Abstract
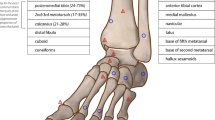
Stress fractures of the ankle and hindfoot are common injuries in athletes. Most of these fractures can be treated nonoperatively, but certain factors including fracture location, vascular supply, lower leg alignment, and return to play considerations may require surgical intervention. Stress fractures of the ankle and hindfoot can also be classified as low risk and high risk in regard to propensity to heal with conservative treatment. Generally, low-risk fractures include the distal tibia, distal fibula, talus, and calcaneus. High-risk fractures include the medial malleolus and navicular. The treating health-care provider should have a low index for suspicion for stress fracture and be able to identify risk factors in regard to bone health, structural alignment, and training/competing issues in order to achieve a quick recovery and minimize the chance for recurrence of injury.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Stress fractures of the ankle and hindfoot are injuries that require a high index of suspicion. Bone stress injuries account for 15% of all musculoskeletal foot and ankle injuries in elite collegiate athletes [1] and 10% of injuries in recreational and competitive athletes [2].
Stress fractures of the ankle and hindfoot can be a result of intrinsic and extrinsic factors. Intrinsic factors relate to the patient’s anatomy and biology. Females have been shown to have a higher incidence of stress fracture with a reported incidence of 3% versus 9.2% in males in military populations and 6.5% versus 9.7% in athletes [3]. Other intrinsic factors can include poor bone density, vascular supply, foot structure such as cavus feet or forefoot varus, tarsal coalition, hormonal imbalances, or heel cord contractures. Extrinsic factors can include training regimen, specific sport, improper footwear, and exercise terrain. Intrinsic factors are more difficult to modify than extrinsic factors.
The clinical evaluation of patients with a potential stress fracture should start with a thorough history and physical examination. Most fractures can be attributed to a recent change in training regimen or shoe wear. Athletes will typically complain of an insidious onset of pain or swelling over the past few weeks and can sometimes be difficult for the athlete to localize. The pain is usually activity related and relieved by rest. A thorough history should include recent training, diet, and any risk factors for low bone density. Physical examination starts with a weight-bearing assessment of both lower extremities for alignment and comparison of any differences in swelling. A single-limbed heel rise can help localize the anatomic area of pain. Inspection of gait, range of motion, and strength testing is performed. Tenderness to palpation is not always indicative of location of stress injury. Standing radiographs of the foot or ankle are often negative if symptoms are less than a few weeks old, with a sensitivity of 10% reported for the detection of stress injury at initial presentation [4], which increases up to 30–70% after 3 weeks [5]. Radiographic findings are dependent on the chronicity, specific bone involved, and even location within each bone. If a stress fracture occurs in cancellous bone, such as the calcaneus, initial radiographic finding is a faint trabecular sclerosis due to microcallus formation [6]. In contrast, if the cortex of a long bone is involved, the initial radiographic finding is a subtle cortical lucency followed later by a periosteal reaction and endosteal callous formation [6]. In more high-grade injuries, a frank cortical break will be evident. In chronic presentation, evidence of sclerosis can be seen at fracture line on radiographs. However, often these fractures are difficult to visualize radiographically and may result in a delay in diagnosis.
The decision to proceed with further imaging if radiographs are negative is dependent on the specific suspected fracture and the potential for altering the treatment plan. For instance, a suspected calcaneal stress fracture can likely be managed with a walking boot and follow-up with repeat radiographs in 2 weeks which by then will likely be positive. However, for a suspected navicular stress fracture in an athlete, further imaging would be recommended to further clarify the extent of injury to determine whether surgical intervention is indicated.
MRI is the preferred imaging technique when initial radiographs are negative. MRI findings of stress fracture include periosteal and bone marrow edema, with intracortical signal changes or intramedullary, low-signal intensity fracture line only able to be visualized relatively late in the pathogenesis of stress fractures [6]. A stress reaction represents a clinical syndrome thought to be due to early accumulation of microdamage and likely represents an early stress injury [6]. The MRI findings of stress reaction include bone marrow edema like signal without a distinct fracture line. A stress reaction becomes a stress fracture once a cortical break develops. Not all bone marrow edema, however, predisposes athletes to later stress fracture. One study of 21 asymptomatic college distance runners demonstrated a 43% incidence of bone marrow edema on MRI [7].
