Abstract
Polymyalgia rheumatica (PMR) is one of the most common inflammatory rheumatologic condition occurring in older adults. It is characterized by proximal pain and stiffness in the shoulders, neck, and/or pelvic girdles in individuals over 50 years of age along with increased markers of inflammation. Although the above clinical symptoms are very characteristic for the condition, it is considered a diagnosis of exclusion as other autoimmune, as well as infectious, malignant and endocrine disorders can present with similar symptoms. It can co-occur with elderly-onset RA (EORA) or giant cell arteritis (GCA), and these should be explored as alternative diagnoses in every patient. The cornerstone of PMR treatment is low to medium doses of glucocorticosteroids (GC), but while there is typically a swift response to GC treatment, response rate is variable and flare-ups occurs in 50% of patients while tapering down GC doses. Because GC treatment itself is associated with a myriad of deleterious side effects, screening and management of patient comorbidities which may be affected by GC treatment should be carried out on a regular basis during treatment, with novel immunosuppressive agents such as interleukin (IL)-6 blocking agents being developed as GC-sparing agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Epidemiology and Immunopathogenesis
Polymyalgia rheumatica, a condition first reported as early as 1888 and later named by Barber in 1957 [1], is the most common inflammatory disorder affecting the elderly. It most commonly occurs in women of Northern European ancestry but can occur in any ethnic group, with increasing age and female sex being the two main risk factors. It almost never occurs in patients younger than 50 years of age with peak incidence occurring at age 70–75 years with 66–75% of patients being female with lifetime risk of 2.4% for women and 1.7% for men [2,3,4]. While the etiology of PMR remains unclear, its occurrence seems to stem from an interplay between genetic and environmental factors with a role for immunosenescence, the latter representing a series of changes that occur in the immune system of the elderly.
Unlike the overlapping condition giant cell arteritis (GCA), where HLA-DRB1∗04 genotype is a known risk factor for disease development, no clear HLA association has been shown in PMR [5]. However, even in PMR, genetic polymorphisms may play a role, such as polymorphisms of IL-6, as recent reports show relevance of this mediator in PMR immunopathogenesis (see below). Additional immunopathogenic factors involve the aging immune system, with a decrease in production of T-cells due to thymic involution. This, in turn, causes a reduction in the output of thymic regulatory T cells, leading to immune dysregulation and autoimmunity [6]. A consequence of immune dysregulation is an increase in pro-inflammatory cytokine production such as IL-6 in older adults [7, 8]. Indeed, elevated IL-6 levels have been shown to correlate with PMR disease activity, [5] and IL-6 blocking agents are currently being trialed in PMR after having shown efficacy in GCA management [9].
Aside from genetic factors, environmental factors are also purported to play a role in PMR development. Indeed, cyclic fluctuations have been reported to correspond with incidence peaks of GCA during the winter months in the setting of mycoplasma, chlamydia pneumonia, and parvovirus B19 epidemics [10], and herpes zoster occurrence was recently found to be associated with an increased risk of GCA in two large GCA patient cohorts [11]. However, no infectious organism has been clearly associated with PMR occurrence to date [10].
2 Clinical and Laboratory Manifestations
Clinical signs and symptoms of PMR include abrupt onset (typically ranging between 2 weeks and up to 2 months, though a more subtle, progressive course can also occur) of pain and stiffness typically affecting the neck and one or both proximal girdles – the shoulder girdle, and less commonly, the pelvic girdle. Although symptoms may be unilateral on initial presentation, clinical manifestations are later bilateral [12]. Stiffness is particularly debilitating and is typically present for more than 30 minutes in the morning, making it difficult for patients to lift their arms to complete their activities of daily living (ADLs) including brushing their teeth, combing their hair, or in putting on a bra while getting dressed. Similarly, it may be difficult for patients affected by pelvic girdle stiffness to get out of bed or rise from a chair without assistance. Resting stiffness, in which stiffness worsens after periods of rest, may also occur. In some patients, the pain in the shoulder and pelvic regions may radiate to the elbows, hips, and even to the knees. Characteristic clinical signs of systemic inflammation, such as low-grade fever, anorexia and weight loss, and/or malaise and fatigue occur in approximately 40–50% of patients and are particularly common in patients over 70 years of age [4].
