Abstract
Infections of cardiac implantable electrical device (CIEDI) are an emerging medical issue. They can be distinguished as infections limited to the pocket (more common) or systemic CIEDI (endocarditis or bacteremia). A correct and early microbiological diagnosis is essential for proper management of these patients. In this chapter we will present the various clinical patterns of CIEDI. Later we will describe the pathogens involved in CIEDI, as reported by literature, and their specific characteristics focusing in particular on biofilm production, its role for development of CIEDI, and the importance for treatment/prevention of device infections. We will deeply discuss both standard culturing and novel approaches for identification of the causing agent, like sonication and broad-range sequencing of bacterial DNA which are promising techniques to improve the diagnostic sensitivity. Later we will focus on antibiotic treatment of CIEDI in accordance to pathogens and clinical manifestation. Finally we will conclude by providing the premises for prevention of CIEDI on the bases of the pathogens involved.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Epidemiology and Clinical Presentation
Cardiovascular implantable electronic device infections (CIEDI) are increasing worldwide. In the United States (USA), according to the Nationwide Inpatient Sample database, the number of hospitalized patients with CIEDI increased from 5308 in the year 2003 to 9948 in 2011 [1]. During the same time period, the incidence of CIED infection increased by 210% [2]. Several factors underlie this increasing trend in CIEDI prevalence. First, with the broadening of indications, the number of cardiovascular implantable electronic device (CIED) implants is growing year by year. Second, the improved life expectancy has led to a dramatic increase of number of fragile patients treated with CIED implant, including elderly, immunocompromised, and comorbid patients [3, 4]. In addition to morbidity for patients, CIED infection has been linked to increase of both short-term and long-term mortality [5, 6] and to a significant increase of healthcare costs [5] (for a complete perspective on CIEDI epidemiology, costs, and outcomes, see also Chap. 1).
Commonly, CIEDI should be distinguished as pocket-related infections (Fig. 2.1) or CIED-related endocarditis (Fig. 2.2). In fact, these two groups of infections show complete different clinical presentation, management, and outcome [7, 8]. Another distinctive feature of these two groups of infection is the prevalence. A retrospective review of 189 cases of patients with CIED-infections admitted to Mayo Clinic Rochester from 1991 to 2003 revealed that generator pocket infection constituted the 69% of cases, while device-related endocarditis was diagnosed in 23%. In another study conducted at the Cleveland Clinic, among 412 cases of CIED infections, 59% involved only the device pocket, whereas 41% of cases had an endovascular involvement [3]. The different presentation may be related to the time of onset after the index procedure. In fact, a study evaluating early- versus late-onset infection found that the former were more likely to be pocket infections as compared with the latter [9].
A different clinical manifestation of CIEDI is the presence of Staphylococcus aureus bacteremia (SAB) without evidence of CIED involvement. A prospective study suggested that the overall prevalence of CIEDI in patients presenting with SAB may be as high as 45% and may reach 71% of cases when SAB occur within 1 year after device placement [10]. In 60% of these patients, no local signs or symptoms are commonly identified [10]. Among all cases of SAB occurring in CIED carrier, the risk of underlying a CIED infection is higher in the case of carriers of permanent pacemaker (vs. defibrillator), presenting a more prolonged bacteremia and those with history of repeated CIED procedures [11]. According with this data, any patients carrying a CIED and developing a SAB should undergo extensive evaluation that includes follow-up blood cultures, echocardiography, and screening for septic embolization with either computed tomography or fluorodesossiglucose-positron emission tomography [12,13,14,15,16]. Similar studies conducted in patients with CIED developing gram-positive bacteremia, other than SAB, found similar results in terms of prevalence of CIEDI [17]. By contrast, an association between gram-negative bacteremia and either endocarditis or pocket infection was not confirmed [18].
2.2 Microbiology: Available Methods and Etiology
A key point for a correct management of CIEDI is the achievement of a microbiological diagnosis. The main component of a successful microbiological diagnosis relies on correct sampling and good microbiological methods (Table 2.1). Sterile technique for sampling, fast submission to the microbiology laboratory, and seeding of the removed hardware are essential to optimize the management of CIEDI. Different studies compared the diagnostic yields of blood cultures, pocket swab, and hardware culturing after removal. In a Japanese study of 208 patients with CIEDI, blood culture, lead culture, and swab culture were positive in 27%, 81%, and 73% of cases, respectively [19]. In an older study conducted in Italy and including 118 lead extractions, 87% of which due to infection, lead cultures were positive in 92% and 100% in patients presenting with decubitus/fistula or local acute infection, respectively. Blood cultures were positive in 58% of patients presenting with sepsis. Despite concordance between blood cultures and lead cultures was high especially in the case of S. aureus isolation, concordance between lead or tip and pocket cultures was less satisfactory [20].
