Abstract
Exercise, at appropriate volumes, shows promise as a strategy to optimize functional capacity, symptom management, and quality of life in those with advanced cancer. Specifically, the research evidence suggests that exercise may prevent or delay declines in aerobic fitness and strength and help the survivor to maintain adequate physical function to perform daily activities.
The purpose of this chapter is to outline the role of exercise for survivors with advanced cancer, who have metastatic disease, or who are receiving palliative care. In this setting, the challenges facing the exercise specialist are complex and involve identifying symptoms, functional impairments, and co-pathologies that may impact exercise risk and tolerance. The need for ongoing modification of exercise programming should be anticipated to ensure that exercise participation is safe and effective. The goal of exercise for survivors with terminal cancer and limited life expectancy is to help maintain function as the focus of care shifts to living as well as possible in the short term. At this stage of disease, an interdisciplinary team approach is paramount to address symptom management and optimize independence.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Approximately 50% of all individuals with a cancer diagnosis will ultimately die from disease progression [1]. The World Health Organization endorsed palliative care as a global health issue in 1990 and defined palliative care as “the active total care of patients whose disease is not responsive to curative treatment. Control of pain, of other symptoms, and of psychological, social and spiritual problems, is paramount. The goal of palliative care is achievement of the best possible quality of life for patients and their families” [2]. In parallel with the rising incidence of cancer and improved treatment, there is a growing population of survivors living months to years longer with advanced chronic cancer, survivors who are not yet palliative or appropriate for end-of-life care. Maintaining function and control of symptoms is necessary for these survivors to live well for as long as possible [3].
Timely rehabilitation and exercise, at appropriate volumes, shows promise as a strategy to optimize functional capacity, symptom management, independence, and quality of life (QoL) [3,4,5]. Evidence suggests that exercise may help to prevent or delay declines in aerobic fitness and strength, and maintain adequate physical function to perform daily activities [3]. Moreover, performance status, which is used to guide decision-making regarding appropriate treatment for survivors with advanced cancer, heightens the importance of exercise to maintain or attenuate declines in function and quality of life [6]. The purpose of this chapter is to outline the role of exercise in survivors with advanced cancer, who have metastatic disease, or who are receiving palliative care. Our aim is to focus on the unique needs of these survivors and to highlight the differences in terms of exercise screening, testing, and training from those of survivors with curative disease. Table 16.1 includes the definitions for the terms survivor, palliative care, advanced cancer, metastatic disease, and terminal (end of life) cancer.
Palliative Care
Modern definitions of palliative care are reflective of the ever-changing landscape of how palliative care is delivered worldwide. Whereas traditional models of palliative care were focused on caring for survivors solely in the terminal stage of illness, there is a proliferation of evidence in support of integration of palliative care early in the disease trajectory [7]. In their 2018 Cochrane review of seven randomized and cluster-randomized controlled trials involving a total of 1614 participants, Haun et al. showed that early palliative care interventions had beneficial effects on quality of life and symptom intensity in survivors with advanced cancer, compared to usual/standard cancer care alone; despite small effect sizes, these findings were felt to be clinically relevant in survivors with advanced stage disease and limited prognosis [8]. In their 2017 updated Clinical Practice Guideline, the American Society of Clinical Oncology recommends that advanced cancer survivors receive dedicated palliative care services, early in the disease course and alongside active treatment of their cancer; early palliative care involvement was defined as within 8 weeks of the initial diagnosis of advanced cancer [9].
In response to the growing evidence base, the 2017 Lancet Commission argued for a new definition that “explicitly rejects any time or prognostic limitation on access to palliative care, includes complex chronic or acute, life-threatening, or life-limiting health conditions, and considers all levels of the health-care system from primary to specialized care and all settings where palliative care can be delivered” (p. 1400) [10]. Hence, the International Association for Hospice and Palliative Care (IAHPC) developed its own international consensus-based definition of palliative care, which is the “active holistic care of individuals across all ages with serious health-related suffering due to severe illness, and especially of those near the end of life” with the overall aim of improving quality of life for survivors, caregivers, and families [11]. The IAHPC contends that palliative care is applicable throughout the course of an illness, can be provided concurrently with disease modifying therapies, and can positively influence the course of illness.
Advanced Cancer
Differentiating between the concepts of advanced, metastatic, and terminal (end of life) disease remains challenging [12]. The American Society of Clinical Oncology defines advanced cancer as incurable, malignant disease [13]. This definition is congruent with that of the National Cancer Institute, for whom advanced cancer is “cancer that is unlikely to be cured or controlled with treatment” [14]. The proportion of cases of advanced cancer at diagnosis for lung cancer, for example, is approximately 75% [15]. Other reported cancers with high proportions of advanced cancers at diagnosis include pancreatic cancer (79%) and non-Hodgkin’s lymphoma (64%) [15]. There are clinical circumstances, however, for which these definitions do not apply. Locally advanced cancer, or cancer that has grown beyond its initial primary site but has not yet spread to distant sites in the body, may be curable, depending on the cancer primary [16]. For example, many locally advanced prostate cancers are curable [17], whereas most locally advanced pancreatic cancers are not curable [18].
Metastatic Disease
The Canadian Cancer Society defines metastatic cancer as the “spread of cancer cells from the primary tumor to other parts of the body, by means of the blood or lymphatic system” [19]. This definition is congruent with that of the National Cancer Institute, for whom metastatic cancer is “the spread of cancer cells from the place where they first formed to another part of the body” [20]. Metastasis involves breakdown of intercellular cohesion, tumor cell migration, angiogenesis, access to and survival in the systemic circulation, evasion of local immune responses, and growth in distant organs [21]. Again, there are clinical circumstances wherein metastatic cancer may not necessarily imply incurable or terminal disease. For example, greater than 80% of cases of metastatic testicular cancer are curable [22].
Terminal Disease
There is significant ambiguity and a lack of definitional clarity in the terms “actively dying,” “end of life,” “terminally ill,” and “terminal care” [12]. In their 2014 systematic review, Hui et al. highlighted the key defining features of the terms “end of life,” “terminally ill,” and “terminal care,” as being “life-limiting disease with irreversible decline and expected survival in terms of months or less” [23]. There are clinical circumstances, however, where terminal cancer implies neither advanced nor metastatic disease. For example, survivors with high-grade gliomas may have terminal disease and no metastases [24].
Research Evidence Supporting Exercise
Emerging evidence supports the benefits of exercise for survivors with advanced cancers [3, 25, 26]. The number and quality of studies looking at the relationship between exercise and advanced cancer have greatly increased in number over the last decade. Three recent systematic reviews have been performed examining the benefits of exercise in advanced cancers (Table 16.2); these reviews comprised 23 distinct randomized controlled trials (RCTs) and a total of 1787 participants. Of these 23 RCTs, 9 studies were performed with mixed cancer types, 5 with lung cancer, 4 with hematological cancers, 2 with breast cancer, 1 with gastrointestinal cancers, and 2 studies involved survivors with bone metastases. The heterogeneity among studies in tumor types, definitions of advanced or terminal cancer, inclusion criteria, and exercise prescription variables precludes synthesis of findings, thus limiting overall conclusions on benefits. In general, however, findings support feasibility of exercise as assessed by safety (e.g., no serious adverse events), recruitment and completion rates, and adherence (e.g., attendance). The most important findings include improvements in cardiopulmonary fitness, muscle strength, and physical function [3, 25]. The benefits of exercise alone for quality of life and symptoms such as fatigue and dyspnea remain unclear. However, studies involving interdisciplinary rehabilitation interventions, for example, have shown benefit for fatigue [27, 28], chemotherapy-induced peripheral neuropathy [29], body weight management [30], and quality of life [30].
Given the heterogeneity among trials to date, more research is needed to explore the optimal regimen in terms of the stage of disease (advanced, metastatic, or end of life), as well as exercise mode and parameters of frequency, intensity, time, and type. Studies, to date, have mostly involved supervised exercise programs. Home-based exercise is an attractive option for survivors with advanced cancer given advantages of time, eliminating the need for travel, and favoring activities of daily living; however, concerns exist over the feasibility of this type of programming [31, 32]. Wearable activity monitors, smartphone applications, and virtual interventions show promise as e-health solutions for the delivery of supported home-based interventions that also allow for exercise supervision and survivor (and caregiver) connectedness [33].