On clinical examination, the clinician may observe reduced active and passive ranges of motion, especially in shoulder elevation or hip flexion. It is noteworthy that while patients may experience a sensation of proximal muscle weakness, actual muscle weakness or findings supporting myopathy are lacking. Distal musculoskeletal manifestations may occur in 25–50% of patients, most frequently as transient, nonerosive asymmetrical arthritis primarily affecting the knee or wrist in up to 39% of patients [12,13,14]. Tenosynovitis, such as carpal tunnel syndrome, is also common [4, 14]. Pitting edema can affect the hands, wrists, ankles and feet, and occasionally is the presenting finding.
Among laboratory signs of inflammation, elevated erythrocyte sedimentation rate (ESR) typically over 40 mm/h, and elevated C-reactive protein (CRP) level are highly characteristic of PMR and are detected in more than 90% of patients [4]. Both tests should be ordered as they may be discordant when one test performs better than the other in certain individuals. Notably, both ESR and CRP may be normal in a small percentage of patients [4]. Additional common laboratory markers of inflammation, including anemia of chronic disease, thrombocytosis, or elevated ferritin level can also be seen. Mild elevation of liver enzymes, especially alkaline phosphatase, may also occur [4].
3 Diagnosis
The diagnosis of PMR in the elderly can pose a significant challenge for treating clinicians because no universally accepted diagnostic criteria currently exist; and signs, symptoms and laboratory studies associated with PMR are all non-specific. Moreover, older adults may await seeking medical advice for several months due to attribution of musculoskeletal aches and pains to aging. PMR may also not be obvious in the elderly due to multiple co-morbidities [15]. For instance, dementia, which is more common in the elderly, may make history-taking and clinical examination more challenging. Older patients may also present with nonclassical features due to other co-existing disorders, further complicating the clinical picture. Especially notable in this regard is rotator cuff pathology or osteoarthritis (OA) of the shoulders and cervical or lumbar spine in older adults, which should normally be excluded when diagnosing PMR but which commonly co-exist with PMR in elderly patients [15]. Moreover, similar to other conditions in the elderly, PMR may present primarily as acute-onset functional impairment, which can be seen in a large variety of medical conditions as diverse as infection or stroke, thus making diagnosis more difficult. Last, other rheumatic inflammatory conditions which primarily affect older adults, such as elderly-onset rheumatoid arthritis (EORA) or Remitting Seronegative Symmetrical Synovitis with Pitting Edema (RS3PE) may present initially like PMR [4, 15, 16].
Because of multiple mimics of PMR, the 2015 EULAR/ACR management guidelines recommend that, at a minimum, a basic workup to exclude alternative diagnoses of PMR and to establish a baseline for monitoring of therapy should include complete blood count, inflammatory markers, thyroid function tests, bone profile (vitamin D, calcium, alkaline phosphatase levels), kidney and liver function tests, creatine kinase level, rheumatoid factor (RF) and/or anti-citrullinated protein antibodies (ACPA), urinalysis, and protein electrophoresis. Depending on clinical signs and symptoms and likelihood of alternative diagnoses, additional testing such as anti-nuclear antibodies (ANA), anti-cytoplasmic neutrophil antibodies (ANCA) or tuberculosis testing may be warranted [17]. Ultrasonography can be particularly useful in patients with typical proximal symptoms but normal inflammatory markers.
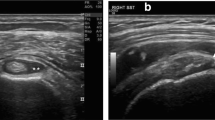
In 2012, the European League Against Rheumatism and the American College of Rheumatology jointly developed the Provisional Classification Criteria for Polymyalgia Rheumatica (Table 19.1) [18]. Ultrasonographic findings of shoulder or hip inflammation can be used to enhance specificity of these criteria (Fig. 19.1). These criteria, which were devised for clinical research and not for diagnostic purposes, include essential manifestations of PMR; when tested, they were found to have 66–68% sensitivity and 65–88% specificity, depending on the differentials being tested and whether ultrasonography was being used. Unfortunately, because these criteria are not of high sensitivity or specificity, they make it difficult to distinguish between PMR and other rheumatic conditions. For instance, they can distinguish PMR from other shoulder conditions with only 86% specificity, with even lower specificity of 65% when aiming to discriminate between PMR and RA [18].
4 Differential Diagnosis of PMR in the Elderly
Due to the nonspecific nature of its signs and symptoms and non-specific laboratory findings, PMR is considered a diagnosis of exclusion. Several categories of illnesses can present similarly to PMR (Table 19.2). Therefore, careful history taking and clinical examination must be conducted when considering PMR. Importantly, because of the overlap between PMR and GCA, this form of large vessel vasculitis must be considered in every patient presenting with PMR.