A novel microbiological method for device-related infection is the use of sonication. This technique is mainly applied in the management of prosthetic joint infection, but it may be used in any device-related infection [21]. Sonication is the process of converting an electrical signal into a physical vibration that can be directed toward a substance. In microbiology, and more specifically in device-related infections, the use of low-intensity ultrasounds to remove biofilms from hardware and subsequently fluid culture is a novel promising method to improve sensibility of cultural methods. The main advantages of sonication are that the sampling site is directly the surface of the device allowing the detection of larger number of microorganisms. In this case, additional susceptibility test may be performed in different colonies consenting the detection of hetero-resistance, particularly for S. aureus strains [22]. In a study enrolling 42 patients undergoing lead extraction for non-infectious cause and 35 patients with CIED infection, use of sonication was compared with conventional cultures. In the group of patients with infection, significant bacterial growth was observed in 54% of sonicate fluids, significantly greater than the sensitivities reported for pocket swab culture (20%), device swab culture (9%), or peri-device tissue culture (9%) [23].
Broad-range sequencing of bacterial 16S ribosomal DNA represents an alternative approach for establishing the underlying organism in device-related infections. Unfortunately, it has been poorly studied in CIEDI. In studies performed in patients with infective endocarditis, the use of broad-range 16S rDNA polymerase chain reaction(PCR)-sequencing for molecular diagnosis shows that heart valve PCR may improve microbiological diagnosis in up to 20% of patients and may be associated to high sensitivity and specificity [24, 25]. Advantages of molecular methods rely on rapid turnaround time and high sensitivity also in patients previously exposed to antimicrobial treatment which, in turn, may be paradoxically a limitation. In fact, PCRs are exposed to contamination and may result in false-positive results. Contamination can occur through environmental DNA or from PCR reagents despite using nucleic acid-free compounds. False-positive PCR findings can be due to circulating cell-free DNA from dead bacteria or fungal DNA in the absence of infection—the so-called DNAemia rather than a true bacteremia or fungemia [26, 27]. In addition, an infection successfully controlled by the immune system or by an efficient anti-infectious therapy will release pathogenic DNA that can persist several days in the blood.
Another limitation of conventional cultures is the poor concordance between different microbiological methods as demonstrated by different studies [20]. More specifically, acceptable concordance was found for isolation of S. aureus, gram-negatives, mycobacteria, and fungi. However, unsatisfactory concordance was found especially for other common skin contaminants [19, 20]. Additionally, colonization of device may occur without clinical relevant infection. In a study including 115 lead extractions for non-infectious cause, devices were analyzed with standard swab cultures and device sonication. Of the 115 devices analyzed, 44 (38%) resulted positive in sonication fluid cultures and 30 (27%) in swab cultures. Most of the pathogen found were CoNS and Propionibacterium acnes [28].
Detailed bacterial etiology of CIEDI is summarized in Fig. 2.3. Commonly gram-positive bacteria are responsible for more than 90% of infections. Coagulase-negative staphylococci (CoNS) are cultured in 33–69%. Among CoNS, Staphylococcus epidermidis is found in 70–81% of cases. S. aureus is the second more important pathogen, being found in 13–27% of cases. Lastly, negative cultures may occur in about 9–13% of cases [4, 19, 29,30,31]. Few studies compared microbiology of early- versus late-onset infection defined as infection diagnosed 1 year after last CIED-related procedure (for non-infectious cause). In the study of Welch et al., S. aureus was found more frequently in early infection, and by comparison CoNS were more frequent in late infection [32]. Similarly, in the study of Jan et al., S. aureus was isolated in 11.5% of early infection and in 6.9% of late infections [33]. This finding is not surprising as early device-related infections are commonly caused by more virulent strains. In fact, early infections are more likely to present with pocket erythema, swelling, and pain, whereas late infections were more likely have pocket erosion and valvular vegetations [32]. Late infections are also more likely to be caused by methicillin-susceptible strains [33]. Studies comparing etiology of pocket infection with CIED-associated endocarditis did not report significant differences [33].
