Abstract
We present the current understanding on the diversification of the Mesoamerican genus Sphenarium, a group of generalist-herbivorous grasshoppers that could play a major role on the evolution of defense mechanisms and life history traits of plants along to their distribution range. We discuss their phylogenetic relationships and how geological and climatic history, as well as environmental variation, could favor their expansion and diversification. Furthermore, in a phylogenetical framework, we considered future directions on the study of their interactions with the plants with which their populations have evolved.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The diversity of herbivores, their host plants, and the defensive adaptations of plants to herbivory are postulated to have arisen by a long history of coevolution that has affected the food web links between these trophic levels (Ehrlich and Raven 1964). The understanding of the macroevolutionary history of interactions requires the integration of ecology, evolution, and the role of history in shaping the diversification or decline of lineages (Futuyma and Agrawal 2009; Reznick and Ricklefs 2009). Phylogenies allow us to infer a major component of evolutionary history, namely, the sequence of divergence of lineages as portrayed in phylogenetic trees (Futuyma and Agrawal 2009). They play a fundamental role to understanding the dynamic of communities (Kraft et al. 2007; Buckley et al. 2010; Gerhold et al. 2015); the phylogenetic evidence allows us to examine the structure of community assemblages, exploring the basis of community niche structure and adding a community context to studies of trait evolution and biogeography (Webb et al. 2002; Cavender-Bares et al. 2012; Villalobos et al. 2016).
The Evolutionary History of the Genus Sphenarium
The Genus Sphenarium, Its Higher-Level Phylogenetic Relationships, and Biogeographic Origin
The genus Sphenarium Charpentier, 1842 comprises a monophyletic group of 17 fusiform flightless grasshopper species (Sanabria-Urbán et al. 2017). This genus belongs to the family Pyrgomorphidae (Orthoptera: Caelifera), an ancient lineage of grasshoppers of Gondwanan origin around 141 mya (Mariño-Pérez and Song 2019). This family comprises 482 extant species in 149 genera mainly distributed across the tropics of the world (Mariño-Pérez and Song 2018). However, most pyrgomorphs are found in the Old World (Africa, Asia and Australia), whereas only 41 species in 13 endemic genera are found in the New World, where Sphenarium is the most diverse genus (Cigliano et al. 2019).
Various phylogenetic and biogeographic hypotheses for the origin of the New World Pyrgomorphidae, including Sphenarium, have been proposed (Kevan 1977; Mariño-Pérez and Song 2019). However, only currently these hypotheses have been tested by Mariño-Pérez and Song (2019) using molecular information. They found that the New World Pyrgomorphidae consist of at least three separate clades spread throughout the phylogeny of the family . The first clade includes the genera in the South American endemic tribe Omurini and the genus Jaragua restricted to the Caribbean, the second clade comprises the genera in the Mexican endemic tribes Ichthiacridini and Ichthyotettigini, and the last clade includes the Mesoamerican genera Sphenarium and Prosphena . Interestingly, this last clade, Sphenarium-Prosphena, is more closely related to the African genera Ochrophlegma and Tanita than to other American pyrgomorphs. Moreover, these four genera (Sphenarium-Prosphena-Ochrophlegma-Tanita) are most closely related to the Mexican Ichthiacridini-Ichthyotettigini clade within the phylogeny of the family.
Given these phylogenetic relationships Mariño-Pérez and Song (2019) have inferred a complex biogeographic history for the New World Pyrgomorphidae that implicate at least two colonization events to America. The first and oldest event could involve vicariance or dispersal from Africa of the common ancestor of the South American and Caribbean lineage (Omurini-Jaragua) during the Cretaceous (112–81 mya). In both cases a subsequent dispersal event from South America to the Caribbean in the late Cretaceous (~68 mya) is also inferred. The second wave of colonization came by dispersal from West Africa to northern South America and then to North America and Africa, when the continents were already separated. The common ancestor that colonized South America for the second time in the late Cretaceous (~69 mya) gave rise over there to the clades Sphenarium-Prosphena, Ochrophlegma-Tanita, and Ichthiacridini-Ichthyotettigini. The common ancestors of these clades then dispersed to North America (Sphenarium-Prosphena and Ichthiacridini-Ichthyotettigini) and back to Africa (Ochrophlegma-Tanita), before the entire lineage became extinct in South America during the early Paleogene (60–50 mya). According with this scenario, the ancestors of Sphenarium could establish in North America since the Eocene (~ 50–40 mya), which may imply a relatively long history of evolution of Sphenarium grasshoppers in this region.
