Abstract
Several studies provided evidence that family-level insect diversity remained flat throughout the initial mid-Cretaceous angiosperm radiation 125–90 million years ago. As this result has engendered considerable commentary, a reanalysis was done of a new dataset of 280 plant-associated insect families spanning the 174 million year interval of the Jurassic–Paleogene periods from 201 to 23 million years ago. Lineage geochronologic ranges were determined, and feeding attributes were characterized by: (i) dominant feeding guild (herbivore, pollinator, herbivore–pollinator, pollinator–mimic, xylophage); (ii) membership in one of eight functional feeding groups; and (iii) dominant plant host or host transition (cryptogam/fern only, cryptogam/fern → angiosperm, gymnosperm only, gymnosperm → angiosperm, angiosperm only). A time-series plot of insect lineages and their dominant plant–host affiliations resulted in four conclusions. First, insect lineages with dominant gymnosperm hosts reached a level of 95 families in the 35 million years preceding the initial angiosperm radiation. Second, earlier insect lineages with gymnosperm → angiosperm host transitions and newly originated insect lineages that developed dominant associations with emerging angiosperms rapidly diversified during the angiosperm radiation, later establishing a plateau of 110 families during a 20 million year interval after the initial angiosperm radiation. Third, these two diversity maxima were separated during the angiosperm radiation by a diversity minimum, the Aptian–Albian gap, indicating major turnover and time-lag effects associated with the extirpation and acquisition of plant associations. Last, insect lineages most affected during this interval were herbivores and pollinators, exophagous feeders, and those hosting gymnosperms, angiosperms and gymnosperm → angiosperm transitions. These data largely explain the flat or even decreased level of insect diversity immediately before, during, and after the initial angiosperm radiation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aptian–albian gap
- Cretaceous
- Feeding guild
- Fern
- Functional feeding group
- Gymnosperm
- Host–plant preference
- Plant–insect interactions
- Stasis
- Time lags
1 Introduction
In 1993, Labandeira and Sepkoski published a report in Science documenting the fossil diversity of insects from an assessment of their family-level lineage diversity through time. One of the results of this study was the clear presence of stasis in the rate of origination of insect diversity immediately before, during, and after the initial radiation of angiosperms in the mid-Cretaceous (Fig. 13.1). Some of the reaction to this discovery was negative, particularly from some paleoentomologists and paleobotanists. The discontent was attributable to the view that a slackening of insect diversity contravened a well-received view that the diversity of associated herbivores and pollinators should have significantly increased in concert with sharply increased diversification of angiosperm lineages. This “coevolutionary” view presumed that an expected, coordinated evolution would occur between angiosperms and their various insect associates in a multiplicative and opportunistic manner during an interval of resource expansion that would include food, shelter, mating sites, and other features essential for survival of insects and their host–plants. An unexpressed corollary to this view was that earlier gymnosperms were largely unavailable to insect lineages that potentially could interact with plants.
A reproduction of Fig. 4 from Labandeira and Sepkoski (1993), showing insect family-level diversity increasing monotonically from the Middle Triassic to the Late Jurassic, stabilizing throughout the Cretaceous during the ascendancy of the angiosperms, and rising again during the Paleogene, indicated by the Baltic amber spike. The vertical axis is semilogarithmic and the dashed line is interpretive, indicating a divergence from exponential diversification during the Cretaceous. The only change to this figure has been relabeling of the abandoned “Tertiary Period” to reflect its modern division into an earlier Paleogene Period (Pg) and later Neogene Period (Ng). Reproduced with permission from American Association for the Advancement of Science
During the early 1990s to mid-2000s not much was known about the Mesozoic fossil record of insects and land plants. This ignorance affected understanding of gymnosperm relationships with insects prior to the major emergence of angiosperms during the Aptian to Turonian stages from 125 to 90 million years ago. There was limited evidence for the consumption of live plant tissues (Labandeira 2013), although a few studies documented several Eurasian, mid-Mesozoic insect lineages with pollen as gut contents (Krassilov et al. 2007). There was isolated documentation, overwhelmingly from paleobotanists, that evidence for insect herbivory was present on gymnospermous plants typical of mid-Mesozoic floras, such as puncture wounds on cheirolepidiaceous conifers (Watson 1977), borings in conifer woods (Zhou and Zhang 1989), and galls on bennettitalean foliage (Harris 1942), but these reports were too few to provide any convincing conclusion that preangiospermous floras had insects that used gymnosperms appreciably for food. Only recently have gymnosperm-dominated floras been systematically studied to document broad patterns of herbivory within specific habitat settings (Ding et al. 2014).
During this time, there were additional examinations of insect family-level diversity in the fossil record (Jarzembowski and Ross 1993, 1996; Alekseev et al. 2001). These reports confirmed that relative stasis existed for insect family-level diversity throughout the same interval of the angiosperm radiation, even though the data came from largely separately assembled datasets (Dmitriev and Zherikhin 1988; Rasnitsyn 1988; Labandeira 1994; Jarzembowski and Ross 1996). After publication of these studies and during the 2000s, a different approach was pursued—examination of the evidence for preangiospermous plant–insect interactions, focusing on herbivory, pollination, and eventually mimicry. Long-term projects with multiple colleagues were initiated to examine diverse preangiospermous, gymnosperm-dominated floras (Labandeira 2006; Ding et al. 2014). Current preliminary studies of Mesozoic herbivory use a similar methodology as those for the early Permian (Labandeira and Allen 2007; Schachat et al. 2014), the Cretaceous–Paleogene (K-Pg) boundary (Labandeira et al. 2002; Wilf et al. 2006), and the mid-Paleogene climate events (Wilf et al. 2005; Wappler et al. 2012; Currano et al. 2009). In addition, studies of mid-Mesozoic pollination have centered principally on the preangiospermous mid-Mesozoic (Ren et al. 2009; Labandeira 2010; Peñalver et al. 2012). Recently, there has been detection of mid-Mesozoic mimicry (Wang et al. 2012b). The purpose of these efforts in examining different aspects of plant–insect interactions and associations with gymnosperms in floras prior to the angiosperm radiation was to establish independent lines of evidence for understanding why there was no increase in diversity commensurate with the angiosperm radiation.
In this contribution, a comprehensive summary of family-level, plant-associated lineages with dominantly cryptogam/fern, gymnosperm, and angiosperm host relationships is provided for an interval of time encompassing the Jurassic, Cretaceous, and Paleogene periods from 201 to 23 million years ago. The major focus of this report is to assess the diversity pattern and effects that the initial rise of angiosperms had on insect families that previously hosted cryptogams, ferns, and gymnosperms, and whether the subsequent shift of insect lineages toward angiosperm hosts is associated with a marked increase in their diversity. It is hoped that this exercise would spur other examinations of insect diversity patterns, such as assessments at the genus rank, through this formative time interval that resulted in much of the modern terrestrial world.