Other emerging pathogens should be always kept in mind when dealing with CIED infection. Even if very rare, rapidly growing mycobacteria are increasingly reported and may be associated to outbreaks in the setting of major heart surgery or electrophysiology. In a recent review of 32 cases reported in the literature, the most common mycobacteria associated to CIED infection belong to the Mycobacterium fortuitum group followed by Mycobacterium abscessus, Mycobacterium smegmatis, and Mycobacterium chelonae [34,35,36]. All these pathogens are characterized by challenging diagnosis and treatment as may not be detected by standard cultures or require prolonged incubation. Correct identification of these agents is relevant for effective treatment since it entails long-term antibiotic treatment in addition to device removal [34].
2.3 Role of Biofilm
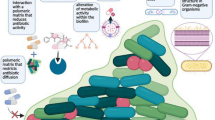
Biofilm development is an ancient prokaryotic adaptation [37] and represents a mode of growth that allows bacteria to survive in hostile environments and to colonize new niches by various dispersal mechanisms [37]. Biofilm is a multicellular community held together and embedded in a hydrated matrix of extracellular polymeric substances [38]. The formation of biofilm occurs when prokaryotic cells encounter a surface such as a foreign body or a medical device [39]. Classically the formation of biofilm can be divided into different stages that include adhesion to the surface, growth of a heterogenous multilayer slime, and detachment [Fig. 2.4]. Both and non-mutually exclusive genetic predisposition and environmental adaptation are involved in this process.
Biofilm formation on medical devices. At first, the surface of medical devices is coated with a layer of proteins and glycoproteins (1), and then cellular colonization takes place (2) with adhesion to the surface of the coated medical device (3) and subsequent release of signaling molecules with increased up-regulation of transcription due to the high concentration of (“quorum sensing”) (4). This results in an increased production of extracellular polymeric substance (5) and progressive maturation of biofilm (6). After its formation, biofilm parts may detach and be carried by bloodstream, possibly leading to secundarism of infection (7) (Reproduced from Zhang, Z, Wagner, V, Antimicrobial Coatings and Modifications on Medical Devices, Edited by Springer, 2017, page100 with permission)
There are several hypotheses to explain the benefit of biofilm formation and its association with surfaces, such as devices. Surfaces offer a stable environment to grow, and biofilm formation offers the opportunity to defend from environmental challenges such us UV exposure [40], acid exposure [41], and phagocytosis [42]. In addition, biofilm growth is associated to antimicrobial tolerance for several reasons. First, cells included in the biofilm are metabolically heterogenous, comprise nutritionally variant colonies, and therefore can be hardly detected through conventional cultures [43]. Second, metabolically variant colonies and more specifically cells that result in a stationary-phase dormancy may be unaffected to antibiotic therapy [44]. Third, the diffusion of antimicrobial agents in the matrix is impaired, and therefore the proportion of drug that may reach the cell is reduced. Similarly, biofilm cells may produce efflux pump or other antibiotic-degrading enzymes. Factors that may influence the antibiotic activity are cell density and biofilm age which are strongly correlated [45]. Studies on Pseudomonas aeruginosa showed that the activity of antimicrobials is greater in younger cells than older cells, especially for beta-lactams [46, 47]. Similarly, a meta-analysis of different studies showed that the efficacy of antimicrobials in biofilm-related infection is reduced for large or dense biofilm [45]. All of these factors link the production of biofilm with clinical failure or relapse when attempts of conservative treatment with antimicrobial therapy alone were tested.
2.4 Antibiotic Treatment of CIEDI
As stated in the previous paragraph, the formation of biofilm, which is common in device-related infection, hampers any conservative approach consisting in antibiotic treatment alone. Whenever feasible, device removal should be primarily considered for CIEDI. Attempts of conservative treatment can be considered only when there are strong contraindications to device removal. Choice of antibiotic treatment should be based on clinical presentation and diagnosis of CIEDI. As previously mentioned, CIEDI should be divided into pocket-related CIEDI and CIED-related endocarditis. Beyond, these two classical presentations, several patients may exhibit bacteremia without underlying clinically significant signs of device involvement. In observational studies of patients having a CIED and presenting SAB without clinical signs of device pocket infection, an actual CIED involvement was found in 34–40% of cases [10, 11]. A higher proportion of CIEDI are reported in the case of CoNS bacteremia [17]. In accordance, a different therapeutic management for each of these three situations should be considered. Clinical severity should also be considered in order to select the correct timing of antibiotic administration. Lastly, duration of antimicrobial treatment is strongly correlated by therapeutic approach, being different in conservative treatment or when device removal is carried out. Figure 2.5 represents a possible diagnostic and therapeutic algorithm for pocket-related infection, CIED endocarditis, and patients with SAB.
