Abstract
A cyclodextrin-triazole-titanium based nanocomposite (CD.COM) was successfully synthesized and fully characterized. CD.COM exhibited a good adsorption performance for removal of Zn+2, Cd2+, and Pb2+ ions from aqueous solutions by batch technique. The adsorption parameters such as pH, contact time, adsorbent dosage, temperature, metal ions concentration, coexisting ions and the regenerability of CD.COM were investigated. The Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherm models were evaluated using adsorption equilibrium data. The Langmuir model showed the best agreement with the experimental data, resulting in the maximum adsorption capacities of 147.1, 158.7, and 200.0 (mg . g−1) for Zn+2, Cd+2, and Pb+2 ions, respectively. Among the pseudo-first order, pseudo-second order, intraparticle diffusion and Elovich models, the kinetic data was well fitted with the pseudo-second order model. Thermodynamic parameters indicated a spontaneous and endothermic adsorption process. The bioadsorbent showed high selectivity for Pb(II) ions in the presence of other coexisting metal ions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
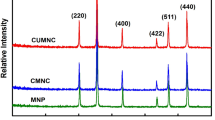
Clean water is the most essential need for human’s life and removing the metal ions pollution from water is one of the most vital research area which has attracted global attention due to adverse effect of pollution on the environment and human health [1]. Environmental benign nanocomposites with high surface area, more active sites and good adsorption capacity have gained a great deal of attraction [2]. In this context, CD.COM was synthesized via nucleophilic reaction of amino functionalized TiO2 with click modified cyclodextrin and its structure was characterized by FTIR, TGA, FESEM, TEM, EDX-MAP, XRD, BET, and ICP-OES analyses. The adsorption behavior of CD.COM was investigated for removal of Zn(II), Cd(II), and Pb(II) ions from aqueous solutions.
2 Experimental
CD.COM was synthesized according to the synthetic pathway shown in Scheme 1.
3 Batch Adsorption Experiments
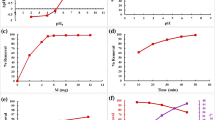
Adsorption isotherm, kinetic and thermodynamic data were evaluated by batch adsorption experiments. The effects of pH, contact time, adsorbent dosage, temperature and initial metal ions concentration on the adsorption efficiency of CD.COM were investigated. The removal percentage (R%) and adsorption capacity (Q) of CD.COM were calculated according to Eqs. (1) and (2):
Where, C0 and Ce (mg . L−1) are the initial and final concentrations of metal ions, Q (mg . g–1) is the adsorption capacity, V (L) is the volume of the metal ion solution and M (g) is the weight of adsorbent.
4 Results and Discussion
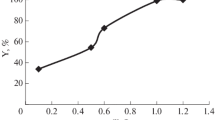
In order to gain more detail of the adsorption mechanism, different isotherms (Langmuir, Freundlich, Temkin and Dubinin-Radushkevich) and kinetic models (pseudo-first order, pseudo-second order, intraparticle diffusion and Elovich) were examined to fit the experimental data. The adsorption data were best fitted with the Langmuir isotherm and pseudo-second order kinetic models (Table 1), suggesting that the adsorption mechanism could be a monolayer chemical adsorption. CD.COM showed a preferential Qmax (mg . g−1) for Pb2+ (200.0) compared with Zn2+ (147.1) and Cd2+ (158.7) ions. The maximum adsorption capacities of CD.COM for these metal ions are higher than those for many cyclodextrin-based adsorbents reported in the literature, due to the synergistic effects of cyclodextrin, triazole and TiO2 moieties. The calculated thermodynamic parameters (ΔG°, ΔH°, and ΔS°) together with Ea demonstrated an endothermic, spontaneous and chemical adsorption process. Competitive adsorption efficiencies of CD.COM for Zn+2, Cd+2, and Pb+2 ions in single and ternary metal ion-containing solutions with equal concentration were compared to evaluate the selectivity of CD.COM towards Pb+2 ions, due to the larger ionic radius and lower hydration energy of Pb+2 ions (Fig. 1). CD.COM can be easily recovered and regenerated with HCl (0.5 M) and/or EDTA (0.05 M) solutions and reused at least five recycles without significant loss of adsorption efficiency (Fig. 2).
5 Conclusion
CD.Com exhibited to be an efficient biosorbent for adsorption of Zn+2, Cd+2, and Pb+2 ions from aqueous solutions. The presence of multiple –OH, –NH functional groups, nitrogen atoms of 1, 2, 3-triazole ring as active binding sites and capability of cyclodextrin moiety for host-guest inclusion complex formation, makes the CD.COM as an effective nanoadsorbent for removal of metal ions. CD.COM with merits such as high adsorption capacity, good selectivity, high reusability and environmentally friendly has great potentials for removal of pollutants from water and waste water.
References
Yidong, Z., et al.: J. Mater. Chem. A 4, 14170–14179 (2016)
Fallah, Z., Nasr Isfahani, H., Tajbakhsh, M., Tashakkorian, H., Amouei, A.: Cellulose 25, 639–660 (2018)
Acknowledgement
We wish to express our gratitude to the University of Mazandaran and research council of Shahrood University of technology for their supports.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Fallah, Z., Isfahani, H.N., Tajbakhsh, M. (2020). Adsorption Behavior of Cyclodextrin-Triazole-Titanium Based Nanocomposite for Heavy Metal Ions from Aqueous Solution. In: Mirzadeh, H., Katbab, A. (eds) Eco-friendly and Smart Polymer Systems. ISPST 2018. Springer, Cham. https://doi.org/10.1007/978-3-030-45085-4_62
Download citation
DOI: https://doi.org/10.1007/978-3-030-45085-4_62
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45084-7
Online ISBN: 978-3-030-45085-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)