Ecology and Natural History of the Genus Sphenarium
The species of Sphenarium are distributed from central Mexico to northwestern Guatemala (Fig. 15.1), where the major mountain ranges delimit their parapatric distribution (Sanabria-Urbán et al. 2017). Sphenarium grasshoppers are found in a wide variety of plant communities, including xerophytic, temperate, tropical deciduous, and rain forests. These insects are mainly found in the border vegetation and in sunny areas, where they feed on weeds, shrubs, and even trees of a wide variety of seasonal and perennial plants species. At least 47 species in 25 families of vascular plants and ferns are known to be eaten by S. purpurascens (Table 15.1) (Cano-Santana and Castellanos-Vargas 2009; and references therein).
Geographic distribution ranges of the species of Sphenarium. (After Sanabria-Urbán et al. 2017)
The diet breadth of the other species of Sphenarium has remained unstudied. However, these other species are commonly found in the same plant species and/or families in nature (Márquez 1962, 1965a, b; Descamps 1975; Oyama et al. 1994; Sanabria-Urbán, pers. obs.), suggesting that in general these insects are polyphagous herbivories.
The diet composition of the grasshoppers of Sphenarium could be determined by a mix of their food preferences and the encounter probability with their host plants. Moreover, the population densities of these insects can modulate their diet breadth. For instance, in low population densities, their diet breadth can be narrow, tending to feed mostly on soft tissue plants (e.g., seasonal Asteraceae), whereas when they reach high densities, their diet breadth can expand to include plants species with harder tissues, such as cactus (e.g., Opuntia sp.) (Cueva del Castillo, pers. obs.). Similar behaviors have been documented in other grasshoppers (Otte and Joern 1976; Bernays and Chapman 1977; Joern 1979), which can be explained by the quality and quantity of nutriments and the difficulty to obtain them from these types of plants.
Besides being polyphagous herbivorous, the species of Sphenarium can be very abundant and even show populations outbreaks (Kevan 1977). For instance, S. purpurascens can represent up to 95% of the dry biomass of the epiphytic arthropods in xerophytic habitats in central Mexico (Ríos-Casanova and Cano-Santana 1994). During their populations outbreaks, these insects can infest several crop plant species. Indeed, Sphenarium grasshoppers have long been regarded as one of the most severe agricultural pests of corn and beans in central Mexico (Cerritos and Cano-Santana 2008). But at the same time, they have been used as food since pre-Colombian times for Mexican people (Ramos-Elorduy and Moreno 1989). Other crop pest species are also recognized in at least ten genera of Pyrgomorphidae, but all of them in the Old World, mainly in Africa and Asia (Table 15.2).
Even though studies on the phenology of Sphenarium grasshoppers have focused mainly in one species, S. purpurascens (Cano-Santana and Castellanos-Vargas 2009; and the references therein), several lines of evidence suggest that all species in the genus are phenologically similar (Márquez 1962, 1965a, b; Descamps 1975; Sanabria-Urbán et al. 2015, 2017). These grasshoppers are univoltine. Their nymphs emerge mainly in the beginning of the rainy season (June–July), and they become adults and reproduce mainly during the fall (from mid-September to mid-December). After reproduction the oviposition and the highest adult mortality occur during the winter (approximately from mid-December to mid-February) (Sanabria-Urbán et al. 2015, 2017). However, the species of Sphenarium show extensive variation at inter- and intraspecific levels on body size and life history traits (Kevan 1977; Sanabria-Urbán et al. 2015, 2017). These traits are common targets of natural selection, and their geographic variation suggest high levels of adaptation to environmental heterogeneity across their distribution (Sanabria-Urbán et al. 2015). Moreover, morphological and behavioral traits appear to be under strong sexual selection (Cueva del Castillo and Nunez-Farfan 1999, 2002; Cueva del Castillo et al. 1999). For instance, in S. purpurascens, larger males have advantage in accessing females (Cueva del Castillo et al. 1999) and show prolonged female guarding behavior (spending up to 22 days mounted on females) that may suggest strong sperm competition (Cueva del Castillo 2003).
Most Sphenarium species exhibit apparently cryptic coloration patterns, but in some species (e.g., S. purpurascens, S. histrio, S. mexicanum, and S. mixtecum) brightly colorations are relatively common (Sanabria-Urbán et al. 2017), resembling aposematic pyrgomorphs from the Old World (Mariño-Pérez and Song 2018). Aposematism and the ability to sequester secondary compounds from toxic plant have been documented in about 10% of the species of Pyrgomorphidae (Mariño-Pérez and Song 2018). However, it seems that these traits have not evolved in the group. For instance, morphological and molecular specializations related with aposematisms in pyrgomorphs have not been observed in Sphenarium grasshoppers (Mariño-Pérez and Song 2018; Yang et al. 2019). In fact, these grasshoppers are heavily predated by multiple species of arthropods, lizards, birds, and mammals, including humans (Cano-Santana and Castellanos-Vargas 2009; Sanabria-Urbán, pers. obs.). Thus, it seems unlikely that these grasshoppers are toxic for their predators. Nevertheless, some species in the genus (e.g., S. purpurascens and S. rugosum) can feed on toxic plants, such as Datura stramonium (Castillo et al. 2014; Sanabria-Urbán, pers. obs.), and generate enormous damage to them (Núñez-Farfán and Dirzo 1994; Fornoni et al. 2003; Castillo et al. 2014), despite of D. stramonium has well-known defense mechanisms against herbivores (e.g., trophane alkaloids and trichomes) (Valverde et al. 2001). So far, it remains largely unknown what are the mechanisms that have allowed Sphenarium species to feed on their multiple host plant species.