Common clinical presentation of cardiovascular implantable electronic device infection and its medical management. CIED infection management algorithm. Suspect of CIED infection may be posed for the evidence of local signs of pocket infection/fever of unknown origin (left side of the diagram) or by the evidence of positive blood cultures. An assessment of the risk of sepsis, or sepsis severity score worsening, should be performed. The presence of endocarditis should be also investigated (echocardiogram, blood cultures), and the need of screening for septic emboli must be decided accordingly. Lastly, the need of lead extraction should be assessed. CIED cardiac electronic implantable device, SOFA Sequential Organ Failure Assessment (SOFA) Score
Based on microbiological data, empirical treatment should include coverage for methicillin-resistant S. aureus (MRSA), especially in area with high prevalence of MRSA. Vancomycin is considered the treatment of choice for MRSA infection in most cases. Although superiority of other drugs versus vancomycin was poorly demonstrated in clinical trials, observational studies suggest that alternative regimens could be associated with improved outcome in specific situations [48, 49]. More specifically, with the spread of strains with reduced susceptibility to vancomycin, treatment failure with this drug was reported [50]. In a meta-analysis including 22 studies, higher mortality was reported in infections caused by MRSA strains with vancomycin MIC ≥2 mg/mL, especially in the case of BSI [51].
Daptomycin is a lipopeptide characterized by high bactericidal activity and good biofilm penetration. In one case-control study of patients with S. aureus bacteremia, use of daptomycin was associated to improved outcome compared to vancomycin [48]. Daptomycin activity seems to be enhanced by combination with beta-lactams, fosfomycin, or rifampin and using higher dosage, especially in device-related infection. Dosages of daptomycin have been recently debated. Daptomycin exhibits a concentration-dependent bacterial killing. That means that higher dosage is associated to higher antimicrobial activity and daptomycin resistance [52, 53]. In a study of patients enrolled in the CORE database (a multicenter retrospective register of patients treated with daptomycin), the efficacy of high-dose daptomycin (≥8 mg/kg/day) was evaluated. The clinical success rate for MRSA infection was 83% among patients receiving high-dose daptomycin [54]. Similarly, in a large multicenter retrospective study including patients treated with high-dose daptomycin as salvage treatment after failing vancomycin therapy, clinical and microbiological success was assessed in 84% and 80% of cases, respectively [55]. In a single-center study focused on 25 cases of CIED infection, daptomycin was administered with a median dose of 8.3 mg/kg. Clinical cure was observed in 80% of cases and microbiological success in 92% of cases [56].
Combination treatment of vancomycin and daptomycin with beta-lactams or other drugs is a matter of debate as well. Some authors suggest that activity of both vancomycin and daptomycin may be enhanced by use of a companion drug. In a pilot randomized trial of 60 patients, vancomycin plus flucloxacillin was associated to a shorter duration of MRSA bacteremia compared with vancomycin alone [57]. In addition, a synergy of daptomycin with beta-lactams, rifampin, and other drugs were observed in both in vitro studies or limited clinical experiences [58,59,60,61,62].
Enterococcus spp. may be also an important pathogen related to CIED infection or CIED-related endocarditis. The majority of enterococcal infection are caused by Enterococcus faecalis which is commonly susceptible to ampicillin. Ampicillin alone however may be associated to clinical failure, and therefore the combination treatment with gentamycin or ceftriaxone should be considered as first-line treatment for patients with CIED endocarditis caused by E. faecalis. In the case of Enterococcus faecium, vancomycin or teicoplanin should be administered. Recently, vancomycin-resistant enterococci (VRE) have emerged as an important threat. The options for treating vancomycin-resistant enterococcus infections are linezolid, daptomycin, or tigecycline. Well-designed comparative studies are not available to assess the best treatment for VRE. However a meta-analysis of 10 retrospective studies comparing outcome of patients treated with linezolid or daptomycin for VRE bacteremia found an increased risk of mortality in patients receiving daptomycin [63]. More recently a US nationwide retrospective cohort study comparing daptomycin and linezolid for the treatment of VRE bacteremia found a significant higher rate of treatment failure among patients receiving linezolid [64]. This controversy in results of observational studies may be related to the dose of daptomycin used. In fact, a study comparing different dosages of daptomycin demonstrated a clinical benefit of higher dose of daptomycin (≥9 mg/kg) compared with low-dose daptomycin for the treatment of bacteremia caused by VRE [65].
