Abstract
Antibiotics are vital for the healthcare of human and animals; these products are used to prevent, alleviate and cure disease and give a better way of living. However, after administration a large fraction of these products are excreted unchanged into water bodies, and nobody knows about the ultimate destination of these chemical compounds. Similarly the possible adverse effects due to the presence of these chemical to environment and human health are unknown due to lack of information. Most of the antibiotics products are reached to water bodies unaltered and known as active pharmaceutical ingredient. These products are transformed into metabolites and even into some other compounds through natural process which occurs in aquatic environment. Currently different technologies such as physical, chemical and biological or advance oxidation processes are used to treat the antibiotics. Some of these technologies are time consuming, ineffective and non-adequate for the emerging contaminants; furthermore few methods like AOPs (Advance Oxidation Processes) are innovative and efficient but they are expensive, need high energy and produced reactive or unstable oxidant which are not able to remove refractory contaminants. Therefore it needs an innovative green technologies which enable to decontaminate the water containing antibiotics. The electrochemical technologies offer an alternative way to treat these pollutants; the major process are electro-oxidation, electro-reduction, electrocoagulation, electro-Fenton, photoelectron-Fenton, sono-electrochemical, etc. The electrochemical technologies have some advantages over the other oxidation processes, like easily operation, high efficiency, coupling with other process, and low temperature required for its operation; moreover it can be powered with the help of solar panel to decrease the energy consumption. However still exist some challenges to overcome like designing and cost of electrode, improving the basic scientific understanding and in some cases the production of toxic intermediates. This study explores the electrochemical technologies and their application towards treatment of antibiotics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
All living organisms depend on water and the terrestrial life, in particular depends on fresh water resources. However, more than 97% of total water reservoir is saline, which does not support life on land. 75% of fresh water are confined in ice caps and glaciers. Spring, stream, pond, lakes and rivers are the major sources of potable water, comprising 0.01% of the total water (Baird 1999). Recently the global climate is drastically changed which put a high tension on water supplies. Heretofore, some regions are facing water scarcity and Pakistan is among top 15 countries with serious water dearth (https://www.un.org.pk/water-insecurity/). As life on earth completely depends on water, therefore the quality and quantity should be managed properly. At the same time, the fast industrialization and urbanization have resulted in serious surface water pollution with organic compounds such as dyes, pesticides, herbicides, cleansing agents, foot stabilizer, their metabolites and transformation products.
Recently, the focus of environmental scientist is diverting from conventional organic pollutants and is mainly focused on the micro-organic pollutants, which are known as emerging contaminants. These chemicals are found at level of (μg L−1) in the water bodies and are determined with the help of more sophisticated analytical instrumentations (Reemtsma and Jekel 2006; Barceló and Petrovic 2008). The recent advances in ultra-performance chromatography coupled with mass allow the detection of such emerging pollutants like pharmaceuticals, metabolites, and transformation (Petrovic and Barceló 2006). There is no common description not a comprehensive inventory that could be clearly included under the term “emerging contaminant”. Many of these chemicals, in ppb levels or below, are responsible for contamination of aquatic environment. Because of such lower concentration they are often called micro-pollutants. Many of these have been found to form intermediates often more complex and toxic than the parent compound because they pass through various chemical, photolytic, hydrological, and microbial transformations in the aquatic environment (Fig. 17.1).
These contaminants might also be emerging because it discover from a novel source or it may reach to human through new route (Fig. 17.1) or it may be due to its sensing and decontamination methods (U.S. Department of Defense (DoD) 2006). Emerging pollutants can be classified into some broad categories like pharmaceuticals and personal care products (PPCPs), endocrine disruptor chemicals (EDCs), perfluorochemicals (PFCs), polychlorinated naphthalenes (PCNs), polydimethylsiloxanes (PDMSs), bisphenol A (BPA), polychlorinated alkanes (PCAs) quaternary ammonium compounds (QACs), triclosan (TCS), triclocarban (TCC), pesticides, benzothiazoles, benzotriazoles, and engineered nano-materials (Lapworth et al. 2012). Figure 17.2 represents simple classification of ECs and its properties.
The data-related presence, risk assessment and ecotoxicological are not available, so it is hard to explore adverse health or environmental consequences (Barceló 2003). In many countries these contaminants are in the process of legislation; however still their synergistic effects are unknown because of the lack of information. Some of these emerging contaminants have been discussed in detail below.
17.2 Endocrine Disrupting Chemicals (EDCs)
Among the emerging pollutants, endocrine disrupters affect animal reproduction (Ghiselli and Jardim 2007; Bila and Dezotti 2007). These substances may have synthetic (Xenoestrogens) or natural origins (phytoestrogens) and can disturb the endocrine system, even at low concentrations. The effects of these substances have been found in various situations such as feminization of fish, reduced hatching of eggs of various species of animals (Grover et al. 2009), cancer in humans, and disorder in the reproductive system. The endocrine system is one of the vital systems of the animal body consisting of glands, hormones and receptors found in various parts of the body. The endocrine system is important because hormones have an important regulatory function in various organs such as testis, ovary, pancreas, adrenals, thyroid, parathyroid, pituitary and thalamus glands. Some features are directly controlled by hormones, as the activities of organs, levels of salts, sugars and lipids in the blood, storage of energy, sexual characteristics, etc. There are various possible sources of EDCs; it can be synthesized or can come as by-products of emissions from pulp and paper industries, steel foundries and motor vehicles and incineration of products, especially those containing chlorine like PVC. However, there are several other products including cosmetics, sun blocks, fragrances, contraceptive pills, solvents, surfactants, pesticides, herbicides, plastics, etc. that may contain endocrine disrupters. Several substances classified (Maia and Dezotti 2007) as endocrine disrupters include phthalates (Haarstad and Borch 2004), flame retardant (Wensing et al. 2005), alkylphenols, organochlorine compounds, bisphenol (Abdelmalek et al. 2008; Alonso-Magdalena et al. 2006), polycyclic aromatic hydrocarbons, pesticides (Africa et al. 2006; Birkhøj et al. 2004), biphenyls compounds, drugs, phytoestrogens, natural estrogens and heavy metals. The endocrine disrupters are accumulated in fatty tissues in infected animal’s body. The combination of EDCs with another chemical may have a synergistic effect and the toxicity is often higher than that of each substance separately. In case of herbicides, there are cases where the sub-product is more hazardous than original substance and the mixture between the two make them even more toxic (Richard et al. 2005; Yousef et al. 1995).
Pesticides are also considered endocrine disruptors and have been found to cause infertility, cancer of the testis, prostate, breast and ovary, etc. The estrogenic activity of several pesticides has been a concern. Some organochlorines pesticides such as DDT and its metabolites, the methoxychlor, dieldrin and mirex have been found to have this disrupting action (Meyer et al. 1999).
Pharmaceutical products are the important class of chemical serving and improving the standards of human life. It can be categorized according to the biological action and purport such as the use of antibiotics to treat bacterial infections, analgesic to reduce pain, and antineoplastic in anticancer therapy. The antibiotics have potential towards bacteria resistance and that is why they are of specific interest; after administration these pharmaceuticals undergo structural changes in human and animals bodies and get converted into metabolites which still acts as API (active pharmaceutical ingredient).
This variation seldom complete and a specific proportion of the parent compound API is normally discharged alongside the metabolite. For example, few antibiotics are broken down to 95%, while other antibiotics are only 5%. An investigation of the intake and throughput rates of APIs demonstrated that 75% of the antibiotics utilized in Germany were discharged unaltered, i.e. still active APIs (Kümmerer and Henninger 2003). From way back, the manufacturing and use of chemicals and pharmaceuticals have caused severe environmental pollution and severe health effects. The APIs used in medicine and additives can enter the environment via various non-fixed sources, pharmaceutical industries, sewage treatment plant effluents and domestic wastes. Pharmaceuticals is utilized in veterinary for treatment of diseases, as growth promoter, and a huge amount is discharged as waste and ultimately enter the water bodies (Kümmerer 2010). Figure 17.1 shows the possible routes of such pharmaceutical products. If they become part of soil components, they can reach groundwater and some can also be transported from surplus to surface water in case of heavy rain.
17.3 Antibiotics
Antibiotics are an important group of pharmaceuticals. It is used for the bacterial infection. They are widely used as a medicine for human, animals as well as for marine life. The basic purpose of antibiotics is the prevention and treatment of microbial contagion, whereas it is also used for promotion of animal growth. The standard definition of an antibiotic is a chemical that microorganism produce to disturb the growth of other organism. It may be classified on the basis of chemical structure or the way of microorganism prevention. It is widely used in medicine, veterinary, agriculture and marine inhibits for the obviation and treatment of diseases. Some antibiotics like streptomycines are utilized in fruit crops, while others are utilized in bee keeping (Kümmerer 2008).
Antibiotic is the class of pharmaceuticals that can be subgrouped into β-lactams, quinolones, tetracycline, macrolides, sulphonamides and others. Active compound may be neutral, ionic, or dipolar ions and come under the category of complex molecule with various functions. Physical, chemical and biological characteristic may vary with pH due to various functionalities in single molecule (Kümmerer 2008). Pharmaceuticals active compound, in particular antibiotics, are mostly complex molecules relative to the chemicals of industrial and environmental concern (Trivedi and Vasudevan 2007). Antibiotics may be metabolized more or less broadly by humans and animals. Human antibiotics are defecated in metabolic waste and can reach the sewage treatment plant (STP). As a still active compound, the non-metabolized fraction is excreted. From the survey of all compounds, approximately 75% of antibiotics consumed in Germany are unchanged (Kümmerer and Henninger 2003). In STP, antibiotics are only incompletely removed, and if it is not completely removed, it may reach the environment and mostly the water bodies. Its some amount may also touch the surface water or ground water. Liquid manure may also loose active substance during the raining from the top of soil.