The Phylogenetic Relationships Among the Species of Sphenarium
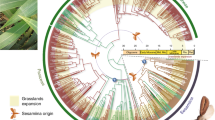
The most comprehensive phylogenetic study on Sphenarium until now was conducted by Sanabria-Urban et al. (2017). They found that after the divergence from its sister genus Prosphena, the common ancestor of Sphenarium gave raise to three major clades that diversified subsequently (Fig. 15.2). These clades are geographically restricted to particular regions across the distribution of the genus. The first and most basal clade is just composed by the species S. borrei, which is restricted to the inner highlands of western-central Mexico. The second clade is composed by the species S. totonacum, S. occidentalis, S. mexicanum, and S. histrio that are distributed in the costal lowlands and the highlands of southern Mexico and northwestern Guatemala. The third clade comprises all the species distributed in the inner basins and highlands of central Mexico: S. infernalis, S. adelinae, S. mixtecum, S. planum, S. minimum, S. macrophallicum, S. crypticum, S. rugosum, S. tarascum, S. zapotecum, S. variabile, and S. purpurascens (Fig. 15.2). A closer phylogenetic relationship is recovered between the last two clades but with poor support.
Phylogenetic relationships and mean divergence times between the species of Sphenarium based on a Bayesian species tree analysis of the genus (after Sanabria-Urban et al. 2017). The geographic distribution and species within the three major clades of Sphenarium are represented by the gray shapes and rectangles. Each species is represented by different colored squares (same as in Fig. 15.1). Nodes with posterior probability values ≥0.8 are indicated with open circles. The temporal occurrence of the second major formation of the Mexican Volcanic Belt (FMVB) and Quaternary climatic fluctuations (QCF) is denoted by the light-colored areas on the chronogram
The species within the last two clades integrate into different nested monophyletic groups. In the second clade, the basal position is occupied by S. totonacum, followed by S. occidentalis, which is closely related to S. mexicanum and S. histrio. In the third clade, sister relationships were recovered between S. adelinae and S. mixtecum, S. crypticum and S. macrophallicum, and S. rugosum and S. tarascum, and between S. purpurascens, S. variabile, and S. zapotecum. The last seven species conform a monophyletic group along with S. minimum and S. planum. Nonetheless, the phylogenetic relationships between these groups of species and the other species in the clade are poorly supported. The basal positions in this last monophyletic group are occupied by the species in the western range of the clade (S. adelinae, S. mixtecum, and S. infernalis), followed by the species distributed in the Tehuacan Valley (S. planum), the Balsas River Basin (S. crypticum, S. macrophallicum, S. rugosum, and S. tarascum), and in the southern Sierra Madre Oriental (S. minimum), whereas the most recently derived species are those distributed in the highlands of central and southern Mexico (S. purpurascens, S. variabile, and S. zapotecum) (Fig. 15.2). The low phylogenetic resolution of some of the basal divergences, as well as the fact that some morphologically different sister species were found to be paraphyletic in their genetic lineages (S. histrio-S. mexicanum; S. macrophallicum-S. crypticum; S. rugosum-S. tarascum; and S. purpurparcens-S. variabile-S. zapotecum), can be explained by incomplete lineage sorting associated with relatively rapid and/or simultaneous cladogenetic events.
Historical Biogeography of the Genus
According with Sanabria-Urbán et al. (2017), the initial divergences in Sphenarium (between the clades and the basal lineages within them) occurred between 2.91 and 7.22 mya, whereas most of the cladogenetic events within the clades occurred between 0.19 and 2.51 mya (Fig. 15.2). These two major episodes of diversification correlate temporally and spatially with the third major formation of the Mexican Volcanic Belt (MVB), around 3–7.5 mya (Ferrari et al. 2012), and the Quaternary climatic fluctuations around 0.01–2.6 mya. These historic events are recognized as some of the most important drivers of lineage diversification in other co-distributed taxa in Mexico (Bryson et al. 2011, 2012; Duennes et al. 2012; Mastretta-Yanes et al. 2015).