Several studies to date examine the reasons for initial misdiagnosis of PMR. In a retrospective case series by González-Gay et al., the most common finding present in cases initially misdiagnosed as PMR was lack of rapid response to GC treatment in most of these cases compounded with a lack of consideration of alternative diagnoses [21]. In another study, persistently elevated ESR despite GC treatment was a clue pointing to an alternative diagnosis [16]. Such studies highlight the significance of PMR being first and foremost a diagnosis of exclusion.
5 Conditions Commonly Co-occurring with PMR
Several conditions so frequently present with initial symptomology of PMR that an active effort to exclude these conditions is warranted.
5.1 Giant Cell Arteritis (GCA)
GCA, a form of large and medium vessel vasculitis, shares both epidemiological and immunological similarities with PMR. The clinical connections between PMR and GCA suggest that they may be different manifestations of the same disease process: both conditions have similar age and sex distributions and both present with increased levels of serum acute-phase reactants with swift response to GC [13]. Population based studies show that PMR is two to three times more common than GCA (PMR incidence 52.5/100,000 aged 50+ in Olmsted County, Minnesota where GCA incidence is 20/100,000) [10]. Importantly, 16–21% of PMR patients present with clinical features of GCA; conversely, about 40% of patients with GCA have symptoms of PMR before, concomitantly, or following diagnosis of GCA [13]. Interestingly, both pathology and imaging studies reveal that subclinical vasculitis without clinical features of GCA may be detected in a subset of patients with PMR. For instance, a positive temporal artery biopsy was described in up to 9% of patients with PMR [22], and evidence of vasculitis was found in up to 31% of PMR patients undergoing 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) at diagnosis [23]. Signs of vasculitis were also found on ultrasound examination of the temporal arteries in 8% of patients with PMR [24]. Based on these findings, it has been suggested that PMR may represent GCA with incompletely developed vascular involvement [25]. Because patients with pure manifestations of PMR do not develop the characteristic clinical complications of GCA, no universal screening recommendations for subclinical vasculitis currently exist [22], but patients with PMR should be educated about signs and symptoms of GCA and asked about symptoms of GCA during followup.
5.2 Remitting Seronegative Symmetrical Synovitis with Pitting Edema (RS3PE) and Elderly-Onset RA (EORA)
PMR, EORA, and RS3PE are a triad of clinically-overlapping inflammatory conditions affecting individuals over 60 years of age. Importantly, both PMR and EORA can initially present as RS3PE and both PMR and RS3PE can be initial presentations of EORA [4]. By definition, RS3PE is a symmetrical polyarthritis in which pitting edema of the hands and/or feet is a prominent feature [26]. As in PMR, IL-6 has been implicated in the pathogenesis of RS3PE. RS3PE has been reported as an isolated syndrome, as a paraneoplastic syndrome in solid and hematologic malignancies, or in association with infectious agents or rheumatologic conditions [27]. In the case of EORA first presenting as PMR or RS3PE, it usually has sudden onset and is typically accompanied by elevated acute phase reactants and negative RF and ACPA [26]. Any one of these three conditions can be treated by GC doses varying between 10 and 20 mg/day with gradual taper. However, when difficulty arises in tapering down GC, the clinician should consider the onset of EORA [28].
6 Immune Checkpoint Inhibitors and Onset of PMR
Immune Checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are currently being successfully used to increase survival in many cancers. However, this treatment is associated with immune-related adverse events (IrAE). Recently, several case series reported PMR occurring following ICI treatment [29,30,31,32].
7 Management of PMR in the Elderly
In contrast to GCA, where GC therapy is started immediately because of possible risk of vision loss, patients with apparently isolated PMR should have a basic diagnostic work-up prior to starting therapy.
Goals of therapy in the majority of elderly patients with PMR are to treat and control chronic PMR symptoms and prevent disease relapse, to optimize and preserve activity level, and to optimize musculoskeletal function and improve quality of life with minimal adverse effects from medications. Long-term treatment goals include reduction in decline of mobility over time which may occur in older adults with PMR and reduction of cardiovascular complications [12, 15].
7.1 Non-pharmacologic Interventions
Physical therapy and range-of-motion exercises for the shoulder and hips are important for maintaining good physical function and mobility in PMR patients. Patient education on PMR and side effects of GC therapy is essential to good quality of patient care. Influenza and pneumococcal vaccinations are recommended for all patients receiving immunosuppressive medications, including PMR patients [33].
