), 689–701 (2018). https://doi.org/10.1177/0885328217739562
Nithya, S., Nimal, T.R., Baranwal, G., Suresh, M.K., Anil Kumar, C.P.A., Gopi, V., Mohan, C., Jayakumar, R., Biswas, R.: Preparation, characterization and efficacy of lysostaphin-chitosan gel against Staphylococcus aureus. Int. J. Biol. Macromol. 2018110, 157–166. https://doi.org/10.1016/j.ijbiomac.2018.01.083
Ajish, J.K., Ajish Kumar, K.S., Chattopadhyay, S., Kumar, M.: Glycopolymeric gel stabilized N-succinyl chitosan beads for controlled doxorubicin delivery. Carbohydr. Polym. 144, 98–105 (2016). https://doi.org/10.1016/j.carbpol.2016.01.067
Sohrabi, S., Haeri, A., Mahboubi, A., Mortazavi, A., Dadashzadeh, S.: Chitosan gel-embedded moxifloxacin niosomes: an efficient antimicrobial hybrid system for burn infection. Int. J. Biol. Macromol. 85, 625–633 (2016). https://doi.org/10.1016/j.ijbiomac.2016.01.013
Frank, L.A., Sandri, G., D'Autilia, F., Contri, R.V., Bonferoni, M.C., Caramella, C., Frank, A.G., Pohlmann, A.R., Guterres, S.S.: Chitosan gel containing polymeric nanocapsules: a new formulation for vaginal drug delivery. Int. J. Nanomed. 9, 3151–3161 (2014). Published 2014 June 28. https://doi.org/10.2147/IJN.S62599
Morán, M.C., Jorge, A.F., Vinardell, M.P.: Sustainable DNA release from chitosan/protein based-DNA gel particles. Biomacromol 15(11), 3953–3964 (2014). https://doi.org/10.1021/bm501039g
Johnston, T.P., Miller, S.C.: Inulin disposition following intramuscular administration of an inulin/poloxamer gel matrix. J. Parenter Sci. Technol. 43(6), 279–286 (1989)
Carrera, G.V.S.M., Raymundo, A., Fernandes, F.M.B., Jordão, N., Sousa, I., da Ponte, M.N., Branco, L.C.: Tetramethylguanidine-based gels and colloids of cellulose. Carbohydr. Polym. 169, 58–64 (2017). https://doi.org/10.1016/j.carbpol.2017.03.084
Demilecamps, A., Beauger, C., Hildenbrand, C., Rigacci, A., Budtova, T.: Cellulose-silica aerogels. Carbohydr. Polym. 122, 293–300 (2015). https://doi.org/10.1016/j.carbpol.2015.01.022
Rai, V.K., Yadav, N.P., Sinha, P., Mishra, N., Luqman, S., Dwivedi, H., Kymonil, K.M., Saraf, S.A.: Development of cellulosic polymer based gel of novel ternary mixture of miconazole nitrate for buccal delivery. Carbohydr Polym. 103, 126–133 (2014). https://doi.org/10.1016/j.carbpol.2013.12.019
Keshavarz, M., Kaffashi, B.: The ability of retention, drug release and rheological properties of nanogel bioadhesives based on cellulose derivatives. Pharm. Dev. Technol. 19(8), 952–959 (2014). https://doi.org/10.3109/10837450.2013.846371
Martins, M.L., Calabresi, M.F., Quini, C., Matos, J.F., Miranda, J.R., Saeki, M.J., Bordallo, H.N.: Enhancing the versatility of alternate current biosusceptometry (ACB) through the synthesis of a dextrose-modified tracer and a magnetic muco-adhesive cellulose gel. Mater. Sci. Eng. C Mater. Biol. Appl. 48, 80–85 (2015). https://doi.org/10.1016/j.msec.2014.11.059
Celebi, D., Guy, R.H., Edler, K.J., Scott, J.L.: Ibuprofen delivery into and through the skin from novel oxidized cellulose-based gels and conventional topical formulations. Int. J. Pharm. 514(1), 238–243 (2016). https://doi.org/10.1016/j.ijpharm.2016.09.028
Schestakow, M., Karadagli, I., Ratke, L.: Cellulose aerogels prepared from an aqueous zinc chloride salt hydrate melt. Carbohydr. Polym. 137, 642–649 (2016). https://doi.org/10.1016/j.carbpol.2015.10.097
Ji, J.A., Ingham, E., Wang, J.Y.: Effect of EDTA and methionine on preventing loss of viscosity of cellulose-based topical gel. AAPS Pharm. Sci. Tech. 10(2), 678–683 (2009). https://doi.org/10.1208/s12249-009-9258-6
Kim, U.J., Kuga, S.: Ion-exchange separation of proteins by polyallylamine-grafted cellulose gel. J. Chromatogr. A 955(2), 191–196 (2002). https://doi.org/10.1016/s0021-9673(02)00246-7
Gallego, R., Arteaga, J.F., Valencia, C., Franco, J.M.: Rheology and thermal degradation of isocyanate-functionalized methyl cellulose-based oleogels. Carbohydr. Polym. 98(1), 152–160 (2013). https://doi.org/10.1016/j.carbpol.2013.04.104