2 Methods and Definitions
2.1 Herbivory: Dominant Feeding Guilds, Functional Feeding Groups, and Plant Hosts
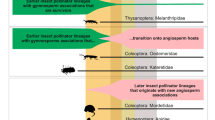
The first of these dietary habits of insects is the feeding guild (Table 13.1, Fig. 13.2). Five major feeding guilds are considered to encompass the variety of insect relationships with plants that are specified in the primary documentation (Table 13.1, Fig. 13.2). These dominant feeding guilds are herbivory, pollination, herbivory–pollination, pollination–mimicry , and xylophagy . The feeding guilds characterize family-level lineages of the eleven major orders of plant-associated insects during the Jurassic to Paleogene periods: Orthoptera, Phasmatodea, Thysanoptera, Hemiptera, Neuroptera, Coleoptera, Trichoptera, Lepidoptera, Mecoptera, Diptera, and Hymenoptera. These principal insect lineages constitute a broad variety of habitus types affiliated with orthopteroid, hemipteroid, and holometabolous developmental modes.
Distribution of the eleven major, plant-associated insect lineages and their host–plant assignments during the Jurassic to Paleogene interval. The 35 million year-long angiosperm radiation encompasses the Aptian through Turonian stages of the mid Cretaceous as a vertical gray column at center. Major plant–host associations of herbivory, pollination, xylophagy, herbivory–pollination, and herbivory–mimicry, and their dominance in cryptogam/fern (cr/fn), cryptogam/fern → angiosperm, gymnosperm (gymno), gymnosperm → angiosperm and angiosperm (angio) hosts are indicated in the inset. Darker hues indicate gymnosperm hosts; lighter hues indicate angiosperm hosts. Data are from Table 13.1
Herbivory is an antagonistic interaction defined as the consumption of live, photosynthetic plant tissues such as foliage, stems, and other organs. Although pollination is an interaction that may include the consumption of photosynthetic or non-photosynthetic tissues such as seeds, it is primarily characterized as the transfer of pollen from the pollinate to the ovulate reproductive organ of a conspecific plant host. When an insect taxon harbors two dominant plant-interactional strategies, such as an immature insect instar (nymph, larva) feeding on foliar tissues and an adult actively pollinating a different suite of host plants, such an interaction is herbivory–pollination. Similarly, a few pollinators possess the nonfeeding interaction of mimicry to deter or otherwise avoid insect predation but frequently are pollinators as well, in which case they are pollinator–mimics. Last, xylophagy is the consumption of wood, but for this study the feeding guild obligately includes the consumption of associated live tissue, such as subcortical cambia, pith parenchyma, or other meristematic tissues that include live, actively dividing cells.
The second dietary habit is the functional feeding group. The data are divided into eight functional feeding groups for a more discrete, ecologically different characterization (Tables 13.1 and 13.2). The functional feeding groups are the modes of access to food that are effected principally through the action of mouthparts. External feeding is the consumption of foliage such as skeletonization and margin feeding in which the insect is outside of the tissue being consumed. Piercing and sucking consists of puncturing host tissues by specialized, stylate mouthparts and the subsequent sucking of fluid food. Surface fluid feeding is where surface fluids, such as pollination drops, floral or extrafloral nectar, or other plant exudates, are imbibed without inflicting a wound. Palynivory, or consumption of pollen, can be achieved by a variety of insect mouthpart types, in which ingestion may represent punctured pollen grains or entire to highly fragmented pollen clusters. Most pollinating insects are surface fluid feeders or palynivores.
The four previous functional feeding groups are ectophagous, occurring with the insect to the outside of the tissue consumed; by contrast, the following four interactions are endophytic, whereby the insect, typically an immature such as a larva or nymph, is lodged within plant tissues. Galling is a complex interaction whereby an insect immature inhabits a chamber surrounded by tumor-like plant tissues of newly created, inner, nutritive tissue for larval sustenance, outer hardened tissues for protection, and vascular tissue for food and water supply. Gall interactions are essentially parasitic and the galler arthropod hormonally controls the plant–host tissue and organ development adjacent the gall. Leaf mining is another endophytic interaction wherein an egg hatches into a larva that begins to consume foliage tissue, leaving a distinct frass trail and a leaf-mine with features such as successive width enlargements and a terminal chamber often used for pupation. Seed predation represents a variety of herbivore feeding types that have the common effect of consuming the embryonic and sustaining tissues of an ovule or seed. Wood boring consists of consumption of live meristematic tissues and parenchyma, often associated with tunneling through wood, the fabrication of borings, galleries and pupal chambers, and the consumption of associated fungi.
The third major characterization of dietary attributes of insect lineages in Table 13.1 is the dominant plant hosts and host transitions, linked to Fig. 13.2. We present five principle hosts and host transitions during the 174 million year interval from the Triassic–Jurassic to the Paleogene–Neogene boundaries. First, some insect lineages cryptogam or fern hosts (cryp/fern) only. Second, other cryp/fern insect lineages have transitioned from cryp/fern to angiosperm hosts (cryp/fern → angio). Third, many insect lineages have always had dominantly gymnosperm hosts in their history. Fourth, some insect lineages on gymnosperms have switched their dominant hosts to angiosperms (gymno → angio). Fifth, more recent insect lineages have always had angiosperms (angio) as their dominant hosts.
Cryptogams included the familiar groups of liverworts, mosses, and lycopods; ferns consist of horsetails and marattialean and filicalean ferns. By contrast, gymnosperms include a diverse spectrum of extinct lineages (Taylor et al. 2009), such conifers, caytonialean and corystospermalean seed ferns, diverse ginkgophytes, bennettitaleans, and pentoxylaleans. Most gymnosperm sublineages became extinct during the angiosperm radiation, although several lineages now are known to have survived into the Gondwanan Paleogene, such as cheirolepidiaceous conifers (Barreda et al. 2012), corystosperm seed ferns (McLoughlin et al. 2008), Mesozoic-style ginkgoaleans (Hill and Carpenter 1999), and bennettitaleans (McLoughlin et al. 2011). By the close of the angiosperm radiation, all major groups of angiosperm lineages were established, including basal “paleoherb” lineages, monocots, Chloranthaceae, eumagnoliids, and core eudicots (Friis et al. 2010), and achieved ecological prominence in local habitats (Crane 1987).
2.2 Data Collection
Several initial conventions were used to provide a chronology of the summarized data (Table 13.1, Fig. 13.2). The 174 million year interval from the Triassic–Jurassic boundary at 201 Ma to the Paleogene–Neogene boundary at 23 Ma was used to document time durations of all identified plant-associated lineages. This time interval, consisting of the Jurassic, Cretaceous, and Paleogene periods, is divided into ca. 75 million years before the beginning of the angiosperm radiation at 125 Ma, and ca. 75 million years after its end at 90 Ma, providing a sufficiently long interval to record lineage turnover, long-term host–plant associations and major host transitions. These host associations occurred during the 35 million year-long angiosperm radiation from 125 Ma (ca. Barremian–Aptian boundary) to 90 Ma (ca. Turonian–Coniacian boundary). Insect lineage occurrence data were plotted at the midpoint for each geologic stage in which the insect lineage occurred. The range-through method was used (Labandeira and Sepkoski 1993), in which the first occurrence datum and last occurrence datum defined the continuous presence of the lineage, whether or not it has been recorded in intervening stages. Occurrence data for the Jurassic and Cretaceous periods were plotted at the level of the geologic stage, whereas Paleogene stage-level data were amalgamated at the more inclusive level of the geologic epoch. The most recent, internationally approved, standardized geochronology was used (Gradstein et al. 2012).