CT scans are useful in distinguishing a fracture line better than MRI, and in fractures of the medial malleolus and navicular, they can help determine the need for surgical intervention. Ultrasound imaging is becoming more assessable in the office setting and can identify a cortical break, but evaluation of marrow space is not possible.
Medial Malleolus
Stress fractures of the medial malleolus are relatively uncommon injuries, accounting for only 0.6–4.1% of all lower extremity fractures [8]. Shelbourne first described stress fractures of the medial malleolus with the presentation of chronic or subacute pain over the medial malleolus, tenderness to palpation along the medial ankle, and a history of running activity at the time of injury or running activities aggravating the pain [9]. Because athletes with this injury often present with nonspecific ankle pain and normal radiographs, clinicians should include this fracture in their differential diagnosis of medial ankle pain in the running or jumping athlete. Failure to diagnose in the athlete can result in fracture progression, nonunion, chronic pain, and extended delay in return to athletic activity. Radiographs may appear normal for up to 2 months after symptoms appear [10]. When there is clinical suspicion of a medial malleolar stress fracture with normal-appearing radiographs, MRI is recommended for further imaging evaluation which typically shows bone marrow edema localized to the medial malleolus. CT scan is then helpful to determine whether a fracture line is present in the setting of extensive medial malleolar edema and can help in surgical planning (Fig. 18.1). The majority of medial malleolar stress fractures are vertically oriented, and the fracture line typically extends proximately from the junction of the tibial plafond and medial malleolus [11]. Jowett and colleagues indicated that a major intrinsic risk factor in professional athletes is the presence of anteromedial distal tibial osteophytes [12]. The location of these osteophytes was shown in an anatomic study to involve the non-weight-bearing anteromedial cartilage of the distal tibia, which extends up to 3 mm proximal to the tibiotalar joint line [13]. The initiation of these osteophytes is thought to be caused by repetitive trauma to the cartilage, which then responds by the formation of scar tissue and subsequent calcification [14]. Damage to this cartilage can also be caused by supination trauma, particularly on the medial side in the case of ankle instability, and can lead to osteophyte formation [15]. These bone growths are theorized to impart rotational forces to the medial malleolus during end dorsiflexion of the ankle. Foot alignment, specifically cavus foot, has been a proposed intrinsic risk factor for medial malleolar stress fractures by transferring similar forces to the medial tibia. Medial malleolar stress fractures can be treated either conservatively with immobilization and reduction in weight-bearing activities or with surgery. However, conservative treatment has been associated with prolonged healing times and tendency toward nonunion [16]. With conservative treatment, fracture union and return to full activity can take as long as 6 months. With surgical treatment, return to play can be expected as early as 3 months following surgery [18]. Historically, the operative treatment of medial malleolar fractures has been placing two cancellous screws perpendicular to the fracture line to prevent superior displacement of the fracture. A recent study has found that an anti-glide plate construct provides the stiffest initial fixation while withstanding higher load to failure for vertical medial malleolar fractures when compared to unicortical and bicortical screw fixation alone [17]. The senior author O’Malley has recently reported on the results of six professional basketball players treated with surgical fixation and iliac crest bone marrow aspirate graft, with five of the six players able to return to play by the 12th postoperative week [18] (Fig. 18.2). Additionally, it is important to address any tibial or talar osteophytes arthroscopically or by open treatment in addition to fixation of the fracture. Calder reported on 16 professional soccer players treated with surgical fixation and arthroscopic osteophyte debridement [19]. All the 16 patients had bone osteophytes on the tibia and/or talus. Ten patients had spurs on both the tibia and the talus, while six patients had isolated tibial spurs.