Sulphonamides and trimethoprim are bacteriostatic agents that synergistically target and inhibit two pathway steps in bacterial folic acid synthesis (Masters et al. 2003; Skold 2001). Both these are very effective toward various potential bacterial infections. Sulphonamides are not entirely utilized and may excrete in parts into sewage in the form of source compounds and metabolites (Gobel et al. 2005; Hirsch et al. 1999). N4-acetylated is the main metabolite of sulphonamides, a biologically inactive product, that transform back to the active parent compound in period of sewage handling (Gobel et al. 2005). So, this may lead to inefficient subtraction of sulphonamides, especially sulphamethoxazole amid the biological treatment of waste (Gobel et al. 2007).
Sulphamethoxazole is one of the most commonly observed municipal sewage sulphamides (Brown et al. 2006; Kim et al. 2007; Gobel et al. 2007; Levine et al. 2006; Yang et al. 2005). Sulphamethoxazole, for instance, was noted to have concentration of up to 7.91 mg L−1 in sewage impacts in China, and this is among the 15 best selling medicine (Peng et al. 2006). Metabolites of some antibiotic like SMX (acetylated, N4 acetyle SMX) have been found in more concentration than parent antibiotic SMX in wastewater treatment plant.
SMX partially destroyed in conventional wastewater plants and ends up in water bodies with the possibility to unfavourably effect both marine and terrain organisms. Sulphamethoxazole in range of (0–1000 μg L−1) has been found and can cause adverse effects on the plant kingdom (Brain et al. 2004). Adverse effects to aquatic bacteria (Vibrio fischeri), freshwater invertebrates (Daphnia magna) and Japanese medaka fish (Oryzias latipes) have been reported for sulphamethoxazole (Kim et al. 2007). As mentioned above, conventional techniques, e.g. biotreatment and physicochemical treatment, including coagulation, volatilization, adsorption, sedimentation and filtration, cannot completely remove the SMX in STPs and also show the need of efficient removal of antibiotics and their metabolites from the water bodies to prevent its adverse effects on the environmental life (Jones et al. 2005; Rahman et al. 2009; Suárez et al. 2008).
The most important environmental problem in the present scenario is to safeguard the integrity of our water resources from the dangerous concentration of SMX. Today, the development of innovative and economical wastewater technologies aimed at reducing the daily amounts of antibiotics released into the environment or transforming them into less toxic or more biodegradable intermediates is increasingly important. A wide range of chemical and physical methods for the degradation of antibiotics in waste water are available. The methods of treatment included in this work are described in detail here.
17.4 Electrochemical Technologies for Wastewater Treatment
Electrochemical treatment is one of oxidation processes that can mineralize organic materials into water, carbon dioxide and inorganic molecules. The electrochemical oxidation process has proved fruitful in recent years for the treatment of wastewater, mainly because of its efficiency and ease of operation. The use of electrochemistry for environmental protection was the subject of a number of books and reviews. Somewhere else (Ibanez et al. 1994) the main features of electrochemical technologies are explained in detail. The electrochemical technologies are flexible and should be applied directly or indirectly and could be easily scale up from microliter to millions of litres, and it can be easily coupled with other processes like photochemical, sonochemical, bio-degradation or photo catalysis. The process is also an energetically efficient and work on minimum temperature and pressure and there is less power wastage during operation. Similarly the process is easy to operate and automate and only electrically controlled variables are used in the process. Along with all these characteristic the electrochemical process is also environmentally compatible and produce only electrons during the process, which are high potential particles whose reactivity can be adjusted by selecting an appropriate electro catalyst to avoid unwanted metabolites from being produced. Furthermore, it is cost effective in term of operation, space and labours; however some electrode materials are expensive which increase the capital cost of the process. Therefore intensive research aims to discover more efficient electrode materials, electro-catalyst and optimized parameters for electrochemical applications. In the last two decades, electrochemical technologies have become a focus of scientist around the world. Not only are the electrochemical technologies cost comparable to other technologies, they are sometimes more efficient and compact.
Electrochemical technologies offer a versatile way to treat the wastewater. Broadly speaking, it can be used to separate the pollutant from wastewater such as electrocoagulation and electro-filtration and it used to decompose and destroy the organic pollutant into harmless product in water. The degradation of pollutants may be done by direct electrochemical process or indirect electrochemical process by some oxidant species generating at electrode surface. The electrochemical processes which produced (•OH) are called electrochemical advanced oxidation processes (EAOPs). As we already discussed, electrochemical processes are versatile and may be coupled with some other advanced oxidation processes. Some of the most common electrochemical processes used for the treatment of antibiotic are shown in Fig. 17.3.
17.5 Electrochemical Oxidation
17.5.1 Electrochemical Oxidation without NaCl
The application of electrochemical oxidation for wastewater effluent is not new; it has root back in the nineteenth centuries. Initially Kuhn studies oxidative degradation of some cyanides, thiocyanates and phenol compounds using both anodic and chlorine oxidizing (Kuhn 1971). After that, Nilsson investigated the oxidation of simple, monohydric phenols at a PbO2 anode in aqueous sulphuric acid (Nilsson et al. 1973). Afterwards some others extensively investigated the synthetic wastewater containing phenol and aniline. However in the 1990s Feng and Johnson moved forward to explore the mechanism of electrochemical oxidation (Feng and Johnson 1990).
They reveal that upon the discharge of H2O on the electrode surface (S) produced hydroxyl radicals (•OH) which adsorbed on electrode surface represent as S[OH] (Eq. 17.1). It is further proposed that these S[OH] may be shifted to oxidation product through oxygen transfer mechanisms (Eq. 17.2) or oxidize the organic compound (R) into (RO) as shown in equation (Eq. 17.3).
Furthermore, in the 1990s Comninellis elaborated a simplified mechanism for electrochemical oxidation of organic compound using different electrode materials (Pt, Ti/lrO2, Ti/SnO2). He proposed that oxide anodes (MOx) forming the so-called higher oxide MOx + 1 and degradation occurs with electrodes at the surface where hydroxyl radical accumulated (Comninellis 1994). Comninellis thoroughly investigated the electrochemical conversion mechanism of organic compound using metal and metal oxide electrodes and proposed that water molecule has adsorbed on the electrode surface and generate adsorbed hydroxyl radicals as in Eq. (17.4).
However it is strongly depend on anode materials and hence we differentiate them into two classes, i.e. active and nonactive anodes.
-
1.
In case of active anode the Eq. (17.4) followed by interaction of oxygen of oxide electrode with MOx (HO•) and transformed it into higher oxide state as followed by Eq. (17.5).
$$ {\mathrm{MO}}_x\left({\mathrm{OH}}^{.}\right)+\mathrm{R}\to {\mathrm{MO}}_{x+1}+{\mathrm{H}}^{+}+{e}^{-}. $$(17.5)The activation of surface redox pair MOx + 1/MOx, often referred as active oxygen which may chemisorb active oxygen (MOx + 1). If there is no organic species in the premises, the physisorbed MOx (HO•) and chemisorbed MOx + 1 converted into dioxygen following (Eqs. 17.6 and 17.7)
$$ {\mathrm{MO}}_{x+1}\to {\mathrm{MO}}_x+\frac{1}{2}\kern0.33em {\mathrm{O}}_2 $$(17.6)$$ {\mathrm{MO}}_x\left({\mathrm{O}\mathrm{H}}^{.}\right)\to {\mathrm{MO}}_x+\frac{1}{2}{\mathrm{O}}_2+{\mathrm{H}}^{+}+{e}^{-}+{\mathrm{MO}}_x $$(17.7)However if organic compound are present, then the chemisorbed oxygen act as mediator for the selective conversion of pollutants on electrode surface.
$$ {\mathrm{MO}}_{x+1}+\mathrm{R}\to {\mathrm{MO}}_x+\mathrm{R}\mathrm{O} $$(17.8) -
2.
In case of nonactive anode no higher oxide is formed and adsorbed hydroxyl radicals non-selective degraded the organic pollutants into CO2 as represented in Eq. 17.8.
$$ {\mathrm{MO}}_x\left({\mathrm{OH}}^{.}\right)+\mathrm{R}\to {\mathrm{MO}}_x+m{\mathrm{CO}}_2+{\mathrm{H}}^{+}+{e}^{-} $$(17.9)Nevertheless, “active oxygen” or “active electrode” by both chemisorbtion and physisorbtion also lead an aggressive side reaction such as oxygen production that results in reduction in the proficiency of the anodic process. Generally, anodes with low oxygen production potential such as carbon, graphite, IrO2, RuO2 or platinum have an “active” nature, and may only partially oxidize pollutants, while anodes with high oxygen production potential, such as antimony-doped tin oxide, lead dioxide or BDD, have “non-active” behaviour and leads to almost the total mineralization of organic pollutants. It is therefore considered ideal electrodes for the wastewater treatment (Comninellis 1994). These different types of electrode (anodes) with their oxidation potential are present in Table 17.1.
Organic pollutant can be degraded electrochemically by either direct or indirect oxidation. The mechanism of direct or indirect Ibanez in Fig. 17.4(1)) and (2).1 and oxidation mechanism has strongly depended on the electrode material construction, operating condition and supporting electrolyte (Panizza 2010) (Fig. 17.4).