The several volcanic episodes during the formation of the MVB (Ferrari et al. 2012) probably sundered ancestral populations of Sphenarium, causing to the divergences between the three major clades, whereas Quaternary climatic changes probably caused several vicariance events within the clades by promoting recurrent distribution shifts of the ancestral populations across the mountain ranges and the costal lowlands of Mexico. In addition, the fact that strongly supported monophyletic groups of Sphenarium are geographically restricted to well-defined biogeographic provinces (Figs. 15.1 and 15.2) indicates that vicariance events could have played a fundamental role on the diversification of the group. Therefore, the current parapatric distribution of the species, along with their narrow sympatric zones, has probably resulted from secondary dispersal events. Moreover, the phylogenetic relationships among the lineages of Sphenarium suggest that probably the common ancestor of the group occupied initially the outer lowlands and that younger lineages have more recently colonized inner basins and highlands of central Mexico.
The Mechanisms of Differentiation Among the Species
The genus Sphenarium is an assemblage of lineages with different levels of morphological and genetic divergence suggesting a complex interplay between evolutionary forces during the evolution of the genus. There are three broad patterns of differentiation that reflect the relative importance of evolutionary forces on the diversification of Sphenarium (Sanabria-Urbán et al. 2017). Firstly, despite some species pairs are very close genetically (e.g., S. histrio-S. mexicanum; S. macrophallicum-S. crypticum; S. rugosum-S. tarascum; and S. purpurascens-S. variabile), they strongly differ from each other by male genital morphology. Because male genitalia are known to be under strong sexual selection (Eberhard 2010), and sexually selected characters tend to diverge very rapidly (Hosken and Stockley 2004), sexual selection may have played a major role in the divergence among these Sphenarium species. A second pattern is found among species that are morphologically similar (e.g., S. miztecum-S. adelinae; S. histrio-S. occidentalis; and S. infernalis-S. rugosum) but strongly differ genetically, reveling cryptic diversity in the genus. In these cases, evolutionary processes different from sexual selection on male genitalia could have played an important role in the divergence of lineages. Recurrent isolation events and genetic drift in ancestral populations could have generated genetic rather than morphological divergence. The last pattern involves species that differ both morphologically and genetically (e.g., S. borrei and S. totonacum), which suggest an interplay between different evolutionary forces (e.g., drift and sexual selection) in driving the species differentiation. In addition, Sanabria-Urbán et al. (2015) found correlative evidence suggesting that natural selection on body size in response to altitudinal climatic variation could have also promoted the diversification of the genus Sphenarium. Despite phylogenetic relationships have heavily affected the body size and the climatic niche of Sphenarium species, they have diverged considerably in size, and large species are associated with high temperatures during the winter (Sanabria-Urbán et al. 2015). This climatic body size cline probably reflects the life history adaptability of Sphenarium grasshoppers. In lowlands, during benign winters, the window for development and reproduction may increase, allowing grasshoppers to achieve larger body sizes. Conversely, when mean temperatures are lower, body sizes become smaller. Similar body size clines associated with decreasing temperatures have been observed in other insects at higher latitudes (Roff 1980; Dingle et al. 1990; Berner et al. 2004). Smaller body sizes at low temperatures are commonly explained by natural selection favoring faster development by decreasing development time (reducing the number of nymphal instars or diapause (Dingle et al. 1990) or increasing growth rates (Hodkinson 2005). However, decreasing the time to maturity at low temperatures may have negative effects on the fitness of individuals by reducing reproductive success via small body sizes (Mousseau and Roff 1987; Abrams et al. 1996; Morbey 2013). Therefore, the smallest species of Sphenarium (S. purpurascens, S. minimum, S. planum, S. variabile, and S. tarascum) probably have lower fecundity than larger species, but they have been able to colonize highlands.
The geologic and climatic events over the last 10 mya in Mexico had a profound impact on the diversification of the genus Sphenarium causing the vicariance of the ancestral lineages within this genus along their distribution. These historic events also determinate in great extent the complex mosaic of environmental heterogeneity to which Sphenarium species have adapted. The low mobility of these univoltine and flightless grasshoppers, plus the combination of strong natural selection on adult body size and maturation times, could enhance the genetic isolation and consequently the speciation of these Neotropical grasshoppers.