A second set of guidelines circumscribed the early angiosperm fossil record. Background information for the Jurassic through Paleogene record of land plants originated from several sources, including the mutually consistent and occasionally rich palynological, mesofossil, and macrofossil records (Friis et al. 2011). Of relevance to data collection is the origin of angiosperms during the early Cretaceous Period, consistent with a wide variety of paleobotanical and plant-morphological evidence (Crane et al. 1995), and increasingly with molecular evidence (Bell et al. 2010; Magallón 2010). The origin of angiosperms is taken as no earlier than the mid-Hauterivian stage at ca. 135 Ma (Friis et al. 2011). The subsequent, primary diversification interval of angiosperms occurred during the 35 million year interval from the Barremian–Aptian stage boundary to the Turonian–Coniacian stage boundaries (Hughes 1994).
A third group of procedures were employed to establish the presence of fossil insect lineages. Several compendia were consulted to determine occurrence data for fossil insect lineages (Dmitriev and Zherikhin 1988; Rasnitsyn 1988; Carpenter 1992; Ross and Jarzembowski 1993; Labandeira 1994; Evenhuis 1994; Rasnitsyn and Quicke 2002; Grimaldi and Engel 2005; Sohn et al. 2012), buttressed by updates from recent taxonomic insect literature and the online Paleobiology Database (PBDB, 2014), accessed through the Fossil Works portal. As many of the earlier compendia had spurious occurrences, it was essential to consult considerably more modern sources to rectify synonymies, delete unvetted data, add new occurrences, and provide more current time-range extensions or contractions. After these filters were used, the culled dataset consisted of 280 family-level fossil insect lineages. The family was the focal taxonomic rank of interest. Alternative, more modern, classifications occasionally demote families to subfamily rank, a consequence that was taken into account in constructing Fig. 13.2. The insect lineage dataset consisted of 36.8 % Hemiptera, by far the most represented group; ca. 14 % each of Coleoptera and Lepidoptera; ca. 10 % each for Diptera and Hymenoptera; ca. 4–6 % each for Orthoptera and Thysanoptera, and 1–1.5 % each for the least abundant lineages of the Phasmatodea, Neuroptera, Trichoptera, and Mecoptera (Table 13.1, Fig. 13.1).
2.3 Establishing Feeding Guild, Functional Feeding Group and Plant–Host Assignments
Eight criteria were used to establish plant–host assignments of herbivory, pollination, xylophagy, and mimicry. These criteria can be divided into habitat-related ecological features and insect-specific morphological attributes. For broad-scale ecological features, the first consideration consists of broad, host–plant affiliations and related ecological attributes of modern descendant taxa, particularly if significant agricultural, entomological, or botanical information is available (Labandeira 1998). This process is taxonomic uniformitarianism (Dodd and Stanton 1990), and assumes that no or minimal host–plant shifts have occurred since the earliest fossil occurrence of the insect lineage in question. A second criterion involves the taxonomic spectrum of herbivorized plants of the flora in which an insect taxon co-occurs. Obviously, the host preferences of an insect in a preangiospermous flora can be safely attributed to a cryptogam, fern or gymnosperm. Conversely, an insect occurring in a diverse flora and consisting only of angiosperms can reasonably be associated with an angiosperm host. A third criterion involves specification of a particular damage type (Labandeira et al. 2007b) that could be attributed to a certain, family-level taxon. An example is the assignment of distinctive leaf mines occurring on angiosperm leaves of a sycamore host species (Platanaceae) from the early Paleocene of Montana, United States, to the dipteran family Agromyzidae (Winkler et al. 2010).
Four additional criteria indicate that host affiliations may be based on morphological features. The fourth criterion is the mouthpart structure of a representative insect taxon from the group in question (Labandeira 1997), which in some instances can be linked to particular types of herbivore damage, pollinator access, or wood boring in the same flora. An example would be the distinctive and specialized phytophagous mouthparts of weevils from the Yixian Formation in northeastern China (Davis et al. 2013), that also would imply gymnospermous plant hosts. Fifth, is presence of gut contents consisting of plant material (Rasnitsyn and Krassilov 2000) or pollen (Krassilov et al. 2007), which provides direct evidence of host affiliations of the insect consumer. Sixth, for pollinator assignment to plant hosts, certain features can be important, such as pollen plastered or attached to the mouthparts, or ventral aspect of the head capsule the associated insect with specialized, pollen-gathering structures such as bee corbiculae (Engel 2000) or thrips ring setae (Peñalver et al. 2012). The seventh criterion, also applicable to pollinators, is the presence of particular plant features that would indicate pollination (Labandeira et al. 2007a). For gymnosperm pollinators of the mid-Mesozoic, probed structures such as integumental tubes, deep funnels, and channels in ovulate organs were used by long-proboscid insects to access nectar-like pollination drops (Ren et al. 2009).
Last, in the case of mimicry, occasionally plant foliage shares an uncanny, detailed resemblance (the models) to particular co-occurring insect species (the mimics). Examples include strong resemblance of wings from one neuropteran species to a particular fern pinnule (Wang et al. 2010); or the entire body of another neuropteran species to a particular ginkgophyte leaf (Wang et al. 2012b).
2.4 Rationale for Understanding Gymnosperm-to-Angiosperm Host Transitions
The initial phase of angiosperm diversification established all major angiosperm lineages during a 35 million-year-long interval that encompassed the four mid-Cretaceous stages of the Aptian, Albian, Cenomanian, and Turonian. It would have been during this time interval that many insect lineages associated with gymnosperm hosts but known to have angiosperm-dominant associations in the more recent part of the geologic record would have shifted to angiosperm hosts (Tables 13.1, 13.2, and 13.3). Given that the angiosperm radiation is represented by four geologic stages during which the shift occurred, transfer ratios were allocated to each of the four constituent stages to represent a linear, monotonic shift from gymnosperm to angiosperm hosts. For the Aptian stage, 25 % of 60 insect lineages were transferred from gymnosperm → angiosperm hosts (column D of Tables 13.2 and 13.3, in bold lettering) to angiosperm-only hosts (column E of Tables 13.2 and 13.3); analogous values for the Albian stage were 50 % of 56 families; for the Cenomanian, 75 % of 63 families; and for the Turonian, 100 % of 64 families, after which all families with gymnosperm-to-angiosperm host transitions were tabulated in the angiosperm-only host column. These transfers are independent of the gymnosperm-only host column which retained dominantly gymnosperm hosts but never acquired dominantly angiosperm hosts.
In a manner parallel to that of the gymnosperms detailed above, insect lineages associated with cryptogams and ferns were evaluated during the angiosperm radiation (column A of Tables 13.2 and 13.3). Similarly, lineages were assessed that possessed dominant cryptogam or fern associations which shifted to angiosperm-dominant associations during the angiosperm radiation (column B of Tables 13.2 and 13.3).