The pollutant has been adsorbed on the electrode surface and then oxidized, the phenomenon is so-called direct oxidation (Fig. 17.4(1)) Low potential favours the direct electro-oxidation, but remediation rate is usually low and depending on the anode’s electrocatalytic activity. High degradation has been reported by György et al. (1997) for the electrodes, namely Platinum and Palladium and metal oxide anodes like IrO2, RuO2−TiO2 and Ir-TiO2. The major problem associated with anodic electro-oxidation is decrease in catalytic activity at fixed potential before the evolution of oxygen. It is because of the development of organic film on the anode surface and commonly termed as the deactivation of electrodes. The proposal of indirect oxidation Fig. 17.4(2) is to prevent the fouling of the electrode by avoiding the exchange of direct electrons between the organic and the anode. Therefore, pollutants are oxidized in indirect electrolysis through the generation of certain redox species (Fig. 17.4(3)), which provide a path for access of electron to the organics pollutants. Following are the key concerns for achieving high efficiency in indirect electrolytic processes:
-
The potential of generated redox species should not be close to the potential for the evolution of oxygen.
-
The generated redox species should be high.
-
The reaction rate of the generated redox species and the contaminant should be higher than the competition rate.
-
Adsorption of pollutants must be controlled.
Boron-doped diamond (BBD), a non-active electrode, has been examined broadly for the remediation of organic compounds in recent years. High O2 over potential of BBD electrodes makes it better than metal oxide anodes and show efficient results for the direct oxidation of contaminants. Higher degradation activity towards organic compounds of BBD electrodes is due to the formation of weakly adsorbed ·OH radical from the electrolysis of water and has been presented in Eq. (17.10) (Comninellis 1994).
BBD anodes also generate many oxidizing species such as ozone, H2O2 and ferrate (Sáez et al. 2008; Sáez et al. 2008). BBD anodes can produce peroxydicarbonate (C2O62−), peroxydiphosphate (P2O84−) and peroxydisulphate (S2O82−), oxidants in the existence of ions, for example, CO3−2, PO43− and SO4−2. Peroxydisulphate radical production occurs in two stages (Eqs. 17.11–17.14). First, the sulphate radical forms from the reaction of sulphate containing compound with electrogenerated ·OH, and in second stage, two sulphate radicals combine and produce peroxydisulphate (Serrano et al. 2002; Cañizares et al. 2009).
To strengthen the AO process efficiency, a great attention has been attracted towards the selection of suitable substrate (Wang et al. 2013). The criteria for suitable substrate is: good conductance of electricity, sufficient body strength, electrochemical inertness and easy development of protective films on the surface of the substratum through passivation (Chen et al. 2003). For this objective, various substrates have been studied for the BBD anode like Si, Ti, Nb, etc. Si substrate has generally been used for the BBD film deposition. But the delicate nature and variable conductivity due to experimental environment of Si create hurdles for the selection as a suitable substrate (Sun et al. 2011). Ti was found the suitable substrate for the BBD film deposition (Chen et al. 2003; (Sun et al. 2011; Chen 2004).
The removal and degradation of antibiotics has been studied by various researchers. Mora-Gomez et al. studied the degradation of Norfloxacin in a boron-doped diamond (BDD) or a novel Sb-doped SnO2 ceramic anode (non-active anode). The process of degradation on both electrodes was found to be first order and BDD showed good oxidizing power towards antibiotic norfloxacin, and up to 92% TOC has been removed (Mora-Gomez et al. 2019). Kaur et al. reported the degradation of antibiotic amoxicillin using active (Titanium coated ruthenium oxide) Ti/RuO2 and investigated various operating parameters. They demonstrated that 60% amoxicillin degraded and 48% TOC has been removed and finally they also proposed the degradation pathway (Kaur et al. 2019).
17.5.2 Electrochemical Oxidation with NaCl
Electrochemical oxidation exhibits different behaviour in the presence of some active oxidizing species like chlorine, chlorine (Cl2) , hypochlorous acid (HOCl) and hypochlorite ions (OCl−1) . However, the mechanism of electro-oxidation using inorganic mediator is still not explored completely. Definitely, oxidation of organic molecules has been either accomplished at the electrode surface through adsorbed oxychloro species (i.e. chloro and oxychloro radicals) or in the main bulk solution through long-lasting oxidant (i.e. Chlorine, hypochlorous acid, or hypochlorite) by electro-oxidation of mediated chloride ion, referring reaction (Eq. 17.15–17.18):
The formation of intermediacy active chlorine species and the mechanism of its reaction have been recommended by Bonfatti et al. (2000b) and Bonfatti et al. (2000a), (Fig. 17.5).
They expanded the idea of Comninellis (1994) for the chlorine medium electro-oxidation process. They replaced mediated oxychloro species from chloride ions instead of hydroxyl radicals. The potential of anodic reactivity for the adsorbed chloride-oxychloride radicals increases in the chloride ion mediation by resisting the oxygen-evolution reaction. The widely used anode materials for onsite generation of active chloride oxidants depend on the platinum or on mixed metal oxides (such as, IrO2, TiO2, RuO2,). These materials have high electro-catalytic property for chlorine production, and also long haul mechanical and chemical strength. Well-known dimensionally stable anodes DSA® has excellent capacity to generate electrochemically chlorine and hypochlorite by the electrolysis of NaCl which is widely used as a supporting electrolyte for the treatment of wastewater (Rajkumar and Kim 2006; Chen et al. 2011). The use of dimensionally stable anodes (DSA®) has been in the degradation of organic compounds such as atrazine (Malpass et al. 2010), carbaryl (Malpass et al. 2009) and dyes (Malpass et al. 2008), mainly due to their flexibility, electro-catalytic efficiency, chemical strength and good lifetime.
Traditionally strong oxidants species like chlorine (Cl2), hypochlorous acid (HOCl) and hypochlorite ions (OCl−1) were traditionally used for industrial wastewater treatment (Rajeshwar and 1997; Tarr 2003). These agents are also widely used for drinking water disinfection (Martínez-Huitle and Brillas 2008). Electrochemical technology provide a substitutive indirect electro-oxidation process for the treatment of organic pollutants with active chlorine species from direct chloride ion oxidation at appropriate anodes. This strategy contrasts from EO in the utilization of contaminated chloride containing solution amid electrolysis to create active chlorine on site. However the major problem associated with indirect oxidation is the generation of chlorinated organic compound during the process, which subsequently increases the toxicity of wastewater. Panizza and Cerisola (2003) observed the chlorinated organic compound during the oxidation of 2-naphthol and surfaced to maximum at about 9 Ah dm−3, which vanished during the electrolysis. On the other hand, it has also been reported that, by selecting suitable operating parameters, an organic compound, i.e. glucose, can be combusted in the chlorine medium without generation of organo-chlorine compounds (Bonfatti et al. 2000b; Bonfatti et al. 2000a).
Hussain et al. (2015) thoroughly elaborate an antibiotic sulphamethoxazole (SMX) using DSA electrode in sodium chloride medium. The work explain the kinetics and electrochemical degradation route under different experimental conditions. They also explain the NaCl, current density and pH have prominent effect on the degradation of sulphamethoxazole. Figure 17.6 reveals that mixed anode are capable of generating active chlorine species at different concentration of NaCl which efficiently degrade and mineralize antibiotic sulphamethoxazole.
The study also demonstrate the possible route of degradation of SMX and almost nine major intermediates of the reaction were identified by LC-ESI-QTOF- MS which contain some lower molecular weight organic intermediates like C6H9NO2S (m/z = 179), C6H4NOCl (m/z = 141), and C6H6O (m/z = 110)). The degradation of SMX proceeds via different routes involving rapture of oxazole and benzene rings by hydroxle radicals and active chlorine. Similarly the study also reveals and depicted in Fig. 17.7 that degradation was pH dependent between 2.5 to 9 and reveal that acidic pH is more favourable for degradation, at this pH higher oxidation potential species like HOCl are responsible for the SMX degradation. Rajkumar et al. 2007), Figure 17.6 presents the effect of pH on the electrochemical degradation and mineralization of SMX.
The material of electrode plays a vital role in electrochemical degradation process, and lead oxide anode is an attractive, low-cost and better electrochemical activity towards organic oxidation (Chen et al. 2014; Dai et al. 2016). The electrocatalytic performance of PbO2 anode can be enhanced by adding some internal layers like Sb-SnO2, α-PbO2, F−, Co2+ and Ce3+.
Dai et al. has successfully prepared rare earth Lanthanum (La) and Yttrium (Y), co-doped Ti/Sn-SbOx/PbO2 electrodes (La-Y-PbO2 electrode) that showed a high performance for the degradation and mineralization of aspirin (Dai et al. 2016). After that they utilized the modified PbO2 anode for the levofloxacin and electrode showed high treatment efficiency and low cost (Xia and Dai 2018). The anodic degradation was dependent on current density 30 mA cm−2, pH 3 and initial LFX (levofloxacin) concentration 800 mg L−1. Different aromatic intermediate products of LFX degradation were found with different structure to the LFX molecule including pepiperazinyl hydroxylation, decarboxylation and defluorination. They also found some inorganic species like CO2, H2O, NH4+, NO3− and F− during mineralization of levofloxacin.
