Abstract
In this paper, preparation, characterization and adsorption properties of a new biocompatible cellulose-titania based nanocomposite (Cell-Com) made by the click reaction were investigated. FTIR, XRD, FESEM, BET, EDX and ICP-OES analyses were used to characterize the structure of Cell-Com. The adsorption behavior of the Cell-Com for the removal of Pb2+, Cd2+ and Zn2+ ions from aqueous solutions was performed by batch experiments. The effects of pH, contact time, adsorbent dose, initial metal ion concentration, temperature, coexisting ions and the regeneration performance of synthesized nanocomposite were evaluated. The optimal adsorption conditions was determined to be at pH 7.0, 60 min contact time, 10 mg adsorbent dose and 20 ppm initial metal ion concentration at 298 K. The equilibrium data was fitted well with the Langmuir isotherm among the examined isotherms and the maximum adsorption capacity of the bioadsorbent for Zn2+, Cd2+ and Pb2+ ions found to be 102.04, 102.05 and 120.48 mg g−1, respectively. The pseudo-second order model was the best kinetic model to explain the adsorption kinetic data, suggesting chemical sorption as the rate-determining step of sorption mechanism. The ΔG°, ΔH° and ΔS° values were estimated from Van’t Hoff plot together with activation energy (Ea) determined using Arrhenius equation, displayed that the adsorption process is spontaneous and endothermic in nature. The synthesized nanocomposite showed high selectivity and interference resistance from coexisting ions for the adsorption of Pb2+ ion. The Cell-Com can be easily regenerated and reused without significant loss of adsorption capacity. Recovery and reusability of Cell-Com were performed after 4–5 repeated adsorption/desorption cycles in HCl or in EDTA solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water contamination by heavy metals has become an increasingly serious environmental problem in recent years (Li et al. 2016a). Some toxic heavy metals, such as lead, cadmium and zinc are common contaminants in the industrial wastes (Gurgel and Gil 2009b). These heavy metals are extremely toxic to humans even at low concentrations and can cause major health disorders because of their difficulty in biodegradation, procreate reactive radicals and tendency to accumulate in the body (Godt et al. 2006; Järup 2003; Jomova and Valko 2011). Over the past years, various methods have been developed for the elimination of metal ions from aqueous solutions, among which adsorption processes have gained much attention because of their accessibility, effectiveness, flexibility and low operating expenses (Yang et al. 2014). Nanostructured adsorbents possess unique properties including increased active adsorption sites and high specific surface area which appeared as a fascinating area of interest for efficient potential in water treatment (Mahfoudhi and Boufi 2017; Olivera et al. 2016; Shahadat et al. 2015; Zhang et al. 2017). Among metal oxide nanoparticles, TiO2 nanoparticles have found many applications in various fields of chemistry (Bai and Zhou 2014; Dahl et al. 2014; Park et al. 2013; Seidlerová et al. 2016). Moreover, TiO2 nanoparticles offer additional advantages such as nontoxicity, low cost, stability, availability, high specific surface area, good affinity toward metal ions and other excellent physical and chemical properties (Li et al. 2015b; Zhong et al. 2008). Nevertheless, the use of TiO2 nanoparticles encounters some drawbacks such as loss of activity due to agglomeration, difficulty of recovery and environment risks caused by the lapsed-particles (Hua et al. 2012; Li et al. 2016b). An efficient technique to overcome these problems is to fabricate a nanocomposite by immobilizing TiO2 nanoparticles onto polymer support (Xiong et al. 2017; Zhao et al. 2011). Moreover, it has been shown that cellulose and its derivatives could be deserved as attractive templates for the synthesis of hybrid materials (Anirudhan et al. 2012), not only for their renewability, biocompatibility, biodegradability and sustainability, but also because of their low density, good stability and reactive surfaces (Karim et al. 2017; Luo et al. 2014). In fact, the multi-hydroxyl functionalities on the surface of cellulose can offer many active sites for removing of metal ions (d’Halluin et al. 2017; Klemm et al. 2011). Cellulose nanocomposites have efficient potential as adsorbent for environmental remediation and water treatment (Bossa et al. 2017; Li et al. 2017; O’Connell et al. 2008). During the last decade, various synthetic routes have been developed to immobilize nanoparticles onto polymer matrix (Jamshaid et al. 2017; Zhao et al. 2011). The TiO2 nanoparticles could be functionalized with coupling agents, to make them compatible with polymer matrix and increase the possibility of chemical bonding with the polymer (Pujari et al. 2014; Zhou et al. 2014). This could be achieved by anchoring the titania nanoparticles with amine functionality. For instance, tetrazole (Massin et al. 2014) and triazole (Ghaemy et al. 2013; Tao et al. 2013) moieties have been used as new anchoring groups for attachment of titanium nanoparticles onto polymer matrix. Therefore, it is desirable to combine and benefit from the advantages of the both systems by designing a natural bead bearing these two functionalities. We reasoned that click chemistry would be a method of choice as it provides a simple approach for the chemical grafting of modified nanoparticles with functionalized cellulose (Meng and Edgar 2016). Click reactions supply modular, mild and effective modification routes in generating derivatives with novel properties, functionalities and designs which are difficult to get through conventional procedures (Liang and Astruc 2011; Moses and Moorhouse 2007; Yadav et al. 2015). Besides, triazole rings generated from the click reaction are expected to have additional adsorbing sites for metal ions (Jin et al. 2017), due to characteristic structure containing –C=N–(azomethine) and –NH–(imino) groups which are capable of chelating metal ions (Lamanna et al. 2017; Urankar et al. 2010). In this respect, a new nanocomposite containing cellulose biopolymer, triazole and TiO2 moieties was designed for removal of heavy metal ions with high adsorption capacity. The nanocomposite (Cell-Com) was synthesized by reacting alkyne terminated TiO2 nanoparticles with functionalized cellulose through a click reaction. The azide terminated cellulose was obtained by functionalization of cellulose with tosyl chloride followed by reacting with sodium azide. The alkyne terminated TiO2 was prepared by treating TiO2 nanoparticles with 3-glycidoxypropyltrimethoxysilane followed by reacting with propargylamine. The synthesized Cell-Com was characterized by FTIR, XRD, FESEM, EDX, BET and ICP-OES analyses. The ability of Cell-Com as a porous and high functionalized adsorbent for the removal of Pb2+, Cd2+ and Zn2+ ions from aqueous solutions was investigated by batch adsorption experiments. The effect of various parameters including pH, contact time, adsorbent dose, initial metal ion concentration, temperature, coexisting ions and the regeneration efficiency of Cell-Com were studied. Three different isotherms and kinetic models were examined to determine the isotherm parameters and the adsorption kinetics. High relative surface area and surface porosity, presence of nitrogen and oxygen hetero atoms together with triazole rings capable of chelating with metal ions in Cell-Com, provide a fast adsorption rate, high adsorption capacity with good selectivity and regenerability.
Experimental
Materials
Microcrystalline cellulose (MCC), titanium tetrachloride (TiCl4), p-toluene sulfonyl chloride (TS-Cl), lithium chloride (LiCl), 3-glycidyloxypropyltrimethoxysilane (GPS ≥ 98%), sodium azide (NaN3 ≥ 99.9%), copper(II) sulfate pentahydrate (CuSO4·5H2O), propargylamine (PA ≥ 98%), ascorbic acid and solvents were purchased from Sigma-Aldrich and Merck Companies and used without further purification.
Dimethylacetamide (DMAC) and dimethylformamide (DMF) were distilled in the presence of calcium hydride for 24 h and stored over molecular sieves before use. Toluene was dried with sodium metal previous to use. Microcrystalline cellulose and anhydrous lithium chloride were dried under vacuum for 24 h at 80 °C before application. The aqueous solution of lead, cadmium and zinc ions were prepared from their stock nitrate salt solutions (1.0 g/L), which was then diluted to desired concentrations. All other chemicals were analytical grade and consumed as received.