Calejo, M.T., Cardoso, A.M., Marques, E.F., Araújo, M.J., Kjøniksen, A.L., Sande, S.A., de Lima, M.C., Jurado, A.S., Nyström, B.: In vitro cytotoxicity of a thermoresponsive gel system combining ethyl(hydroxyethyl) cellulose and lysine-based surfactants. Colloids Surf. B Biointerf. 102, 682–686 (2013). https://doi.org/10.1016/j.colsurfb.2012.09.033
Van Damme, L., Govinden, R., Mirembe, F.M., Guédou, F., Solomon, S., Becker, M.L., Pradeep, B.S., Krishnan, A.K., Alary, M., Pande, B., Ramjee, G., Deese, J., Crucitti, T., Taylor, D.: CS study group. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission [published correction appears in N. Engl. J. Med. 359(8), 877, 2008 Aug 21]. N. Engl. J. Med. 359(5), 463–472 (2008). https://doi.org/10.1056/NEJMoa0707957
Hauptstein, S., Hintzen, F., Müller, C., Ohm, M., Bernkop-Schnürch, A.: Development and in vitro evaluation of a buccal drug delivery system based on preactivated thiolated pectin. Drug Dev. Ind. Pharm. 40(11), 1530–1537 (2014). https://doi.org/10.3109/03639045.2013.836213
Günter, E.A., Markov, P.A., Melekhin, A.K., Belozerov, V.S., Martinson, E.A., Litvinets, S.G., Popov, S.V.: Preparation and release characteristics of mesalazine loaded calcium pectin-silica gel beads based on callus cultures pectins for colon-targeted drug delivery. Int. J. Biol. Macromol. 120(Pt B), 2225–2233 (2018). https://doi.org/10.1016/j.ijbiomac.2018.07.078
Gunter, E.A., Melekhin, A.K.: Prikl Biokhim Mikrobiol. 51(1), 59–64 (2015). https://doi.org/10.7868/s0555109915010055
Liu, J., Zhang, L., Jia, Y., Hu, W., Zhang, J., Jiang, H.: Preparation and evaluation of pectin-based colon-specific pulsatile capsule in vitro and in vivo. Arch. Pharm. Res. 35(11), 1927–1934 (2012). https://doi.org/10.1007/s12272-012-1109-4
Preibisch, I., Niemeyer, P., Yusufoglu, Y., Gurikov, P., Milow, B., Smirnova, I.: Polysaccharide-based aerogel bead production via jet cutting method. Materials (Basel) 11(8), 1287 (2018). https://doi.org/10.3390/ma11081287
El-Gibaly, I.: Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int. J. Pharm. 232(1–2), 199–211 (2002). https://doi.org/10.1016/s0378-5173(01)00903-6
Yu, L.L., Jiang, L.N., Wang, S.Y., Sun, M.M., Li, D.Q., Du, G.M.: Pectin microgel particles as high adsorption rate material for methylene blue: Performance, equilibrium, kinetic, mechanism and regeneration studies. Int. J. Biol. Macromol. 112, 383–389 (2018). https://doi.org/10.1016/j.ijbiomac.2018.01.193
Chen, K., Zhang, H.: Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 136, 936–943 (2019). https://doi.org/10.1016/j.ijbiomac.2019.06.138
Günter, E.A., Popeyko, O.V., Istomina, E.I.: Encapsulated drug system based on the gels obtained from callus cultures modified pectins. J. Biotechnol. 289, 7–14 (2019). https://doi.org/10.1016/j.jbiotec.2018.11.005
Chen, H., Gan, J., Ji, A., Song, S., Yin, L.: Development of double network gels based on soy protein isolate and sugar beet pectin induced by thermal treatment and laccase catalysis. Food Chem. 292, 188–196 (2019). https://doi.org/10.1016/j.foodchem.2019.04.059
Sriamornsak, P., Asavapichayont, P., Nunthanid, J., Luangtana-Anan, M., Limmatvapirat, S., Piriyaprasarth, S.: Wax-incorporated emulsion gel beads of calcium pectinate for intragastric floating drug delivery. AAPS Pharm. Sci. Tech. 9(2), 571–576 (2008). https://doi.org/10.1208/s12249-008-9082-4
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kirtania, M.D., Kahali, N., Maity, A. (2020). Biopolymeric Gels in Drug Delivery. In: Nayak, A., Hasnain, M. (eds) Advanced Biopolymeric Systems for Drug Delivery. Advances in Material Research and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-46923-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-46923-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46922-1
Online ISBN: 978-3-030-46923-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)