Perspectives
The current understanding of the evolutionary history of Sphenarium grasshoppers provides the opportunity to investigate many aspects of the interaction between these grasshoppers and their host plant species. Given that the species of Sphenarium are polyphagous insects that can reach high population densities; they can represent a strong selective force for several plant species across their distribution range. However, the strength of their selective pressure on their host plants might differ geographically and temporally depending on both, the variation in their own populations’ densities and the variation on the composition and abundance of the plant in their communities. In those areas where these grasshoppers are more abundant, they might impose a stronger selective pressure on the plants. On the other hand, the distribution and abundances of their different host plant species also vary geographically and temporally. Thus, for some plant species and in some of their populations, these insects can be a more important selective factor than in others. Even though Sphenarium are generalist herbivorous, perhaps they have evolved in a complex mosaic of ecological interactions, generating places with high and low levels of coadaptation between them and their host plants. A recent study has depicted part of this complexity detecting geographic variation in defensive characteristics of the toxic plant D. stramonium associated with the abundance of S. purpurascens (Castillo et al. 2014). In some areas this grasshopper species exerts a strong selective pressure in D. stramonium toward reduction of the alkaloid atropine, whereas in other populations, a more derived alkaloid confers a greater defense. These results are congruent with geographic mosaic of adaptation in D. stramonium, in which Sphenarium grasshoppers are involved. Similar studies in other plant species could reveal similar responses, adding to our understanding of the relative importance that these grasshoppers have had on the evolution of defense mechanisms in plants. In this context, it should be considered the biogeographic history of the genus Sphenarium, and how it has influenced the geographic distribution of its host plant species. The distribution of Sphenarium species can generate geographic variation on the levels of herbivory which could explain the geographic distribution of some plant species. For instance, the sunflower (Helianthus annuus) reaches its southern distribution limit in the Mexican tropics, where Sphenarium species and other herbivores exert a tremendous herbivory pressure on the species (Lentz et al. 2008). The plants’ ability to colonize a new area can be constrained by its native generalist herbivores because the foreign plants may be poorly adapted to defend themselves against them (Avanesyan and Culley 2015). However, coadaptation to these herbivores may eventually happen allowing the foreign plants to expand their distribution ranges in the new areas (Schaffner et al. 2011). Therefore, dominant generalist herbivores, such as Sphenarium grasshoppers, might restrict more strongly the distribution of recently established plant linages, in comparison with older clades that have coexisted with these insects during their evolution. These predictions can be tested by obtaining phylogenetic and biogeographic reconstructions of the different host plant lineages of Sphenarium. On the other hand, phylogenetic and biogeographic reconstructions of both, Sphenarium grasshoppers and their host plants, would allow to infer how old the interactions between Sphenarium species and their host plants are. In addition, the phylogenetic information would provide the bases to search for common adaptations among different host plants on defense and/or tolerance against these generalist grasshoppers. Finally, this would help to better understand how the evolutionary history of Sphenarium has been influenced by the diversification of their host plants and vice versa.
References
Abrams PA, Leimar O, Nylin S, Wiklund C (1996) The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. Am Nat 147:381. https://doi.org/10.1086/285857
Adamu RS, Dike MC, Ogunlana MO (1999) Insects associated with soybean (Glycine max (L) Merr.) in Northern Nigeria. J Sustainable Agricult Environ 1:272–278
Ane N, Hussain M (2015) Diversity of insect pests in major rice growing areas of the world. J Entomol Zool Stud 4:36–41
Avanesyan A, Culley TM (2015) Herbivory of native and exotic North-American prairie grasses by nymph Melanoplus grasshoppers. Plant Ecol 216:451–464. https://doi.org/10.1007/s11258-015-0449-9
Bernays EA, Chapman RF (1977) Deterrent chemicals as a basis of oligophagy in Locusta migratoria (L.). Ecol Entomol. https://doi.org/10.1111/j.1365-2311.1977.tb00861.x