3 Results
Figure 13.2 depicts range-through occurrences of 280 vertically arrayed, family-level lineages that represent eleven plant-associated insect orders along a Jurassic through Paleogene time series. The insect lineages are characterized by the dominant feeding modes of herbivore, pollinator, herbivore–pollinator, pollinator–mimic, and xylophage, and whether their dominant hosts are cryptogams/ferns (purple), gymnosperms (darker hue), or angiosperms (lighter hue), as indicated in the legend insets. Major gymnosperm-to-angiosperm host–plant transitions during the angiosperm radiation (gray vertical column) are indicated. The data in Fig. 13.2 are restated in Table 13.3, which is a geochronologic stage-by-stage summary of the raw data in Table 13.1. Summary Fig. 13.3 details the trivariate relationship between (i) the diversity of fossil insect families in the vertical axis), (ii) their major plant–host associations of cryptogams/ferns (purple), gymnosperms (dark green), and angiosperms (light green) in the field of the figure, and (iii) stage-level geologic time in the horizontal axis. While not expressed graphically, the functional-feeding-group data in Table 13.1 is presented in summary form in the middle columns of Table 13.2. These data provide a qualitative description of functional feeding strategies for each insect lineage that are not apparent from their role in a dominant feeding guild or from their host–plant associations.
Plot of the major plant hosts (field of view) associated with plant-associated fossil insect families (vertical axis) versus geologic time (horizontal axis). Data are derived from Table 13.1, summarized in Table 13.3. The purple color indicates cryptogam and fern hosts; dark green indicates gymnosperm hosts; light green indicates angiosperm hosts. The vertical column indicates the interval of time represented by the initial angiosperm radiation
3.1 Plant-Feeding Features of Jurassic to Paleogene Insect Lineages
The dataset of 280 plant-associated insect families are categorized by order and partitioned into three feeding-related ecological attributes (Table 13.2). The first feeding attribute is the dominant feeding guild, the second is the major functional feeding group, and the third is the dominant plant hosts and their transitions. For the dominant feeding guild, the most frequently encountered category are herbivores (56.4 % of all occurrences), then herbivore–pollinators (19.6 %), pollinators (17.5 %), xylophages (5.0 %), and pollinator–mimics (1.4 %). Of these five categories, herbivory dominates all other interactions in the dataset.
For the functional feeding group (Table 13.2), the most frequently encountered mode is piercing and sucking (34.6 % of all occurrences), then external feeding (28.2 %), surface fluid feeding (14.3 %), galling (6.4 %), wood boring (5.7 %), leaf mining (4.3 %), palynivory (3.6 %) and seed predation (2.9 %). These proportions indicate that external (ectophagous) feeding predominates for ca. four-fifths of the occurrences whereas internal (endophagous) feeding contributes to only one-fifth of the data.
The third attribute is the identity of the dominant plant hosts and the amount of host switching among the dominant insect families. Those lineages with angiosperms as the dominant host represent 42.9 % of all families, whereas those with gymnosperms as the dominant hosts consisted of 29.3 %. Lineages hosting gymnosperm hosts and existing prior to the advent of angiosperms but later shifting to angiosperm-dominant hosts provided 24.3 % of families. Cryptogams and ferns played a minor role as major hosts of insect lineages, consisting of 1.4 % of all cryptogam/fern-only occurrences and similarly 1.1 % of all cryptogam/fern lineages transitioning onto angiosperms.
3.2 Gymnosperm Versus Angiosperm Host Use Before, During, and After the Angiosperm Radiation
Data from Table 13.3 are plotted in Fig. 13.3. Shown in purple for Fig. 13.3 are insect families that retained their dominant cryptogam/fern hosts to the end of the Paleogene Period (trajectory A), recorded from column A of Table 13.3; and those that shifted dominantly to angiosperms during the angiosperm radiation (trajectory B), recorded from column B of Table 13.3. Likewise, shown in dark green are insect families that have kept their dominantly gymnosperm hosts, recorded from column C of Table 13.3, to which are added those insect lineages that transitioned from earlier gymnosperm-dominant hosts to angiosperm-dominant hosts after the angiosperm radiation, recorded from column D of Table 13.3 in bold lettering (See Sect. 13.2.4 for details). Insect family-level diversity with gymnosperm hosts thus represent the summation of columns C and D in Table 13.3, plotted as trajectory C + D in Fig. 13.3. Insect families with dominantly angiosperm hosts and originating during or after the angiosperm radiation are shown in light green and provide the most sustained increase of a host-affiliated insect group (trajectory E).
Two derivative features involving insect families with particular plant–host affiliations are depicted in Fig. 13.3. First, insect families with gymnosperm-dominant plant hosts, shown in trajectory C + D, form a distinct diversity plateau of ca. 95 families during the 20 million year-long Berriasian to Barremian interval, perhaps extending back in time to a decreased level of ca. 85 families to the Oxfordian stage another 20 million years earlier. After the Barremian stage, and the angiosperm radiation, insect lineages with gymnosperm hosts decrease linearly and monotonically to a flat diversity level of 10–14 insect families. By contrast, insect families with angiosperm-dominant hosts of trajectory E increase linearly and monotonically commencing at the angiosperm radiation, and reaching a sustained plateau of 110 families for the ca. 20 million years of the Turonian through Maastrichtian stages. Thereafter, insect families with angiosperm-dominant hosts increase dramatically into the late Paleogene.
Other than these two diversity plateaus, Fig. 13.3 illustrates a distinctive gap between the trajectories of C + D and E. Before and after the crossover between the gymnosperm-dominant and angiosperm-dominant family diversity curves of insects, there is a collective diversity minimum, the Aptian–Albian gap. The Aptian–Albian gap spans the angiosperm radiation and represents a significant decrease of 45 % from the earlier gymnosperm plateau of 95 families and 53 % of the later angiosperm plateau of 110 families.
4 Discussion
Three broader aspects of these findings deserve an extended mention. An obvious issue is to what extent does the data presented here explain the presumed “counterintuitive” result reported in Labandeira and Sepkoski (1993) that there was no increase in insect diversity during the formative interval of initial angiosperm diversification (Fig. 13.1)? Secondly, what is the meaning of the earlier gymnosperm and later angiosperm plateaus that bracket the angiosperm radiation, and do they have any relationship to the intervening Aptian–Albian gap (Fig. 13.3)? Last, is there a broader message about attempting to understand the role of fossil insect diversity vis-à-vis the angiosperm radiation, and vice versa, by using multiple approaches of investigation (Fig. 13.4).
Three mecopteran and a thysanopteran insect association with mid-Mesozoic gymnosperms. a The mecopteran long-proboscid pollinator, Lichnomesopsyche gloriae (Mesopsychidae, entry 224 of Table 13.1 and Fig. 13.2), with proboscis entering an integumental channel in the ovulate organ of Caytonia sewardi (Caytoniaceae) from the Callovian of Inner Mongolia, China. b Another mecopteran long-proboscid pollinator, Vitimopsyche kozlovi (Mesopsychidae, also entry 224 of Table 13.1 and Fig. 13.2), bearing Classopollis pollen and probing the ovulate organ catchment funnel of Alvinia bohemica (Cheirolepidiaceae) from the Barremian of Liaoning, China. c The mecopteran leaf mimic, Juracimbrophlebia ginkgofolia (Cimbrophlebiidae, entry 223 of Table 13.1 and Fig. 13.2), resembling a multilobed Ginkgoites leaf of Yimaia capituliformis (Ginkgoaceae) from the Callovian of Inner Mongolia, China. d The thysanopteran punch-and-suck pollinator, Gymnopollisthrips minor (Melanothripidae, entry 25 of Table 13.1 and Fig. 13.2), with Cycadopites sp. pollen grains on the ovulate organ of Nehvezdyella bipartita (Nehvezdyellaceae) from the Aptian of Spain. Drawings reprinted with permission: (a) and (b) courtesy of Mary Parrish, N.M.N.H. Department of Paleobiology in Washington, DC, USA; (c) courtesy of Wang Chen, C.N.U. College of Life Sciences in Beijing, China; and (d) courtesy of Enrique Peñalver, Instituto Geológico y Minero de España in Madrid, Spain)
4.1 Reasons for the Mid-Mesozoic Constancy of Insect Family-Level Diversity
There are several, independent explanations that could explain the relative stasis of family-level insect diversity during the angiosperm radiation. One reason, based on evidence from this report, is that an expectation of elevated insect diversification during the angiosperm radiation that would range from diffuse to intimate coevolution (Friis et al. 2011), needs to be balanced by evidence indicating equally high associational diversity between insects and gymnosperms prior to the angiosperm radiation. Given recent developments in understanding the associational diversity between gymnosperms and insects prior to and during the angiosperm radiation (e.g., Ratzel et al. 2001; Ren 1998; Ren et al. 2009; Labandeira 2010; Wang et al. 2012b; Peñalver et al. 2012; Ding et al. 2014), it is highly likely that gymnosperm–insect interactions preceding the angiosperm radiation were almost or just as diverse as angiosperm interactions that followed the event.