Goals of the Prescribed Physical Activity or Exercise Program
For survivors living with advanced, metastatic, or terminal cancer, exercise goals are often quite different from those with early-stage curative disease [34]. Symptom control, physical function, and maintenance of independence are primary reasons for seeking out and taking part in exercise interventions [3]. Exercise has the potential to improve function even if the disease is advanced; however, the focus of exercise may vary by the cancer type, the survivor’s physical fitness and functioning, presenting impairments related to cancer or cancer treatment, and the presence of other illnesses or conditions. A survivor with advanced lung cancer, for example, may struggle with shortness of breath on exertion limiting activities of daily living, while a survivor with a brain tumor may have deficits in balance, placing them at risk of falling. Thus, exercise programs need to be personalized and flexible. Moreover, exercise goals may need to be revisited periodically as the treatment approach changes, and the disease progresses. Importantly, the exercise professional must be able to recognize issues and determine how best to modify the exercise program as the survivor’s situation changes [5]. Outside of the physical and functional goals of exercise, making the most of the potential psychosocial benefits from exercise participation should be considered, particularly the benefits of social interaction with other survivors in a group exercise setting.
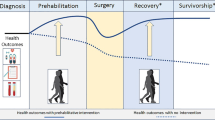
For advanced cancer survivors with stable disease who are not receiving active treatment, the goals of exercise training may be to maintain or improve fitness and function, as well as to manage any effects of the disease and its prior treatments. Exercise during this period can also be used as a prehabilitation strategy to optimize performance status in preparation for future cancer treatments, that may be necessary either prophylactically, or to address eventual disease progression.
The goal of exercise for survivors with terminal cancer and limited life expectancy is to help maintain independence as the focus of care shifts to living as well as possible in the short term. At this stage, an interdisciplinary approach is paramount to address symptom management, and consideration should be given to integrating exercise into activities of daily living.
Screening for Exercise Testing and Participation
The goal of exercise screening in survivors with advanced cancer is to reduce the risk of any adverse events during fitness assessments or exercise training [35]. Screening of the survivor is important to identify existing risks related to exercise and should include a medical history covering the cancer diagnosis, current disease status and prognosis, treatment received and in process [36, 37], presenting impairments, and other potential comorbid conditions [38]. The screening process allows the exercise professional to (i) anticipate potential side effects and adverse events; (ii) identify the appropriate level of exercise supervision; (iii) identify the type of monitoring needed during exercise sessions; and (iv) identify the optimal type and intensity of exercise.
Suggested steps for screening and communication around exercise safety are outlined in the Screening and Triage Decision Tree in Fig. 16.1. We recommended that medical clearance be obtained prior to the survivor beginning an exercise program. This clearance should be sought from health care providers (HCP) who provide cancer care (e.g., oncologist, palliative care specialist) and/or who manage comorbid conditions (e.g., family physician, cardiologist). Any pre-exercise evaluation completed as part of the medical clearance is at the discretion of the medical provider and may include a physical examination, medical imaging, and/or laboratory tests [36]. Comprehensive screening, as outlined in Fig. 16.1, can effectively determine the most appropriate treatment pathway. For example, higher-risk or more complex individuals, or those experiencing severe or multiple cancer-related adverse effects, may require interdisciplinary cancer-specific rehabilitation support.
Objective Physical Fitness Assessment
Ideally, all aspects of health-related fitness should be assessed prior to the survivor beginning an exercise program including testing aerobic fitness, muscular strength, flexibility, and balance. Functional assessments such as the Timed Up and Go test, sit-to-stand, or Short Physical Performance Battery can easily be performed in the clinical setting and are helpful to inform the survivor’s ability to carry out activities of daily living [39, 40]. A number of factors related to the survivor’s status should be considered when selecting tests, such as functional status, cancer and treatment status, and the presence and extent of any comorbid conditions. These factors should be matched with the abilities, effort, and overall demand to complete the selected fitness assessments. Moreover, the overall goals of the exercise training program should be taken into account to determine what information is actually needed for effective exercise prescription, with tests then selected to match the needs and goals of the survivor. While gold standard, maximal type fitness assessments can be safely completed in individuals with advanced cancer [41, 42], when considering which tests to complete, the focus should be on avoiding any undue risk or burden to the survivor while still producing sufficient and accurate information to assist in the exercise prescription and to monitor changes over time. Depending on the survivor’s status, additional rest time between fitness tests and spreading the assessments over multiple days may be necessary.
Additional Special Tests and Monitoring
The physical assessment of the survivor with advanced cancer should start with assessment of vital signs [43]. For a survivor whose status may fluctuate on a daily basis (i.e., on treatment or with active progressive disease), we recommend monitoring of key vital signs at each visit. These resting physiological measures include heart rate, blood pressure, respiratory rate, and oxygen saturation; however, consideration should be given to incorporation of additional measures to monitor symptoms, as indicated [36]. For example, it may be useful to obtain heart rate and blood pressure readings in the supine, sitting, and standing positions for a survivor reporting symptoms of dizziness. This information can inform fall risk and the need for modifications in positioning of exercises (e.g., upright positions versus on a floor mat), the order of exercises (e.g., avoiding unnecessary or minimizing the number of position changes), and teaching the survivor strategies to move safely when changing positions (e.g., slowly changing position and pausing with each position change to allow for adaptation).
Body weight changes also have implications on the ability of the survivor to participate in and gain benefit from exercise. Gains in fat mass, and losses in muscle mass related to prescribed corticosteroids, for example, may negatively impact function and increase fall risk. A survivor with advanced cancer may become cachexic, leading to poor exercise tolerance and increased post-exercise fatigue [44]. Across cancer types, negative changes in body weight and composition add complexity to the survivor’s programming and necessitate close collaboration with the dietician as well as the interdisciplinary team [18]. Furthermore, collaboration with specialists in physical medicine and rehabilitation and/or physical therapy may be needed for assessment and treatment of other impairments such as peripheral neuropathy; lymphedema; gait, ambulation, and fall risk and to address comorbid conditions such as arthritis.
Principles of Exercise
Many of the guiding principles of exercise prescription hold true in the advanced cancer exercise setting, with the most important considerations being individualization, progressive overload, specificity, and recovery [45]. Individualization of the exercise prescription to the survivor’s current status, and modifying the program to match any change, is crucial to safe and beneficial exercise. The training overload (total volume of training), with FITT parameters of Frequency, Intensity, Time/duration, and Type of training, should be considered, as would normally be done in other populations [36]. Progression of the total training volume should be gradual and symptom-limited and is best guided by the survivor’s response to training. Determining the appropriate overload and progression of exercise is a challenge in the advanced cancer setting, as cancer treatments and disease progression may have a profound effect on physiological systems [46]. Specificity of the training program should address areas of need or weakness as determined from the initial screening and fitness assessment. Incorporating longer rest time between exercises and sets is important to avoid exacerbating symptoms such as fatigue, and the number of planned training sessions per week should allow for adequate rest and recovery, while also considering the time burden of appointments.
While currently available exercise guidelines for cancer survivors provide a reasonable framework for exercise prescription, a survivor with advanced cancer may need and prefer to exercise at lower total volumes and intensities of exercise [41, 47]. Applying the principles of exercise training and prescription becomes even more complex as disease and treatment factors tend to have a greater and growing impact on the survivor’s ability to both complete and recover from exercise training. Ideally, some combination of aerobic, resistance, core, balance, and flexibility exercises should be included in the training program to benefit all aspects of the survivor’s health-related fitness and functional abilities. We recommend that setting of intensities be conservative, particularly when the ability to prescribe specific training intensities (e.g., % maximum heart rate or one repetition maximum) is not possible from data obtained from the fitness assessment. The survivor should finish exercise sessions feeling better, possibly more energized or comfortably tired, and not be exhausted, or require a nap. Depending on the survivor’s presentation and risk for adverse events, exercises that are high impact, high intensity, and/or of longer duration may need to be avoided [46]. The goal of the exercise prescription overall is to create a balance between having a sufficient exercise stimulus to positively impact the health and fitness of the survivor and not increase fatigue, cause pain or injury or exacerbate symptoms. The survivor’s function, symptoms, or the disease itself may ultimately limit progression of exercise, and, in fact, the survivor do better with dose reduction over time.