Berner D, Körner C, Blanckenhorn WU (2004) Grasshopper populations across 2000 m of altitude: Is there life history adaptation? Ecography (Cop) 27:733–740. https://doi.org/10.1111/j.0906-7590.2005.04012.x
Bryson RW, García-Vázquez UO, Riddle BR (2011) Phylogeography of Middle American gophersnakes: mixed responses to biogeographical barriers across the Mexican Transition Zone. J Biogeogr 38:1570–1584. https://doi.org/10.1111/j.1365-2699.2011.02508.x
Bryson RW, García-vázquez UO, Riddle BR (2012) Molecular phylogenetics and evolution relative roles of Neogene vicariance and quaternary climate change on the historical diversification of bunchgrass lizards (Sceloporus scalaris group) in Mexico. Mol Phylogenet Evol 62:447–457. https://doi.org/10.1016/j.ympev.2011.10.014
Buckley LB, Davies TJ, Ackerly DD et al (2010) Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc R Soc B Biol Sci 277:2131–2138. https://doi.org/10.1098/rspb.2010.0179
Cano-Santana Z, Castellanos-Vargas I (2009) Historia natural y ecología de Sphenarium purpurascens (Orthoptera : Pyrgomorphidae). In: Biodiversidad del Ecosistema del Pedregal de San Ángel. Universidad Nacional Autonoma de Mexico, pp 337–346
Castillo G, Cruz LL, Tapia-López R et al (2014) Selection mosaic exerted by specialist and generalist herbivores on chemical and physical defense of Datura stramonium. PLoS One 9. https://doi.org/10.1371/journal.pone.0102478
Cavender-Bares J, Ackerly DD, Kozak KH (2012) Integrating ecology and phylogenetics: the footprint of history in modern-day communities. Ecology
Cerritos R, Cano-Santana Z (2008) Harvesting grasshoppers Sphenarium purpurascens in Mexico for human consumption: a comparison with insecticidal control for managing pest outbreaks. Crop Prot 27:473–480. https://doi.org/10.1016/j.cropro.2007.08.001
Cigliano MM, Braun H, Eades DC, Otte D (2019) Orthoptera species file. Version 5.0/5.0. [1/8/2018]. http://orthoptera.speciesfile.org/Common/basic/Taxa.aspx?TaxonNameID=1109732
Cueva del Castillo R (2003) Body size and multiple copulations in a neotropical grasshopper with an extraordinary mate-guarding duration. J Insect Behav 16:503–522. https://doi.org/10.1023/A:1027303323242
Cueva del Castillo R, Nunez-Farfan J (1999) Sexual selection on maturation time and body size in Sphenarium purpurascens (Orthoptera : Pyrgomorphidae): correlated response to selection. Evolution (NY) 53:209–215
Cueva del Castillo R, Núñez-Farfán J (2002) Female mating success and risk of pre-reproductive death in a protandrous grasshopper. Oikos 2:217–224. https://doi.org/10.1034/j.1600-0706.2002.960203.x
Cueva del Castillo R, Núñez-Farfán J, Cano-Santana Z (1999) The role of body size in mating success of Sphenarium purpurascens in Central Mexico. Ecol Entomol 24:146–155. https://doi.org/10.1046/j.1365-2311.1999.00188.x
Debbarma A, Jayaraj J, Chandramani P, Senthil N, Ananthan M, Prabakaran K (2017) A survey on occurrence and diversity of insect pests of cauliflower in Dindigul and Theni districts of Tamil Nadu, India. Int J Curr Microbiol App Sci 6(8):2495–2505
Descamps M (1975) Etude du peuplement acridien de L’etat de Veracruz (Mexique). Folia Entomológica Mex 31:3–98
Dingle H, Mousseau TA, Scott SM (1990) Altitudinal variation in life cycle syndromes of California populations of the grasshopper, Melanoplus sanguinipes (F.). Oecologia 84:199–206. https://doi.org/10.1007/BF00318272
Duennes MA, Lozier JD, Hines HM, Cameron SA (2012) Geographical patterns of genetic divergence in the widespread Mesoamerican bumble bee Bombus ephippiatus (Hymenoptera: Apidae). Mol Phylogenet Evol 64:219–231
Eberhard WG (2010) Evolution of genitalia: theories, evidence, and new directions. Genetica 138:5–18. https://doi.org/10.1007/s10709-009-9358-y
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution (NY) 18:586. https://doi.org/10.2307/2406212
Fassakin K (1991) Studies on the effect of sowing depth and planting density on vegetative growth and leaf yield of two local cultivars of Ceratotheca sesamoides. Master of Science Project. University of Ilorin, Ilorin
Ferrari L, Orozco-Esquivel T, Manea V, Manea M (2012) The dynamic history of the Trans-Mexican Volcanic Belt and the Mexico subduction zone. Tectonophysics 522:122–149
Fornoni J, Valverde PL, Núñez-Farfán J (2003) Quantitative genetics of plant tolerance and resistance against natural enemies of two natural populations of Datura stramonium. Evol Ecol Res
Futuyma DJ, Agrawal AA (2009) Evolutionary history and species interactions. Proc Natl Acad Sci 106:18043–18044. https://doi.org/10.1073/pnas.0910334106