A second reason involves the Mesozoic Lacustrine Revolution, which evidently changed food-web structure of lotic and lentic ecosystems during the late Jurassic to early Cretaceous (Buatois et al. 2015). The environmental context of this transformation involves the change from detritivore based, typically hypotrophically stratified water bodies (Zherikhin et al. 1999), to herbivore dominated, typically pseudoligotrophically stratified water bodies (Sinitshenkova 2002). This physiochemical and biological turnover in aquatic ecologic structure occurred during the mid-Cretaceous and is synchronous with an aquatic insect extinction event (Sukatsheva 1991; Buatois et al. 2014). Approximately 20 family-level insect lineages became extinct at the Mesozoic Lacustrine Revolution (Buatois et al. 2014), supplemented by an additional 30 % of the plant-associated insect families during the same time interval.
A third cause for the constancy of diversity involves the parasitoid diversification of especially Hymenoptera, and to a lesser extent Diptera, during the Jurassic and Early Cretaceous (Rasnitsyn 1980). This major radiation of major, high-ranked lineages (Labandeira 2002) had a major effect not only on top to down regulation of herbivores in terrestrial webs, but significantly increased Jurassic and Early Cretaceous insect diversity that are captured in global compendia (Table 13.1, footnote 2) and in derivative plant–insect studies (Labandeira and Sepkoski 1993; Jarzembowski and Ross 1996; Alekseev et al. 2001). The inclusion of parasitoid insect families prior to the angiosperm radiation would have the effect of increasing insect diversity and balancing diversity levels after the angiosperm radiation.
Last, there is considerable evidence from modern molecularly based phylogenetic analyses that some plant-associated insect lineages diversified preceding the angiosperm radiation. Evidence for this comes from the major hyperdiverse clades such as the Hemiptera (Moran et al. 2005; Cocroft et al. 2008; Wang et al. 2012a), Hymenoptera (Rasnitsyn 1980; Davis et al. 2010), Coleoptera (Farrell 1998; McKenna et al. 2009; Wang et al. 2013), Diptera (Ren 1998; Labandeira 2005), and Lepidoptera (Imada et al. 2011; Zhang et al. 2013). The deeper extensions suggest that these lineages were diverse and actively consuming live tissues of cryptogams, ferns, and gymnosperms from millions to a few tens of millions of years before the initial angiosperm diversification interval.
Contributions of family-level insect taxa from these four data sources would provide relative stasis in family-level insect diversity throughout the late Jurassic and into the early Paleogene (Fig. 13.1). However, of these data sources, plant–host-associated families of insects likely were most important.
4.2 Host Switching, Diversity Plateaus and the Aptian–Albian Diversity Low
The pattern of gymnosperm-to-angiosperm host–plant dominance throughout the 100 million year interval from the Callovian (166 Ma) to the K-Pg extinction event at the end of the Cretaceous (66 Ma) potentially reveals the family-level insect dynamics associated with this shift (Fig. 13.3). An upper limit of 95 families was reached for insect lineages whose hosts were dominantly to exclusively gymnosperms, supplemented by a minor level of cryptogam and fern associations. This plateau disappeared at the beginning of the angiosperm radiation, as major older insect lineages with gymnosperm (and cryptogam and fern) hosts shifted onto new angiosperm hosts (Peñalver et al. 2012; Ding et al. 2014), or became extinct, and as new major insect lineages initially hosting angiosperms increased dramatically in diversity (Labandeira et al. 1994; Hartkopf-Fröder et al. 2011). By the Santonian stage (85 Ma) of the Late Cretaceous, an upper limit of 110 families was established, which remained until a dramatic diversity increase following the K-Pg event.
Separating the earlier plateau of gymnosperm-dominated families from subsequent and somewhat more elevated plateau of angiosperm-dominated families is the Aptian–Albian gap, which represents an interval of time characterized by transition from gymnosperm to angiosperm hosts. The probable cause of this gap is time lags that occur between when a food resource is available and when it becomes herbivorized. Geochronologic lags have been demonstrated at time intervals such as the appearance of vascular plant tissues in the earlier Devonian, and when they are later herbivorized during the mid-Paleozoic (Labandeira 2007); additionally, time delays occur in the colonization of eudicot plant hosts by lepidopteran leaf-mining genera during the late Cretaceous and Cenozoic (Lopez-Vaamonde et al. 2006). This downturn in plant-associated insect diversity is evident in the coarse-grained epoch-level analysis of Jarzembowski and Ross (1993) and the fine-grained stage-level analyses of Alekseev et al. (2001), although the gap appears phase-shifted toward the late Cretaceous by a stage or two. An Aptian–Albian minimum also may be present in aquatic insect lineages (Buatois et al. 2014), but no relevant analysis of family-level aquatic insect diversity has been made for this interval.
The presence of two, successive upper bounds for insect lineages with gymnosperm- and angiosperm-dominant hosts separated by a diversity minimum (Fig. 13.3), suggests a global event and major plant–host replacement during this 35 million-year interval. Such an event would represent a significant scaling up from considerably more spatiotemporally and taxonomically confined host shifts illustrated between plants and insects from a variety of modern habitats (Pellmyr and Seagraves 2003; Cocroft et al. 2008). One particular system is nymphaline butterflies (fritillaries) and their angiosperm lamialean (mints and relatives) and asteraceous (daisies and relatives) hosts. One study (Nylin and Wahlberg 2008) indicates that host shifts of angiosperm food plants by fritillary butterflies likely were associated with a previous extensive period of polyphagy, wherein multiple, unrelated, colonized plant lineages were replaced by a major shift to a novel host unrelated to the previous spectrum of consumed plants. This host shift was accelerated by a major plant extinction event at the K-Pg boundary, which restricted the range of new potential hosts available to certain fritillaries (Nylin and Wahlberg 2008). Significant range expansions of post-event surviving fritillary taxa may have enhanced the probability of new host shifts (Weingartner et al. 2006). Such an event, between fritillaries and their dicot hosts, when multiplied and writ large geographically, could provide a model for understanding the extensive, global gymnosperm-to-angiosperm shift of many insect lineages during the mid-Cretaceous.