Distal Tibia
The posterior medial tibial shaft is the most common location for stress fractures, most typically reported in military recruits and running athletes. Hard surfaces pose a higher risk for stress fractures [20]. Tibial strain rates in runners were 48–285% higher when running over ground compared with running on treadmills [21]. Worn running shoes may increase the risk for stress fracture because of decreased shock absorption. A distal tibial stress injury can initiate as a stress reaction where no fracture line has developed and progress to a frank cortical fracture [10]. The location along the posterior medial tibia is a result of repetitive impaction and muscular forces. Compressive repetitive forces from the gastrocnemius-soleus complex and pull of the deep plantar flexors have been thought to be mechanical factors [22]. Athletes will report a pain along the medial distal tibia that is worse with impact. Typically, patients will have tenderness along the posterior medial distal tibia to palpation. Radiographs initially are normal or can show a subtle cortical lucency followed by periosteal reaction and cortical thickening [10] (Fig. 18.3). MRI is recommended with clinical suspicion of stress fracture and is often diagnostic (Fig. 18.4). Treatment is almost always conservative as these injuries have a high likelihood of healing with rest and immobilization. One study has looked at gait retraining to reduce lower extremity loading in runners, which resulted in 20% decrease in vertical force impact peak and 30% decrease in vertical force loading rates which were maintained at 1 month follow-up [23]. This decrease in forces may reduce their risk of tibial stress fractures. MRI grading is described according to the Fredricson classification (and Kijowski modifications) and can be helpful in estimating time to return to athletic activities [24]. The shortest time to return is in a grade 1 injury, which presents on MRI as a periosteal tibial edema with normal marrow signal, and results as a mean time of return of 16 days, compared to a grade 4B injury, which demonstrates a linear cortical fracture line, and the longest time of 71 days to return to play. Distal tibial stress fractures have also been reported in adolescent athletes as a stress fracture of the distal tibial physis, for example, in a 9-year old female gymnast and dancer [25]. She was made non-weight-bearing for 6 weeks and then allowed to ambulate in a walking boot. She was not able to return to activities until 6 months after presentation. The distal tibial stress fracture is different from the anterior cortical tibial stress fracture which is described elsewhere in this book.
Distal Fibula
Stress fractures of the distal fibula most commonly affect the lateral cortex of the fibula and are commonly reported in the military and athletic populations [26]. Distal fibular stress fractures have been reported in 6.6% of all stress fractures in athletes [27]. Stress fractures of the distal one-third of the fibular are more common than stress fractures of the proximal two-thirds with the majority of these fractures occurring 4–7 cm proximal to the lateral malleolus. Devas and Sweetham proposed that the mechanism of injury in these fractures was related to running on hard ground and that recurrent contraction of the plantar and long toe flexors transmitted stress through their origin on the fibula, approximating the fibula to the tibia and creating a bending moment that results in the stress injury [28]. Alternatively, it is thought that the area of the fibula just proximal to the syndesmotic ligaments is susceptible to increased forces of running and impact activities. Athletes will complain of lateral ankle pain, and the pain is most common after increasing or changing exercise regimen. The differential diagnosis includes peroneal tendon pathology and lateral ankle ligament injury. Initially radiographs are normal within the first 3–4 weeks of symptoms but then will show a periosteal reaction [10]. Intramedullary sclerosis, callous formation, or discrete fracture in a transverse pattern may be seen later (Fig. 18.5). Treatment is generally conservative with walking boot immobilization, and return to activity is usually in 6–8 weeks. MRI and further imaging often are often unnecessary unless one is concerned about tendon or ligament pathology but can confirm diagnosis (Fig. 18.6).
A separate mechanism exists for distal fibula stress fractures in the patient or athlete with a flat foot. Patients or athletes with a posterior tibial tendon dysfunction can result in a degeneration and elongation of the posterior tibial tendon, which then results in a flatfoot deformity. The lateralization of the load axis of the lower leg then contributes to weight-bearing across the fibula [29]. The fibula typically plays a secondary role in weight-bearing with approximately 6.4–17.2% of total body weight applied to the fibula [30]. One study demonstrated a lateral shift of contact area and peak pressure in a flatfoot model and suggested this causes a transfer of load off the talar dome [31]. The increased load in a flatfoot deformity concentrates stresses on the fibula and can lead to a stress injury. Initial treatment of these fractures is similar to the non-flatfoot fibular stress fracture, but longer-term treatment involves orthotic and shoe wear modifications and possible surgical intervention to address the posterior tibial tendon and foot deformity.