7.2 Pharmacologic Interventions
7.2.1 Glucocorticosteroids (GC)
GC therapy with slow taper is considered the mainstay of PMR treatment, with mean length of treatment of 1.8 years [14]. Response to GC is usually rapid, occurring within days of initiation of therapy. The British Society for Rheumatology Guidelines define a rapid response to GC as a patient-reported global improvement of ≥70% within a week of starting GC treatment with normalization of inflammatory markers within 4 weeks [34]. However, response may also be more gradual with studies showing that about one-third of patients have incomplete response at 4 weeks [4]. Because steroid-responsiveness is not a feature specific to PMR, an empirical GC ‘test of treatment’ is not recommended to aid in PMR diagnosis.
The 2015 EULAR/ACR recommendations for PMR management emphasize that there is no ideal GC regimen suitable for all patients, so that GC dosing and tapering schedule should be based on individual patient characteristics, including disease severity, comorbidities, other prescribed medications, risk of GC-related adverse events, and patient preference. Clinicians should aim at the minimum effective starting single-daily dose of GC within a range of 12.5–25 mg prednisone equivalent [17, 35]. Prednisone dose should be tapered to an oral dose of 10 mg/day prednisone equivalent within 4–8 weeks with progressive taper when response seems favorable. Relapse is common, occurring in about 50% of patients [4, 5], with female sex, ESR > 40 mm/h and peripheral arthritis indicating a higher risk for relapse [4, 17]. When relapse occurs, oral prednisone dose should be increased to the pre-relapse dose with gradual decrease within 4–8 weeks to the dose at which the relapse occurred. Once remission is achieved, prednisone should be tapered by 1 mg every 4 weeks (alternate day schedules can be used) until discontinuation when the patient is asymptomatic from their polymyalgic symptoms [17]. A more rapid tapering regimen is often associated with a high relapse rate and should thus be avoided [15, 35]. Elevated acute phase reactants in the absence of PMR symptoms is not an indication for continuation of steroid therapy; however, this finding should require further investigation [15]. The use of intramuscular (IM) methylprednisolone acetate has also been trialed but is not recommended due to its limited availability and due to limited studies with this agent [4, 36].
7.2.2 Conventional Disease-Modifying Antirheumatic Drugs (cDMARDs)
Because of need for long-term treatment of PMR with GC, GC-sparing agents have been trialed in PMR management. Of cDMARDs, methotrexate (MTX) is the only immunosuppressive agent that has been evaluated in randomized clinical trials [4, 37, 38], with most studies showing benefit with regards to relapse rate, cumulative GC dose used and ability to discontinue GC treatment [4, 37, 38]. Accordingly, EULAR/ACR recommendations call for early use of MTX in individual patients at high risk of relapse (female patients, ESR > 40 mm/h, peripheral arthritis), in relapsing disease, or in patients with GC-related adverse events or comorbidities that might be exacerbated by GC use [17]. There are also reports of using azathioprine (AZA) [39] and leflunomide (LEF) [40, 41] in PMR patients.
7.2.3 Biologic Disease-Modifying Antirheumatic Drugs (bDMARDs)
Unlike GCA, the use of novel bDMARDs in PMR has been challenging due to the lack of proper animal models for PMR. In recent years, the interleukin-6 (IL-6) blocking agents have been successfully trialed in GCA [9], and given the overlap between PMR and GCA, they are currently being trialed in PMR [42, 43].
7.3 Monitoring PMR Disease Activity
Clinicians should closely monitor patients with PMR for clinical and laboratory signs of disease activity and evidence of GC-related toxicity. Monitoring is suggested every 4–8 weeks in the 2–4 months after treatment is started and then every 4–12 weeks during the first year of disease. In the second year, monitoring should be done every 8–12 weeks and as indicated in cases of relapse during tapering of GC or other immunosuppressive agents [4].
Currently, there is no generally accepted definition of remission or relapse in PMR, but the absence of PMR symptoms, particularly morning stiffness, in conjunction with normal ESR and CRP, has often been used to define remission in clinical studies [44]. Conversely, the reappearance of clinical signs of PMR, with or without ESR or CRP elevation, is considered to indicate relapse, as a PMR flare in the absence of an increase in markers of inflammation may be observed in up to 25% of patients even if these markers were abnormal at time of diagnosis [4, 44].
8 Prognosis
Epidemiological studies attest to PMR having a benign course without affecting patient survival, with median duration of the disease running up to 11 months (range, 2–54 months) [45]). The main morbidity related to PMR actually involves complications of GC therapy, as noted below.