Duration of treatment may depend on the baseline clinical picture. Patients with local infection with negative blood cultures and negative echocardiography may be treated with a 7- to 10-day antibiotic treatment after device removal. In the case of S. aureus bloodstream infection, a course of 2–4 weeks of antibiotic treatment should be ensured. Lastly, patients with endocarditis should receive at least 4–6 weeks of treatment [8, 66, 67]. Timing of new device implantation may depend on urgency of pacing and underlying patient condition. The commonest and most safe procedure is to perform a 2-stage procedure consisting in device and lead removal, temporal pacing, and new definitive device insertion. In this case, blood cultures should be negative for at least 72 h before reimplantation [7, 8]. Notably, in a study evaluating 68 patients treated with 1-stage removal and contralateral implant, no relapse of infection involving the new device was detected after a long-term follow-up [68]. However, larger studies should be performed to confirm the safety of a similar approach.
When patients present major contraindication to device removal, usually very old and fragile patients, infection management is more challenging, and the outcomes are poor. In most of the cases, chronic suppression therapy is necessary [7, 8]. In a retrospective study, among 660 cases of CIED infection, 48 patients were treated with chronic suppression antibiotic therapy. The median age was 78 years, and the most preferred drugs were trimethoprim-sulfamethoxazole, penicillin, and amoxicillin. The estimated median overall survival was 1.43 years, and 18% of survivors developed relapse within 1 year [69].
2.5 Prevention
Prevention of CIEDI is extremely important since it is associated with high mortality and increased healthcare costs [5]. Risk factors for CIEDI have been described in the literature. Older and comorbid patients, such as those with congestive heart failure, malignancies, or renal failure, and those receiving corticosteroids are at risk to develop CIEDI. Prevention should include, whenever possible, the reduction of patients’ modifiable risk factors including control of blood sugar levels, reduction of international normalized ratio (INR), and discontinuation of steroids [70,71,72,73].
One key factor of CIED infection is microbial contamination during device placement. This can occur (a) during manufacture or packaging, (b) before CIED implantation, (c) during CIED implantation, (d) secondary to surgical site infection, (e) via hematogenous seeding from a distant site (especially as a consequence of a SAB) [66, 70], or (f) via contamination after erosion through the skin [70]. Microbial contamination during manufacture is rare but should be considered when there is an outbreak of infection caused by the same organism especially when an environmental, uncommon organism is involved. Even if contamination of CIED during manufacture or packaging is poorly reported in literature, a recent outbreak of Mycobacterium chimaera infection was reported in several healthcare facilities performing major heart surgery. In this case, contamination during manufacture of a heater-cooler device used for cardiac surgery was found after extensive investigation [74]. A second important pathophysiological pathway to CIED infection is contamination during implantation or as a consequence of skin erosion or surgical site infection. According to this pathway, inpatients receiving emergent procedure with longer time of implant can be considered at higher risk for infection when compared with outpatients undergoing shorter elective procedures. In this scenario common skin contaminants such as CoNS, P. acnes, and diphtheroids are involved [75, 76]. In addition, subsequent device revisions have been linked to augmented probability of infection confirming that multiple manipulation confers higher opportunity for contamination [73, 77, 78]. Strategies to prevent CIED infection according with this mechanism are listed in Table 2.2 (for a complete review of available strategies for CIEDI prevention, see also Chap. 11).
References
Sridhar AR, Lavu M, Yarlagadda V, Reddy M, Gunda S, Afzal R, et al. Cardiac implantable electronic device-related infection and extraction trends in the U.S. PACE. 2017;40(3):286–93.
Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6.
Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7(8):1043–7.
Gandhi T, Crawford T, Riddell J. Cardiovascular implantable electronic device associated infections. Infect Dis Clin North Am. 2012;26(1):57–76.
Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171(20):1821–8.
Rizwan Sohail M, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. PACE. 2015;38(2):231–9.
Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–77.
Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al. 2017 hrs expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–e51.
Sohail MR, Hussain S, Le KY, Dib C, Lohse CM, Friedman PA, et al. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol. 2011;31(2):171–83.
Chamis AL, Peterson GE, Cabell CH, Corey GR, Sorrentino RA, Greenfield RA, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001;104(9):1029–33.
Sohail MR, Palraj BR, Khalid S, Uslan DZ, Al-Saffar F, Friedman PA, et al. Predicting risk of endovascular device infection in patients with Staphylococcus aureus bacteremia (predict-sab). Circ Arrhythm Electrophysiol. 2015;8(1):137–44.
Fowler VG Jr, Li J, Corey GR, Boley J, Marr KA, Gopal AK, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. 1997;30(4):1072–8.
Vos FJ, Kullberg BJ, Sturm PD, Krabbe PF, van Dijk AP, Wanten GJ, et al. Metastatic infectious disease and clinical outcome in Staphylococcus aureus and Streptococcus species bacteremia. Medicine. 2012;91(2):86–94.
Graziosi M, Nanni C, Lorenzini M, Diemberger I, Bonfiglioli R, Pasquale F, et al. Role of (1)(8)f-fdg pet/ct in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging. 2014;41(8):1617–23.
Bonfiglioli R, Nanni C, Morigi JJ, Graziosi M, Trapani F, Bartoletti M, et al. (1)(8)f-fdg pet/ct diagnosis of unexpected extracardiac septic embolisms in patients with suspected cardiac endocarditis. Eur J Nucl Med Mol Imaging. 2013;40(8):1190–6.
Goto M, Schweizer ML, Vaughan-Sarrazin MS, Perencevich EN, Livorsi DJ, Diekema DJ, et al. Association of evidence-based care processes with mortality in Staphylococcus aureus bacteremia at veterans health administration hospitals, 2003-2014. JAMA Intern Med. 2017;177(10):1489–97.
Madhavan M, Sohail MR, Friedman PA, Hayes DL, Steckelberg JM, Wilson WR, et al. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia caused by gram-positive cocci other than Staphylococcus aureus. Circ Arrhythm Electrophysiol. 2010;3(6):639–45.
Uslan DZ, Sohail MR, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis. 2006;43(6):731–6.
Fukunaga M, Goya M, Nagashima M, Hiroshima K, Yamada T, An Y, et al. Identification of causative organism in cardiac implantable electronic device infections. J Cardiol. 2017;70(5):411–5.
Golzio PG, Vinci M, Anselmino M, Comoglio C, Rinaldi M, Trevi GP, et al. Accuracy of swabs, tissue specimens, and lead samples in diagnosis of cardiac rhythm management device infections. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S76–80.
Liu H, Zhang Y, Li L, Zou HC. The application of sonication in diagnosis of periprosthetic joint infection. Eur J Clin Microbiol Infect Dis. 2017;36(1):1–9.
Esteban J, Sorli L, Alentorn-Geli E, Puig L, Horcajada JP. Conventional and molecular diagnostic strategies for prosthetic joint infections. Expert Rev Mol Diagn. 2014;14(1):83–96.
Nagpal A, Patel R, Greenwood-Quaintance KE, Baddour LM, Lynch DT, Lahr BD, et al. Usefulness of sonication of cardiovascular implantable electronic devices to enhance microbial detection. Am J Cardiol. 2015;115(7):912–7.
Marin M, Munoz P, Sanchez M, del Rosal M, Alcala L, Rodriguez-Creixems M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine. 2007;86(4):195–202.
Millar B, Moore J, Mallon P, Xu J, Crowe M, McClurg R, et al. Molecular diagnosis of infective endocarditis—a new duke’s criterion. Scand J Infect Dis. 2001;33(9):673–80.
Vernon SD, Shukla SK, Conradt J, Unger ER, Reeves WC. Analysis of 16s rRNA gene sequences and circulating cell-free DNA from plasma of chronic fatigue syndrome and non-fatigued subjects. BMC Microbiol. 2002;2:39.
Opota O, Jaton K, Greub G. Microbial diagnosis of bloodstream infection: towards molecular diagnosis directly from blood. Clin Microbiol Infect. 2015;21(4):323–31.
Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, et al. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation. 2010;121(15):1691–7.
Bongiorni MG, Tascini C, Tagliaferri E, Di Cori A, Soldati E, Leonildi A, et al. Microbiology of cardiac implantable electronic device infections. Europace. 2012;14(9):1334–9.
Rodriguez DJ, Afzal A, Evonich R, Haines DE. The prevalence of methicillin resistant organisms among pacemaker and defibrillator implant recipients. Am J Cardiovasc Dis. 2012;2(2):116–22.
Dy Chua J, Abdul-Karim A, Mawhorter S, Procop GW, Tchou P, Niebauer M, et al. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. PACE. 2005;28(12):1276–81.
Welch M, Uslan DZ, Greenspon AJ, Sohail MR, Baddour LM, Blank E, et al. Variability in clinical features of early versus late cardiovascular implantable electronic device pocket infections. PACE. 2014;37(8):955–62.
Jan E, Camou F, Texier-Maugein J, Whinnett Z, Caubet O, Ploux S, et al. Microbiologic characteristics and in vitro susceptibility to antimicrobials in a large population of patients with cardiovascular implantable electronic device infection. J Cardiovasc Electrophysiol. 2012;23(4):375–81.
Phadke VK, Hirsh DS, Goswami ND. Patient report and review of rapidly growing mycobacterial infection after cardiac device implantation. Emerg Infect Dis. 2016;22(3):389–95.
Giannella M, Valerio M, Franco JA, Marin M, Bouza E, Munoz P. Pacemaker infection due to mycobacterium fortuitum: the role of universal 16s rrna gene pcr and sequencing. Diagn Microbiol Infect Dis. 2007;57(3):337–9.
Verghese S, Mullaseri A, Padmaja P, Subhadra AC, Cherian KM. Pacemaker implant site infection caused by atypical mycobacteria. Indian Heart J. 1998;50(2):201–2.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108.
Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13(1):20–6.
Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209.
Espeland EM, Wetzel RG. Complexation, stabilization, and uv photolysis of extracellular and surface-bound glucosidase and alkaline phosphatase: implications for biofilm microbiota. Microb Ecol. 2001;42(4):572–85.
McNeill K, Hamilton IR. Acid tolerance response of biofilm cells of Streptococcus mutans. FEMS Microbiol Lett. 2003;221(1):25–30.
Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186(11):6585–96.
Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–43.
Walters MC 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47(1):317–23.
Stewart PS. Antimicrobial tolerance in biofilms. Microbiol Spectr. 2015;3(3)
Tanaka G, Shigeta M, Komatsuzawa H, Sugai M, Suginaka H, Usui T. Effect of the growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents: Beta-lactams and fluoroquinolones. Chemotherapy. 1999;45(1):28–36.
Shigeta M, Komatsuzawa H, Sugai M, Suginaka H, Usui T. Effect of the growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents. Chemotherapy. 1997;43(2):137–41.
Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54(1):51–8.
Claeys KC, Zasowski EJ, Casapao AM, Lagnf AM, Nagel JL, Nguyen CT, et al. Daptomycin improves outcomes regardless of vancomycin mic in a propensity-matched analysis of methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2016;60(10):5841–8.
Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46(2):193–200.
van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54(6):755–71.
Akins RL, Rybak MJ. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2001;45(2):454–9.
Rose WE, Leonard SN, Rybak MJ. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2008;52(9):3061–7.
Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. Safety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapy. Ann Pharmacother. 2009;43(7):1211–9.
Kullar R, Davis SL, Levine DP, Zhao JJ, Crank CW, Segreti J, et al. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy. 2011;31(6):527–36.
Durante-Mangoni E, Casillo R, Bernardo M, Caianiello C, Mattucci I, Pinto D, et al. High-dose daptomycin for cardiac implantable electronic device-related infective endocarditis. Clin Infect Dis. 2012;54(3):347–54.
Davis JS, Sud A, O’Sullivan MVN, Robinson JO, Ferguson PE, Foo H, et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis. 2016;62(2):173–80.
Dhand A, Sakoulas G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin Ther. 2014;36(10):1303–16.
Miro JM, Entenza JM, Del Rio A, Velasco M, Castaneda X. Garcia de la Maria C et al. high-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 2012;56(8):4511–5.