Measurements
A PHS-3C pH-meter (Germany) was used for pH measurement. Fourier-transform infrared spectroscopy (FTIR) spectra were recorded on a Bruker Tensor 27 spectrometer, using KBr pellets for solids (Germany). X-ray diffraction patterns were recorded by X-ray diffractometer (XRD, D8 ADVANCE-BRUKER) with CuKα radiation (1.5406 Å) operating at 35.4 kV and 28 mA in the 2θ range of 5°–80° with a scanning rate of 5° min−1 at room temperature. Particle surface morphology was examined using field emission scanning electron microscopy (FESEM; MiRA3 TESCAN, Switzerds). Energy dispersive X-Ray (EDX) was recorded on a SANX (UK). The Brunauer–Emmett–Teller (BET) survey was determined by a BELCAT-A instrument. Inductively coupled plasma optical emission spectroscopy (ICP-OES) 730-ES, Varian (λ = 334.941 nm) was used for measurement of Ti content in the Cell-Com. The concentration of metal ions in aqueous solution was measured using a flame atomic absorption spectroscopy (AAS) (Hewlett-Packard 3510).
Methods
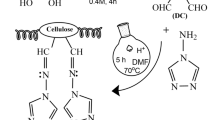
The step-wise procedure for the synthesis of Cell-Com is illustrated in Scheme 1.
Preparation of anatase TiO2 nanoparticles (1, TiO2 NPs)
TiO2 NPs were synthesized via sol–gel method reported in the literature (Li et al. 2005). Detailed synthesis procedure can be found in the ESI. †.
Preparation of 3-glycidyloxypropyl titania (2, Ti/GPS)
TiO2 NPs (1 g) dispersed in anhydrous toluene (15 mL) and ultrasonicated for 20 min. The mixture was then stirred for a further 15 min GPS (2 mL) was added and stirred at 80 °C for 24 h under nitrogen atmosphere. The mixture was centrifuged and washed with toluene and methanol alternatively for at least 5 cycles to remove excessive silane. The centrifuged particles in toluene were ultrasonicated for 20 min and centrifuged again. The modified particles were dried in an oven at 100 °C for 24 h under vacuum (Zhao et al. 2012).
Preparation of titania/silane coupling agent/propargylamine (3, Ti/GPS/PA)
Ti/GPS (1 g) dispersed in anhydrous DMF (15 mL) and ultrasonicated for 30 min. Propargylamine (1 mL, 15.61 mmol) was added to this solution and stirred at 80 °C for 24 h. The reaction mixture was centrifuged and washed with DMF and EtOH successively to remove unreacted materials, then dried in a vacuum oven at 80 °C.
Pretreatment and dissolution of microcrystalline cellulose (4, MCC)
MCC (1 g) was suspended in MeOH (10 mL) by stirring at room temperature for 30 min. The suspension was filtered and the residue was suspended in acetone and DMAC (10 mL) subsequently under the same conditions. Finally, the pretreated MCC was dried at 80 °C under vacuum for 24 h (Ishii et al. 2007). LiCl (3 g) was dissolved in DMAC (30 mL) at 80 °C, then the pretreated MCC (1 g) was added and stirred at 70 °C for 2 days under N2 atmosphere in the DMAC/LiCl solution. After the dissolution completed, a clear solution with light amber color was obtained (Elchinger et al. 2012b).
Preparation of cellulose p-toluenesulfonate (5, Cell-OTS)
Triethylamine (5.16 mL, 37 mmol) in DMAC (25 mL) was added to a cellulose solution (1 g in 3 gr/30 mL LiCl/DMAC) under continuous stirring at 5–10 °C. After stirring for 1 h, a solution of TS-Cl (3.52 g, 18.51 mmol) in DMAC (5 mL) was added dropwise within 30 min and stirring was continued for 24 h at 5–10 °C. The reaction mixture was slowly poured into ice water (500 mL). The white precipitate was filtered off and washed with hot water (1000 mL) and hot EtOH (500 mL), then dissolved in 25 mL of DMF and reprecipitated in ~ 400 mL of distilled water. After filtration and washing with EtOH, the product was dried in vacuum at 80 °C for 24 h (mass yield: 120%, DSs: 1.02) (ESI. †) (Liu and Baumann 2005).
Preparation of 6-azido-6-deoxy cellulose (6, Cell-N3)
Cell-OTS (1 g) was dissolved in dry DMF (30 mL). After addition of NaN3 (1 g, 15.38 mmol), the mixture was stirred at 80 °C for 24 h under N2 atmosphere. The product was separated by precipitation in cold water (200 mL). The precipitate was filtered off, washed with distilled water (500 mL) and EtOH (250 mL), then dissolved in DMF (20 mL), reprecipitated in distilled water (300 mL) and finally dried at 40 °C under vacuum overnight (mass yield: 94%, DSN: 0.84, DSS: 0.18) (ESI. †) (Pierre-Antoine et al. 2012).
Preparation of cellulose nanocomposite (7, Cell-Com)
A mixture of Cell-N3 (1 g), copper(II) sulfate pentahydrate (0.1 g, 0.4 mmol, in 1 mL of water), ascorbic acid (0.3 g, 1.7 mmol, in 1 mL of water) and Ti/GPS/PA (0.35 g) in DMSO (10 mL) was stirred at 80 °C for 48 h. The reaction mixture was cooled to room temperature, filtered off and washed with deionized water (500 mL). The gray powder was stirred in NH4OH (50 mL, 25%) for 2 h at room temperature to remove the copper salts. The mixture was filtered, washed with water and dried in an oven at 70 °C under vacuum to give Cell-Com as a pale brown solid (mass yield: 93%, Ti content: 11.69 wt%) (ESI. †).
Batch adsorption experiments
The heavy metal ion solution at predetermined concentrations (20-80 ppm) were prepared from their corresponding stock solutions. Cell-Com (10 mg) was placed in 100 mL erlenmeyer flask containing of the heavy metal ion solution (20 mL) and each container was placed on a platform shaker at 300 rpm at room temperature (~ 25 °C) until equilibrium was reached (60 min for Pb2+, Cd2+ and Zn2+ ions). Then, the adsorbent was removed by filtration and the metal concentration in the filtrate was measured using atomic absorbance spectrophotometer (AAS). The various factors including of pH, contact time, adsorbent dosage, temperature and initial concentrations of heavy metal ions on the adsorbent capacity for removal of Pb2+, Cd2+ and Zn2+ ions from aqueous solution were investigated.
The percent sorption of each metal ion (A %) and adsorption capacity of Cell-Com (Qe) were determined by Eqs. (1) and (2).
where A is the adsorption percentage, Qe (mg g−1) is the equilibrium adsorption capacity, C0 and Ce (mg L−1) are the initial and residual metal concentrations, V (L) is the volume of the metal ion solution and m (g) is the adsorbent mass.
The range of solution pH in this study was 2.0–8.0 and it was adjusted by using HCl or NaOH (0.1–1.0 mol L−1) solutions. The contact time (10–100 min), adsorbent dosage (5–30 mg), temperature range (5–55 °C) and initial metal ion concentration (20–80 mg L−1) also were studied. The Langmuir, Freundlich and Temkin isotherm models and the pseudo-first order, pseudo-second order and Weber-Morris intra-particle diffusion kinetic models were used to determine the parameters and capacity of adsorption for removal of heavy metal ions from aqueous solutions by Cell-Com.
Results and discussion
Structural characterization
FTIR
Figure 1 shows the FTIR spectra of TiO2 (a), Ti/GPS (b), Ti/GPS/PA (c), MCC (d), Cell-OTS (e), Cell-N3 (f), and Cell-Com (g).
TiO2 (1)
The broad absorption band in the region of 400–700 cm−1 is assigned to the stretching vibration of Ti–O and Ti–O–Ti. The stretching vibration of hydroxyl groups on the surface of TiO2 nanoparticles is observed at 3200–3500 cm−1 (Li et al. 2005). The absorption peak around 1625 cm−1 is attributed to the bending mode of hydroxyl groups (Fig. 1a) (Bezrodna et al. 2004).
Ti/GPS (2)
The main characteristic peak of Si–O–C is observed at 1450 cm−1. The symmetrical and asymmetrical stretching vibration of Si–O–Si appear at 800 and 1110 cm−1, respectively. The absorption peaks at 1250–1260 cm−1 is related to the stretching vibrations of C–O in epoxy ring. The asymmetrical and symmetrical stretching vibrations of the C–H bond in methylene group are observed at 2925 and 2850 cm−1, confirming the successful grafting of GPS (Fig. 1b) (Sabzi et al. 2009).