The need for ongoing modification of programming should be anticipated to ensure that exercise participation is safe and effective. Each training session should begin with a conversation with the survivor regarding the response to the previous workout in terms of impact on fatigue and symptoms (immediately post-exercise and 24 and 48 hours later) to determine any immediate need for modifications to programming. Further, the survivor must understand the need to communicate any concerns during sessions to allow for in-training modifications. The perceived exertion scale is helpful for monitoring both exercise intensity and symptoms (e.g., dyspnea, fatigue, pain scales) [48]. The exercise professional should explain the proper use of the rating of perceived exertion scale at the commencement of the survivor’s programming. Once trained and practiced, the survivor can effectively perceive their level of exertion, muscle fatigue, pain, or breathlessness [49]. Close attention to the survivor’s perceived exertion can help to determine if the training volume is set at a manageable level. Given the survivor’s potential fluctuating status, this information is beneficial to also obtain feedback on perceived exertion when performing activities of daily living [49].
Exercise Professional Training
Exercise professionals should have adequate cancer education and experience to ensure the safety of the survivor and quality of exercise programming. Professionals with certifications, such as the Canadian Society of Exercise Physiology – Certified Exercise Physiologist and American College of Sports Medicine (ACSM) Clinical Exercise Physiologist, have the ideal combination of education and experience and approved scope of practice to work with survivors with advanced cancers [36]. Additional cancer-specific practical experience or formal training, such as ACSM/American Cancer Society Certified Cancer Exercise Trainer, is recommended. Other health professionals (medical doctors, nurse practitioners, physical therapists, and other rehabilitation staff) working with cancer survivors who also have experience or training in exercise are well suited to deliver exercise training in an advanced cancer setting.
Special Considerations
Cancer-Related Fatigue (CRF)
Cancer-related fatigue is a symptom subjectively experienced as a physical, emotional, and/or cognitive tiredness or exhaustion secondary to cancer or cancer treatment, which interferes with usual functioning, and that is distressing, persistent, and not proportional to recent activity [50]. There is wide variability in the clinical presentation of cancer-related fatigue, whose underlying pathophysiology is multifactorial and involves somatic, psychosocial, cognitive, and emotional variables [51]. Originating in the central nervous system, central fatigue presents as the inability to complete physical and mental tasks requiring self-motivation and internal cues, in the absence of motor weakness or cognitive failure [52]. Putative central mechanisms include the vagal afferent nerve [53], dysregulation of cytokines [54] or serotonin [55], and disruption of circadian rhythm [56] or the hypothalamic-pituitary-adrenal axis [57]. Peripheral fatigue, on the other hand, presents as the inability of muscle to perform a task in response to central stimulation, either at the level of muscle or the neuromuscular junction [58]. Putative peripheral mechanisms include adenosine triphosphate dysregulation [59], contractile properties [60], and muscle metabolism [61].
Fatigue is the most common and distressing symptom. A common physical complaint of CRF is the onset of tiredness and/ or weakness upon sustained exertion or during repetitive tasks [62, 63]. Cancer-related fatigue occurs in up to 40% of survivors at the time of cancer diagnosis, in up to 80% of survivors treated with chemotherapy, and in up to 90% of survivors treated with radiotherapy [64]. In a systematic review of symptom prevalence in older cancer survivors receiving palliative care, fatigue was the most prevalent symptom, occurring in at least 50% [65]. Cancer-related fatigue negatively impacts activities of daily living and overall quality of life [63] and affects survivors more and for longer than any other symptom, including pain [66]. In a systematic review of symptoms associated with cancer-related fatigue, psychosocial distress had higher overall correlations with cancer-related fatigue than symptom distress [67].
Cancer-related fatigue management starts with comprehensive assessment of the symptom, its impact on the survivor’s experience, and treatment of any potential contributors. Uncontrolled symptoms, such as pain, dyspnea, nausea, anxiety, and depression, can exacerbate fatigue in the cancer survivor [68]. Deconditioning due to prolonged bed rest and immobility, overexertion, infection, anemia, autonomic dysfunction, cachexia, polypharmacy, hypoxia, dehydration, metabolic/endocrine disorders, and renal/hepatic/cardiac comorbidities can all further contribute to symptoms of fatigue [69].
Commonly used pharmacological agents for the management of cancer-related fatigue include corticosteroids, megestrol acetate, psychostimulants, and investigational agents (e.g., eicosapentaenoic acid, thalidomide, L-carnitine, testosterone, melatonin) [70]. Non-pharmacological strategies for the management of fatigue include psychological interventions, physiotherapy, and occupational therapy [71]. The survivor with moderate to severe CRF may benefit from involvement of the interdisciplinary rehabilitation team where interventions may include, for example, nutrition counseling and occupational therapy education on energy conservation and maximization. For the exercise specialist, careful attention to symptom flares and excessive post-exercise fatigue allows for adjustment of the exercise prescription [72, 73]. With appropriately prescribed exercise, and monitoring of perceived exertion to inform activity pacing, the survivor should notice improved ability to complete daily tasks and meaningful activities, even if overall fatigue persists [73]. In a meta-analysis comparing pharmacological, psychological, and exercise treatments for cancer-related fatigue, exercise and psychological interventions were shown to be effective in reducing cancer-related fatigue both during and after cancer treatment and were significantly better than the available pharmacological options [74].
Bone Metastases
Bone is the third most frequent site of metastasis, after lung and liver. Bone metastases are nearly always multiple, and their distribution within the axial skeleton is primarily attributed to the red bone marrow therein. Although the overall incidence of bone metastases is not known, the incidence of bone metastases is highest in multiple myeloma (up to 95%), followed by prostate cancer (up to 90%), breast cancer (up to 75%), lung cancer (up to 64%), thyroid cancer (60%), melanoma (up to 45%), bladder cancer (40%), and renal cell carcinoma (up to 25%) [75]. After diagnosis of bone metastases, median survival is highest in prostate cancer (up to 53 months), followed by thyroid cancer (48 months), breast cancer (up to 25 months), renal cell carcinoma (12 months), bladder cancer (up to 9 months), lung cancer (up to 7 months), and melanoma (6 months) [76]. Table 16.3 provides information on diagnostic imaging methods for the detection and evaluation of bone metastases.
There are three main types of bone metastases, which are classified according to the putative mechanism of interference with bone remodeling [77]. Osteolytic metastases are characterized by osteoclast-mediated destruction of normal bone and involvement of parathyroid hormone-related peptide [78]; osteolytic metastases comprise the majority of bone metastases in breast cancer and are present in multiple myeloma, renal cell carcinoma, melanoma, non-small cell lung cancer, non-Hodgkin’s lymphoma, and thyroid cancer [76]. Osteoblastic (sclerotic) metastases are characterized by new bone deposition with transforming growth factor, bone morphogenic proteins, and endothelin-1 contributing to osteoblast generation [79]; osteoblastic metastases are present in prostate cancer, carcinoid, small cell lung cancer, Hodgkin’s lymphoma, and medulloblastoma. Mixed bone metastases refer to the survivor having both osteolytic and osteoblastic lesions or if an individual metastasis is comprised of both components; mixed bone metastases can occur in breast cancer, gastrointestinal cancer, and squamous cell cancers.
Bone metastases confer significant morbidity and mortality in people with advanced cancer [80]. Bone pain, the most common type of pain from cancer, may have inflammatory (e.g., local release of cytokines and chemical mediators by the tumor cells) and mechanical (e.g., related to pressure or mass effect of tumor tissue within the bone) components [81]. The high prevalence and incidence of skeletal-related events (SREs), which include pathological fractures, hypercalcemia, and spinal cord injury, contribute to poor performance status and decreased quality of life in cancer survivors [82]. Sudden onset back pain and neurological deficits are ominous for spinal cord compression, which is a medical emergency requiring magnetic resonance imaging, high-dose corticosteroid therapy, and urgent referral for surgical decompression and spinal stabilization [83].