Gerhold P, Cahill JF, Winter M et al (2015) Phylogenetic patterns are not proxies of community assembly mechanisms (they are far better). Funct Ecol 29:600–614. https://doi.org/10.1111/1365-2435.12425
Gupta SK and Chandra K (2013). Annotated list of orthopteran insect pests in India. Bionotes: 117–122
Heinrichs EA, Barrion AT (2004) Rice-feeding insects and selected natural enemies in West Africa: biology, ecology and identification. International Rice Research Institute and Abidjan (Côte d’Ivoire): WARDA–The Africa Rice Center, Los Baños
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev Camb Philos Soc 80:489–513. https://doi.org/10.1017/S1464793105006767
Hosken DJ, Stockley P (2004) Sexual selection and genital evolution. Trends Ecol Evol 19:87–93. https://doi.org/10.1016/j.tree.2003.11.012
IITA (1984) Annual report for 1982. IITA, Ibadan
Jago ND (1998) The world-wide magnitude of orthoptera as pests. J Orthoptera Res 7:117–124
Jarvis E (1927) Notes on insects damaging sugar cane in Queensland. Division of Entomology. Bureau of Sugar Experiment Stations, 9–11 pp
Joern A (1979) Feeding patterns in grasshoppers (Orthoptera: Acrididae): Factors influencing diet specialization. Oecologia 38:325–347. https://doi.org/10.1007/BF00345192
Kekeunou S, Weise S, Messi J (2006) Insect pest incidence and variation due to forest landscape degradation in the humid forest zone of southern Cameroon: farmers’ perception and need for adopting an integrated pest management strategy. Afri J Biotech 5:555–562
Kevan DKM (1977) The American Pyrgomorphidae (Orthoptera). Rev la Soc Entomológica Argentina 36:3–28
Kevan DKM, Hsiung C-C (1985) The tropical and southern African species of Pyrgomorpha Audinet-Serville, 1838, other than the P. conica-group (Orthoptera: Acridoidea: Pyrgomorphidae). J Ent Soc Sth Afr
Khaemba BM, Mutinga MJ (1982) Insect pests of sunflower (Helianthus annuus l.) in Kenya. Insect Sci Applicts 3:281–286
Kobayashi T, Hasegawa T, Kegasawa K (1972) Major insect pests of leguminous crops in Japan. Trop Agric Res Ser, Japan 6:109–126
Kraft NJB, Cornwell WK, Webb CO, Ackerly DD (2007) Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat 170:271. https://doi.org/10.2307/4541080
Lee DW, Park JC, Kim DS, Kim CS, Choo HY (2007) Kinds and occurring time of insect pests in medicinal plant Garden. https://agris.fao.org/aos/records/KR2008003318
Lentz DL, Bye R, Sánchez-Cordero V (2008) Ecological niche modeling and distribution of wild sunflower (Helianthus annuus L.) in Mexico. Int J Plant Sci 169:541–549. https://doi.org/10.1086/528754
Mahabir S (1980) Relative toxicity of some insecticides to Chrotogonus trachypterus Blanchard (Orthoptera: Pyrgomorphidae). Pesticides 14:14–15
Mariño-Pérez R, Song H (2018) Phylogeny of the grasshopper family Pyrgomorphidae (Caelifera, Orthoptera) based on morphology. Syst Entomol 43:90–108. https://doi.org/10.1111/syen.12251
Mariño-Pérez R, Song H (2019) On the origin of the New World Pyrgomorphidae (Insecta: Orthoptera). Mol Phylogenet Evol 139. https://doi.org/10.1016/j.ympev.2019.106537
Márquez C (1962) Estudios de las especies del género Sphenarium basado en sus genitalia (Acrididae; Orthoptera), con la descripción de una nueva especie. An Inst Biol UNAM Ser Zool 33:247–258
Márquez C (1965a) Contribución al estudio de ortópteros de México, III. Estudios ecológicos preliminares de ortópteros del valle de Mezcala, Guerrero. An Inst Biol UNAM Ser Zool 35:87–93
Márquez C (1965b) Contribución al estudio de ortópteros de México, IV. Ortópteros del Pedregal de San Ángel, Villa Orbegón, DF. An Inst Biol UNAM Ser Zool 39:107–122
Mason JB (1979) Acridoidea of south west Angola (Orthoptera). Eos 53:91–132
Mastretta-Yanes A, Moreno-Letelier A, Pinero D et al (2015) Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Trans-Mexican Volcanic Belt. J Biogeogr 42:1586–1600
Morbey YE (2013) Protandry, sexual size dimorphism, and adaptive growth. J Theor Biol 339:93–99. https://doi.org/10.1016/j.jtbi.2013.05.009
Mousseau TA, Roff DA (1987) Natural selection and the heritability of fitness components. Heredity (Edinb) 59(Pt 2):181–197. https://doi.org/10.1038/hdy.1987.113
Núñez-Farfán J, Dirzo R (1994) Evolutionary ecology of Datura stramonium l. in central Mexico: natural selection for resistance to herbivorous insects. Evolution (NY) 48:423–436. https://doi.org/10.1111/j.1558-5646.1994.tb01321.x
Otte D, Joern A (1976) On feeding patterns in desert grasshoppers and the evolution of specialized diets. Proc Acad Nat Sci Philadelphia 128:89–126. https://doi.org/10.1038/scientificamerican09231854-16
Oyama K, Cano-Santana Z, Careaga S (1994) Estudios sobre la interacción herbívoro-planta en el Pedregal de San Ángel, México, D. F. In: Reserva ecológica behaviour “El Pedregal” de San Ángel: ecología, historia natural y manejo. Universidad Nacional Autónoma de México, Mexico City, pp 301–311