4.3 Questions of the Fossil Record That Only Can Be Answered by Multidisciplinary Data
Back in 1993, the study by Labandeira and Sepkoski was purely an exploratory venture toward understanding the insect fossil record. One of the patterns noted by some and engendering significant negative animus was the pattern of long-term stasis of insect families that encompassed the considerable stretch of time from the Late Jurassic to the early Paleogene, notably including the angiosperm radiation. This result provided an opportunity to subsequently pursue alternative research to test the conclusion of Labandeira and Sepkoski (1993) that the angiosperm radiation had no effect on family-level insect diversity. One approach assessed features of suspect insect pollinators in preangiospermous floras to determine whether gymnosperms were being actively pollinated (Fig. 13.4a, b, d) (Labandeira et al. 2007a; Ren et al. 2009; Labandeira 2010; Peñalver et al. 2012). A second tack involved the presence, extent, and type of mimicry in preangiospermous biotas (Fig. 13.4c) (Wang et al. 2010, 2012b). A third opportunity allowed investigation of early angiospermous and older gymnospermous Mesozoic floras to establish quantitative levels of herbivory diversity and intensity before and after the angiosperm radiation (Ding et al. 2014). A fourth procedure is the examination of ecosystem food-web structure before (currently no data) and after (Dunne et al. 2014) the angiosperm radiation. And last, modern molecularly based evolutionary biology studies indicate that significant radiations of plant-associated insect lineages occurred earlier than the angiosperm radiation (Farrell 1998; Davis et al. 2010). These approaches suggest that the ecological and evolutionary biological infrastructure of insect lineages associated with gymnosperm hosts was a 40 million-year-long feature of Late Jurassic to mid-early Cretaceous terrestrial habitats. This process was rivaled by insect lineages occurring on diverse angiosperm hosts that persisted for a 20 million-year interval during the Late Cretaceous.
5 Conclusions
This report should be seen as a first attempt in addressing the reciprocal roles of insects and angiosperms during the initial radiation of angiosperms. Although there are three major conclusions derived from the data presented in this report, additional analyses with more improved, taxonomically resolved and geochronologically constrained data would go far to ferret out further these patterns.
-
1.
The angiosperm radiation. Herbivory is one of the fundamental attributes of insects, and the host plants consumed by insects are resources that have an important fossil record. One of the major episodes in the evolution of insect herbivory is the transition from gymnosperm- to angiosperm-dominant hosts during the initial diversification of angiosperms 125 to 90 million years ago. Exploring the patterns and evolutionary and ecological mechanisms responsible for this global taxonomic shift in consumer resources is a goal of this report.
-
2.
The pattern. Evidence indicates that plant-associated insect families that hosted gymnosperms prior to the angiosperm radiation consisted of a sustained peak of ca. 95 families for 40 million years. This was followed by a switchover by insect lineages that acquired angiosperm hosts, eventually reaching a level of ca. 110 lineages for a 20 million-year interval during the Late Cretaceous. After this stasis in diversity, there was a rapid increase in angiosperm-hosted insect lineages well into the Paleogene Period. Thus, a major gap occurred during these two diversity maxima levels present on both sides of the angiosperm radiation, attributable to turnover in the plant–host preferences of insect lineages and time-lag effects resulting in the shift from gymnosperm to angiosperm hosts. Notably, the plateau established by earlier insect lineages with gymnosperm hosts was 86.4 % that of the later insect lineages with angiosperm hosts.
-
3.
Implications. The pattern and inferred processes outlined here indicates that modern insect lineages retain only a very minor legacy of their former Middle Jurassic to mid-Cretaceous gymnosperm hosts. By contrast, insect lineages with dominantly gymnosperm hosts during the preangiospermous Late Jurassic to Early Cretaceous rivaled in diversity insect lineages with dominantly angiosperm hosts after the mid-Cretaceous angiosperm radiation and throughout the Late Cretaceous. The ecology of interactions between these older insect lineages and their dominantly gymnosperm hosts needs to be explored further to establish an entrée into this earlier world devoid of angiosperms.
References
Alekseev AC, Dmitriev VY, Ponomarenko AG (2001) The evolution of taxonomic diversity. Geos, Moscow
Barreda VD, Cúneo NR, Wilf P, Currano ED, Scasso RA, Brinkhuis H (2012) Cretaceous/Paleogene floral turnover in Patagonia: drop in diversity, low extinction, and a Classopollis spike. PLoS ONE 7(12):e52455
Bell CD, Soltis DE, Soltis PS (2010) The age and diversification of the angiosperms re-revisited. Am J Bot 97:1296–1303
Buatois LA, Labandeira CC, Cohen AC, Mángano G, Voigt S (2015) The fossil history of continental aquatic taxa and the Mesozoic lacustrine revolution. In: Buatois LA, Mángano G (eds) The trace-fossil record of major evolutionary events, in review. Springer, Berlin (in review)
Carpenter FM (1992) Superclass Hexapoda. In: Moore RC, Kaesler RL, Brosius E, Keim J, Priesner J (eds) Treatise on invertebrate paleontology, part R Arthropoda 4, vol 3 and 4. Geological Society of America, Boulder, and University of Kansas, Lawrence
Cocroft RB, Rodriguez RL, Hunt RE (2008) Host shifts, the evolution of communication, and speciation in the Enchenopa binotata species complex of treehoppers. In: Tillmon KJ (ed) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, Berkeley, pp 88–100
Crane PR (1987) Vegetational consequences of the angiosperm diversification. In: Friis EM, Chaloner WG, Crane PR (eds) The origins of angiosperms and their biological consequences. Cambridge, New York, p 107–144
Crane PR, Friis EM, Pedersen KR (1995) The origin and early diversification of angiosperms. Nature 374:27–33
Currano ED, Labandeira CC, Wilf P (2009) Fossilized insect folivory tracks temperature for six million years. Ecol Mon 80:547–567
Davis RB, Baldauf SL, Mayhew PJ (2010) The origins of species richness in the Hymenoptera: insights from a family-level supertree. BMC Evol Biol 10:109
Davis SR, Engel MS, Legalov A, Ren D (2013) Weevils of the Yixian Formation, China (Coleoptera: Curculionoidea): phylogenetic considerations and comparison with other Mesozoic faunas. Syst Entomol 11:399–429