External beam radiation therapy is the primary treatment modality for symptomatic bone metastases to reduce pain, achieve local tumor control, and improve quality of life [84]. Radionuclide therapy is the systemic use of radioisotopes for palliation of painful bone metastases; radiopharmaceuticals, such as strontium-89, are preferentially taken up at sites of bone formation, thus likely being most efficacious for osteoblastic metastases [85]. Pathological fractures occur in up to 30% of all cancer survivors, with the most common fracture site being proximal parts of the long bones, particularly the femur [86]. Movement-exacerbated pain is predictive for impending fracture, with primary internal stabilization followed by radiotherapy being the treatment of choice. Percutaneous vertebroplasty, wherein polymethylmethacrylate is injected into bone by percutaneously inserted needles under radiologic guidance, can alleviate pain from and stabilize pathological vertebral body fractures [87]. Locoregional techniques such as radiofrequency ablation [88], cryotherapy [89], photodynamic therapy [90], endovascular embolization [91], or chemical ablation [92] can facilitate tumor debulking. In select cancer survivors, surgery can correct and prevent further deformity through spine stabilization and nerve decompression [93].
The most common pharmacological agents for management of bone metastases are bisphosphonates, which inhibit osteoclastic bone resorption and are the primary treatment for hypercalcemia of malignancy [94]. Recent Cochrane reviews have shown that bisphosphonates appear to reduce bone pain, decrease the risk of developing SREs, and delay the median time to SRE in women with metastatic breast cancer that has spread to the bone [95]; reduce pathological vertebral fractures, SREs, and pain in multiple myeloma survivors [96]; and probably decrease SREs and disease progression in advanced prostate cancer survivors [97]. There is increasing evidence for the use of denosumab, a RANK-ligand inhibitor, in preventing SREs in multiple myeloma survivors [98]. Systemic opioids are the mainstay analgesic therapy for painful bony metastases [99]; corticosteroids, such as dexamethasone, are commonly prescribed as adjuvant therapy in survivors with limited life expectancy and painful bone metastases [100].
There is emerging evidence in support of exercise as a potential non-pharmacological intervention in the advanced cancer survivor with bone metastases. In a randomized controlled trial of a modular multimodal exercise program in 57 prostate cancer survivors with bone metastases, there were statistically significant improvements in self-reported physical function and objectively measured lower body muscle strength, with no skeletal complications or increased bone pain [101]. In a randomized controlled trial of guided isometric resistance training of the paravertebral muscles in 60 survivors with spinal bone metastases undergoing radiotherapy, there were statistically significant improvements in functional capacity and fatigue [102]. Larger, high-quality randomized controlled trials are needed to establish the efficacy of exercise training in advanced cancer survivors with bone metastases. Table 16.4 includes considerations for screening and exercise training for the survivor with bone metastases.
Dyspnea
Dyspnea is a symptom experienced as “breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” and is the consequence of complex interactions between psychological, physiological, social, and environmental factors [103]. Multiple sensory inputs can contribute to the subjective experience of dyspnea, including (i) the sensations of work or effort, (ii) tightness which is specific to bronchoconstriction and stimulation of airway receptors, and (iii) air hunger/unsatisfied inspiration that arises from imbalances between inspiratory drive, efferent activation, and feedback from afferent receptors through the respiratory system [104]. A person with advanced cancer can experience both a chronic background level of continuous dyspnea and intermittent, acute episodes of breathlessness [105]. Dyspnea can only be perceived by the person experiencing it; therefore, self-report is a critical component of dyspnea assessment.
Dyspnea is present in up to 40% of patients at the time of diagnosis of advanced cancer [106], increases with disease progression [107], and occurs in up to 70% of patients in the last 6 weeks of life [108, 109]. Dyspnea is among the most feared symptom, as many report that they are unable to catch a breath or feel they are suffocating or drowning [110]. Dyspnea has been associated with fatigue, anxiety, and depression and can cause significant suffering in patients and their families [111, 112]. Dyspnea negatively impacts quality of life, including physical functioning and interfering with daily life activities [113].
The underlying etiology of dyspnea in people with advanced cancer is multifactorial. Pulmonary causes of dyspnea include airway obstruction, atelectasis, infection, interstitial lung disease, lymphangitic carcinomatosis, metastatic disease, pleural effusion, and pulmonary embolism [114]. Systemic causes of dyspnea include anemia, congestive heart failure, deconditioning, hypoxemia, pericardial effusion/pericarditis, pulmonary hypertension, muscle weakness, neuromuscular conditions, sepsis, and uremia [115]. Psychogenic causes of dyspnea include panic disorder, anxiety, and psychosocial distress [116]. Dyspnea may also be caused by adverse effects of anticancer treatment, including chemotherapy or radiotherapy-induced pneumonitis and fibrosis [117, 118].
Dyspnea management starts with comprehensive assessment of the symptom, its impact on the patient’s experience, and treatment of any reversible etiologies [119]. Commonly used pharmacological agents for the management of dyspnea include systemic opioids, benzodiazepines, steroids, and oxygen therapy [120]. Non-pharmacological strategies for the management of dyspnea include anxiety reduction training, relaxation techniques, breathing exercises, environmental modification, and activity pacing and energy conservation [121]. Complex interventions that are administered by an interdisciplinary team, and which combine pulmonary rehabilitation with cognitive and behavioral management techniques, may be of benefit for advanced cancer patients with dyspnea [122, 123].
Exercise is one potential non-pharmacological strategy for the management of dyspnea in people with advanced cancer. In their 2019 Cochrane review of the effects of exercise training in adults with advanced lung cancer, Peddle-McIntyre et al. showed that upon completion of the intervention period, there was no significant difference in dyspnea between the intervention and control groups [25]; the evidence was graded as “low certainty” due to small sample sizes and significant risk of bias across the five studies [124,125,126,127,128]. Larger, high-quality randomized controlled trials are needed to establish efficacy of exercise training for dyspnea in patients with advanced lung cancer. Table 16.5 describes key considerations for screening, evaluation, as well as exercise testing and training.
Venous Thromboembolism
Venous thromboembolism (VTE) is a clinical syndrome characterized by blood clot formation in the veins and is comprised of two types, deep venous thrombosis (DVT) and pulmonary embolism (PE) [129]. DVT is the formation of blood clot in the body’s large veins, most commonly in the lower limbs; PE results from dislodgement of the blood clot from the blood vessel where it formed and subsequently getting blocked in the lung [130]. Risk factors for VTE can be classified into two main categories: (i) idiopathic, primary, and unprovoked and (ii) secondary and provoked [131]. Idiopathic, primary, and unprovoked risk factors include age >65 years, air pollution, cigarette smoking, hypertension, long-haul travel, metabolic syndrome, obesity, thrombophilia (factor V Leiden or prothrombin gene mutation), and no apparent cause. Secondary and provoked risk factors include cancer, acute medical illness (e.g., pneumonia, congestive heart failure), immobilization, oral contraceptives, postmenopausal hormonal replacement, surgery, pregnancy, and trauma.
VTE presentation can be asymptomatic. DVT can present with nonspecific symptoms like leg pain/ache/discomfort, sensation of warmth, tenderness, swelling, or discoloration, whereas PE can present with chest pain, dyspnea, palpitations, or sudden collapse [130]. Diagnosis of VTE begins with a clinical probability assessment, which incorporates clinical history (e.g., individual and familial presentation), physical examination (e.g., abnormalities in oxygen saturation, symptoms, or signs), and diagnostic imaging (e.g., abnormalities in chest radiography or electrocardiography) [132]. In survivors identified as having low clinical probability, VTE diagnosis can be ruled out by a blood D-dimer test; in those having intermediate or high clinical probability, compression ultrasonography or multidetection CT angiography is warranted. For the initial treatment, early maintenance, and long-term treatment of established VTE, low-molecular-weight heparins are the mainstay pharmacological therapy [133].