Page WW, Harris JRW, Youdeowei A (1980) Defoliation and consequent crop loss in cassava caused by the grasshopper (L.) (Orthoptera: Pyrgomorphidae) in southern Nigeria. Bull Entomol Res 70(1):151–163
Paraïso AA, Douro-kpindu OK, Onzo A et al (2012) The acridoidea of Benin (Orthoptera): an annotated checklist. Inter J Sci Advan Tech 2:22–52
Patra S, Rahman Z, Bhumita P, Saikia K, Thakur NSA (2013) Study on pest complex and crop damage in maize in medium altitude hill of Meghalaya. Bioscan 8(3):825–828
Ramos-Elorduy J, Moreno JMP (1989) Los Insectos comestibles en el México antiguo: estudio etnoentomológico. AGT, Mexico City
Reznick DN, Ricklefs RE (2009) Darwin’s bridge between microevolution and macroevolution. Nature 457:837–842. https://doi.org/10.1038/nature07894
Ríos-Casanova L, Cano-Santana Z (1994) Análisis cuantitativo de los artrópodos epifitos del Pedregal de San Ángel. In: Rojo A (ed) Reserva Ecológica “El Pedregal” de San Ángel: Ecología. Historia Natural y Manejo. Universidad Nacional Autonoma de Mexico, Mexico City, pp 275–281
Roff D (1980) Optimizing development time in a seasonal environment: The “ups and downs” of clinal variation. October 45:202–208. https://doi.org/10.1007/BF00346461
Sanabria-Urbán S, Song H, Oyama K et al (2015) Body size adaptations to altitudinal climatic variation in neotropical grasshoppers of the genus Sphenarium (Orthoptera: Pyrgomorphidae). PLoS One 10:e0145248. https://doi.org/10.1371/journal.pone.0145248
Sanabria-Urbán S, Song H, Oyama K et al (2017) Integrative taxonomy reveals cryptic diversity in neotropical grasshoppers: Taxonomy, phylogenetics, and evolution of the genus Sphenarium Charpentier, 1842 (Orthoptera: Pyrgomorphidae). Zootaxa 4274:1–86. https://doi.org/10.11646/zootaxa.4274.1.1
Schaffner U, Rindenour WM, Wolf VC et al (2011) Plant invasions, generalist herbivores, and novel defense weapons. Ecology 92:829–835. https://doi.org/10.2307/41739278
Seino RA, Ghogomu RT, Kekeunou S, Chifon RN, Manjeli Y (2013) An inventory of short horn grasshoppers in the Menoua Division, West Region of Cameroon. Agric Biol J N Am 4(3):291–299
Seino RA, Njoya MTM (2018) Species diversity of Pyrgomorphidae (Orthoptera: Caelifera) grasshoppers in the north west region of Cameroon. Int J Zool Appl Biosci 3:104–109
Soomro I, Sultana R, Wagan Ms, Kumar S, Solangi FH (2014) Mating strategies of Poekilocerus pictus (Fabricus, 1775) (Pyrgomorphidae: Acridoidea: Orthoptera). Pak J Entomol 29:21–25
Swamy BCH, Rajagopal D, Farooqi AA, Chakcravarthy AK (1993) Insect pests of Costus speciosus, a medicinal plant. Myforest 29:101–103
Thakur SK, Thakur MS (2011) Orthopteran crop-pest relationship in roper wetland and its environ Punjab, India. Int J Plant Anim Environ Sci 2:52–54
Tandon SK (1986) Grasshoppers of economic importance in India. In: Manual: Collection, preservation of insects and mites of economic importance. Zool Surv India, Calcutta: 35-46.
Valverde PL, Fornoni J, Núñez-Farfán J (2001) Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. J Evol Biol 14:424–432. https://doi.org/10.1046/j.1420-9101.2001.00295.x
Villalobos F, Carotenuto F, Raia P, Diniz-Filho JAF (2016) Phylogenetic fields through time: temporal dynamics of geographical co-occurrence and phylogenetic structure within species ranges. Philos Trans R Soc B Biol Sci 371:20150220. https://doi.org/10.1098/rstb.2015.0220
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Syst 33:475–505. https://doi.org/10.1146/annurev.ecolsys.33.010802.150448
Yang L, Nitin R, Marino-Perez R, et al (2019) Predictability in the evolution of Orthopteran cardenolide insensitivity. Philos Trans R Soc B. https://doi.org/10.6084/m9.figshare.c.4472423
Acknowledgments
We thank to Dr. Ken Oyama (Laboratory of Genetic and Molecular Ecology, ENES Morelia) and Dr. Hojun Song (The Song Lab of Insect Systematics and Evolution, Texas A&M University) for all the facilities provided. This research was funded by the projects CONACYT 57009 and PAPIIT-UNAM granted to RCC, as well as by the Theodore J. Cohn Research Fund granted to SSU.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sanabria-Urbán, S., Cueva del Castillo, R. (2020). The Evolution and Diversification of Neotropical Generalist Herbivores: The Evolutionary History of the Grasshopper Genus Sphenarium Charpentier, 1842. In: Núñez-Farfán, J., Valverde, P. (eds) Evolutionary Ecology of Plant-Herbivore Interaction. Springer, Cham. https://doi.org/10.1007/978-3-030-46012-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-46012-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46011-2
Online ISBN: 978-3-030-46012-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)