8.1 Assessing for GC-Induced Damage
Patients being treated for PMR should be monitored regularly not only for disease activity, but also for GC-induced complications, which occur in up to 65% of patients [46]. Management of comorbidities including cardiovascular risks such as hypertension, diabetes, and hyperlipidemia as well as osteoporosis is necessary throughout the entire course of disease. Studies show that three variables independently increase the risk of adverse events among PMR patients: age at PMR diagnosis, a cumulative dose of prednisone ≥1800 mg, and female sex [46]. Indeed, population studies reveal that long-term GC treatment in PMR patients carries with it a 2–5 times greater risk of diabetes mellitus, osteoporotic fractures (vertebral fractures, femoral neck fractures, and hip fractures) compared with age- and sex-matched individuals [46]. Given this risk, osteoporosis prophylaxis should be initiated along with initiation of GC treatment. ACR 2017 recommendations [47] underline that calcium intake (1000–1200 mg/day) and vitamin D intake (600–800 IU/day) should be optimized in all patients starting on GC therapy along with lifestyle modifications and consideration of bisphosphonate therapy.
Bibliography
Barber HS. Myalgic syndrome with constitutional effects; polymyalgia rheumatica. Ann Rheum Dis. 1957;16(2):230–7.
Michet CJ, Matteson EL. Polymyalgia rheumatica. BMJ. 2008;336(7647):765–9.
Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633–9.
Matteson EL, Dejaco C. Polymyalgia rheumatica. Ann Intern Med. 2017;166(9):ITC65–80.
Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology. 2017;56(4):506–15.
Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp Gerontol. 2006;41(4):339–45.
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54.
Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011;13(4):231.
Stone JH, Klearman M, Collinson N. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377(15):1494–5.
Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med. 1995;123(3):192–4.
England BR, Mikuls TR, Xie F, Yang S, Chen L, Curtis JR. Herpes zoster as a risk factor for incident giant cell arteritis. Arthritis Rheumatol. 2017;69(12):2351–8.
Castañeda S, García-Castañeda N, Prieto-Peña D, Martínez-Quintanilla D, Vicente EF, Blanco R, et al. Treatment of polymyalgia rheumatica. Biochem Pharmacol. 2019;165:221–9.
Salvarani C, Macchioni PL, Tartoni PL, Rossi F, Baricchi R, Castri C, et al. Polymyalgia rheumatica and giant cell arteritis: a 5-year epidemiologic and clinical study in Reggio Emilia, Italy. Clin Exp Rheumatol. 1987;5(3):205–15.
Caylor TL, Perkins A. Recognition and management of polymyalgia rheumatica and giant cell arteritis. Am Fam Physician. 2013;88(10):676–84.
Patil P, Dasgupta B. Polymyalgia rheumatica in older adults. Aging Health. 2013;9(5):483–95.
Ceccato F, Uña C, Regidor M, Rillo O, Babini S, Paira S. [Conditions mimicking polymyalgia rheumatica]. Reumatol Clin. 2011;7(3):156–60.
Dejaco C, Singh YP, Perel P, Hutchings A, Camellino D, Mackie S, et al. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheumatol. 2015;67(10):2569–80.
Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71(4):484–92.
Slobodin G, Rimar D, Boulman N, Kaly L, Rozenbaum M, Rosner I, et al. Acute sacroiliitis. Clin Rheumatol. 2016;35(4):851–6.
Gazitt T, Kibari A, Nasrallah N, Abu Elhija M, Zisman D. Polymyalgia rheumatica: the great imitator. Isr Med Assoc J. 2019;21(9):627–8.
González-Gay MA, García-Porrúa C, Salvarani C, Olivieri I, Hunder GG. Polymyalgia manifestations in different conditions mimicking polymyalgia rheumatica. Rheumatol Clin. 2011;18(6):755–9.
Gonzalez-Gay MA. Giant cell arteritis and polymyalgia rheumatica: two different but often overlapping conditions. Semin Arthritis Rheum. 2004;33(5):289–93.
Blockmans D, De Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: a prospective study in 35 patients. Rheumatology. 2007;46(4):672–7.
Schmidt WA, Gromnica-Ihle E. Incidence of temporal arteritis in patients with polymyalgia rheumatica: a prospective study using colour Doppler ultrasonography of the temporal arteries. Rheumatology. 2002;41(1):46–52.
Salvarani C, Cantini F, Hunder G. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372(9634):234–45.