Effect of pH (■ 2.5,  5,
5,  7,
7,  9) on (a) degradation; (b) TOC removal of SMX (condition: pH = 3, temperature = 25 °C, initial concentration = 200 mg L−1; current density = 40 mA cm−2 (Hussain et al. 2015)
9) on (a) degradation; (b) TOC removal of SMX (condition: pH = 3, temperature = 25 °C, initial concentration = 200 mg L−1; current density = 40 mA cm−2 (Hussain et al. 2015)
17.6 Indirect Electrochemical Oxidation
17.6.1 Electro-Fenton Process
The Fenton process is a simple process for producing (•OH) through Eq. (17.19). The hydroxyl radicals perform an important role in electro-Fenton and Photo Electro-Fenton process, which degrade unselectively the organic compounds into carbon dioxide and water by hydroxylation and dehydrogenation reactions because of high reduction potential of (•OH) Eo = 2.8 V vs. SHE. Ferrous ion is vital for the Fenton process; however some other metal ions lik Cu2+ may also be used in Fenton like process to produce (•OH) from H2O2.
The H2O2 may also be decomposed into (•OH) by ultraviolet radiation in photo-Fenton process (Eq. 17.20), leading to some propagation (Eqs. 17.21–17.23) and termination reactions (Eqs. 17.24–17.26) (Bataineh et al. 2012; Keenan and Sedlak 2008; Lee et al. 2013; Liu et al. 2018).
However the Fe3+ slow down the production of •OH in Fenton process, and subsequently decrease the H2O2 degradation and generate unwanted hydroperoxyl radical (HO2•) (Eq. 17.27).
The hydrogen peroxide is not only the way for producing •OH, likewise in electrochemical process these radicals are formed at the electrode solution interface from H2O discharge reaction (Eq. 17.28) or in the bulk of solution by adding a suitable catalyst like Fe2+ and electro-generated hydroxyl radical (Eq. 17.29) (Brillas et al. 2009)
The electro-Fenton process produce •OH using electrochemical assisted Fenton process and H2O2 is regularly produced in bulk of solution by reduction of dissolved oxygen (Eq. 17.30) and small quantity of a catalyst like Fe2+ is added to produce •OH in simple Fenton process (Eq. 17.19). Fe3+ is reduced straightforward to Fe2+ at cathode (Eq. 17.31) (Brillas et al. 2009).
The electrolyte played an important role in any electrochemical process, and during EF process Na2SO4 is generally used as electrolyte due to its low activity, Though some other electrolytes like NaCl may also be used for EF process, NaCl produced Cl2 and HOCl in EF process which start reaction with •OH and consumed these strong oxidant (Eq. 17.32–17.37).
The electro-Fenton process generate electrochemically H2O2 using two or three electrodes in a single or separated cell. Generally graphite, carbon felt, boron doped diamond (BDD), Ti4O7 and gas diffusion electrode (GDE) are employed as cathode (Brillas and Sirés 2012) (Ganiyu et al. 2017) (Zhou et al. 2018) (Salazar et al. 2017). Sopaj et al. (2015) performed comparative study of different anode materials for degradation of sulphamethazine (SMT) in EF system. The author tested Pt, BBD, dimensionally stable anode (DSA, Ti/RuO2-IrO2) and graphite felt (GF). The author observed that GF is the most effective anode for the degradation of SMT at low applied current density. It cannot be used at higher current density as it burns for long operation time. The author concluded BBD as the best anode that achieved almost complete mineralization (98.5% TOC removal).
The use of EF process offers many advantages than simple Fenton process. In EF process, Fenton reagent is generated in situ and due to this in situ generation of Fenton reagents, the cost of transportation and storage and risk associated with it decreases. The reaction loss of Fenton regents also decreases; as in the EF process, a control amount of Fenton reagents is produced and consumed. So, as a result sludge formation also reduces. It provides a high rate of organic degradation due to Fe2+ electrochemical regeneration, that catalyses the reaction of Fenton (Sirés et al. 2014; Rodrigo et al. 2014; Oturan and Oturan 2018; Zhu et al. 2011).
There are a several working parameters engaged with the effectiveness of the EF process that have been examined for quite a while (Nidheesh and Gandhimathi 2012; Martínez-Huitle et al. 2015; Oturan and Aaron 2014; Brillas et al. 2009). The main parameters are: applied current density, type and concentration of catalyst, pH of contaminated water, distance between the electrode, supply of oxygen and more importantly the electrode materials (Liu et al. 2018).
The pH is an essential parameter and it is usually recommended to carry out the EF process at about pH 3 (Boye et al. 2003). H2O2 has been generated in the acidic medium as in (Eq. 17.28). However, pH value below 2 leads to conversion of H2O2 into peroxonium (H3O2+) which is less reactive towards Fe2+ and less •OH radical is produced via Fenton reaction (Mousset et al. 2017). H2O2 decomposes to water and oxygen at a high pH level. Fe3+ is precipitated as Fe (OH)3 at pH above 4, and at pH below 1, Fe2+ forms H2O2 complexes (Pignatello 1992).
The voltage is also an essential factor in EF process because the rate of applied current promotes the production of •OH. This factor therefore considerably influences the oxidation and/or mineralization proficiency of the process (Oturan and Aaron 2014; (Sankara Narayanan et al. 2003). However, high current stimulates unnecessary side reaction (reduction or oxidation of water to H2 or O2) or wasting (recombination or oxidation reactions) in the electrolytic cell (Zhang et al. 2007; Brillas et al. 2009; Sirés et al. 2014).
The nature or concentration of the catalyst is another important parameter. Among the different catalyst tried for the EF process, iron (Fe2+/Fe3+) has been appeared to be extraordinary compared to others in light of the fact that it isn’t unsafe, has low expenses and requires low concentration (Brillas et al. 2009). The optimal value of Fe2+ concentration depends on the cathode used. It has been reported that the optimal value of Fe2+ as a catalyst for carbon-felt cathode is in range of 0.1–0.2 mM, while for gas diffusion cathode, 0.5 mM has been reported in lots of literature (Brillas et al. 2009; Oturan and Aaron 2014; Sirés et al. 2014). In general, high Fe2+ concentrations are avoided as they promote the waste reaction as in (Eqs. 17.38 and 17.39) (Oturan and Aaron 2014; Panizza and Cerisola 2001; Babuponnusami and Muthukumar 2014).
The Cu2+/Cu+ pair was similarly examined as a catalyst in the EF process and conflicting results have reported. For a case, a current report has revealed that copper can be more effective and cost-proficient than iron with a greater rate of TOC reduction (Panizza and Cerisola 2001). Conversely, different analysts have accentuated that iron as a catalyst offers a superior mineralization productivity than Cu ion particles (Panizza and Cerisola 2001). Recently Zhou et al. (2018) studied the oxidation of levofloxacin and trimethoprim by EF process and compared the degradation of these two antibiotics in the presence of Pd-Fe3O4 and Fe3O4 as a catalyst. The author observed that levofloxacin shows high oxidation in the presence of Pd-Fe3O4 instead of Fe3O4. The rate constant increased from value of 0.156 to 0.243 min−1 by substituting Pd-Fe3O4 instead of Fe3O4 as a catalyst, while trimethoprim shows no improvement by changing catalyst.
Similarly, Barhoumi et al. (2017) also compared two different catalysts, i.e. chalcopyrite with conventional EF process for degradation of tetracycline. The author observed that the presence of chalcopyrite shows high degradation compared to conventional EF process. Almost complete mineralization (98% TOC removal) was achieved in the presence of chalcopyrite in 360 min of operation under optimum operating condition. Likewise, Chen et al. (2017) compared different catalytic degradation of antibiotic ciprofloxacin (CIP) in EF system. The author first studied the effect of Fe2+ and Fe3+ dosage for the oxidation and mineralization of CIP. Almost 73% and 72% TOC were removed by Fe2+ and Fe3+ at optimum condition. Then Fe3+-CIP chelate was tested for the degradation of CIP. The author observed that Fe3+-CIP chelate had no significant effect on the degradation and mineralization of CIP in EF process.
Air supply and air diffuser are additionally critical parameters which impact oxygen exchange and hydrodynamic conditions in the reactors altogether. The low oxygen stream rate, the solution fed up unsaturated and oxygen exchange can turns into the controlling step, influencing the effectiveness of H2O2 production and consequently •OH generation through Fenton’s reaction.
The temperature has direct relation with the kinetics of reaction, and therefore it can be a crucial factor for EF process. It can also impact the mass transfer parameters (coefficient of diffusion, etc.). Temperature between 20 and 30 °C is commonly reported in the literature (Panizza and Cerisola 2001). As the temperature increase, it decreases the solubility of O2 and enhances the decomposition rate of H2O2. Similarly, the type of electrolyte also affects the EF process. Ghoneim et al. (2011) conducted a study on the effect of nature of electrolyte. The author studied three different electrolytes, i.e. Na2SO4, NaCl and KCl. The author concluded that improved efficiency of SO4−2 shows that SO4−2 gives the higher conductivity compared to Cl−. Similarly, Ghoneim et al. (2011) also compared the KCl and NaCl and the author concluded that higher degradation rate of KCl shows that K+ gives higher conductivity compared to Na+. It is also observed that sodium sulphate gives slower degradation rate of pollutant that NaClO4 or NaNO3 as a supporting electrolyte and it is due to formation of iron sulphate complexes in EF process. The presence of electrolyte in the solution decreases the ohmic potential drop and, as a result, the energy consumption of the process decreases. Furthermore, it has also been reported that too high concentration of electrolyte can also promote side reaction, and due to this the TOC reduction may decrease.