Ti/GPS/PA (3)
The absorption band at 1167 cm−1 is assigned to the C–OH stretching vibration of the coupling agent (Xue 1987). The appearance of absorption bands at 3310 cm−1 (ν Calkyne–H), 2190 cm−1 (ν Calkyne–C) and a shoulder at around 1575 cm−1 (ν bending –NH group) together with the higher intensity of ν CH2– modes at 2930 and 2856 cm−1, indicate that the amine groups open the epoxy rings (Fig. 1c) (Martina et al. 2014).
MCC (4)
The absorbance bands at 3429 (stretching vibration of hydroxyl group), 2922 (ν C–H), 1626 (bending vibration of hydroxyl group) and 1200–1000 cm−1 (ν C–O) are assigned to the glycoside ring. The absorption peaks at 1450 and 1300 cm−1 are related to the vibration modes of –CH and –CH2 groups. The adsorptions at 710–617 cm−1 are associated with native cellulose (Fig. 1d) (Hao et al. 2017; Mohammadinezhad et al. 2014; Peng et al. 2012).
Cell-OTS (5)
FTIR spectrum of cellulose tosylate shows the typical absorptions of the cellulose backbone as well as absorptions of the tosylate group at 3068 (ν as C–Harom.), 1598, 1495 (ν C–Carom.), 818 (ν s C–Harom.), 1371 (ν as SO2) and 1177 cm−1 (v s SO2). A significant decrease in the intensity of the hydroxyl stretching vibration at 3429 cm−1 can be attributed to the consumption of OH group during the modification reaction (Fig. 1e) (Liu and Baumann 2005).
Cell-N3 (6)
The FTIR spectrum of Cell-N3 shows a strong absorption at 2115 cm−1, confirming the replacement of tosylate by azide group. However presence of the weak aromatic and tosyl groups absorptions, show that the totality of tosyl moieties has not been replaced by azide ones, and only the primary tosylate moieties which positioned on C-6 could be easily substituted by NaN3 during the nucleophilic displacement reaction (Fig. 1f) (Sadeghifar et al. 2011).
Cell-Com (7)
The FTIR spectrum of Cell-Com exhibits all the expected absorptions of modified cellulose and TiO2. The disappearance of alkyne absorption peaks at 3310 and 2190 cm−1, significant decrease in the azide peak at 2115 cm−1 and appearance of absorption bands of C=N stretching at 1628 cm−1, N=N stretching at 1580 and 1445 cm−1, C–N–C stretching at 1379 cm−1, ring vibration at 1285 cm−1 and in-plane ring C–H bending at 1066 cm−1 confirming successful click reaction (Fig. 1g).
Microstructural and morphological study
XRD
The XRD patterns of MCC, Cell-OTS, Cell-N3, TiO2, Ti/GPS/PA and Cell-Com are presented in Fig. 2. The XRD pattern of MCC shows strong diffractions at 2θ = 14.88°, 16.68°, 22.74° and 34.5° corresponding to (1-10), (110), (200) and (004) lattice planes of cellulose Iβ (French 2014; Gu and Hsieh 2015; Li et al. 2015a). X-ray diffraction maxima of Cell-OTS and Cell-N3 show only the broad signal at 2θ = 20.45 and 22°, respectively, indicating the disruption of the supramolecular order resulted in reducing the cellulose crystalline structure to form an amorphous structure (Elchinger et al. 2012a). The XRD pattern of TiO2 exhibit peaks at 2θ = 25.3°, 37.9°, 48.1°, 53.92°, 55.1°, 62.8°, 68.8°, 70.89° and 75.38° A which related to (101), (004), (200), (105), (211), (204), (116), (220) and (215) lattice planes of anatase TiO2, respectively (Shirke et al. 2011). The XRD pattern of Ti/GPS/PA is similar to that of TiO2 NPs, indicating that chemical modification of TiO2 NPs does not change the crystal structure of TiO2. The diffraction peaks of TiO2 and cellulose are observed in the XRD pattern of Cell-Com 1. The peak intensity of cellulose became weak significantly in Cell-Com 1 which might be due to titania coating on cellulose which could act as a shield against X-ray radiation (Karimi et al. 2010). The similarity of the diffraction pattern of Cell-Com 2 with TiO2 together with the absence of characteristic peaks of cellulose suggesting that with increasing of TiO2 content, the anatase TiO2 faces dominates (Morawski et al. 2013) and cellulose might have been exfoliated and dispersed to form integrity type matrix (Jun et al. 2009; Thommes et al. 1995). However Cell-Com 1 was studied for removal of metal ions from aqueous solution in this work.
The crystallinity index (CrI) of the cellulose was estimated by using the following Eq. (3):
where I 200 is the diffraction intensity for the crystalline portion of cellulose at about 2θ = 22.74° and I am represents the diffraction intensity for the amorphous portion of cellulose at about 2θ = 18° (French and Santiago Cintrón 2013). The estimated value for the crystallinity of MCC was found to be ~ 52%.
The average crystalline size (D) were calculated from the most intense diffraction peak (200) using the Scherrer’s Eq. (4):
where λ is the wavelength of the X-ray radiation (0.154 nm), K is the Scherrer’s constant taken as 0.94, β is the line width at half maximum height and θ is Bragg’s angle in degree (Jiang et al. 2013; Scherrer 1918). The average crystalline size of TiO2, MCC and Cell-Com particles were calculated to be 19.7, 6.1 and 20.3 nm, respectively.
FESEM
Figure 3 shows the FESEM images of MCC (a), Cell-OTS (b), Cell-N3 (c), TiO2 (d), Ti/GPS (e), Ti/GPS/PA (f), Cell-Com at different magnifications (g–i) and Cell-Com before and after adsorption of Cd(ΙΙ) (j, k). Figure 3a shows the MCC particles are in an irregular aggregated fragments (Vanhatalo et al. 2016) with probably a low aspect ratios (Miao and Hamad 2013). FESEM images of Cell-OTS (b) and Cell-N3 (c) are nearly similar to each other and composed of interconnected particles which provide a porous structures indicating the reduction of cellulose surface order after the chemical modification. This probably increases the contact area and would improve the adsorption capacity. FESEM image of the prepared anatase TiO2 NPs (d) shows that the particles are spherical shape and uniformly distributed (Karimi et al. 2010). The average particle size is about 20 nm, in agreement with the result obtained from XRD. After modification of TiO2 NPs, partial changes are observed in the surface morphology of modified TiO2 NPs (e, f). FESEM images of Cell-Com (g–j) with different magnifications exhibit that the modified TiO2 NPs are captured into the network of functionalized MCC with some degree of agglomeration (Li et al. 2015b). However, inspection of FESEM image after Cd(ΙΙ) adsorption showed that the surface porosity of Cell-Com was somewhat reduced as depicted in Fig. 3k.
BET surface area
Specific surface area is an important index for evaluating the adsorption capacity of adsorbents. As shown in the BET experimental results (Table S1 and Fig. S1, ESI. †), specific surface area, pore volume and pore size are affected when cellulose are covalently bonded to the TiO2 NPs. These values for Cell-Com are apparently decreased in comparison with TiO2 NPs. These changes are clearly related to a reduction in accessible volume caused by the presence of the bulky cellulose (Martina et al. 2014).
The reason of high metal ion adsorption of bionanocomposite might be related to the high surface area, specially exposed facets of TiO2 NPs, synchronization effect between TiO2 NPs and modified microcrystalline cellulose and high density of functional groups on the accessible pore channels (Li et al. 2015b).
EDX
As shown in Fig. S2 (ESI. †), presence of appropriate elements in the EDX spectra of MCC, Cell-OTS, Cell-N3, TiO2, Ti/GPS, Ti/GPS/PA and Cell-Com (Fig. S2 a–g), confirm the successful synthesis of each compound. Moreover, the presence of Cd(II) in the structure of Cell-Com after adsorption was confirmed by EDX analysis as shown in Fig. S2 h.
ICP-OES
The content of titanium in the Cell-Com structure was measured by ICP-OES at wavelength of 334.941 nm. The amount of Ti in Cell-Com was found to be 11.69 wt% which is in agreement with the result obtained from TGA analysis (12.72 wt%) (Fig. S3, ESI. †).