Talus
Stress injuries of the talus are relatively uncommon with mostly case reports in the literature. McGlone was the first to report on a stress fracture of the talus in 1965 [32]. The precise mechanism for stress injury to the talus is unclear. Proposed theories include the increased compression of the talar body against the navicular during pushoff [33] and excessive subtalar pronation and plantar flexion causing the lateral process of the calcaneus to impinge on the posterolateral corner of the talus [34]. One retrospective study reviewed MRI findings in military recruits with foot or ankle pain and reported that 51 recruits exhibited bone stress injuries in the talus during the study period of 96 months [34]. This yielded a person-based incidence of 4.4 per 10,000 person-years. Bilateral injuries were seen in five cases, and in 86% of the cases with talar bone edema, there was also bone marrow edema in other tarsal bones. The diagnosis can be difficult to make as the athlete typically will complain of a vague and nonspecific pain, and it is often difficult to elicit any focal tenderness on physical examination. Radiographs are usually unremarkable, and MRI typically demonstrates bone marrow edema (Fig. 18.7). Of the 56 bone stress injuries reported in military recruits, 40 occurred in the head, 15 in the body, and 5 in the posterior part of the talus [34]. The median time from the reported onset of pain to the date of diagnosis of talar stress injury on MRI was 62 days. Treatment of talar stress injuries is generally a walking boot and some period of non-weight-bearing if a fracture line is visible on imaging. Bone marrow edema, however, can be a nonspecific finding that can be present in infections, osteonecrosis, malignancies, and bone contusion. In an MRI study of 12 random professional ballet dancers, 75% demonstrated bone marrow edema of the talus [35]. Studies describing the incidence and outcome of actual stress fractures of the talus with demonstrable fracture line on MRI are even less common. In a study following eight military recruits with a talar fracture line visible on MRI, five had mild to moderate symptoms after a mean follow-up time of 45 months [36]. All recruits were treated with reduced activity or weight-bearing restrictions based on initial symptoms and were symptom free at an average of 64 days. Five patients displayed subchondral degeneration and edema near the original area in the follow-up MRI, and in two of these patients, the degeneration was also visible on the plain radiographs.
Stress fractures have also been described of the lateral process of the talus in a runner [37] and in a competitive tennis player [38]. Both athletes had a history of greater than 1 year of vague lateral foot pain and had multiple prior diagnoses. The runner had a supinated foot which has been shown to increase pressures along the lateral talus. Stress fracture of the talus has also been reported after resection of a talocalcaneal coalition with a new onset of medial ankle pain 3 months post-surgery [39].
Calcaneus
Stress fractures of the calcaneus are quite common and reportedly comprise up to 20% of all stress fractures of the foot [40]. They are often associated with running and jumping sports and are correlated with heel strike and non-cushioned shoe wear and hard training surfaces. The pull of the Achilles tendon insertion in resisting plantar flexion of the foot is also thought to contribute. The athlete will present with posterior heel pain, most often after an increase in training activity. The examination is usually positive for tenderness with simultaneous compression of both medial and lateral aspects of the calcaneus. The differential diagnosis can include insertional Achilles tendinopathy, plantar fasciitis, and distal tarsal tunnel syndrome. Calcaneal stress fracture has been reported in injuries observed in the minimalist runners [41]. Calcaneal stress fractures can be visualized on radiographs as soon as 10 days after the onset of symptoms and appear as a sclerotic line perpendicular to the trabeculae which run in arcs perpendicular to the posterior cortex of the calcaneus [10] (Fig. 18.8). MRI will demonstrate low signal intensity line with surrounding edema (Fig. 18.9). In an MRI study of military recruits, 26% of calcaneal stress fractures occurred in the anterior region of the calcaneus, 18% in the middle, and 56% in the posterior calcaneus [42]. A total of 79% occurred in the upper region of the bone and 21% in the lower region of the calcaneus. Fifty-nine percent of the injuries were of a higher grade with a fracture line that was visible on MRI. A total of 22 of the 30 cases were associated with stress injuries of the talus, navicular, or cuboid. Treatment is conservative and involves protected weight-bearing in walking boot until symptoms diminish which generally takes 6–8 weeks. With the high association of other associated stress injuries of the foot, treatment plans can be altered.
Stress fractures of the anterior process of the calcaneus are rare. There have been two reports associated with a calcaneonavicular coalition, with the lack of normal motion from a coalition leading to increased pressure along the anterior process. In one case, the bar was resected and a screw placed across the calcaneal stress fracture [43]. A case report of a 14-year-old female basketball player described a stress fracture of an elongated anterior process and was subsequently treated with drilling of the fracture after failure of conservative care [44].
Navicular
Stress fractures of the navicular are high-risk fractures commonly seen in track and field [45], tennis [46], and basketball athletes. First reported in the orthopedic literature by Towne in 1970 [47], navicular stress fractures have been described to account for almost 35% of all bone stress fractures [48]. These fractures can have significant effect on the athlete’s career. Anderson reported on players at the NFL combine with a history of navicular stress fracture, and overall only 28.6% of players with fracture played over 2 years in the NFL compared to 69.6% that did not have a navicular injury [49]. Talonavicular arthritis was present in 75% of athletes with injury.