17.6.2 Photo-Electro-Fenton and Solar Photo-Electro-Fenton
Brillas’ group broadly studied the phenomenon of EF treatment of organic compound assisting with irradiation by means of artificial UV radiation or solar radiation and termed as PEF or SPEF process (Brillas et al. 2009; Brillas et al. 1998; Boye et al. 2003; Flox et al. 2007; Flox et al. 2007; Garcia-Segura et al. 2014; Thiam et al. 2015). The Fe(III)-hydroxyl complexes formed in electro-Fenton process (Eq. 17.38) should be photo-catalytically reduced and should regenerate.OH and Fe2+ (Eq. 17.40) (Sun and Pignatello 1993), which result in the production of regeneration. Complexes are formed between Fe3+ and some organics due the direct photolysis according to the general (Eq. 17.41) (Vogler 1993; Zuo and Hoigne 1992; Faust and Zepp 1993). It allows the formation of weak oxidizing species such as superoxide anion radical, carbon dioxide anion radical and H2O2 and as a result Fe2+ regenerate in parallel.
PEF process utilizes the UVA of wavelength in range of 315–400 nm, UVB of wavelength in range of 280–315 nm and UVC wavelength in range of <280 nm radiation. Artificial lamps radiation may be utilized in PEF treatments. The wavelength and intensity of radiation source may affect the degradation mechanism. The degradation rate can be increased by increasing the intensity of radiation source. When the radiation source emits light in range of wavelength that pollutant can absorb radiation efficiently, the phenomenon is termed as direct photolysis. Among synthetic lights, the UVA lights have been the most generally utilized (Brillas et al. 2008; Flox et al. 2006; Borràs et al. 2013; El-Ghenymy et al. 2013; Moreira et al. 2015).
The PEF process may also be facilitated by using Fe3+ carboxylate complex; Vilar et al. recently degraded antibiotic trimethoprim in a lab-scale reactor equipped with BDD and carbon-PTFE cathode. The study revealed that the presence of complex promotes the generation of Fe3+ to Fe2+ at high pH and also decreased the formation of unwanted species like chloride, chlorate or sulphate in PEF process (Moreira et al. 2015). The iron for the EF or PEF may also be provided by non-conventional reagent; an antibiotic tetracycline has been removed in EF process utilizing chalcopyrite as a source of iron, and the study showed 90% of mineralization of recalcitrant antibiotic tetracycline (Barhoumi et al. 2017).
Due to the high electrical cost of PEF method, it limits the application of degradation of organic pollutants. However, SPEF process can be utilized to overcome the limitation of PEF method by utilizing solar light instead of UV light. A self-sustainable solar-assisted photo electro-Fenton (SPEF) system applied to degrade antibiotic trimethoprim (TMP) and SPEF process is then compared with other processes like anodic oxidation (RuO2/Ti), AO-H2O2 and EF; it was demonstrated that SPEF mineralized the TMP antibiotic up to 8% in 6 h of treatment (Zhang et al. 2016). Furthermore SPEF was able to degrade sulphamethoxazole (SMX) in pilot plant; a study conducted elsewhere showed up to complete removal and 90% TOC was eliminated in SPEF process (Murillo-Sierra et al. 2018).
17.6.3 Bio-Electro-Fenton
There are different bio-electrochemical systems such as microbial fuel cell, microbial electrolysis cell and microbial reverse-electro-dialysis cells which are used to treat the wastewater. The biodegradable waste could be easily degraded in these systems at anodic chamber; however bio-refractory materials like antibiotic could not be degraded by bacteria. Initially Zhu and Ni used microbial fuel cell electro-Fenton system for the degradation of refractory compound in cathodic chamber, and named the process Bio-electro-Fenton (Zhu and Ni 2009).
The electrons are released due to oxidation of organic matter (like acetate ion) through microorganisms in an anodic chamber (Eq. 17.42), and then moved to cathodic chamber by electrical connection, where H2O2 is produced by oxygen reduction reaction (Eq. 17.43). Fe2+ is produced there using some iron source, which consequently generated •OH to treat the contaminant (Eqs. 17.43 and 17.44) as described in Fig. 17.8.
Some researchers coupled EF and biological system (Olvera-Vargas et al. 2016a; Ganzenko et al. 2018; Olvera-Vargas et al. 2017) and applied for the degradation of pharmaceuticals such as metoprolol, furosemide, ranitidine and real pharmaceutical wastewater. EF may be coupled with biological system either pre-treatment or post treatment.
The Bio-electro-Fenton was broadly classified as microbial fuel cell electro-Fenton (MFC), microbial electrolysis cell (MEC) electro-Fenton and microbial reverse-electro-dialysis cell (MREC) Electro-Fenton system and these systems are self-sustainable in terms of electricity. The anode and cathode materials are important in any electrochemical process; similarly in Bio-Electro-Fenton (BFE) mostly a cost-effective anode is used like activated carbon, graphite, carbon felt, carbon mesh, and carbon cloth (Li et al. 2018). The previous studies showed that anode must have strong compatibility with microorganism, good conductivity, chemical stability and high active surface area. Similarly, the cathode material in BEF process plays a vital role because the production of H2O2 through oxygen oxidation reaction takes place at cathode. The graphite (Zhang et al. 2015), carbon felt (Zhu and Ni 2009) and gas-diffusion electrodes (Rozendal et al. 2009) are widely used as cathode in BEF process. Recently some other carbon materials like graphene or reduced graphene oxide and some iron containing electrode like Fe@Fe2O3/carbon felt (Xu et al. 2011), Fe@Fe2O3/graphite (Yong et al. 2017), Fe@Fe2O3/NCF (Xu et al. 2013), CNT/FeOOH (Feng et al. 2010), Fe2O3/active carbon felt (Ling et al. 2016) and Carbon felt/FeOOH (Wang et al. 2014) have been applied ac cathode material for BEF. Mansour et al. (2014) studied sulphamethazine degradation by combined EF-biological system of 0.2 mM. Batch biological treatment was performed under continuous stirring in the presence of activated sludge at 25 °C. While the EF process was performed in 1 L of undivided cell holding carbon felt cathode and a tube-shaped platinum anode. The solution was continuously stirred and oxygen was supped at 450 mL min−1. EF process was initiated in the presence of 0.5 mM Fe2+, 0.05 M sodium sulphate, pH 3 and 500 mA at 18 °C. After 6 h of EF process alone, the TOC removal was 6.5% with an optimum condition of 0.1 mM pollutant 0.1 mM Fe2+ and 200 mA, while 0% TOC removal was observed only for the biological system. After 0.5 h of operation, the TOC removal for the combined EF-biological system was 61.4% while the TOC removal was 78.8% and 93.9%, respectively, after 1 and 4 h of operation. Biodegradability was also assessed in the combination degradation process and was <0.4 after 0.5 h of operation and 0.5 after 1 h of operation.
Mansour et al. (2015) treated Tunisian pharmaceutical wastewater with 200 ppm of sulphamethazine. Fresh activated sludge was introduced in a batch reactor for the biological degradation investigation. The EF process was performed in 1 L undivided cell holding carbon felt cathode and a tube-shaped platinum anode. The solution was continuously stirred and oxygen was supplied at 450 mL min−1. EF oxidative treatment was initiated in the presence of 0.5 mM Fe2+, 0.05 M sodium sulphate, pH 3 and 500 mA at 18 °C. The TOC removal percentage for the EF system alone is 7.5%, while the TOC removal percentage for the combined EF-biological system is 81.4%, while BOD5/COD is 0.35 after 100 min.
Olvera-Vargas et al. (2016b) studied the 0.1 mM Ranitidine synthetic solution by combining the EF-biological system. Biological degradation was performed in batch reactor at 30 °C for 7 days, while 300 mL of undivided cell holding carbon felt cathode and BDD anode was used for EF oxidation. The solution was continuously stirred and dense air was supplied perpetually. The process was initiated in the presence of 0.1 mM Fe2+, 0.05 M sodium sulphate, pH 3 and 500 mA applied current. 59% TOC removal was observed for the 1 h of EF operation, while, for the combine system, 94% TOC removal was observed for 1 h of operation. BOD5/COD for the EF-biological system is 0.37 for 1 h of operation.
Furthermore, the microorganisms are important for BEF process, because they catalysed the electron from organic waste to anode (Li et al. 2017). A variety of domestic waste, municipal wastewater, brewery wastewater, and sewage sludge contain these electro-genic microorganisms which are able to transfer electron from waste to anode. However some exoelectrogenic microorganisms have been separated such as Geobacter species and Shewanella species which have high potential to generate power density as compared to mixed culture (Wang and Ren 2013). Ferrag-Siagh et al. (2013) reported the oxidation and degradation of tetracycline by coupling EF and biological system. Biological process was carried out from 21 to 25 days. Activated sludge was used as a biological substrate in 500 mL of biological reactor at 25 °C. While, for EF process, a Batch reactor containing carbon felt was used as a cathode and tube-shaped platinum was used as anode. The capacity of the reactor was I L and oxygen was supplied perpetually. The oxidative EF process was initiated in the presence of 0.1 mM Fe2+, 0.05 M sodium sulphate and 300 mA. Efficiency of both processes was evaluated individually and then efficiency of combined EF and biological system was evaluated. It was concluded that EF process alone decreases COD: after 2 h = 66%, after 4 h = 86% and after 6 h = 93%. While, the TOC: after 2 h = 46%, after 4 h = 72%. The TOC removal efficiency of only biological system after 25 days was 10%. While, the TOC removal efficiency of combine system was: after 2 h = 69%, after 4 h = 86%.