Adsorption studies
Adsorption experiments for the removal of metal ions (Pb2+, Cd2+ and Zn2+) from aqueous solutions resulted in the standardization of optimal conditions as: adsorbent dose (10 mg), metal concentration (20 ppm), contact time (60 min), temperature (25 °C) and solution volume (20 ml) at pH 7.0. The removal percent of metal ions: Pb(ΙΙ): 91, Cd(ΙΙ): 82 and Zn(ΙΙ): 79% was obtained. The removal efficiency of MCC, TiO2 and Cell-Com are shown in Fig. 4. As it is clear, Cell-Com exhibits the highest adsorption efficiency at optimal dosage.
The enhanced adsorption capacity of Cell-Com could be explained to relative high surface area, porous structure, presence of 1,2,3-triazolium rings and availability of free –OH and –NH groups as chelating agents thus enabling the electrostatic interactions with the metal ions. The various experimental parameters: pH, contact time, adsorbent dose, temperature and initial metal ion concentration were investigated as is ascribed below:
Effect of pH
pH of aqueous solutions for the removal of metal ions greatly affects the ionic form of metal ions and thus on the adsorption capacity of an adsorbent (Liu et al. 2014). The hydrated metal ions M(OH)n that have higher mobility and smaller effective size under high pH value, results increasing the adsorption of the adsorbent (Gan et al. 2016). Therefore, the maximum solution pH that the metal ions (Pb2+, Cd2+ and Zn2+) may not exist as hydrolyzed species, were calculated using metal ion concentrations and solubility product constants (KSP) (Gurgel and Gil 2009a). KSP for Pb(OH)2, Cd(OH)2 and Zn(OH)2 are 1.43 × 10−20, 7.2 × 10−15 and 3 × 10−16, respectively. Calculated maximum pH values for optimal metal ions concentration are 10, 11 and 10.8, respectively. Thus, the pH range of 2.0–8.0 was selected for adsorption studies, as shown in Fig. 5a. At low pH values (2–4), the adsorption of the three cations is < 40% because the metal ions concentration is less than of the proton (H+) and the active sites of adsorbent mostly filled with H+ ions (Rahman et al. 2016). When the solution pH increases (5–8), the binding sites for metal ions gradually become available, thus the adsorption capacity for metal ions increases (Liu et al. 2014). At higher pH values (> 8), precipitation and adsorption of hydrolyzed species might occur as predicted by KSP (Masoumi et al. 2016), therefore pH 7.0 was selected as the optimal pH for three metal ions (Pb2+, Cd2+ and Zn2+) adsorption experiments. However, the adsorption capacity of Cell-Com for removal of Pb2+ is relatively higher than Cd2+ and Zn2+ ions.
Effect of contact time
Contact time is one of the important parameters that reflecting the adsorption kinetics of an adsorbent. Adsorption time depends on many factors including, morphology and structural property of adsorbent, stirring rate, initial concentration of metal ions and amount of adsorbent (Zhou et al. 2013). The metal ions sorption on Cell-Com was measured as a function of time in the range of 10–100 min to determine the optimum contact time for the adsorption of Pb2+, Cd2+ and Zn2+ ions and the results are shown in Fig. 5b. As it is clear, the adsorption process is fast initially related to high metal ion concentrations and relatively low coverage of adsorbent surface. After 10 min the adsorption process become slow because the metal ion concentrations and the number of free adsorptive sites of adsorbent decrease and then reaching the equilibrium concentration. According to the Fig. 5b, 60 min was considered as the optimum contact time. High removal efficiency and short time period for adsorption equilibrium could be attributed to the large surface area and dispersion of TiO2 NPs on the surface of functionalized cellulose that provides increased number of active sites for metal ions adsorption in aqueous solutions.
Effect of adsorbent dose
The effect of adsorbent dosage on the adsorption of Pb2+, Cd2+ and Zn+2 ions in aqueous solutions was studied and the results depicted in Fig. 5c. As expected the removal rate increases as the amount of adsorbent is added, which could be attributed to the number of free adsorptive sites on the surface of the adsorbent to interact with the metal ions (Kardam et al. 2013). However the maximum adsorption observed at 10 mg adsorbent. Increasing the adsorbent dosage from 10 mg onwards did not increase the metal ion adsorptions significantly which is due to equilibrium between adsorbate and adsorbent under the operating conditions.
Effect of temperature
The effect of temperature on the adsorption of studied metal ions was investigated under isothermal conditions in the range of 5–55 °C (278.15–328.15 K) and at pH 7.0 and the results are illustrated in Fig. 5d. It is clear that the adsorption of Pb2+, Cd2+ and Zn2+ ions by Cell-Com from aqueous solution does not show any significant temperature dependence and temperature has only a marginal effect on adsorption of studied metal ions (Wu et al. 2015). According to these findings room temperature was chosen for adsorption process in this study without decrease of adsorption efficiency.
Effect of initial metal ion concentration
Initial concentrations of metal ions were varied from 20 to 80 mg L−1 in this study. The results in Fig. 6a Show that the limited existing adsorption sites at higher initial concentrations may result in decrease of metal ion adsorption which increasing the adsorption capacity (Fig. 6b) (Rahman et al. 2016).
Adsorption isotherms
Three adsorption isotherm models were applied to fit the experimental data of Cell-Com sorbent for Zn2+, Cd2+ and Pb2+ ions removal from aqueous solution.
The Langmuir equation
The linier Langmuir model is described as Eq. (5):
where Qe (mg g−1), the equilibrium adsorption capacity; Ce (mg L−1), the equilibrium concentration of metal ion; Qmax (mg g−1), the maximum capacity of the adsorbent and KL (L g−1), the Langmuir adsorption constant (Langmuir 1916). Qmax and KL was calculated from the linear plot of Ce/Qe versus Ce (Fig. 7a). The R2 values for Zn2+, Cd2+ and Pb2+ ions were found to be 0.996, 0.997 and 0.999, respectively (Table 1) suggesting the excellent agreement of experimental data with the Langmuir isotherm model. In addition, the calculated Qmax values for Zn2+, Cd2+ and Pb2+ ions determined to be 102.04, 102.05 and 120.48 mg g−1, respectively, explaining the adsorption rate was fast in the initial stage but slowed down, thereafter the binding sites accordingly decrease with the increase of adsorption time. The Langmuir constant (KL) values that are related to the heat of adsorption and affinity to the binding sites were calculated to be in the order of Pb2+> Cd2+> Zn2+.
The RL can be calculated from the constant KL by Eq. (6):
The value of RL describes the tendency of the adsorption process, which is either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0) (Yu et al. 2013). The calculated RL from the Langmuir isotherm for Zn2+, Cd2+ and Pb2+ ions were found to be 0.32, 0.31 and 0.18, respectively, which implies that the adsorption of three metal ions on Cell-Com were a favorable and useful process. Greater affinity of the adsorbent is inferred when RL is smaller (Langmuir 1916). From the RL values, Pb2+ ion with the smallest RL has the highest affinity for adsorption onto the adsorbent surface.
The Freundlich equation
The linear empirical equation of Freundlich isotherm model is described as Eq. (7):
where KF (L g−1) and nF, the Freundlich isotherm constants which are related to the adsorption capacity and the adsorption strength of the adsorbent, respectively (Freundlich 1906). The linear plots of lnQe versus lnCe are shown in Fig. 7b and the isotherm constants predicted from these plots are tabulated in Table 1. As it is clear from Table 1, the R2 values determined to be 0.974, 0.979 and 0.991 for adsorption of Zn2+, Cd2+ and Pb2+ by Cell-Com, respectively. The 1/nF values were calculated to be 0.44, 0.42 and 0.39 for Zn2+, Cd2+ and Pb2+, respectively. In the Freundlich isotherm, the 1/nF values between 0.1 and 0.5 indicate easy adsorption process which may explain that the adsorption of metal ions by Cell-Com could quickly reach equilibrium (~ 60 min) (Qin et al. 2016).
The Temkin equation
The Temkin model is given by the following Eq. (8):
where the equilibrium binding constant, AT (L mg−1); the Temkin constant corresponding to the heat of sorption, BT (J mol−1); the gas constant, R (8.314 J mol−1 K−1) and the absolute temperature, T (K) (Temkin and Pyzhev 1940). The plot of Qe versus lnCe is illustrated in Fig. 7c for all metal ions and the constants resulted from these linear plots are summarized in Table 1. As observed in the Fig. 7c the Qe value increases with the increase of metal ions concentration which may be due to the high driving force for the mass transfer.