Cancer-associated VTE confers significant morbidity and mortality [134]. Cancer survivors are six times more likely to develop VTE than noncancer survivors, and survivors with cancer account for greater than 20% of all new VTE diagnoses [135]. Incident VTE risk is higher in survivors with primary brain tumors and pancreatic, stomach, and lung cancers [136]. The use of systemic chemotherapy [137], indwelling catheters [138], and supportive therapies (e.g., red blood cell transfusions, platelet transfusions, and erythropoiesis-stimulating agents) [139] results in increased VTE risk. Increased tumor burden confers higher risk of VTE, and the presence of VTE is a poor prognostic sign [140]. After the cancer itself, VTE is the second highest cause of death in cancer survivors [140].
The role of exercise in people with cancer-associated VTE is unknown. The association between regular physical activity and incident VTE risk has not been established in healthy populations [141]. In a randomized controlled trial of adults who were receiving therapeutic anticoagulation after a first episode of unprovoked VTE, early initiation of exercise training resulted in improvements in physical activity and fitness, and symptoms related to postthrombotic syndrome, with no adverse events; people with cancer-associated VTE, however, were excluded [142]. In a retrospective study of 422 people postacute PE who participated in an inpatient rehabilitation program, there were no recurrent VTE or severe bleeds, and the individualized exercise program was deemed safe; however, none of the participants had cancer [143]. Further research is needed to determine the feasibility, safety, and efficacy of exercise in the prophylaxis of, and rehabilitation from, VTE in people with cancer.
Nausea
Nausea is an unpleasant, subjective experience which signals imminent vomiting, which may or may not result; vomiting, on the other hand, is an unpleasant symptom objectively experienced as forceful elimination of stomach contents by gastric cardia opening and sustained abdominal muscle contraction [144]. Between 6% and 68% of people with advanced cancer experience nausea [107], for which average intensity scores tend to plateau in the last 6 months of life [112]. In patients with advanced cancer who are admitted to hospice, 62% reported nausea and vomiting, whereas 34% reported isolated nausea and 4% reported isolated vomiting [145]. Vomiting was frequently unbearable (73%) for cancer patients within the last 6 months of life, when symptom intensity overall was low [146]. Nausea and vomiting contribute to dehydration, electrolyte abnormalities, weight loss, and the inability to take medications, and result in complications which interfere with treatment and social interaction [147]. In newly diagnosed cancer patients undergoing combined modality treatment, approximately two-thirds reported co-occurrence of nausea, vomiting, and appetite loss, which synergistically and negatively impacted overall quality of life (including physical functioning, fatigue, and overall health) and psychological distress [148]. Nausea is often undertreated in people with advanced cancer, with detrimental effects on quality of life [149].
Nausea and vomiting are centrally mediated by a diffuse, interconnecting neural network which results in the emetic reflex. Rather than a discrete vomiting center, the emetic complex involves groups of loosely organized neurons which are distributed between the prodromal-sign center (located in the reticular area dorsally adjacent to the semicompact part of the nucleus ambiguous) and the central pattern generator center (located dorsomedial to the retrofacial nucleus) [150]. Together, the prodromal-sign center (PSC) and central pattern generator center (CPGC) integrate afferent input from different areas throughout the brainstem and medulla: the vestibular nuclei and cerebellum, the higher central nervous system (CNS) centers, the nucleus tractus solitarius (NTS), and the chemoreceptor trigger zone/area postrema (CTZ/AP). Located in the floor of the fourth ventricle with no blood-brain barrier, the CTZ/AP contains chemosensitive nerve cell projections, which are directly exposed to noxious agents in the cerebrospinal fluid; drugs/toxins/metabolites in the systemic circulation are detected from the dense vascular network of fenestrated local capillaries therein. The major afferent neural pathway from the body to the central structures is the vagus nerve, with additional contributions from the splanchnic nerves, sympathetic ganglia, and glossopharyngeal nerve [144].
The underlying etiology of nausea and vomiting in people with advanced cancer is complex [151]. Cerebral cortical causes of nausea and vomiting include CNS or meninges tumors, increased intracranial pressure, anxiety or other conditioned responses, and uncontrolled pain [147]. Vestibular/middle ear causes of nausea and vomiting include vestibular disease, middle-ear infections, and motion sickness. CTZ/AP causes of nausea and vomiting include medications (e.g., opioid analgesics, chemotherapy, antibiotics, theophylline, digoxin), metabolic toxins (e.g., renal impairment, liver failure, tumor products), hyponatremia, and hypercalcemia. Gastrointestinal tract (GIT) causes of nausea and vomiting include irritation by medications (e.g., nonsteroidal anti-inflammatory medications, iron, alcohol, antibiotics), tumor infiltration, radiation therapy to the GIT, infection (e.g., candida esophagitis, colitis, history of radiation therapy), constipation/fecal impaction, incomplete tumor obstruction, bowel dysmotility, tube feedings, gag reflex from feeding tube, nasopharyngeal bleeding, and thick secretions [147].
Nausea and vomiting management is a mechanistic approach, which is based on identifying the likely etiology of nausea and vomiting, the putative pathway by which the cause triggers the emetic reflex, and the potentially involved neurotransmitters [152]. Non-pharmacological measures for managing nausea and vomiting include small, frequent meals consisting of food that the person desires, avoiding foods with unpleasant tastes or strong odors, and drinking frequent, small sips of fluid [144]. Treatment of reversible causes of nausea and vomiting includes whole-brain radiotherapy for brain metastases, antibiotics for middle-ear infections, antifungal therapy for gastrointestinal tract infections, reducing tube feeding volumes, laxatives and manual disimpaction for constipation, and hydration and pamidronate for hypercalcemia. Commonly used pharmacological agents for the management of nausea and vomiting in people with advanced cancer include dexamethasone [153], levomepromazine [154], haloperidol [155], olanzapine [156], and metoclopramide [157]. In people with advanced cancer not receiving antineoplastic therapy, pharmacological agents associated with a statistically significant decrease in nausea/vomiting were olanzapine, laxatives, corticosteroids, domperidone, and metoclopramide [158].
There is emerging evidence regarding exercise as one potential non-pharmacological strategy for the management of nausea and vomiting in advanced cancer patients. In a randomized controlled trial of a combined nutrition and exercise program in 58 survivors with metastatic or locally advanced gastrointestinal and lung tumors, nausea and vomiting increased less in survivors of the intervention group than in survivors of the control group (p < 0.01) [159]. Larger, high-quality randomized controlled trials are needed to establish the efficacy of exercise training for nausea and vomiting in people with advanced cancer.
Summary
This chapter has described the background information and practical aspects related to exercise screening and delivery, including special considerations for the survivor with advanced, metastatic, and terminal cancer. The challenges facing the exercise specialist are complex and involve identifying symptoms, functional impairments, and co-pathologies that may impact exercise risk and tolerance and adapting exercise programming to allow the patient to participate safely, comfortably, and effectively.
References
Canadian Cancer Statistics. https://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=on.
WHO. Cancer pain relief and palliative care: report of a WHO Expert Committee. World Health Organization technical report series. Geneva; 1990. p. 11.
Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–32.
Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63(5):295–317.
Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16(3):293–300.
Laird BJ, Fallon M, Hjermstad MJ, Tuck S, Kaasa S, Klepstad P, McMillan DC. Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol. 2016;34(23):2769–75.
Simone CB 2nd. Early palliative care and integration of palliative care models in modern oncology practices. Ann Palliat Med. 2015;4(3):84–6.
Haun MW, Estel S, Rucker G, Friederich HC, Villalobos M, Thomas M, Hartmann M. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev. 2017;6:Cd011129.
Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, Firn JI, Paice JA, Peppercorn JM, Phillips T, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96–112.
Knaul FM, Farmer PE, Krakauer EL, De Lima L, Bhadelia A, Jiang Kwete X, Arreola-Ornelas H, Gomez-Dantes O, Rodriguez NM, Alleyne GAO, et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. Lancet (London, England). 2018;391(10128):1391–454.
Global Consensus based palliative care definition. https://hospicecare.com/what-we-do/projects/consensus-based-definition-of-palliative-care/definition/.
Hui D, Mori M, Parsons HA, Kim SH, Li ZJ, Damani S, Bruera E. The lack of standard definitions in the supportive and palliative oncology literature. J Pain Symptom Manag. 2012;43(3):582–92.
Peppercorn JM, Smith TJ, Helft PR, Debono DJ, Berry SR, Wollins DS, Hayes DM, Von Roenn JH, Schnipper LE. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29(6):755–60.