17.6.3.1 Sono-Electrochemical Process
Ultrasound offer an alternative method for the treatment of contaminants and received a considerable attention in recent years (Serna-Galvis et al. 2019; Dietrich et al. 2017; Yang et al. 2016). The ultrasound is a sound wave with frequencies above 18 kHz, and therefore cannot be heard by human ear. There are two distinct categories of ultrasound: (a) ultrasound of high frequency or diagnostic ultrasound, with frequency between 2 and 10 MHz and is used mainly for medical applications, such as prenatal ultrasound, and (b) the ultrasound of low frequency or power ultrasound, which has frequency between 20 kHz and 2 MHz and is responsible for the phenomenon of cavitation.
The propagation of sound waves in a liquid medium causes cavitation. This is defined as the phenomenon that involves the formation, growth and violent collapse (implosion) of cavities or bubbles of steam and gases at high sound pressure. A sound wave consists of cycles of compression (positive pressure) and expansion (negative pressure) (Serna-Galvis et al. 2019). The formation and growth of bubbles occur in the expansion stage, and during the compression, occurs contraction and implosion of the bubbles. The collapse of bubbles generates highly reactive radical species and heat. On such drastic conditions, oxidizing species are generated by homolytic cleavage of molecules of gas or solvent. HO● radicals and some other reactive oxidizing species are produced from sonolysis of water (Eqs. 17.45–17.49). In these equations “)))” represents the ultrasound waves.
The main advantages of electrochemical processes and sonochemical on other AOPs are: (a) the oxidizing species are generated in situ, and therefore it is unnecessary to add them to the solution being treated, (b) both processes do not require a rigorous control of pH of the solution and (c) at the end of the process, the treated solution can be easily separated from the reaction system and is not necessary to introduce any additional steps.
Regarding the electrochemical process, another advantage is that the electrode can be reused several times. These characteristics show that the combination of electrochemistry with sonochemistry can result in a promising and interesting process for the destruction of organic contaminants in water which is known as sono-electrochemistry.
The hydroxyl radicals produced by electrochemical and sonochemical processes can oxidize organic contaminants. In addition to the generation of ROS, synergistic effects could be expected to couple the sonication and oxidation via electrochemical in one reactor (Ren et al. 2013). In addition, the physical effects of ultrasound such as streaming, microjets and acoustic cavitation (AC) shockwaves contribute to increased mass transfer, reduced electrode passivation and a reduction in the accumulation of gas bubbles in the electrodes (Pollet 2012; Neppolian et al. 2012). In sono-electrochemical process different kinds of electrode have been studied; Ren et al. (2014) degraded Triclosan using Niobium coated diamond electrode under high frequency ultrasound (850 kHz); the electrode gives the efficient result with affirmative synergistic effect which degraded 92% of pollutant in 15 min following pseudo-first-order kinetics. Similarly Tran et al. oxidized carbamazepine using Ti/PbO2 with sonochemical process. The author observed that increasing applied current density enhances the gradation of synergy; however, it also increases the imposed ultrasonic power. Highest ultrasonic power was reported for highest synergy degree value, i.e. 33% at lowest current intensity of 1 A and 40 W of ultrasonic power remove 99.5% pollutant by combined effect of sono-electrochemical process (Tran et al., 2017). On the contrary, Pt and graphite electrochemical system couple with ultrasonic process, which effectively degrade SMX at nominal current density of 20 mA cm−2 in both sodium sulphate and sodium chloride medium. It was interesting to note that combined US-EC system also prevents the generation of complex chlorinated by-product that is normally detected with sodium chloride (Huang et al. 2017). The sono-electrochemical process is also applicable in different matrix; Ren et al. (2019) reported the combined effect of US-EC for the Chlorpyrifos effluent using different matrix. The combined US-EC successfully removed 93.3% chlorpyrifos under 20 V and 200 W US power. The couple process was observed to follow pseudo-first order kinetics.
17.6.4 Photo-Electrochemical Process or Photo-Electrocatalytic Process
The efficacy of an electrochemical process can be magnified by coupling with UV radiation and the process is known as photo-electrochemical or photo-electrocatalytic technique. In simple photocatalytic process various semiconductors such as TiO2, ZnO, and WO3 can be used for the photocalalytic process in suspension form and the process is independent of mass transfer. However the suspension semiconductor needs filtration at final stage, which is a difficult step at industrial scale. The nanocrystalline from anatase of TiO2 is a semiconductor material widely used in photocatalysis for light-initiated destruction of organic contaminants in wastewaters (Peralta-Hernández et al. 2006). It has exceptionally appealing characteristic for this application, for example, minimal effort, low danger and a wide band gape of 3.2 eV, which results in high strength and avoid photo-corrosion (Peralta-Hernández et al. 2006). As the photocatalytic material is irradiated with UV light having greater energy than bandgap of material, the electron in valance band (VB) is activated and promoted into the conduction band (CB), generating charged electron (e−) and hole (h+) (Eq. 17.50). The (h+) has good oxidation ability and degraded the organic pollutants directly or it may react with H2O and produce hydroxyl radical which indirectly oxidized the pollutant (Liu et al. 2019; Banerjee et al. 2015) (Eqs. 17.51 and 17.52).
Similarly, the (e−) reacts with O2 and produces reactive oxygen species and some other species like superoxide O2−•, HO2•, and H2O2 also produced •OH by interaction of hυ and H2O2 (Pillai et al. 2017; Xie and Li 2006) (Eqs. 17.52–17.57).
However these electron (e−) and hole (h+) re-join each other by releasing heat and consequently decrease the efficiency of degradation process (Pillai et al. 2017; Bai et al. 2019; Peralta-Hernández et al. 2006; Robert and Malato 2002) (Eqs. 17.58 and 17.59).
The photo-electrochemical process present different synergetic way to enhance degradation process like it avoid the recombination of these electron (e−) and hole (h+) by applying bias potential through external circuit. So the hole should be available for the sole degradation purpose and enhance the degradation of pollutant (Fig. 17.9). Similarly, the potential between the OER potential and redox potential enhances the direct electro-oxidation pollutant. Similarly, the indirect photocatalytic generated species minimize the electrode fouling. Likewise, the electrochemically generated O2 takes the electrons and may produce H2O2 (Fig. 17.9).
Light-inducted electrochemical process can efficiently treat wastewater. The light-supported method applied either a constant i (current density) or a constant bias anodic potential (Eanode) to a TiO2-based thin film anode such DSA® subjected to UV illumination. In this situation, external electrical circuit constantly release photo-induced electron from the anode which result in the prevention of reaction (Eq. 17.56) (Bessegato et al. 2014) and promote the generation of high sum of holes and heterogeneous •OH from reaction (Eq. 17.51). Thus, there is generation of high photo-induced species, and it also increases the organic destruction relative to the photocatalysis (Bai et al. 2019; Bessegato et al. 2016; Xie and Li 2006; Zanoni et al. 2003; Peralta-Hernández et al. 2006). Commonly, most widely used photo-induced anode is Ti meshes or plates coated with TiO2 (Carneiro et al. 2005; Zainal et al. 2005; Carneiro et al. 2004), or Ti/Ru0.3Ti0.7O2 (Socha et al. 2006; Socha et al. 2005; Socha et al. 2007; Pelegrini et al. 1999; Catanho et al. 2006; Hussain et al. 2017). Different parameters can influence the efficiency of photo-electrochemical degradation, such as pH, pollutant concentration and the most important is supporting electrolyte. Different studies described more efficient removal of pollutant using NaCl medium due to the electroformation of oxidizing species at the anode (Su et al. 2016; Zanoni et al. 2004; Yuan et al. 2011; Hussain et al. 2017). The reaction (Eqs. 17.60–17.65) described the mechanism for generating chlorine radicals during photo-electrochemical process.
These radicals oxidize and mineralize the organic substances, which is more efficient than pure electrochemical process. In addition, photoactive substances can be cleaved into the solution, increasing the speed and efficiency of the oxidation reaction, and also break the bonds between C-Cl in chlorinated organic compounds and generate highly oxidizing species, improving the efficacy of the process (Eq. 17.66–17.68).
The photo-assisted electrochemical process is generally more efficient than electrochemical and can efficiently oxidize the organic contaminants. Many studies have demonstrated that DSA® electrodes can be successfully used in photo-electrochemical oxidation processes (Pelegrini and Bertazzoli 2002; Alves et al. 2010). In a recent study Hussain et al. conducted the photochemical, electrochemical and photo-assisted electrochemical degradation of antibiotic SMX in a UV-illuminated electrochemical flow cell as presented in Fig. 17.10
Photo-electrochemical flow cell for the degradation of SMX antibiotic (Hussain et al. 2017)
The author compare the degradation and TOC removal of SMX in these three processes, and conclude that the photo-assisted electrochemical was more efficient to eliminate 100% of SMX within 10 min, while only 76.6% and 42.3% of the SMX could be removed by electrochemical and photocatalytic processes, respectively, as shown in Fig. 17.11. Similarly, TOC abatement of 16.5%, 28.6% and 49.6% of TOC was eliminated from SMX after 120 min of photocatalytic, electrochemical and photo-assisted electrochemical degradation, respectively. It is clear that combining UV irradiation with the electrochemical process generates a significant synergic effect to the degradation and mineralization of SMX. Furthermore, the energy consumption parameter for electrochemical and photoelectrochemcial process was compared; the electrical energy per order (EEO) increased from 0.67 to 1.06 kW hm−3 order−1 as the current density increased from 10 to 60 mA cm−2 and dropped from 8.82 to 0.57 kW hm−3 order−1 which was less than sole electrochemical process.