From the isotherm adsorption data in Table 1, the Langmuir model fits with the experimental data better than the Freundlich and Temkin models. In this regard, it is assumed that the adsorption of metal ions are identical and energetically equivalent which occurs without any interaction between the adsorbed molecules and adsorption sites (Zhou et al. 2014).
Adsorption kinetic
The kinetic of adsorption process in this study was investigated using pseudo-first order, pseudo-second order and Weber-Morris intra-particle diffusion models to fit the experimental data.
The pseudo-first order model
This kinetic model is explained by Eq. (9):
where Qt and Qe (mg g−1) are the adsorption capacities at time t (min) and at equilibrium, respectively and k1 (min−1) is the rate constant of pseudo-first order (Ho 2004). The plot of Ln(Qe − Qt) versus t is depicted in Fig. 8a. The values K1 and Qe calculated from the slope and intercept of the corresponding plots and tabulated in Table 2.
The pseudo-second order model
This kinetic model is defined as Eq. (10):
where K2 (g mg−1 min−1) is the rate constant of second-order adsorption. The values Qe and K2 for each metal ion is calculated from the related slope and intercept of the plot of t/Qt versus t (Fig. 8b) (Ho 2006; Sun et al. 2014). All the parameters calculated using this model are given in Table 2. The initial adsorption rate h0 (mg g−1 min−1) for pseudo-second order kinetic model (When t → 0) can be written as Eq. (11) (Anirudhan and Rejeena 2011):
The intra-particle diffusion model
This kinetic model can be described as Eq. (12):
where Ki (mg g−1 min−0.5) and C (mg g−1) are the constants which are calculated from the slope and intercept of the linear plot of Qt versus t0.5 (Fig. 8c) (Weber and Morris 1963).
The correlation coefficients (R2) and the calculated adsorption capacity values of the pseudo-first, pseudo-second orders and intra-particle diffusion models for the removal of Pb2+, Cd2+ and Zn2+ ions in Table 2 revealed that the pseudo-second order model gives the best fit with the experimental data. For example, for Pb+2, the R2 value of pseudo-second order kinetic is 0.999, higher than pseudo-first order kinetic (0.988) and intra-particle diffusion model (0.979). In addition, adsorption capacity (Qe, calc.) of Pb2+ for pseudo-second order kinetic 37.17 mg g−1, which is much closer to the experimental data (Qe, exp. = 36.4 mg g−1). Similarly R2 and Qe values for Cd2+ and Zn2+ ions, the pseudo-second order model may be considered the best kinetic model compared with the other two models (Qin et al. 2016). However, comparing the parameter values of intra-particle diffusion model, with those of pseudo-second order model, it is suppose that diffusion and mass transfer may be involved in the adsorption of metal ions onto the Cell-Com. But it would not be the rate limiting step in adsorption process. In view of well-matched pseudo-second order kinetic model with the experimental data, it is postulated that in the Cell-Com adsorption process of Pb2+, Cd2+ and Zn2+ ions from aqueous media, the pseudo-second order mechanism predominant and the adsorption mechanism would be a chemical process. This might be due to the valence force through sharing electrons on the hetero atoms of Cell-Com with the metal ions.
Thermodynamic parameters
Thermodynamic parameters of the adsorption of metal ions by Cell-Com have been estimated based on Van’t Hoff plot using the Eqs. (13– 16 ) (Mansour Lakouraj et al. 2015):
where ΔG°, the free energy changes of adsorption (J mol−1); ΔH°, the standard enthalpy changes (J mol−1); ΔS°, the standard entropy changes (J mol−1 K−1); K (L mol−1) and KL (L g−1), the thermodynamic equilibrium constants; R, the gas constant (J mol−1 K−1); T, the absolute temperature (K) and M is the molar mass of metal ion (g mol−1) (Krishna Kumar et al. 2013). The ΔH° and ΔS° of the adsorption process were determined by the slope and intercept of the linear plots of lnK versus 1/T (Fig. S4, ESI. †). These parameters and the calculated values of ΔG° are tabulated in Table S2 (ESI. †). As it can be seen in Table S2 (ESI. †) adsorption of all three metal ions by the nanoadsorbent are spontaneous and thermodynamically favorable because of the negative values of ΔG° (Gurgel and Gil 2009a). Positive values of ΔH° confirms the endothermic nature of the adsorption process at studied temperatures. Besides, the positive values of ΔS° represents the entropy increase of metal ions in solution due to rising temperature was much greater compared with the entropy decrease of the adsorbed metal ions in the adsorption process and consequently, the ΔS° of the whole adsorption system increased (Su et al. 2015). However, the values in Table S2 shows that at higher temperature adsorption capacity of nanoadsorbent is increased which could be due to the higher mobility of metal ions and faster rate diffusion of adsorbate ions on the surface of Cell-Com. The activation energy (Ea) for the adsorption of metal ions to be Zn2+ = 38.52, Cd2+ = 38.57 and Pb2+ = 38.05 kJ mol−1, were calculated by Arrhenius-type relationship represented by following Eqs. (17) and (18):
where K, the rate constant; A, the pre-exponential factor; R, the universal gas constant and T is the temperature in Kelvin. The positive values of Ea also indicate an endothermic adsorption process (Eligwe et al. 1999).
Competitive adsorption
In general, wastewater contains a lot of different metal ions, so the effect of coexisting ions on adsorption of each ion was studied by adding two other ions. The simultaneous adsorption of each ion without and with coexisting ions are shown in Fig. 9. The effect of coexisting ions (Cd2+ and Zn2+) on Pb2+ adsorption was small compared with the adsorption without coexisting ions. However, the adsorption rate of Cd2+ and Zn2+ decreased more than that for adsorption without coexisting ions. This means that the binding of Pb2+ was relatively unaffected by other metals and the Cell-Com has quite good selectivity for Pb2+ ions. We explain this selectivity to two reasons: (i) the larger ionic radius of Pb2+ compared with Cd2+ and Zn2+ reduces the electrostatic nature of Pb2+ ions, it favors interactions of a covalent nature between Pb2+ and the active sites of adsorbent (Yu et al. 2013; Zhou et al. 2013). (ii) Divalent cations are sorbed preferably with low hydration energies (Peng et al. 2014). The enthalpy of hydration (KJ mol−1) of ions are − 1485 for Pb2+, − 1809 for Cd2+ and − 2047 for Zn2+. The low hydration energy of Pb2+ suggests the preferential adsorption and the selective sequence as Pb2+> Cd2+> Zn2+.
Desorption and reusability
The recovery and reusability of the adsorbents are important factors for their economic value of adsorption process. Therefore, the adsorption–desorption study is important to clarify the behavior of adsorption, recycling of the adsorbent and the recovery of the adsorbent surface, to enhance the economic value of adsorption process. Desorption studies were accomplished in aqueous solution of HCl (30 mL, 0.5 M) and EDTA (30 mL, 0.05 M) at room temperature for 2 h. Desorption results of Cell-Com by HCl and EDTA solution and reusability of the adsorbent are depicted in Fig. 10 and are tabulated in Tables (S3 and S4, ESI. †), respectively. Dilute HCl is one of the most widely used reagents, in techniques which employ acid solutions to reuse of adsorbents, due to the abundant hydrogen ions in the solution, a dominant protonation reaction takes place between hydrogen ions and active sites, so low pH favors the dissociation of the existing complexes, meanwhile M+2 readily reacts with chloride, to form a MCl2 precipitate, that is only slightly soluble in dilute acids or water. EDTA is one of the most widely used complexing agents that extract metals on exchange sites of complexes (Leleyter et al. 2012). The removal efficiency of metal ions decreased after acid treatment, which is caused by the protonation of active sites of adsorbent in the process of acid treatment. Although the treated adsorbent was washed repeatedly to reach neutral for reuse, some H+ occupied the binding sites on the adsorbent surface was irrevocable, which led to the reduction of removal efficiency (Gan et al. 2016). Hence, to release the adsorbed cations it is necessary to use an efficient chelating agent capable to form a strong complex with cations that can no longer be adsorbed on the surface. The high chelating stability constant of divalent metal ions-EDTA explained the better capacity of EDTA to completely release the adsorbed metal ions by the Cell-Com (Maatar and Boufi 2015). This study compares the efficiency of two chemical procedure (using EDTA and HCl as recycling agents) to recycle of adsorbent. We suggest that aqueous solution of EDTA can used rather than dilute HCl to remove metal ions especially lead ion from adsorbents because the cycle number of adsorbent in EDTA solution is more than HCl solution, in addition to, EDTA ligands have a very strong affinity binding with lead ions (Yu et al. 2013).