The navicular is a saddle-shaped bone that articulates with the talus proximally and with the medial, middle, and lateral cuneiforms distally [48]. That poster tibial tendon inserts on the medial tuberosity, and the calcaneonavicular spring ligament inserts along the plantar beak. The foot can be divided into two parallel columns consisting of a more rigid medial column and a more flexible lateral column. The navicular is the keystone of the medial column and provides stability to the longitudinal and transverse charges of the foot [48].
The vascular supply to the navicular comprises medial tarsal branches of the dorsal pedis artery as well has branches from the superficial branch of the medial plantar artery. A recent cadaver study reported that 12% of specimens had an avascular region in the dorsal central third of the bone corresponding to the usual location of navicular stress fracture [50]. The navicular’s decreased vascularity in this region has implications for healing and can result in delayed healing, high risk of nonunion, and prolonged time out of sport. In addition to the vascular properties of the navicular, specific biomechanical properties are thought to contribute to stress fracture at the central one-third. It has been theorized that during the foot strike phase of running, compression forces are generated from distal to proximal across the medial and lateral aspects of the navicular through the first and second metatarsal cuneiforms joints [51]. The forces across the first metatarsal and medial cuneiform are shared by the talar head, where those forces across the second metatarsal and middle cuneiforms are not, and result in a sheer force at the central one-third of the navicular bone. Runners who demonstrate increased rearfoot eversion and reduced forefoot abduction during stance may be at risk of developing navicular stress fractures [52]. The presence of an os supranaviculare , an accessory ossicle at the proximal dorsal cortex of the navicular reported in 1% of individuals, has been implicated in the development of a navicular stress fracture [53]. The typical dorsal navicular depression under the os supranaviculare is localized at the area of maximal stress on the navicular and contributes to the propagation of stress fractures (Fig. 18.10). An osteochondral lesion of the tarsal navicular has also been reported with a stress fracture of navicular in high-level athletes [54].
Delay in diagnosis is common and has been reported up to 6 months on average and in a study by Saxena of up to 8.8 months [55]. Typically the athlete complains of a slow onset of vague medial and dorsal foot pain that radiates along the medial arch of the foot. The pain is worse with activity and generally relieved at rest. Running, jumping, and cutting activities exacerbate the symptoms. Runners often alter their gait to compensate for their pain, minimize their symptoms, and typically have a high threshold for pain.
On physical examination, there is no swelling of the foot, and athletes generally have a normal range of motion and strength. Tenderness to palpation of the central third of the navicular is called the “N” spot, and Torg described the tenderness to palpation in 81% of patients with navicular stress fractures [56]. A single leg heel rise or hop test often elicits pain along the midfoot.
Radiographs are often negative but can evaluate other causes of foot and ankle pain (Fig. 18.11). In a study by Saxena [57], only 2 out of the 22 patients had their fracture visible on plain X-ray. If initial radiographs are negative and there is clinical suspicion of a navicular stress fracture, then MRI is recommended. With a positive MRI for navicular stress fracture, a CT scan is indicated for further clarification of fracture line. Saxena proposed a CT classification and treatment scheme [57]. A type I fracture involves a dorsal cortical fracture of the navicular (Fig. 18.12). A type II fracture extends from the dorsal cortex into the navicular body. A type III fracture penetrates a second cortex (plantar, medial, or lateral.) They later added a type 0.5 to indicate stress reaction.