Likewise another investigation also reported the SMX removal in TiO2/Ti Photo anode (Su et al. 2016). The author observed that when increasing the anodic potential in range of 0–0.5 V, decreasing the pH from 10.1 to 2.7 and increasing the molar concentration of sodium chloride in range of 1–100 mM, the degradation rate of pollutant increases. After 70 min of operation and 0.5 V, almost 100% organic compound was removed.
Different pharmaceuticals and antibiotic have been studied; the degradation of ibuprofen and naproxen by electrochemical process assisted with radiation source (Zhao et al. 2009) using BDD modified with Bi2MoO6 film onto a BDD in cylindrical quartz assisted with light emission source of 150 W Xe lamp (wavelength > 420 nm). Bi2MoO6 has the capacity to absorb the visible light at 460 nm. The photo-assisted electrochemical process degraded both ibuprofen and naproxen in range of visible light radiation. However the degradation rate of these pollutants was greater in combined process than the sole process of photo catalysis and electrochemical process. The ibuprofen and naproxen were also productively mineralized in the combine process. Furthermore Cui et al. (2016) conducted a photo-electrochemical study for the degradation of acetylsalicylic in aqueous solution using Pt/TiO2 NTs photo electrode, then electrochemical process coupled with photo catalytic process by using Xe lamp, power of 6.7 mW cm−2. Almost 98.3% organic compounds was removed in 4 h of photo electrochemical process. Similarly, Li et al. (2016a) investigated same pollutant acetylsalicylic in aqueous solution by photo-electrochemical process using Pd/C-N-S-TiO2 as anode, while electrochemical process was assisted by photo using 350 W Xe lamp. After 2.5 h of operation, nearly 90% organic compound was removed by combined UV+ visible radiation source assisting electrochemical process, while only 50% organic compound was removed by only visible light assisting electrochemical process. Kondalkar et al. (2014) studied cafetaxime as a model pollutant by photoelectrocatalytic process. TiO2 was used as photo anode while UV light source, power of 5 mW cm−2, was used to assist the electrochemical process. In 50 mg L−1 initial concentration of pollutant, 1.5 V and pH in range of 3–10 conditions, almost 60–95% organic compound can be removed. Li et al. (2016b) studied ciprofloxacin by anodic oxidation coupled with light emission source. TiO2 NTs was used as photo anode, while UV light source was used. Changing the initial concentration of pollutant in range of 0.1–10 mg L−1, almost 90–100% organic compound can be removed in 2 h of operation. Similarly, Liu et al. (2016) studied ofloxacin by combined photo electrochemical process. UV lamp with power 15 W was used as light emission source, while TiO2 NTs was used as working photo anode. In 0.05 mM of initial concentration of pollutant, applied current of 8 mA, and pH = 2 conditions, almost 50% organic compound was removed after 2 h of operation by the combined process. Tantis et al. (2015) studied omeprazole using UVA (1.5 mW cm−2) and P25/FTO as a working photo anode. The author observed that omeprazole degraded at rate of 1.4 mg L−1 h−1. ZnFe2O4 have been used as photo anode for the abetment of salicylic acid; the investigation reveals about 49% removal and almost 20 COD decrease was reported (Kumbhar et al. 2015).
17.7 Suggestion and Future Research Challenges
A key challenge in the successful implementation of electrochemical treatment of industrial and environmental application is to reduce the energy consumption and cost. Moreover, enhancing the long-term stability, electrolytic efficiency and reducing the cost of electrode materials and advancement in material science and nanotechnology can be vital. Additionally in some cases where NaCl is used as electrolyte the formation of organochlorine by-products is also one of the main challenges of electrochemical process. Laboratory scale experiment should be performed with real water samples and measurement of AOX (Adsorbable organic halogen), toxicity, chlorate and perchlorate is required when oxidation occurs. It may also concern the presence of low concentration of pollutants that may limit the electrochemical reactivity through mass transfer (such as contaminants concentration below that dissolved salts and organic compounds). Therefore, research should be focused towards authentic condition of wastewater. In addition, it is also necessary for complete evaluation of electrochemical system on the basis of appropriate figure of values that provide complete information on reactor configuration, operating parameters, and electrolytic composition. Electrochemical systems are especially suitable for the harmful organic waste treatment. For a case, aqueous film-foaming solution containing high concentration of fluorinated surfactants can be treated by electrodes such as BBD and Ti/SnO2.
Electrochemical system has many beneficial aspects, such as no need of chemical storage and handling, operation control simplicity, reactor design compactness and the adaptability to varying organic load in wastewater. Referring to these essential benefits, the application of electrochemical process may be addressed in the fragmented treatment of water and wastewater, and also in dispersed point of entry and point of use water treatment.
17.8 Conclusion and Perspectives
Electrochemistry-based treatment technologies have a tremendous power to degrade antibiotic in aqueous solution. Some of these technologies like Electro-Fenton, Bio-electro-Fenton and photo-electrocatalytic obtain good focus in the last decades. Many of these processes efficiently degrade the antibiotics; however some process do not completely mineralize the antibiotics, because of slow degradation rates. Similarly the performance of Fenton process has been improved by coupling with photochemical or sonochemical process or by coupling with biological process, and by using solar radiation the energy of treatment process could be decreased.
Recently the self-sustainable Bio-electro-Fenton process has been developed which degrade the organic contaminants and also produce electricity. Some of them like reverse electro dialysis system (RED) generate electricity from salinity gradient from fresh and saline water, and on the other side it degrades the organic pollutant by providing electron at anodic chamber. Lately some new photocatalytic semiconducting materials have been developed for photoelectrochemical process which degrade the antibiotic wastewater efficiently. However the electrochemical process still has some problem like scale up, electricity demand, electrode cost, and application towards real complex medium, so more work will be needed for full implementation on global scale. Finally it can be concluded that electrochemical technologies offer a promising way to treat wastewater containing antibiotics.
References
Abdelmalek F, Torres RA, Combet E, Petrier C, Pulgarin C, Addou A (2008) Gliding arc discharge (GAD) assisted catalytic degradation of Bisphenol A in solution with ferrous ions. Sep Purif Technol 63(1):30–37
Africa A, Dalvie M, London L (2006) Usage of endocrine disrupting pesticides in south African agriculture. Epidemiology 114(1):s331–s311
Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A (2006) The estrogenic effect of Bisphenol a disrupts pancreatic Beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112
Alves PA, Malpass GRP, Johansen HD, Azevedo EB, Gomes LM, Vilela WFD, Motheo AJ (2010) Photo-assisted electrochemical degradation of real textile wastewater. Water Sci Technol 61:491–498
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2(1):557–572
Bai X, Yang L, Hagfeldt A, Johansson EMJ, Jin P (2019) D35-TiO2 Nano-crystalline film as a high performance visible-light Photocatalyst towards the degradation of Bis-phenol a. Chem Eng J 355:999–1010
Baird C (1999) Environmental chemistry, 2nd edn. Freeman and Company, New York
Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced Hydrophilicity and Photocatalysis. Appl Catal B Environ 176–177:396–428
Barceló D (2003) Emerging pollutants in water analysis. TrAC Trends Anal Chem 22(10):xiv–xvi
Barceló D, Petrovic M (2008) Emerging contaminants from industrial and municipal waste. Springer, Berlin
Barhoumi N, Olvera-Vargas H, Oturan N, Huguenot D, Gadri A, Ammar S, Brillas E, Oturan MA (2017) Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite. Appl Catal B Environ 209:637–647
Bataineh H, Pestovsky O, Bakac A (2012) PH-induced mechanistic changeover from hydroxyl radicals to Iron(Iv) in the Fenton reaction. Chem Sci 3:1594
Bessegato GG, Cardoso JC, da Silva BF, Zanoni MVB (2016) Combination of photoelectrocatalysis and ozonation: a novel and powerful approach applied in acid yellow 1 mineralization. Appl Catal B Environ 180:161–168
Bessegato GG, Guaraldo TT, Zanoni MVB (2014) Enhancement of photoelectrocatalysis efficiency by using nanostructured electrodes. In: Modern electrochemical methods in Nano, Surface and Corrosion Science. InTech. https://doi.org/10.5772/58333