Proposed adsorption mechanism
To gain further insight into sorption mechanism, FT-IR spectra of Cell-Com before and after Pb(II) adsorption were performed (Fig. S5, ESI. †). All the adsorption bands of Cell-Com were shifted to higher frequency. This shift of bands reflects electrostatic interactions between the adsorption sites and metal ions (Jia et al. 2008). The obvious shift of the Ti–O band after uptake of Pb2+ could indicate a strong affinity between Ti–O and Pb2+ ions, result from complex formation of metal ions with Ti–O (Peng et al. 2014). Meanwhile, the new adsorption peak at 651 cm−1 after uptake of Pb2+ demonstrates the formation of Pb–O (Li et al. 2015b). These observations may suggest that the chemical reaction occurs between Pb+2 and adsorption sites on the surface of Cell-Com in agreement with the pseudo-second order kinetic model (Özlem Kocabaş-Ataklı and Yürüm 2013; Zhong et al. 2012).
Conclusions
The synthesized bionanocomposite (Cell-Com) in this work consists of modified anatase TiO2 NPs grafted onto the surface of functionalized microcrystalline cellulose through click reaction. Chemical modification of cellulose and TiO2 NPs along with their chemical connection by click reaction formed an efficient bioadsorbent containing a range of functional groups possessing of nitrogen and oxygen heteroatoms with nonbonding electrons capable of chelating with metal ions, thus enhancing the adsorption capacity. This highly porous nanocomposite with many active sites was then used as an adsorbent for the removal of Pb2+, Cd2+ and Zn2+ ions from aqueous solutions. The highest adsorption capacity was achieved at initial metal ion concentration of 20 ppm, 10 mg of adsorbent dose, pH 7.0 and 20 mL total volume in approximately 60 min at room temperature. The batch experimental data for removal of Pb2+, Cd2+ and Zn2+ ions by Cell-Com from aqueous solutions was well fitted with pseudo-second order kinetic model and Langmuir adsorption isotherm which indicated the existence of chemical bonding of metal ions to Cell-Com. The Cell-Com exhibited a preferential binding capacity of Pb2+> Cd2+ > Zn2+. The estimated thermodynamic values (ΔG°, ΔH° and ΔS°) together with Ea value indicate the adsorption process could be spontaneous, endothermic and thermodynamically favorable. Competitive adsorption of ions in a mixture of three metal ions reveals that the Cell-Com adsorbent is preferentially effective toward Pb2+ removal. The nanocomposite can be readily regenerated by treating with HCl or EDTA solutions. The recovered bioadsorbent exhibited high stability and reusability without significant loss of its original performance. The Cell-Com with high efficiency, good selectivity, recyclability and environmental friendliness could be considered as a good candidate for the removal of heavy metal ions from wastewater.
References
Anirudhan TS, Rejeena SR (2011) Thorium(IV) removal and recovery from aqueous solutions using tannin-modified poly(glycidylmethacrylate)-grafted zirconium oxide densified cellulose. Ind Eng Chem Res 50:13288–13298. https://doi.org/10.1021/ie2015679
Anirudhan TS, Nima J, Sandeep S, Ratheesh VRN (2012) Development of an amino functionalized glycidylmethacrylate-grafted-titanium dioxide densified cellulose for the adsorptive removal of arsenic(V) from aqueous solutions. Chem Eng J 209:362–371. https://doi.org/10.1016/j.cej.2012.07.129
Bai J, Zhou B (2014) Titanium dioxide nanomaterials for sensor applications. Chem Rev 114:10131–10176. https://doi.org/10.1021/cr400625j
Bezrodna T, Puchkovska G, Shymanovska V, Baran J, Ratajczak H (2004) IR-analysis of H-bonded H2O on the pure TiO2 surface. J Mol Struct 700:175–181. https://doi.org/10.1016/j.molstruc.2003.12.057
Bossa N, Carpenter AW, Kumar N, de Lannoy C-F, Wiesner M (2017) Cellulose nanocrystal zero-valent iron nanocomposites for groundwater remediation. Environ Sci Nano. https://doi.org/10.1039/C6EN00572A
d’Halluin M, Rull-Barrull J, Bretel G, Labrugère C, Le Grognec E, Felpin F-X (2017) Chemically modified cellulose filter paper for heavy metal remediation in water. ACS Sustain Chem Eng 5:1965–1973. https://doi.org/10.1021/acssuschemeng.6b02768
Dahl M, Liu Y, Yin Y (2014) Composite titanium dioxide nanomaterials. Chem Rev 114:9853–9889. https://doi.org/10.1021/cr400634p
Elchinger P-H, Faugeras PA, Zerrouki C, Montplaisir D, Brouillette F, Zerrouki R (2012a) Tosylcellulose synthesis in aqueous medium. Green Chem 14:3126–3131. https://doi.org/10.1039/c2gc35592b
Elchinger P-H, Montplaisir D, Zerrouki R (2012b) Starch-cellulose crosslinking—towards a new material. Carbohydr Polym 87:1886–1890. https://doi.org/10.1016/j.carbpol.2011.09.027
Eligwe CA, Okolue NB, Nwambu CO, Nwoko CIA (1999) Adsorption thermodynamics and kinetics of mercury(II), Cadmium(II) and lead(II) on lignite. Chem Eng Technol 22:45–49
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
French AD, Santiago Cintrón M (2013) Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 20:583–588. https://doi.org/10.1007/s10570-012-9833-y
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem 57A:385–470
Gan W, Gao L, Zhan X, Li J (2016) Preparation of thiol-functionalized magnetic sawdust composites as an adsorbent to remove heavy metal ions. RSC Adv 6:37600–37609. https://doi.org/10.1039/c6ra02285e
Ghaemy M, Qasemi S, Ghassemi K, Bazzar M (2013) Nanostructured composites of poly (triazole-amide-imide) s and reactive titanium oxide by epoxide functionalization: thermal, mechanical, photophysical and metal ions adsorption properties. J Polym Res 20:278. https://doi.org/10.1007/s10965-013-0278-2
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:22. https://doi.org/10.1186/1745-6673-1-22
Gu J, Hsieh YL (2015) Surface and structure characteristics, self-assembling, and solvent compatibility of holocellulose nanofibrils. ACS Appl Mater Interfaces 7:4192–4201. https://doi.org/10.1021/am5079489
Gurgel LVA, Gil LF (2009a) Adsorption of Cu(II), Cd(II) and Pb(II) from aqueous single metal solutions by succinylated twice-mercerized sugarcane bagasse functionalized with triethylenetetramine. Water Res 43:4479–4488. https://doi.org/10.1016/j.watres.2009.07.017
Gurgel LVA, Gil LF (2009b) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by succinylated mercerized cellulose modified with triethylenetetramine. Carbohydr Polym 77:142–149. https://doi.org/10.1016/j.carbpol.2008.12.014
Hao J, Xu S, Xu N, Li D, Linhardt RJ, Zhang Z (2017) Impact of degree of oxidation on the physicochemical properties of microcrystalline cellulose. Carbohydr Polym 155:483–490. https://doi.org/10.1016/j.carbpol.2016.09.012
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater B 136:681–689. https://doi.org/10.1016/j.jhazmat.2005.12.043
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016
Ishii D, Kanazawa Y, Tatsumi D, Matsumoto T (2007) Effect of solvent exchange on the pore structure and dissolution behavior of cellulose. J Appl Polym Sci 103:3976–3984. https://doi.org/10.1002/app.25424
Jamshaid A et al (2017) Cellulose-based materials for the removal of heavy metals from wastewater—an overview. ChemBioEng Rev 4:240–256. https://doi.org/10.1002/cben.201700002