Rose WE, Berti AD, Hatch JB, Maki DG. Relationship of in vitro synergy and treatment outcome with daptomycin plus rifampin in patients with invasive methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 2013;57(7):3450–2.
Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, et al. Beta-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother. 2012;56(12):6192–200.
El Haj C, Murillo O, Ribera A, Vivas M, Garcia-Somoza D, Tubau F, et al. Daptomycin combinations as alternative therapies in experimental foreign-body infection caused by meticillin-susceptible Staphylococcus aureus. Int J Antimicrob Agents. 2015;46(2):189–95.
Balli EP, Venetis CA, Miyakis S. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother. 2014;58(2):734–9.
Britt NS, Potter EM, Patel N, Steed ME. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of veterans affairs patients. Clin Infect Dis. 2015;61(6):871–8.
Chuang YC, Lin HY, Chen PY, Lin CY, Wang JT, Chang SC. Daptomycin versus linezolid for the treatment of vancomycin-resistant enterococcal bacteraemia: Implications of daptomycin dose. Clin Microbiol Infect. 2016;22(10):890e1–7.
Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70(2):325–59.
Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312(13):1330–41.
Nandyala R, Parsonnet V. One stage side-to-side replacement of infected pulse generators and leads. PACE. 2006;29(4):393–6.
Tan EM, DeSimone DC, Sohail MR, Baddour LM, Wilson WR, Steckelberg JM, et al. Outcomes in patients with cardiovascular implantable electronic device infection managed with chronic antibiotic suppression. Clin Infect Dis. 2017;64(11):1516–21.
Branch-Elliman W. A roadmap for reducing cardiac device infections: a review of epidemiology, pathogenesis, and actionable risk factors to guide the development of an infection prevention program for the electrophysiology laboratory. Curr Infect Dis Rep. 2017;19(10):34.
Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17(5):767–77.
Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116(12):1349–55.
Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45(2):166–73.
Perkins KM, Lawsin A, Hasan NA, Strong M, Halpin AL, Rodger RR, et al. Notes from the field: Mycobacterium chimaera contamination of heater-cooler devices used in cardiac surgery—United States. MMWR Morb Mortal Wkly Rep. 2016;65(40):1117–8.
El Rafei A, Desimone DC, Sohail MR, Desimone CV, Steckelberg JM, Wilson WR, et al. Cardiovascular implantable electronic device infections due to propionibacterium species. PACE. 2016;39(6):522–30.
Pichlmaier M, Marwitz V, Kuhn C, Niehaus M, Klein G, Bara C, et al. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace. 2008;10(9):1067–72.
Lekkerkerker JC, van Nieuwkoop C, Trines SA, van der Bom JG, Bernards A, van de Velde ET, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95(9):715–20.
Ann HW, Ahn JY, Jeon YD, Jung IY, Jeong SJ, Joung B, et al. Incidence of and risk factors for infectious complications in patients with cardiac device implantation. Int J Infect Dis. 2015;36:9–14.
Turagam MK, Nagarajan DV, Bartus K, Makkar A, Swarup V. Use of a pocket compression device for the prevention and treatment of pocket hematoma after pacemaker and defibrillator implantation (stop-hematoma-i). J Interv Card Electrophysiol. 2017;49(2):197–204.
Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
de Oliveira JC, Martinelli M, Nishioka SA, Varejao T, Uipe D, Pedrosa AA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2(1):29–34.
Branch-Elliman W, Stanislawski M, Strymish J, Baron AE, Gupta K, Varosy PD, et al. Cardiac electrophysiology laboratories: a potential target for antimicrobial stewardship and quality improvement? Infect Control Hosp Epidemiol. 2016;37(9):1005–11.
Koerber SM, Turagam MK, Winterfield J, Gautam S, Gold MR. Use of antibiotic envelopes to prevent cardiac implantable electronic device infections: a meta-analysis. J Cardiovasc Electrophysiol. 2018;29(4):609–15.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bartoletti, M., Viale, P. (2020). Microbiological Background: Biofilm, Culturing, and Antibiotics. In: Diemberger, I., Boriani, G. (eds) Infections of Cardiac Implantable Devices. Springer, Cham. https://doi.org/10.1007/978-3-030-46255-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-46255-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46254-3
Online ISBN: 978-3-030-46255-0
eBook Packages: MedicineMedicine (R0)