Treatment for navicular stress fractures in the athlete remains a topic of debate. Nonoperative treatment relies on immobilization and protected weight-bearing in a cast. Torg et al. treated 10 patients with non-weight-bearing cast for 6–8 weeks and had a 100% healing rate without complications, but with return to activity an average of 3–6 months [56]. In a cohort treated with a walking cast, 78% could not resume sports because of pain. Khan also demonstrated a significantly worse return to full activity in athletes who used a weight-bearing cast compared to non-weight-bearing cast [57]. Surgery has been proposed for nondisplaced fractures involving two cortices, displaced fractures, fractures with sclerotic changes, and athletes who failed conservative treatment or cannot tolerate a long recovery course. Saxena and Fulham [58] found that there were no clinical differences in those patients who were treated nonoperatively versus those who underwent surgery for fixation. Surgery was recommended for type II and III fractures, and return to activity was similar for both populations at 3.9 months. A meta-analysis that evaluated outcomes of navicular stress fractures treated with surgery versus non-surgical non-weight-bearing management concluded that there was no statistical significant difference [59]. Weight-bearing as a conservative treatment was shown to be significantly less effective than either non-weight-bearing or surgical treatment. Mallee reviewed 200 stress fractures of the navicular in athletes but did not perform a statistical analysis comparing success of immobilization. However, the researchers did note that the weighted mean time to return to sports was 16.4 weeks in those treated with surgery versus 21.7 weeks in patients treated conservatively with non-weight-bearing cast for greater than 6 weeks [60].
Surgery should be strongly considered with athletes with type II and III navicular stress fractures, especially those with cystic changes, sclerosis, or osteonecrosis. These fractures have a frequency of delayed union and refracture which can result in unpredictable healing times. A shortest time to return to play is important for athletes and is often the determining factor when deciding on treatment recommendations. Saxena described outcomes of navicular fractures in athletes using their protocol of non-weight-bearing for type I fractures, and surgery for type II and III fractures resulted in greater than 90% of athletes being able to return to activity at their preinjury level [54]. All 21 elite or professional athletes were able to return to activity. Patients who underwent open reduction and internal fixation had a return to activity of 4.56 months compared to those who had undergone a nonoperative treatment who had an average return to activity of 3.97 months.
For type I navicular stress fractures treated surgically, percutaneous fixation with solid screw (but cannulated technique) placed lateral to medial is recommended (Fig. 18.13). For type II and III fractures, an open dorsal approach with autograft bone, iliac bone marrow aspirate, and two screws placed lateral to medial through a separate lateral incision can be utilized (Fig. 18.14). The screws should be placed perpendicular to the fracture line, and intraoperative CT scan can aid in technique if available. For refractures or nonunions, a localized bone graft technique that was described by Nunley should be performed [61] (Figs. 18.15 and 18.16).
References
Hunt KJ, Hurwit D, Robel K, Gatewood C, Botser IB, Matheson G. Incidence and epidemiology of foot and ankle injuries in elite collegiate athletes. Am J Sports Med. 2016;45(2):426–33.
Changstrom BG, Brou L, Khodaee M, Braund C, Comstock D. Epidemiology of stress injuries among US high school athletes, 2005–2006 through 2012–2013. Am J Sports Med. 2014;43(1):26–33.
Wentz L, Liu P, Haymes E, Ilich J. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med. 2011;176(4):420–30.
Matherson O. Stress fractures in athletes: a study of 320 cases. Am J Sports Med. 1987;15:46–58.
Ishibashi Y, Okamura Y, Otsuka H. Comparison of scintigraphy and magnetic resonance imaging for stress injuries of bone. Clin J Sports Med. 2002;12:79–84.
Mandell JC, Khurana B, Smith SE. Stress fractures of the foot and ankle, part1: biomechanics of bone and principles of imaging and treatment. Skelet Radiol. 2017;46:1021–9.
Bergman AG, Fredericson M, Ho C, Matheeson GO. Asymptomatic tibial stress reactions: MRI detection and clinical follow-uop in distancve runners. AJR Am J Roentgenol. 2004;183(3):635–8.
Caesar BC, McCollum GA, Elliott R, Williams A, Calder JDF. Stress fractures of the tibia and medial malleolus. Foot Ankle Clin. 2013;18(2):339–55.
Shelbourne KD, Fisher DA, Rettig AC, McCarroll JR. Stress fractures of the medial malleolus. Am J Sports Med. 1988;16(1):60–3.
Mandell JC, Khurana B, Smith SE. Stress Fractures of the foot and ankle, part 2: site-specific etiology, imaging, and treatment, and differential diagnosis. Skelet Radiol. 2017;46:1065–186.
Drakos MC, Domb B, Starkey C, Callahan L, Allen AA. Injury in the National Basketball Association: a 17-year overview. Sports Health. 2010;2(4):284–90.
Jowett AJL, Birks CL, Blackney MC. Medial malleolar stress fracture secondary to chronic ankle impingement. Foot Ankle Int. 2008;29(7):716–20.