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Jia K et al (2008) Adsorption of Pb2+, Zn2+, and Cd2+ from waters by amorphous titanium phosphate. J Colloid Interface Sci 318:160–166. https://doi.org/10.1016/j.jcis.2007.10.043
Jiang F, Han S, Hsieh YL (2013) Controlled defibrillation of rice straw cellulose and self-assembly of cellulose nanofibrils into highly crystalline fibrous materials. RSC Adv 3:12366–12375. https://doi.org/10.1039/c3ra41646a
Jin C, Zhang X, Xin J, Liu G, Wu G, Kong Z, Zhang J (2017) Clickable synthesis of 1, 2, 4-triazole modified lignin-based adsorbent for the selective removal of Cd(II). ACS Sustain Chem Eng 5:4086–4093. https://doi.org/10.1021/acssuschemeng.7b00072
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87. https://doi.org/10.1016/j.tox.2011.03.001
Jun Z, Shanjing Y, Dongqiang L (2009) Adsorbents for expanded bed adsorption: preparation and functionalization. Chin J Chem Eng 17(4):678–687. https://doi.org/10.1016/S1004-9541(08)60263-3
Kardam A, Raj KR, Srivastava S, Srivastava MM (2013) Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol Environ Policy 16:385–393. https://doi.org/10.1007/s10098-013-0634-2
Karim Z, Hakalahti M, Tammelin T, Mathew AP (2017) In situ TEMPO surface functionalization of nanocellulose membranes for enhanced adsorption of metal ions from aqueous medium. RSC Adv 7:5232–5241. https://doi.org/10.1039/c6ra25707k
Karimi L, Mirjalili M, Yazdanshenas ME, Nazari A (2010) Effect of nano TiO2 on self-cleaning property of cross-linking cotton fabric with succinic acid under UV irradiation. Photochem Photobiol 86:1030–1037. https://doi.org/10.1111/j.1751-1097.2010.00756.x
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466. https://doi.org/10.1002/anie.201001273
Krishna Kumar AS, Kalidhasan S, Rajesh V, Rajesh N (2013) Adsorptive demercuration by virtue of an appealing interaction involving biopolymer cellulose and mercaptobenzothiazole. Ind Eng Chem Res 52:11838–11849. https://doi.org/10.1021/ie400921p
Lamanna M, Leiton L, Vega IN, Rivas BL, D’Accorso N (2017) New copolymers with triazole pendant groups: synthesis, characterization and their application to remove heavy metals. Adv Mater Sci. https://doi.org/10.15761/AMS.1000120
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Leleyter L, Rousseau C, Biree L, Baraud F (2012) Comparison of EDTA, HCl and sequential extraction procedures, for selected metals (Cu, Mn, Pb, Zn), in soils, riverine and marine sediments. J Geochem Explor 116–117:51–59. https://doi.org/10.1016/j.gexplo.2012.03.006
Li G, Li L, Boerio-Goates J, Woodfield BF (2005) High purity anatase TiO2 nanocrystals: near room-temperature synthesis, grain growth kinetics, and surface hydration chemistry. J Am Chem Soc 127:8659–8666. https://doi.org/10.1021/ja050517g
Li MC, Wu Q, Song K, Lee SY, Yan Q, Wu Y (2015a) Cellulose nanoparticles: structure morphology rheology relationship. ACS Sustain Chem Eng 3(5):821–832. https://doi.org/10.1021/acssuschemeng.5b00144
Li Y, Cao L, Li L, Yang C (2015b) In situ growing directional spindle TiO2 nanocrystals on cellulose fibers for enhanced Pb2+ adsorption from water. J Hazard Mater 289:140–148. https://doi.org/10.1016/j.jhazmat.2015.02.051
Li X, Bian C, Meng X, Xiao FS (2016a) Design and synthesis of an efficient nanoporous adsorbent for Hg2+ and Pb2+ ions in water. J Mater Chem A 4:5999–6005. https://doi.org/10.1039/c6ta00987e
Li Y, Li L, Cao L, Yang C (2016b) Promoting dynamic adsorption of Pb2+ in a single pass flow using fibrous nano-TiO2/cellulose membranes. Chem Eng J 283:1145–1153. https://doi.org/10.1016/j.cej.2015.08.068
Li M, Liu Z, Wang L, James TD, Xiao H-N, Zhu W (2017) Glutamic acid-modified cellulose fibrous composite for adsorption of heavy metal ions from single and binary solutions. Mater Chem Front. https://doi.org/10.1039/C7QM00210F
Liang L, Astruc D (2011) The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord Chem Rev 255:2933–2945. https://doi.org/10.1016/j.ccr.2011.06.028
Liu C, Baumann H (2005) New 6-butylamino-6-deoxycellulose and 6-deoxy-6-pyridiniumcellulose derivatives with highest regioselectivity and completeness of reaction. Carbohydr Res 340:2229–2235. https://doi.org/10.1016/j.carres.2005.07.018
Liu J et al (2014) Welan gum-modified cellulose bead as an effective adsorbent of heavy metal ions (Pb2+, Cu2+, and Cd2+) in aqueous solution. Sep Sci Technol 49:1096–1103. https://doi.org/10.1080/01496395.2013.872658
Luo Y, Xu J, Huang J (2014) Hierarchical nanofibrous anatase-titania–cellulose composite and its photocatalytic property. CrystEngComm 16:464–471. https://doi.org/10.1039/c3ce41906a
Maatar W, Boufi S (2015) Poly(methacylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent. Carbohydr Polym 126:199–207. https://doi.org/10.1016/j.carbpol.2015.03.015
Mahfoudhi N, Boufi S (2017) Nanocellulose as a novel nanostructured adsorbent for environmental remediation: a review. Cellulose. https://doi.org/10.1007/s10570-017-1194-0
Mansour Lakouraj M, Norouzian RS, Balo S (2015) Preparation and cationic dye adsorption of novel Fe3O4 supermagnetic/thiacalix[4]arene tetrasulfonate self-doped/polyaniline nanocomposite: kinetics, isotherms, and thermodynamic study. J Chem Eng Data 60:2262–2272. https://doi.org/10.1021/acs.jced.5b00080
Martina K, Baricco F, Berlier G, Caporaso M, Cravotto G (2014) Efficient green protocols for preparation of highly functionalized β-cyclodextrin-grafted silica. ACS Sustain Chem Eng 2:2595–2603. https://doi.org/10.1021/sc500546e
Masoumi A, Hemmati K, Ghaemy M (2016) Low-cost nanoparticles sorbent from modified rice husk and a copolymer for efficient removal of Pb(II) and crystal violet from water. Chemosphere 146:253–262. https://doi.org/10.1016/j.chemosphere.2015.12.017
Massin J, Ducasse L, Toupance T, Cl Olivier (2014) Tetrazole as a new anchoring group for the functionalization of Tio2 nanoparticles: a joint experimental and theoretical study. J Phys Chem C 118:10677–10685. https://doi.org/10.1021/jp502488g
Meng X, Edgar KJ (2016) “Click” reactions in polysaccharide modification. Prog Polym Sci 53:52–85. https://doi.org/10.1016/j.progpolymsci.2015.07.006
Miao C, Hamad WY (2013) Cellulose reinforced polymer composites and nanocomposites: a critical review. Cellulose 20:2221–2262. https://doi.org/10.1007/s10570-013-0007-3
Mohammadinezhad A, Nasseri MA, Salimi M (2014) Cellulose as an efficient support for Mn(salen)Cl: application for catalytic oxidation of sulfides to sulfoxides. RSC Adv 4:39870–39874. https://doi.org/10.1039/c4ra06450j
Morawski AW, Kusiak-Nejman E, Przepiórski J, Kordala R, Pernak J (2013) Cellulose-TiO2 nanocomposite with enhanced UV–Vis light absorption. Cellulose 20:1293–1300. https://doi.org/10.1007/s10570-013-9906-6
Moses JE, Moorhouse AD (2007) The growing applications of click chemistry. Chem Soc Rev 36:1249–1262. https://doi.org/10.1039/b613014n
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol 99:6709–6724. https://doi.org/10.1016/j.biortech.2008.01.036
Olivera S, Muralidhara HB, Venkatesh K, Guna VK, Gopalakrishna K, Kumar Y (2016) Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: a review. Carbohydr Polym 153:600–618. https://doi.org/10.1016/j.carbpol.2016.08.017