McCarty DJ, O’Duffy JD, Pearson L, Hunter JB. Remitting seronegative symmetrical synovitis with pitting edema. RS3PE syndrome. JAMA. 1985;254(19):2763–7.
Varshney AN, Singh NK. Syndrome of remitting seronegative symmetrical synovitis with pitting edema: a case series. J Postgrad Med. 2015;61(1):38–41.
Belloli L, Massarotti M, Marasini B. Polymyalgia rheumatica and elderly onset rheumatoid arthritis. J Clin Rheumatol. 2008;14(1):59.
Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol. 2014;66(3):768–9.
Belkhir R, Burel SL, Dunogeant L, Marabelle A, Hollebecque A, Besse B, Leary A, Voisin AL, Pontoizeau C, Coutte L, Pertuiset E, Mouterde G, Fain O, Lambotte O, Mariette X. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis. 2017;76(10):1747–50.
Garel B, Kramkimel N, Trouvin A-P, Frantz C, Dupin N. Pembrolizumab-induced polymyalgia rheumatica in two patients with metastatic melanoma. Joint Bone Spine. 2017;84(2):233–4.
Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open. 2019;5(1):e000906.
van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70(3):414–22.
Dasgupta B, Borg FA, Hassan N, Barraclough K, Bourke B, Fulcher J, et al. BSR and BHPR guidelines for the management of polymyalgia rheumatica. Rheumatology. 2010;49(1):186–90.
Hernández-Rodríguez J, Cid MC, López-Soto A, Espigol-Frigolé G, Bosch X. Treatment of polymyalgia rheumatica: a systematic review. Arch Intern Med. 2009;169(20):1839–50.
Dasgupta B, Dolan AL, Panayi GS, Fernandes L. An initially double-blind controlled 96 week trial of depot methylprednisolone against oral prednisolone in the treatment of polymyalgia rheumatica. Br J Rheumatol. 1998;37(2):189–95.
Ferraccioli G, Salaffi F, De Vita S, Casatta L, Bartoli E. Methotrexate in polymyalgia rheumatica: preliminary results of an open, randomized study. J Rheumatol. 1996;23(4):624–8.
Caporali R, Cimmino MA, Ferraccioli G, Gerli R, Klersy C, Salvarani C, et al. Prednisone plus methotrexate for polymyalgia rheumatica: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2004;141(7):493–500.
De Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis. 1986;45(2):136–8.
Adizie T, Christidis D, Dharmapaliah C, Borg F, Dasgupta B. Efficacy and tolerability of leflunomide in difficult-to-treat polymyalgia rheumatica and giant cell arteritis: a case series. Int J Clin Pract. 2012;66(9):906–9.
Diamantopoulos AP, Hetland H, Myklebust G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int. 2013;2013:120638.
Lally L, Forbess L, Hatzis C, Spiera R. Brief report: a prospective open-label phase IIa trial of tocilizumab in the treatment of polymyalgia rheumatica. Arthritis Rheumatol. 2016;68(10):2550–4.
Devauchelle-Pensec V, Berthelot JM, Cornec D, Renaudineau Y, Marhadour T, Jousse-Joulin S, et al. Efficacy of first-line tocilizumab therapy in early polymyalgia rheumatica: a prospective longitudinal study. Ann Rheum Dis. 2016;75(8):1506–10.
Dejaco C, Duftner C, Cimmino MA, Dasgupta B, Salvarani C, Crowson CS, et al. Definition of remission and relapse in polymyalgia rheumatica: data from a literature search compared with a Delphi-based expert consensus. Ann Rheum Dis. 2011;70(3):447–53.
Chuang TY, Hunder GG, Ilstrup DM, Kurland LT. Polymyalgia rheumatica: a 10-year epidemiologic and clinical study. Ann Intern Med. 1982;97(5):672–80.
Gabriel SE, Sunku J, Salvarani C, O’Fallon WM, Hunder GG. Adverse outcomes of antiinflammatory therapy among patients with polymyalgia rheumatica. Arthritis Rheum. 1997;40(10):1873–8.
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–37.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gazitt, T., Zisman, D. (2020). Polymyalgia Rheumatica. In: Slobodin, G., Shoenfeld, Y. (eds) Rheumatic Disease in Geriatrics . Springer, Cham. https://doi.org/10.1007/978-3-030-44234-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-44234-7_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44233-0
Online ISBN: 978-3-030-44234-7
eBook Packages: MedicineMedicine (R0)