NCI. Advanced cancer. In: National Cancer Institute Dictionary of Cancer Terms. 2018. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/advanced-cancer.
Cancer Statistics for the UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk.
Understanding advanced cancer, metastatic cancer and bone metastases. https://www.cancer.org/treatment/understanding-your-diagnosis/advanced-cancer/what-is.html.
Klein EA, Kupelian PA, Dreicer R, Peereboom D, Zippe C. Locally advanced prostate cancer. Curr Treat Options in Oncol. 2001;2(5):403–11.
Martin RC 2nd. Management of locally advanced pancreatic cancer. Surg Clin North Am. 2016;96(6):1371–89.
What is metastatic cancer? http://www.cancer.ca/en/cancer-information/cancer-type/metastatic-cancer/metastatic-cancer/?region=on.
Metastasis. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/metastasis.
Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10(2):243–255, vii.
Adra N, Einhorn LH. Testicular cancer update. Clin Adv Hematol Oncol: H&O. 2017;15(5):386–96.
Hui D, Nooruddin Z, Didwaniya N, Dev R, De La Cruz M, Kim SH, Kwon JH, Hutchins R, Liem C, Bruera E. Concepts and definitions for “actively dying,” “end of life,” “terminally ill,” “terminal care,” and “transition of care”: a systematic review. J Pain Symptom Manag. 2014;47(1):21.
Sizoo EM, Pasman HR, Dirven L, Marosi C, Grisold W, Stockhammer G, Egeter J, Grant R, Chang S, Heimans JJ, et al. The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer. 2014;22(3):847–57.
Peddle-McIntyre CJ, Singh F, Thomas R, Newton RU, Galvao DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev. 2019;2:Cd012685.
Heywood R, McCarthy AL, Skinner TL. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support Care Cancer. 2017;25(10):3031–50.
Wu C, Zheng Y, Duan Y, Lai X, Cui S, Xu N, Tang C, Lu L. Nonpharmacological interventions for cancer-related fatigue: a systematic review and Bayesian network meta-analysis. Worldviews Evid-Based Nurs. 2019;16(2):102–10.
Do J, Cho Y, Jeon J. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer. 2015;18(1):87–96.
Zimmer P, Trebing S, Timmers-Trebing U, Schenk A, Paust R, Bloch W, Rudolph R, Streckmann F, Baumann FT. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26(2):615–24.
Gagnon B, Murphy J, Eades M, Lemoignan J, Jelowicki M, Carney S, Amdouni S, Di Dio P, Chasen M, Macdonald N. A prospective evaluation of an interdisciplinary nutrition-rehabilitation program for patients with advanced cancer. Curr Oncol. 2013;20(6):310–8.
Lowe SS, Watanabe SM, Baracos VE, Courneya KS. Home-based functional walking program for advanced cancer patients receiving palliative care: a case series. BMC Palliat Care. 2013;12:22.
Siemens W, Wehrle A, Gaertner J, Henke M, Deibert P, Becker G. Implementing a home-based exercise program for patients with advanced, incurable diseases after discharge and their caregivers: lessons we have learned. BMC Res Notes. 2015;8:509.
Gresham G, Schrack J, Gresham LM, Shinde AM, Hendifar AE, Tuli R, Rimel BJ, Figlin R, Meinert CL, Piantadosi S. Wearable activity monitors in oncology trials: current use of an emerging technology. Contemp Clin Trials. 2018;64:13–21.
Salakari MR, Surakka T, Nurminen R, Pylkkanen L. Effects of rehabilitation among patients with advances cancer: a systematic review. Acta Oncol. 2015;54(5):618–28.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59.
American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 10th ed. Philadelphia: Wolters Kluwer Health; 2017.
American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708.
Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, Pescatello LS. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47(11):2473–9.
McIsaac DI, Saunders C, Hladkowicz E, Bryson GL, Forster AJ, Gagne S, Huang A, Lalu M, Lavallee LT, Moloo H, et al. PREHAB study: a protocol for a prospective randomised clinical trial of exercise therapy for people living with frailty having cancer surgery. BMJ Open. 2018;8(6):e022057.
Bryant AL, Deal AM, Battaglini CL, Phillips B, Pergolotti M, Coffman E, Foster MC, Wood WA, Bailey C, Hackney AC, et al. The effects of exercise on patient-reported outcomes and performance-based physical function in adults with acute leukemia undergoing induction therapy: exercise and quality of life in acute leukemia (EQUAL). Integr Cancer Ther. 2018;17(2):263–70.
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26.
Herdy AH, Ritt LE, Stein R, Araujo CG, Milani M, Meneghelo RS, Ferraz AS, Hossri C, Almeida AE, Fernandes-Silva MM, et al. Cardiopulmonary exercise test: background, applicability and interpretation. Arq Bras Cardiol. 2016;107(5):467–81.
McNeely ML, Dolgoy N, Onazi M, Suderman K. The interdisciplinary rehabilitation care team and the role of physical therapy in survivor exercise. Clin J Oncol Nurs. 2016;20(6 Suppl):S8–S16.
Capozzi LC, Lau H, Reimer RA, McNeely M, Giese-Davis J, Culos-Reed SN. Exercise and nutrition for head and neck cancer patients: a patient oriented, clinic-supported randomized controlled trial. BMC Cancer. 2012;12:446.
McArdle WD. Exercise physiology: energy, nutrition, and human performance. Philadelphia: Lippincott Williams & Wilkins; 2006.
Suderman K, Sellar C, Peddle-McIntyre C, McNeely ML. Implementing cancer exercise rehabilitation: an update on recommendations for clinical practice. Curr Cancer Ther Rev. 2019;15(2):100–9.
Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, McCarthy S, Murnane A, Patchell S, Adams D. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209(4):184–7.
Matsugaki R, Akebi T, Shitama H, Wada F, Saeki S. Immediate effects of exercise intervention on cancer-related fatigue. J Phys Ther Sci. 2018;30(2):262–5.
Buckley J. Exercise physiology and monitoring of exercise in cardiac rehabilitation. In: Thow M, editor. Exercise leadership in cardiac rehabilitation. West Sussex: Wiley; 2006. p. 47–95.
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw: JNCCN. 2015;13(8):1012–39.
O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. 2018;26(10):3353–64.
Davis MP, Walsh D. Mechanisms of fatigue. J Support Oncol. 2010;8(4):164–74.
Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. J Neurosci. 1998;18(6):2247–53.
Jager A, Sleijfer S, van der Rijt CC. The pathogenesis of cancer related fatigue: could increased activity of pro-inflammatory cytokines be the common denominator? Eur J Cancer (Oxford, England: 1990). 2008;44(2):175–81.
Alexander S, Stone P, White S, Andrews P, Nussey S, Bano G. Evaluation of central serotonin sensitivity in breast cancer survivors with cancer-related fatigue syndrome. J Pain Symptom Manag. 2010;40(6):892–8.
Tell D, Mathews HL, Janusek LW. Day-to-day dynamics of associations between sleep, napping, fatigue, and the cortisol diurnal rhythm in women diagnosed as having breast cancer. Psychosom Med. 2014;76(7):519–28.
Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist. 2013;18(10):1135–43.
Prinsen H, van Dijk JP, Zwarts MJ, Leer JW, Bleijenberg G, van Laarhoven HW. The role of central and peripheral muscle fatigue in postcancer fatigue: a randomized controlled trial. J Pain Symptom Manag. 2015;49(2):173–82.
Yavuzsen T, Davis MP, Ranganathan VK, Walsh D, Siemionow V, Kirkova J, Khoshknabi D, Lagman R, LeGrand S, Yue GH. Cancer-related fatigue: central or peripheral? J Pain Symptom Manag. 2009;38(4):587–96.
Kisiel-Sajewicz K, Davis MP, Siemionow V, Seyidova-Khoshknabi D, Wyant A, Walsh D, Hou J, Yue GH. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manag. 2012;44(3):351–61.
Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89.
Curt GA. The impact of fatigue on patients with cancer: overview of FATIGUE 1 and 2. Oncologist. 2000;5(Suppl 2):9–12.