Özlem Kocabaş-Ataklı Z, Yürüm Y (2013) Synthesis and characterization of anatase nanoadsorbent and application in removal of lead, copper and arsenic from water. Chem Eng J 225:625–635. https://doi.org/10.1016/j.cej.2013.03.106
Park H, Park Y, Kim W, Choi W (2013) Surface modification of TiO2 photocatalyst for environmental applications. J Photochem Photobiol C Photochem Rev 15:1–20. https://doi.org/10.1016/j.jphotochemrev.2012.10.001
Peng P, Cao X, Peng F, Bian J, Xu F, Sun R (2012) Binding cellulose and chitosan via click chemistry: synthesis, characterization, and formation of some hollow tubes. J Polym Sci A Polym Chem 50:5201–5210. https://doi.org/10.1002/pola.26371
Peng Q et al (2014) Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J Am Chem Soc 136:4113–4116. https://doi.org/10.1021/ja500506k
Pierre-Antoine F, Francois B, Rachida Z (2012) Crosslinked cellulose developed by CuAAC, a route to new materials. Carbohydr Res 356:247–251. https://doi.org/10.1016/j.carres.2011.10.028
Pujari SP, Scheres L, Marcelis ATM, Zuilhof H (2014) Covalent surface modification of oxide surfaces. Angew Chem Int Ed 53:2–36. https://doi.org/10.1002/anie.201306709
Qin X et al (2016) A green technology for the synthesis of cellulose succinate for efficient adsorption of Cd(II) and Pb(II) ions. RSC Adv 6:26817–26825. https://doi.org/10.1039/c5ra27280g
Rahman ML, Sarkar SM, Yusoff MM, Abdullah MH (2016) Efficient removal of transition metal ions using poly(amidoxime) ligand from polymer grafted kenaf cellulose. RSC Adv 6:745–757. https://doi.org/10.1039/c5ra18502e
Sabzi M, Mirabedini SM, Zohuriaan-Mehr J, Atai M (2009) Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog Org Coat 65:222–228. https://doi.org/10.1016/j.porgcoat.2008.11.006
Sadeghifar H, Filpponen I, Clarke SP, Brougham DF, Argyropoulos DS (2011) Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J Mater Sci 46:7344–7355. https://doi.org/10.1007/s10853-011-5696-0
Scherrer P (1918) Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr Ges Wiss Gottingen 2:98–100
Seidlerová J, Šafařík I, Rozumová L, Šafaříková M, Motyka O (2016) TiO2-Based Sorbent of Lead Ions. Procedia Mater Sci 12:147–152. https://doi.org/10.1016/j.mspro.2016.03.026
Shahadat M, Teng TT, Rafatullah M, Arshad M (2015) Titanium-based nanocomposite materials: a review of recent advances and perspectives. Colloids Surf B 126:121–137. https://doi.org/10.1016/j.colsurfb.2014.11.049
Shirke BS, Korake PV, Hankare PP, Bamane SR, Garadkar KM (2011) Synthesis and characterization of pure anatase TiO2 nanoparticles. J Mater Sci Mater Electron 22:821–824. https://doi.org/10.1007/s10854-010-0218-4
Su Y, Liu J, Yue Q, Li Q, Gao B (2015) Adsorption of lead and nickel ions by semi-interpenetrating network hydrogel based on wheat straw cellulose: kinetics, equilibrium, and thermodynamics. Soft Mater 13:225–236. https://doi.org/10.1080/1539445x.2015.1074923
Sun X, Yang L, Li Q, Zhao J, Li X, Wang X, Liu H (2014) Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): synthesis and adsorption studies. Chem Eng J 241:175–183. https://doi.org/10.1016/j.cej.2013.12.051
Tao P, Viswanath A, Li Y, Siegel RW, Benicewicz BC, Schadler LS (2013) Bulk transparent epoxy nanocomposites filled with poly (glycidyl methacrylate) brush-grafted TiO2 nanoparticles. Polymer 54:1639–1646. https://doi.org/10.1016/j.polymer.2013.01.032
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim URSS 12:217–222
Thommes J, Halfar M, Lenz S, Kula MR (1995) Purification of monoclonal antibodies from whole hybridoma fermentation broth by fluidized bed adsorption. Biotechnol Bioeng 45:205–211
Urankar D, Pinter B, Pevec A, De Proft F, Turel I, Košmrlj J (2010) Click-triazole N2 coordination to transition-metal ions is assisted by a pendant pyridine substituent. Inorg Chem 49:4820–4829. https://doi.org/10.1021/ic902354e
Vanhatalo K, Maximova N, Perander AM, Johansson LS, Haimi E, Dahl O (2016) Comparison of conventional and lignin-rich microcrystalline cellulose. BioResources 11(2):4037–4054
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Wu CH, Kuo CY, Andy Hong PK, Chen MJ (2015) Removal of copper by surface-modified celluloses: kinetics, equilibrium, and thermodynamics. Desalin Water Treat 55(5):1253–1263. https://doi.org/10.1080/19443994.2014.926461
Xiong Y et al (2017) A 3D titanate aerogel with cellulose as the adsorption-aggregator for highly efficient water purification. J Mater Chem A 5:5813–5819. https://doi.org/10.1039/C6TA10638B
Xue G (1987) Chemical reactions of an epoxy-functional silane in aqueous solution. Die Angew Makromol Chem 151:85–93
Yadav P, Chacko S, Kumar G, Ramapanicker R, Verma V (2015) Click chemistry route to covalently link cellulose and clay. Cellulose 22:1615–1624. https://doi.org/10.1007/s10570-015-0594-2
Yang R, Aubrecht KB, Ma H, Wang R, Grubbs RB, Hsiao BS, Chu B (2014) Thiol-modified cellulose nanofibrous composite membranes for chromium(VI) and lead(II) adsorption. Polymer 55:1167–1176. https://doi.org/10.1016/j.polymer.2014.01.043
Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W (2013) Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J Environ Sci 25:933–943. https://doi.org/10.1016/s1001-0742(12)60145-4
Zhang J, Li L, Li Y, Yang C (2017) Microwave-assisted synthesis of hierarchical mesoporous nano-TiO2/cellulose composites for rapid adsorption of Pb2+. Chem Eng J 313:1132–1141. https://doi.org/10.1016/j.cej.2016.11.007
Zhao X, Lv L, Pan B, Zhang W, Zhang S, Zhang Q (2011) Polymer-supported nanocomposites for environmental application: a review. Chem Eng J 170:381–394. https://doi.org/10.1016/j.cej.2011.02.071
Zhao J, Milanova M, Warmoeskerken MMCG, Dutschka V (2012) Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf A Physicochem Eng Asp 413:273–279. https://doi.org/10.1016/j.colsurfa.2011.11.033
Zhong LS, Hu JS, Wan LJ, Song WG (2008) Facile synthesis of nanoporous anatase spheres and their environmental applications. Chem Commun. https://doi.org/10.1039/b718300c
Zhong L, Peng X, Yang D, Sun R (2012) Adsorption of heavy metals by a porous bioadsorbent from lignocellulosic biomass reconstructed in an ionic liquid. J Agric Food Chem 60:5621–5628. https://doi.org/10.1021/jf301182x
Zhou Y, Hu X, Zhang M, Zhuo X, Niu J (2013) Preparation and characterization of modified cellulose for adsorption of Cd(II), Hg(II), and acid fuchsin from aqueous solutions. Ind Eng Chem Res 52:876–884. https://doi.org/10.1021/ie301742h
Zhou Y, Fu S, Zhang L, Zhan H, Levit MV (2014) Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(II). Carbohydr Polym 101:75–82. https://doi.org/10.1016/j.carbpol.2013.08.055
Acknowledgments
The authors wish to express their thanks to the research council of Shahrood University of technology for the partial financial support of research work. The authors also thank University of Mazandaran for its helps carrying out this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fallah, Z., Nasr Isfahani, H., Tajbakhsh, M. et al. TiO2-grafted cellulose via click reaction: an efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 25, 639–660 (2018). https://doi.org/10.1007/s10570-017-1563-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1563-8