Ding Q, Labandeira CC, Ren D (2014) Distinctive insect leaf mines on Liaoningocladus boii (Coniferales) from the Early Cretaceous Yixian Formation of northeastern China. Arthro Syst Phylo (in review)
Dmitriev VJ, Zherikhin VV (1988) Changes in the diversity of insect families from data of first and last occurrences. In: Ponomarenko AG (ed) The Mesozoic-Cenozoic crisis in the evolution of insects. Nauka, Moscow, pp 208–215 (in Russian)
Dodd JR, Stanton (1990) Paleoecology: concepts and applications, 2nd edn. Wiley, New York
Dolling WR (1991) The Hemiptera. Oxford, New York
Dunne JA, Labandeira CC, Williams RJ (2014) Highly resolved middle Eocene food webs show early development of modern trophic structure after the end-Cretaceous extinction. Proc Roy Soc B 281. http://dx.doi.org/10.1098/rspb.2013.3280
Engel MS (2000) A new interpretation of the oldest fossil bee (Hymenoptera: Apidae). Am Mus Novit 3296:1–11
Evenhuis NL (1994) Catalogue of the fossil flies of the world (Insecta: Diptera). Backhuys, Leiden
Farrell BD (1998) “Inordinate fondness” explained: why are there so many beetles? Science 281:555–559
Friis EM, Pedersen KR, Crane PR (2010) Diversity in obscurity: fossil flowers and the early history of angiosperms. Phil Trans Roy Soc B 365:369–382
Friis EM, Crane PR, Pedersen KR (2011) Early flowers and angiosperm evolution. Cambridge, Cambridge
Gauld I, Bolton B (eds) (1988) The Hymenoptera. Oxford, New York
Goulet H, Huber JT (eds) (1993) Hymenoptera of the world: an identification guide to families. Agriculture Canada, Ottawa
Gradstein FM, Ogg JG, Schmitz MD, Ogg G (2012) The geologic time scale 2012. Elsevier, Boston
Grimaldi D, Engel MS (2005) Evolution of the insects. Cambridge, New York
Harris TM (1942) Wonnacottia, a new Bennettitalean microsporophyll. Ann Bot 6:577–592
Hartkopf-Fröder C, Rust J, Wappler T, Friis EM, Viehofen A (2011) Mid-Cretaceous charred flowers reveal direct observation of arthropod feeding strategies. Biol Lett 8:295–298
Hill RS, Carpenter RJ (1999) Ginkgo leaves from Paleogene sediments in Tasmania. Austral J Bot 47:717–724
Hughes N (1994) The enigma of angiosperm origins. Cambridge University Press, Cambridge
Imada Y, Kawakita A, Kato M (2011) Allopatric distribution and diversification without niche shift in a bryophyte-feeding basal moth lineage (Lepidoptera: Micropterigidae). Proc Roy Soc B 278:3026–3033
Jarzembowski EA, Ross AJ (1993) Time flies: the geological record of insects. Geol Today 9:218–223
Jarzembowski EA, Ross AJ (1996) Insect origination and extinction in the Phanerozoic. In: Hart MB (ed) Biotic recovery from mass extinction events. Geol Soc Spec Publ 102:65–78
Krassilov VA, Rasnitsyn AP, Afonin SA (2007) Pollen eaters and pollen morphology: co-evolution through the Permian and Mesozoic. Afr Invert 48:3–11
Labandeira CC (1994) A compendium of fossil insect families. Milwaukee Publ Mus Contrib Biol Geol 88:1–71
Labandeira CC (1997) Insect mouthparts: ascertaining the paleobiology of insect feeding strategies. Annu Rev Ecol Syst 28:153–193
Labandeira CC (1998) Early history of arthropod and vascular plant associations. Annu Rev Earth Planet Sci 26:329–377
Labandeira CC (2002) The paleobiology of predators, parasitoids, and parasites: accommodation and death in the fossil record of terrestrial invertebrates. In: Kowalewski M, Kelley PH (eds) The fossil record of predation. Paleontol Soc Pap 8:211–250
Labandeira CC (2005) Fossil history and evolutionary ecology of Diptera and their associations with plants. In: Yeates DK, Wiegmann BM (eds) The evolutionary biology of flies. Columbia, New York, pp 217–273
Labandeira CC (2006) Silurian to Triassic plant and insect clades and their associations: new data, a review, and interpretations. Arth Syst Phylo 64:53–94
Labandeira CC (2007) The origin of herbivory on land: the initial pattern of live tissue consumption by arthropods. Ins Sci 14:259–274
Labandeira CC (2010) The pollination of mid Mesozoic seed plants and the early history of long-proboscid insects. Ann Missouri Bot Gard 97:469–513
Labandeira CC (2013) A paleobiological perspective on plant–insect interactions. Curr Opin Pl Biol 16:414–421
Labandeira CC, Allen EM (2007) Minimal insect herbivory for the lower Permian coprolite bone bed site of north-central Texas, USA, and comparison to other late Paleozoic floras. Palaeogeogr Palaeoclimatol Palaeoecol 247:197–219
Labandeira CC, Dilcher DL, Davis DR, Wagner DL (1994) Ninety-seven million years of angiosperm-insect association: paleobiological insights into the meaning of coevolution. Proc Natl Acad Sci USA 91:12278–12282
Labandeira CC, Kvaček J, Mostovski MB (2007a) Pollination fluids, pollen, and insect pollination of Mesozoic gymnosperms. Taxon 56:663–695
Labandeira CC, Johnson KR, Wilf P (2002) Impact of the terminal Cretaceous event on plant–insect associations. Proc Natl Acad Sci USA 99:2061–2066
Labandeira CC, Sepkoski JJ Jr (1993) Insect diversity in the fossil record. Science 261:310–315
Labandeira CC, Wilf P, Johnson KR, Marsh F (2007b) Guide to insect (and other) damage types on compressed plant fossils. Version 3.0—Spring 2007). Smithsonian Institution, Washington. http://paleobilogy.si.edu/pdf:insectDamageGuide3.01.pdf
Lawrence JF, Ślipiński A (2013) Australian beetles: Morphology, classification and keys, vol 1. CSIRO, Collingwood
Lewis T (1973) Thrips: their biology, ecology and economic importance. Academic Press, London
López-Vaamonde C, Wikström N, Labandeira CC, Goodman S, Godfray HCJ, Cook JM (2006) Fossil-calibrated molecular phylogenies reveal that leaf-mining moths radiated millions of years after their host plants. J Evol Biol 19:1314–1326
Magallón S (2010) Using fossils to break long branches in molecular dating: a comparison of relaxed clocks applied to the origin of angiosperms. Syst Biol 59:384–399
Marshall SA (2012) Flies: the natural history and diversity of Diptera. Firefly, Buffalo
McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DW (eds) (1981–1989) Manual of Nearctic Diptera vols 1–3. Canadian Government Publishing Centre, Hull, Quebec
McKenna DD, Sequeira AS, Marvaldi AE, Farrell BD (2009) Temporal lags and overlap in the diversification of weevils and flowering plants. Proc Natl Acad Sci USA 106:7083–7088
McLoughlin S, Carpenter RJ, Jordan GJ, Hill RS (2008) Seed ferns survived the end-Cretaceous extinction in Tasmania. Am J Bot 95:465–471
McLoughlin S, Carpenter RJ, Pott C (2011) Ptilophyllum muelleri (Ettingsh.) comb. nov. from the Oligocene of Australia: last of the Bennettitales? Int J Plant Si 172:574–585
Miller NCE (1956) The biology of the Heteroptera. Leonard Hill, London
Moran NA, Tran P, Gerardo NM (2005) Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl Environ Microbiol 71:8802–8810