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–60.
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10.
Van Lancker A, Velghe A, Van Hecke A, Verbrugghe M, Van Den Noortgate N, Grypdonck M, Verhaeghe S, Bekkering G, Beeckman D. Prevalence of symptoms in older cancer patients receiving palliative care: a systematic review and meta-analysis. J Pain Symptom Manag. 2014;47(1):90–104.
Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11(8):971–5.
Oh HS, Seo WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid-Based Nurs. 2011;8(4):191–201.
Okuyama T, Akechi T, Shima Y, Sugahara Y, Okamura H, Hosaka T, Furukawa TA, Uchitomi Y. Factors correlated with fatigue in terminally ill cancer patients: a longitudinal study. J Pain Symptom Manag. 2008;35(5):515–23.
Yennurajalingam S, Bruera E. Fatigue and asthenia. In: Cherny N, Fallon MT, Kaasa S, Portenoy RK, Currow DC, editors. Oxford textbook of palliative medicine. Oxford: Oxford University Press; 2015. p. 409–20.
Mucke M, Cuhls H, Peuckmann-Post V, Minton O, Stone P, Radbruch L. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015;(5):Cd006788.
Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26(6):660–7.
Ogilvy C, Livingstone K, Prue G. Management of cancer-related fatigue. In: Rankin J, Robb K, Murtage N, Cooper J, Lewis S, editors. Rehabilitation in cancer care. Oxford: Blackwell Publishing; 2008. p. 264–79.
McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. J Natl Compr Canc Netw: JNCCN. 2010;8(8):945–53.
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–8.
D’Oronzo S, Coleman R, Brown J, Silvestris F. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol. 2019;15:004–4.
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, Goncalves F. Bone metastases: an overview. Oncol Rev. 2017;11(1):321.
Wu MY, Li CJ, Yiang GT, Cheng YL, Tsai AP, Hou YT, Ho YC, Hou MF, Chu PY. Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem. 2018;46(4):1423–38.
Maurizi A, Rucci N. The osteoclast in bone metastasis: player and target. Cancers. 2018;10(7):218.
Ottewell PD. The role of osteoblasts in bone metastasis. J Bone Oncol. 2016;5(3):124–7.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–9s.
Aielli F, Ponzetti M, Rucci N. Bone metastasis pain, from the bench to the bedside. Int J Mol Sci. 2019;20(2):280.
Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–94.
Lawton AJ, Lee KA, Cheville AL, Ferrone ML, Rades D, Balboni TA, Abrahm JL. Assessment and management of patients with metastatic spinal cord compression: a multidisciplinary review. J Clin Oncol. 2019;37(1):61–71.
Saravana-Bawan S, David E, Sahgal A, Chow E. Palliation of bone metastases-exploring options beyond radiotherapy. Ann Palliat Med. 2019;8(2):168–77.
Dash A, Das T, Knapp FFR. Targeted Radionuclide Therapy of Painful Bone Metastases: Past Developments, Current Status, Recent Advances and Future Directions [published online ahead of print, 2019 Feb 1]. Curr Med Chem. 2019;10.2174/0929867326666190201142814.
Anract P, Biau D, Boudou-Rouquette P. Metastatic fractures of long limb bones. Orthop Traumatol Surg Res: OTSR. 2017;103(1s):S41–s51.
Wenger M. Vertebroplasty for metastasis. Med Oncol (Northwood, London, England). 2003;20(3):203–9.
Ringe KI, Panzica M, von Falck C. Thermoablation of bone tumors. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2016;188(6):539–50.
Masala S, Guglielmi G, Petrella MC, Mastrangeli R, Meschini A, Anselmetti GC, Bartolucci DA, Mammucari M, Manenti G, Simonetti G. Percutaneous ablative treatment of metastatic bone tumours: visual analogue scale scores in a short-term series. Singap Med J. 2011;52(3):182–9.
Fan HT, Wang L, Zhang P, Liu SB. Photodynamic therapy in spinal metastases: a qualitative analysis of published results. Int Surg. 2015;100(4):712–9.
Layalle I, Flandroy P, Trotteur G, Dondelinger RF. Arterial embolization of bone metastases: is it worthwhile? J Belg Radiol. 1998;81(5):223–5.
Gangi A, Kastler B, Klinkert A, Dietemann JL. Injection of alcohol into bone metastases under CT guidance. J Comput Assist Tomogr. 1994;18(6):932–5.
Hammerberg KW. Surgical treatment of metastatic spine disease. Spine. 1992;17(10):1148–53.
Toller CS, Charlesworth S, Mihalyo M, Howard P, Wilcock A. Bisphosphonates. J Pain Symptom Manag. 2019;57(5):1018–30.
O’Carrigan B, Wong MH, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2017;10:Cd003474.
Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Bisphosphonates in multiple myeloma: an updated network meta-analysis. Cochrane Database Syst Rev. 2017;12:Cd003188.
Macherey S, Monsef I, Jahn F, Jordan K, Yuen KK, Heidenreich A, Skoetz N. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12:Cd006250.
Yee AJ, Raje NS. Denosumab for the treatment of bone disease in solid tumors and multiple myeloma. Future Oncol (London, England). 2018;14(3):195–203.
Colvin LA, Fallon MT. Cancer-induced bone pain. In: Cherny N, Fallon MT, Kaasa S, Portenoy RK, Currow DC, editors. Oxford textbook of palliative medicine. Oxford: Oxford University Press; 2015. p. 841–59.
White P, Arnold R, Bull J, Cicero B. The use of corticosteroids as adjuvant therapy for painful bone metastases: a large cross-sectional survey of palliative care providers. Am J Hosp Palliat Care. 2018;35(1):151–8.
Galvao DA, Taaffe DR, Spry N, Cormie P, Joseph D, Chambers SK, Chee R, Peddle-McIntyre CJ, Hart NH, Baumann FT, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. 2018;50(3):393–9.
Rief H, Akbar M, Keller M, Omlor G, Welzel T, Bruckner T, Rieken S, Hafner MF, Schlampp I, Gioules A, et al. Quality of life and fatigue of patients with spinal bone metastases under combined treatment with resistance training and radiation therapy- a randomized pilot trial. Radiat Oncol. 2014;9:151.
American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321–40.
Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–52.
Mercadante S, Fusco F, Caruselli A, Cartoni C, Masedu F, Valenti M, Aielli F. Background and episodic breathlessness in advanced cancer patients followed at home. Curr Med Res Opin. 2017;33(1):155–60.
Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):233–43.
Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manag. 2006;31(1):58–69.
Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manag. 2000;19(5):357–62.
Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manag. 2001;21(2):95–102.
Wilcock A, Crosby V, Hughes A, Fielding K, Corcoran R, Tattersfield AE. Descriptors of breathlessness in patients with cancer and other cardiorespiratory diseases. J Pain Symptom Manag. 2002;23(3):182–9.
Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care. 2003;1(4):337–44.
Seow H, Barbera L, Sutradhar R, Howell D, Dudgeon D, Atzema C, Liu Y, Husain A, Sussman J, Earle C. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29(9):1151–8.
Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Prevalence and screening of dyspnea interfering with daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manag. 2002;23(6):484–9.
Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5(2):90–100.
Manning HL, Mahler DA. Pathophysiology of dyspnea. Monaldi archives for chest disease =. Arch Monaldi Mal Torace. 2001;56(4):325–30.
Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med. 1996;154(1):6–17.
Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clin Chest Med. 2017;38(2):201–8.
Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13(4):242–8.
Chin C, Booth S. Managing breathlessness: a palliative care approach. Postgrad Med J. 2016;92(1089):393–400.
Lok CW. Management of breathlessness in patients with advanced cancer: a narrative review. Am J Hosp Palliat Care. 2016;33(3):286–90.
Bausewein C, Booth S, Gysels M, Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;(2):Cd005623.
Farquhar MC, Prevost AT, McCrone P, Higginson IJ, Gray J, Brafman-Kennedy B, Booth S. Study protocol: phase III single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. Trials. 2011;12:130.
Booth S, Moffat C, Farquhar M, Higginson IJ, Burkin J. Developing a breathlessness intervention service for patients with palliative and supportive care needs, irrespective of diagnosis. J Palliat Care. 2011;27(1):28–36.