Tol JL, van Dijk CN. Etiology of the anterior ankle impingement syndrome: a descriptive anatomical study. Foot Ankle Int. 2004;25:383–6.
Mankin HJ. The resonse of the articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–6.
van Dijk CN, Bossuyt PM, Marti RK. Medial ankle pain after lateral ligament rupture. J Bone Joint Br. 1996;78:562–7.
Donley BG, IIaslan H. Stress fractures of the medial malleolus. Oper Tech Sports Med. 2006;14:252–8.
Wegner AM, Wolinsky PR, Robbins MA, Garcia TC, Maitra S, Amanatullah DF. Antiglide plating of vertical medial malleolus fractures provide stiffer initial fixation than bicortical or unicortical screw fixation. Clin Biomech. 2016;31:29–32.
O’Malley MJ. Medial Malleolar Stress Fractures in the National Basketball Association (NBA). Am J Sports Med, in submission.
Nguyen A, Beasley I, Calder J. Stress fractures of the medial malleolus in the professional soccer player demonstrate excellent outcomes when treated with open reduction internal fixation and arthroscopic spur debridement. Knee Surg Sports Traumatol Arthrosc. 2019;27:2884–9.
Johanson MA. Contributing factors in microtrauma injuries of the lower extremity. J Back Musculoskelet Rehabil. 1992;2:12–25.
Migrom C, Finestone A, Segev S, Olin C, Arndt T, Ekenman I. Are overground or treadmill runners more likely to sustain tibial stress fractures? Br J Sports Med. 2003;37(2):160–3.
Ekenman I, Tsai-Fellander L, Johansson C, O’Brien M. The plantar flexor muscle attachments on the tibia. Scand J Med Sci Sports. 2007;5(3):160–4.
Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26(1):78–83.
Kijowski R, Choi J, Shinki K, Del Rio AM, De Smet A. Validation of MRI classification system for tibial stress injuries. Am J Roentgenol. 2012;198(4):878–84.
Bernholt DL, Garzon-Muvdi J, Chhabra A, McFarland EG. Stress fracture of the distal tibial physis in an adolescent recreational dancer. Am J Sports Med. 2013;41(7):1649–52.
Woods M, Kijowski R, Sanford M, Choi J, De Smet A. Magnetic resonance imaging findings in patients with fibula stress injuries. Skelet Radiol. 2008;37(9):835–41.
Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46–58.
Devas MB, Sweetnam R. Stress fractures of the fibula. J Bone Joint Surg Br. 1956;38B:818–29.
Cheng YC, Yang H, Ni L, Song D, Zhang H. Stress fracture of the distal fibula in flatfoot patients: case report. Int J Clin Med. 2015;8(4):6303–7.
Wang Q, Whittle M, Cunningham J, Kenwright J. Fibula and its ligaments in load transmission and ankle stability. Clin Orthop Relat Res. 1996;330:261–70.
Freidman MA, Draganich LF, Toolan B, Brage ME. The effects of adult acquired flatfoot deformity on tibiotalar joint contact characteristics. Foot Ankle Int. 2001;22:241–6.
McClone JJ. Stress fractures of the talus. J Am Podiatry Assoc. 1965;55:814–7.
Long NM, Zoga AC, Kier R, Kavanaugh EC. Insuffiencey and nondisplaced fractures of the talar head: MRI appearances. AJR Am J Roentgenol. 2012;199:613–7.
Sormaala MJ, Niva MH, Kiuru M, Mattila VM, Pihlajamaki HK. Bone stress injuries of the talus in military recruits. Bone. 2006;39:199–204.
Elias I, Zoga AC, Raiken SM, Peterson JR, Besser MP, Morrison WB, Schweitzer ME. Bone stress injury of the ankle in professional ballet dancers seen on MRI. BMC Musculoskelet Disord. 2008;9:39.
Sormaal MJ, Niva MH, Kiuru MJ, Mattila VM, Pihlajamaki HK. Outcomes of stress fractures of the talus. Am J Sports Med. 2006;34(11):1809–14.
Black KP, Ehlert KJ. A stress fracture of the lateral process of the talus in a runner. J Bone Joint Am. 1994;76A:441–3.
Motto SG. Stress fracture of the lateral process of the talus-a case report. Br J Sports Med. 1993;27(4):375–6.
Stocker B, Bennett JT. Stress fracture of the talus following resection of a talocalcaneal coalition: a case report. Foot Ankle Int. 2001;22(1):56–8.
Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:344–53.
Salzer MJ, Bluman EM, Noonan S, Chiodo CP, de Asla RJ. Injuries observed in minimalist runners. Foot Ankle Int. 2012;33(4):262–6.
Sormaala MJ, Niva MH, Kiuru MJ, Mattaila VM, Pihlajamaki HK. Stress injuries of the calcaneus detected with imaging in military recruits. J Bone Joint Am. 2006;88(10):2237–42.
Pearce CJ, Zaw H, Calder JD. Stress fracture of the anterior process of the calcaneus associated with a calcaneonavicular coalition: a case report. Foot Ankle Int. 2011;32(1):85–8.
Taketomi S, Uchiyama E, Iwaso H. Stress fracture of the anterior process of the calcaneus. Foot Ankle Spec. 2013;6(5):389–92.
Van Meensel AS, Peers K. Navicular stress fractures in high performing twin brothers: a case report. Acta Orthop Belg. 2010;76(3):407–12.
Maquirriain J, Ghisis JP. The incidence and distribution of stress fractures in elite tennis players. Br J Sports Med. 2006;40(5):454–9.
Towne LC, Blazina ME, Cozen LN. Fatigue fracture of the tarsal navicular. J Bone Joint Am. 1970;52(2):376–8.
Khan KM, Bruckner PD, Kearney C, Fuller PJ, Bradshaw CJ, Kiss ZS. Tarsal navicular stress fractures in athletes. Sports Med. 1994;17:65–76.
Vopat B, Beaulieu-Jones BR, Waryasz G, McHale KJ, Sanchez G, Logan CA, Whalen JM, DiGovanni CW, Provencher MT. Epidemiology of navicular injury at the NFL combine and their impact on an athlete’s prospective NFL career. Orthop J Sports Med. 2017;5(8):1–7.
McKeon KE, McCormick JJ, Johnson JE, Klein SE. Intraosseous and extraosseous arterial anatomy of the adult navicular. Foot Ankle Int. 2012;33(10):857–61.
Kitaoka HB, Luo ZP, An KN. Contact features of the talonavicular joint of the foot. Clin Orthop Relat Res. 1996;325:290–5.
Becker J, James S, Osternig L, Chou L. Foot kinematics differ between runners with and without a history of navicular stress fractures. Orthop J Sports Med. 2018;6(4):1–9.
Ingalls J, Wissman R. The os supravaviculare and navicular stress fractures. Skelet Radiol. 2011;40:937–41.
Nunang P, Quah C, Pillai A. A rare case of an osteochondral lesion of the tarsal navicular with a subacute stress fracture in a high level athlete. Foot. 2014;24:213–4.
Saxena A, Behan SA, Valerio DL. Navicular stress fracture outcomes in athletes: analysis of 62 injuries. J Foot Ankle Surg. 2017;56:943–8.
Torg JS, Pavlov H, Cooley LH, Bryant MH, Arnoczky SP, Bergfeld J, Hunter LY. Stress fractures of the tarsal navicular: a retrospective review of 21 cases. J Bone Joint Am. 1982;64(5):700–12.
Khan KM, Fuller PJ, Brukner PD, Kearney C. Outcome of conservative and surgical management of navicular stress fracture in athletes. Am J Sports Med. 1992;20(6):657–66.
Saxena A, Fullem B, Hannaford D. Results of treatment of 22 navicular stress fractures and a new proposed radiographic classification system. J Foot Ankle Surg. 2000;39(2):96–103.
Torg JS, Moyer J, Gaughan JP, Boden BP. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048–53.
Mallee WH, Weel H, van Dijk CN. Surgical versus conservative treatment for high-risk stress fractures of the lower leg: a systemic review. Br J Sports Med. 2015;49:370–6.
Fishman FG, Adams SB, Easley ME, Nunley JA. Vascularized pedicle bone grafting for nonunions of the tarsal navicular. Foot Ankle Int. 2012;33(9):734–9.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hubbard, C.E., O’Malley, M.J. (2020). Stress Fractures of the Ankle and Hindfoot. In: Miller, T.L., Kaeding, C.C. (eds) Stress Fractures in Athletes. Springer, Cham. https://doi.org/10.1007/978-3-030-46919-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-46919-1_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46918-4
Online ISBN: 978-3-030-46919-1
eBook Packages: MedicineMedicine (R0)