Naumann ID, Carne PB, Lawrence JF, Nielsen ES, Spradbery JP, Taylor RW, Whitten MJ, Littlejohn MJ (eds) (1991) The insects of Australia: A textbook for students and research workers, vols. 1, 2. Cornell, Ithaca
Nylin S, Wahlberg N (2008) Does plasticity drive speciation? Host-plant shifts and diversification in nymphaliine butterflies (Lepidoptera: Nymphalidae) during the Tertiary. Biol J Linn Soc 94:115–130
Paleobiology Database (2014) http://paleobiol.org. Last accessed 10 Feb 2014
Pellmyr O, Seagraves K (2003) Pollinator divergence within an obligate mutualism: two yucca moth species (Lepidoptera: Prodoxidae: Tegeticula) on the Joshua tree (Yucca brevifolia; Agavaceae). Ann Entomol Soc Am 96:716–722
Peñalver E, Labandeira CC, Barrón E, Delclòs X, Nel A, Nel P, Taffoureau P, Soriano C (2012) Thrips pollination of Mesozoic gymnosperms. Proc Natl Acad Sci USA 109:8623–8628
Rasnitsyn AP (1980) The origin and evolution of hymenopterous insects. Trans Paleontol Inst 174:1–192 (in Russian)
Rasnitsyn AP (1988) Principles and methods of phylogenetic reconstruction. In: Ponomarenko AG (ed) The Mesozoic-Cenozoic crisis in the evolution of insects. Nauka, Moscow, pp 191–207 (in Russian)
Rasnitsyn AP, Krassilov VA (2000) The first documented occurrence of phyllophagy in pre-Cretaceous insects: leaf tissues in the gut of Upper Jurassic insects from southern Kazakhstan. Paleontol J 34:301–309
Rasnitsyn AP, Quicke DLJ (eds) (2002) History of insects. Kluwer, Dordrecht
Ratzel SR, Rothwell GW, Mapes G, Mapes RH, Doguzhaeva LA (2001) Pityostrobus hokodzensis, a new species of pinaceous cone from the Cretaceous of Russia. J Paleontol 75:895–900
Ren D (1998) Flower-associated Brachycera flies as fossil evidence for Jurassic angiosperm origins. Science 280:85–88
Ren D (ed) (2010) Current research on palaeoentomology. Acta Geol Sin 84(4):655–1010
Ren D, Labandeira CC, Santiago-Blay JA, Rasnitsyn AP, Shih CK, Bashkuev A, Logan MAV, Hotton CL, Dilcher DL (2009) A probable pollination mode before angiosperms: Eurasian, long-proboscid scorpionflies. Science 326:840–847
Ross AJ, Jarzembowski EA (1993) Arthropoda (Hexapoda; Insecta). In: Benton MJ (ed) The fossil record 2. Chapman & Hall, London, pp 363–426
Schachat S, Labandeira CC, Gordon J, Chaney D, Levi S, Halthore MS, Alvarez J (2014) Plant–insect interactions from the Early Permian (Kungurian) Colwell Creek Pond, North-Central Texas: the early spread of herbivory in riparian environments. Int J Pl Sci 175: in press
Schuh RT, Slater JA (1995) True bugs of the world (Hemiptera: Heteroptera). Cornell, Ithaca
Sinitshenkova ND (2002) Ecological history of the aquatic insects. In: Rasnitsyn AP, Quicke DLJ (eds) History of insects. Kluwer, Dordrecht, pp 388–426
Sohn J-C, Labandeira CC, Davis D, Mitter C (2012) An annotated catalog of fossil and subfossil Lepidoptera (Insecta: Holometabola) of the world. Zootaxa 3286:1–132
Sukatcheva ID (1991) The Late Cretaceous stage in the history of the caddisflies (Trichoptera). Acta Hydroentom Lat 1:68–85
Taylor TN, Taylor EL, Krings M (2009) Paleobotany: the biology and evolution of fossil plants, 2nd edn. Elsevier, Amsterdam
Wang B, Szwedo J, Zhang H (2012a) New Jurassic Cercopoidea from China and their evolutionary significance (Insecta: Hemiptera). Palaeontology 55:1223–1243
Wang B, Zhang H, Jarzembowski EA (2013) Early Cretaceous angiosperms and beetle evolution. Front Pl Sci 4:360
Wang Y, Labandeira CC, Ding Q, Shih CK, Zhao Y, Ren D (2012b) An extraordinary Jurassic mimicry between a hangingfly and ginkgo from China. Proc Natl Acad Sci USA 109:20514–20519
Wang Y, Liu Z, Wang X, Shih C, Zhao Y, Engel MS, Ren D (2010) Ancient pinnate leaf mimesis among lacewings. Proc Natl Acad Sci USA 107:16212–16215
Wappler T, Labandeira CC, Rust J, Frankenhäuser H, Wilde V (2012) Testing for the effects and consequences of mid-Paleogene climate change on insect herbivory. PLoS ONE 7:e40744
Watson J (1977) Some Lower Cretaceous conifers of the Cheirolepidiaceae from the U.S.A. and England. Palaeontology 20:715–749
Weingartner E, Wahlberg N, Nylin S (2006) Dynamics of host plant use and species diversity in Polygonia butterflies (Nymphalidae). J Evol Biol 19:483–491
Wilf P, Labandeira CC, Johnson KR, Cúneo NR (2005) Richness of plant–insect associations in Eocene Patagonia: a legacy for South American biodiversity. Proc Natl Acad Sci USA 102:8944–8948
Wilf P, Labandeira CC, Johnson KR, Ellis B (2006) Decoupled plant and insect diversity after the end-Cretaceous extinction. Science 313:1112–1115
Winkler IS, Labandeira CC, Wappler T, Wilf P (2010) Diptera (Agromyzidae) leaf mines from the Paleogene of North America and Germany: implications for host use evolution and an early origin for the Agromyzidae. J Paleontol 84:935–954
Yeates DK, Wiegmann BM (eds) (2005) The evolutionary biology of flies. Columbia, New York
Zhang W, Shih CK, Labandeira CC, Sohn JC, Davis DR, Santiago-Blay JA, Flint O, Ren D (2013) New fossil Lepidoptera (Insecta: Amphiesmenoptera) from the Middle Jurassic Jiulongshan Formation of Northeastern China. PLoS ONE 8(11):e79500
Zherikhin VV, Mostovski MB, Vršanský P, Blagoderov VA, Lukashevich ED (1999) The unique lower Cretaceous locality Baissa and other contemporaneous fossil insect sites in North and West Transbaikalia. In: Proceedings of 1st International Palaeoentom Conference (Moscow, 1998). AMBA Projects, Bratislava, p 185–191
Zhou Z, Zhang B (1989) A sideritic Protocupressinoxylon with insect borings and frass from the Middle Jurassic, Henan, China. Rev Palaeobot Palynol 59:133–143
Acknowledgments
Thanks go to Pierre Pontarotti for inviting CCL to attend the Seventeenth Evolutionary Biology meeting in Marseille France. Finnegan Marsh adroitly crafted the figures. We are grateful for the Missouri Botanical Garden (St. Louis, Missouri), Wang Chen (Capital Normal University, Beijing), and Enrique Peñalver (Museo Geominero, Madrid) for use of images in Fig. 13.4. An anonymous reviewer improved the manuscript. Use of the online Paleobiology Data Base (PBDB) is acknowledged. This is contribution 263 to the Evolution of Terrestrial Ecosystems consortium at the National Museum of Natural History, in Washington, D.C.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Labandeira, C. (2014). Why Did Terrestrial Insect Diversity Not Increase During the Angiosperm Radiation? Mid-Mesozoic, Plant-Associated Insect Lineages Harbor Clues. In: Pontarotti, P. (eds) Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life. Springer, Cham. https://doi.org/10.1007/978-3-319-07623-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-07623-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07622-5
Online ISBN: 978-3-319-07623-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)