Henke CC, Cabri J, Fricke L, Pankow W, Kandilakis G, Feyer PC, de Wit M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22(1):95–101.
Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169–77.
Jastrzebski D, Maksymiak M, Kostorz S, Bezubka B, Osmanska I, Mlynczak T, Rutkowska A, Baczek Z, Ziora D, Kozielski J. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. Adv Exp Med Biol. 2015;861:57–64.
Molassiotis A, Charalambous A, Taylor P, Stamataki Z, Summers Y. The effect of resistance inspiratory muscle training in the management of breathlessness in patients with thoracic malignancies: a feasibility randomised trial. Support Care Cancer. 2015;23(6):1637–45.
Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, Kasymjanova G, Jagoe RT. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer. 2017;25(6):1749–58.
Burwen DR, Wu C, Cirillo D, Rossouw JE, Margolis KL, Limacher M, Wallace R, Allison M, Eaton CB, Safford M, et al. Venous thromboembolism incidence, recurrence, and mortality based on Women’s Health Initiative data and Medicare claims. Thromb Res. 2017;150:78–85.
Thachil J. Deep vein thrombosis. Hematology (Amsterdam, Netherlands). 2014;19(5):309–10.
Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet (London, England). 2012;379(9828):1835–46.
Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M, Pauker SG, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S–418S.
Farge D, Bounameaux H, Brenner B, Cajfinger F, Debourdeau P, Khorana AA, Pabinger I, Solymoss S, Douketis J, Kakkar A. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17(10):e452–66.
Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–22.
Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):381s–453s.
Petterson TM, Marks RS, Ashrani AA, Bailey KR, Heit JA. Risk of site-specific cancer in incident venous thromboembolism: a population-based study. Thromb Res. 2015;135(3):472–8.
Ashrani AA, Gullerud RE, Petterson TM, Marks RS, Bailey KR, Heit JA. Risk factors for incident venous thromboembolism in active cancer patients: a population based case-control study. Thromb Res. 2016;139:29–37.
Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665–75.
Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377–81.
Donnellan E, Khorana AA. Cancer and venous thromboembolic disease: a review. Oncologist. 2017;22(2):199–207.
Evensen LH, Braekkan SK, Hansen JB. Regular physical activity and risk of venous thromboembolism. Semin Thromb Hemost. 2018;44(8):765–79.
Lakoski SG, Savage PD, Berkman AM, Penalosa L, Crocker A, Ades PA, Kahn SR, Cushman M. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: a randomized clinical trial. J Thromb Haemost: JTH. 2015;13(7):1238–44.
Noack F, Schmidt B, Amoury M, Stoevesandt D, Gielen S, Pflaumbaum B, Girschick C, Voller H, Schlitt A. Feasibility and safety of rehabilitation after venous thromboembolism. Vasc Health Risk Manag. 2015;11:397–401.
Hardy JR, Glare P, Yates P, Mannix KA. Palliation of nausea and vomiting. In: Cherny N, Fallon MT, Kaasa S, Portenoy RK, Currow DC, editors. Oxford textbook of palliative medicine. Oxford: Oxford University Press; 2015. p. 661–74.
Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer. 2006;14(4):348–53.
Ruijs CD, Kerkhof AJ, van der Wal G, Onwuteaka-Philipsen BD. Symptoms, unbearability and the nature of suffering in terminal cancer patients dying at home: a prospective primary care study. BMC Fam Pract. 2013;14:201.
Kapo JM, Adams C, Giddings-Connolly RM, Hui F, Putnam AT, Sands R, Shalshin A. Nausea and vomiting. In: Shega JW, Paniagua MA, editors. Unipac 4: nonpain symptom management. Chicago: American Academy of Hospice and Palliative Medicine; 2017. p. 43–54.
Pirri C, Bayliss E, Trotter J, Olver IN, Katris P, Drummond P, Bennett R. Nausea still the poor relation in antiemetic therapy? The impact on cancer patients’ quality of life and psychological adjustment of nausea, vomiting and appetite loss, individually and concurrently as part of a symptom cluster. Support Care Cancer. 2013;21(3):735–48.
Reuben DB, Mor V. Nausea and vomiting in terminal cancer patients. Arch Intern Med. 1986;146(10):2021–3.
Smith HS, Smith EJ, Smith AR. Pathophysiology of nausea and vomiting in palliative medicine. Ann Palliat Med. 2012;1(2):87–93.
Collis E, Mather H. Nausea and vomiting in palliative care. BMJ (Clinical Research ed). 2015;351:h6249.
Glare PA, Dunwoodie D, Clark K, Ward A, Yates P, Ryan S, Hardy JR. Treatment of nausea and vomiting in terminally ill cancer patients. Drugs. 2008;68(18):2575–90.
Vayne-Bossert P, Haywood A, Good P, Khan S, Rickett K, Hardy JR. Corticosteroids for adult patients with advanced cancer who have nausea and vomiting (not related to chemotherapy, radiotherapy, or surgery). Cochrane Database Syst Rev. 2017;7:Cd012002.
Cox L, Darvill E, Dorman S. Levomepromazine for nausea and vomiting in palliative care. Cochrane Database Syst Rev. 2015;(11):Cd009420.
Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):Cd006271.
Sutherland A, Naessens K, Plugge E, Ware L, Head K, Burton MJ, Wee B. Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database Syst Rev. 2018;9:Cd012555.
Walsh D, Davis M, Ripamonti C, Bruera E, Davies A, Molassiotis A. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333–40.
Harder S, Herrstedt J, Isaksen J, Neergaard MA, Frandsen K, Sigaard J, Mondrup L, Jespersen BA, Groenvold M. The nature of nausea: prevalence, etiology, and treatment in patients with advanced cancer not receiving antineoplastic treatment. Support Care Cancer. 2019;27(8):3071–80.
Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, Pless M, Ballmer PE. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr (Edinburgh, Scotland). 2018;37(4):1202–9.
Denlinger CS, Carlson RW, Are M, Baker KS, Davis E, Edge SB, Friedman DL, Goldman M, Jones L, King A, et al. Survivorship: introduction and definition. Clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2014;12(1):34–45.
Heindel W, Gubitz R, Vieth V, Weckesser M, Schober O, Schafers M. The diagnostic imaging of bone metastases. Dtsch Arztebl Int. 2014;111(44):741–7.
Morris J, Belzarena A, Boland P. Bone metastases. In: Stubblefield MD, O’Dell MW, editors. Cancer rehabilitation: principles and practices. New York: Demos Medical Publishing; 2019. p. 780–8.
Rief H, Bruckner T, Schlampp I, Bostel T, Welzel T, Debus J, Forster R. Resistance training concomitant to radiotherapy of spinal bone metastases – survival and prognostic factors of a randomized trial. Radiat Oncol. 2016;11:97.
Rief H, Omlor G, Akbar M, Bruckner T, Rieken S, Forster R, Schlampp I, Welzel T, Bostel T, Roth HJ, et al. Biochemical markers of bone turnover in patients with spinal metastases after resistance training under radiotherapy--a randomized trial. BMC Cancer. 2016;16:231.
Rief H, Omlor G, Akbar M, Welzel T, Bruckner T, Rieken S, Haefner MF, Schlampp I, Gioules A, Habermehl D, et al. Feasibility of isometric spinal muscle training in patients with bone metastases under radiation therapy – first results of a randomized pilot trial. BMC Cancer. 2014;14:67.
Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2015;18(2):196.
Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328–35.
Sheill G, Guinan EM, Peat N, Hussey J. Considerations for exercise prescription in patients with bone metastases: a comprehensive narrative review. PM R. 2018;10(8):843–64.
Rewar S, Al Onazi M, Boudreau K, McNeely ML. A scoping review of combined yoga and resistance exercise for dyspnea in lung cancer survivors. J Yoga Physiother. 2018;5(3):1–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lowe, S.S., Sellar, C., Suderman, K., McNeely, M.L. (2020). Advanced Cancers, Metastatic Disease, and Palliative Care. In: Schmitz, K. (eds) Exercise Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-42011-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-42011-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42010-9
Online ISBN: 978-3-030-42011-6
eBook Packages: MedicineMedicine (R0)