Birkhøj M, Christine N, Kirsten J, Jacobsen H, Andersen HR, Dalgaard M, Vinggaard AM (2004) The combined Antiandrogenic effects of five commonly used pesticides. Toxicol Appl Pharmacol 201(1):10–20
Bonfatti F, Ferro S, Lavezzo F, Malacarne M, Lodi G, De Battisti A (2000a) Electrochemical incineration of glucose as a model organic substrate. II. Role of active chlorine mediation. J Electrochem Soc 147:592–596
Bonfatti F, De Battisti A, Ferro S, Lodi G, Osti S (2000b) Anodic mineralization of organic substrates in chloride-containing aqueous media. Electrochim Acta 46(2–3):305–314
Borràs N, Arias C, Oliver R, Brillas E (2013) Anodic oxidation, electro-Fenton and photoelectro-Fenton degradation of cyanazine using a boron-doped diamond anode and an oxygen-diffusion cathode. J Electroanal Chem 689:158–167
Boye B, Dieng MM, Brillas E (2003) Anodic oxidation, electro-Fenton and Photoelectro-Fenton treatments of 2, 4, 5-Trichlorophenoxyacetic acid. J Electroanal Chem 557:135–146
Brain RA, Johnson DJ, Richards SM, Hanson ML, Sanderson H, Lam MW, Young C, Mabury SA, Sibley PK, Solomon KR (2004) Microcosm evaluation of the effects of an eight pharmaceutical mixture to the aquatic Macrophytes Lemna Gibba and Myriophyllum Sibiricum. Aquat Toxicol 70(1):23–40
Brillas E, Sirés I (2012) Electrochemical remediation technologies for waters contaminated by pharmaceutical residues. In: Environmental chemistry for a sustainable world, pp 297–346
Brillas E, Garrido JA, Rodriguez RM, Arias C, Cabot PL, Centellas F (2008) Wastewaters by electrochemical advanced oxidation processes using a BDD anode and Electrogenerated H2O2 with Fe (II) and UVA light as catalysts. Port Electrochim Acta 26(1):15–46
Brillas E, Sauleda R, Casado J (1998) Degradation of 4-Chlorophenol by anodic oxidation, electro-Fenton, photoelectro-Fenton, and peroxi-coagulation processes. J Electrochem Soc 145(3):759–765
Brillas E, Sirés I, Oturan M a (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109(12):6570–6631
Brown KD, Kulis J, Thomson B, Chapman TH, Mawhinney DB (2006) Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci Total Environ 366:772–783
Bila DM, Dezotti M (2007) Desreguladores endócrinos no meio ambiente: efeitos e conseqüências. Quim. Nova 30, 651–666. https://doi.org/10.1590/S0100-40422007000300027
Cañizares P, Sáez C, Sánchez-Carretero A, Rodrigo MA (2009) Synthesis of novel oxidants by electrochemical technology. J Appl Electrochem 39(11):2143
Carneiro PA, Osugi ME, Sene JJ, Anderson MA, Zanoni MVB (2004) Evaluation of color removal and degradation of a reactive textile Azo dye on Nanoporous TiO2 thin-film electrodes. Electrochim Acta 49(22–23):3807–3820
Carneiro, Patricia a, Marly E. Osugi, Cecílio S. Fugivara, Nivaldo Boralle, Maysa Furlan, and Maria Valnice B. Zanoni. (2005) Evaluation of different electrochemical methods on the oxidation and degradation of reactive blue 4 in aqueous solution. Chemosphere 59(3):431–439
Catanho M, Malpass GRP, Motheo AJ (2006) Photoelectrochemical treatment of the dye reactive red 198 using DSA® electrodes. Appl Catal B Environ 62(3–4):193–200
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38(1):11–41
Chen S, Zheng Y, Wang S, Chen X (2011) Ti/RuO2–Sb2O5–SnO2 electrodes for chlorine evolution from seawater. Chem Eng J 172(1):47–51
Chen X, Chen G, Yue PL (2003) Anodic oxidation of dyes at novel Ti/B-diamond electrodes. Chem Eng Sci 58(3–6):995–1001
Chen Y, Hongyi L, Weijing L, Yong T, Yaohui Z, Weiqing H, Lianjun W (2014) Electrochemical degradation of nitrobenzene by anodic oxidation on the constructed TiO2-NTs/SnO2-Sb/PbO2 electrode. Chemosphere 113:48
Chen Y, Aimin W, Yanyu Z, Ruige B, Xiujun T, Jiuyi L (2017) Electro-Fenton degradation of antibiotic ciprofloxacin (CIP): formation of Fe3+-CIP chelate and its effect on catalytic behavior of Fe2+/Fe3+ and CIP mineralization. Electrochim Acta 256:185–195
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39(11–12):1857–1862
Cui, Yuqi, Qi Meng, Xiaoyong Deng, Qiuling Ma, Huixuan Zhang, Xiuwen Cheng, Xiaoli Li, Mingzheng Xie, and Qingfeng Cheng.(2016) Fabrication of platinum nano-crystallites decorated TiO2 Nano-tube array photoelectrode and its enhanced photoelectrocatlytic performance for degradation of aspirin and mechanism. J Ind Eng Chem 43:177–184
Dai Q, Xia Y, Chen J (2016) Mechanism of enhanced electrochemical degradation of highly concentrated aspirin wastewater using a rare earth La-Y co-doped PbO 2 electrode. Electrochim Acta 188:871–881
Dietrich M, Franke M, Stelter M, Braeutigam P (2017) Degradation of endocrine disruptor bisphenol a by ultrasound-assisted electrochemical oxidation in water. Ultrason Sonochem 39:741–749
El-Ghenymy, Abdellatif, Nihal Oturan, Mehmet A. Oturan, José Antonio Garrido, Pere Luis Cabot, Francesc Centellas, Rosa Maria Rodriguez, and Enric Brillas. (2013) Comparative electro-Fenton and UVA photoelectro-Fenton degradation of the antibiotic sulfanilamide using a stirred BDD/air-diffusion tank reactor. Chem Eng J 234:115–123
Faust BC, Zepp RG (1993) Photochemistry of aqueous Iron (III)-Polycarboxylate complexes: roles in the chemistry of atmospheric and surface waters. Environ Sci Technol 27(12):2517–2522
Feng CH, Li FB, Mai HJ, Li XZ (2010) Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment. Environ Sci Technol 44:1875
Feng J, Johnson DC (1990) Electrocatalysis of anodic oxygen-transfer reactions: Fe-doped beta-lead dioxide electrodeposited on noble metals. J Electrochem Soc 137(2):507–510
Ferrag-Siagh F, Fourcade F, Soutrel I, Aït-Amar H, Djelal H, Amrane A (2013) Tetracycline degradation and mineralization by the coupling of an electro-Fenton pretreatment and a biological process. J Chem Technol Biotechnol 88:1380–1386
Flox C, Cabot P-L, Centellas F, Garrido JA, Rodriguez RM, Arias C, Brillas E (2007) Solar Photoelectro-Fenton degradation of cresols using a flow reactor with a boron-doped diamond anode. Appl Catal B Environ 75(1–2):17–28
Flox C, Ammar S, Arias C, Brillas E, Vargas-Zavala AV, Abdelhedi R (2006) Electro-Fenton and Photoelectro-Fenton degradation of indigo carmine in acidic aqueous medium. Appl Catal B Environ 67(1–2):93–104
Garcia-Segura S, Cavalcanti EB, Brillas E (2014) Mineralization of the antibiotic chloramphenicol by solar Photoelectro-Fenton: from stirred tank reactor to solar pre-pilot plant. Appl Catal B Environ 144:588–598
Ganiyu SO, Oturan N, Raffy S, Esposito G, van Hullebusch ED, Cretin M, Oturan MA (2017) Use of sub-stoichiometric titanium oxide as a ceramic electrode in anodic oxidation and electro-Fenton degradation of the Beta-blocker propranolol: degradation kinetics and mineralization pathway. Electrochim Acta 242:344–354
Ganzenko O, Trellu C, Papirio S, Oturan N, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2018) Bioelectro-Fenton: evaluation of a combined biological—advanced oxidation treatment for pharmaceutical wastewater. Environ Sci Pollut Res 25(21):20283–20292
Ghiselli G, Jardim WF (2007) Interferentes Endócrinos No Ambiente. Química Nova 30(3):695–706
Ghoneim MM, El-Desoky HS, Zidan NM (2011) Electro-Fenton oxidation of sunset yellow FCF Azo-dye in aqueous solutions. Desalination 274(1–3):22–30
Gobel A, Athomsen A, McArdell CS, Joss A, Giger W (2005) Occurrence and sorption behavior of Sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ Sci Technol 39(11):3981–3989
Gobel A, McArdell CS, Joss A, Siegrist H, Giger W (2007) Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci Total Environ 372(2–3):361–371
Grover DP, Zhang ZL, Readman JW, Zhou JL (2009) A comparison of three analytical techniques for the measurement of steroidal Estrogens in environmental water samples. Talanta 78(3):1204–1210
György F, Didier G, Christos C (1997) Anodic oxidation of organics on thermally prepared oxide electrodes. Curr Top Electrochem 5:71–91
Haarstad K, Borch H (2004) Indications of hormonally active substances in municipal solid waste leachate: mobilization and effect studies from Sweden and Norway. J Environ Sci Health A Tox Hazard Subst Environ Eng 39:901–913
Hirsch R, Ternes T, Haberer K, Kratz K-l (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118
Huang Y, Zhou T, Wu X, Mao J (2017) Efficient Sonoelectrochemical decomposition of sulfamethoxazole adopting common Pt/graphite electrodes: the mechanism and favorable pathways. Ultrason Sonochem 38:735–743
Hussain S, Gul S, Steter JR, Miwa DW, Motheo AJ (2015) Route of electrochemical oxidation of the antibiotic sulfamethoxazole on a mixed oxide anode. Environ Sci Pollut Res 22(19):15004–15015
Hussain S, Steter JR, Gul S, Motheo AJ (2017) Photo-assisted electrochemical degradation of Sulfamethoxazole using a Ti/Ru 0.3 Ti 0.7 O 2 anode: mechanistic and kinetic features of the process. J Environ Manag 201:153–162
Ibanez JG, Rajeshwar K, Swain GM (1994) Electrochemistry and the environment. J Appl Electrochem
Jones OAH, Voulvoulis N, Lester JN (2005) Human pharmaceuticals in wastewater treatment processes. Crit Rev Environ Sci Technol 35(4):401–427
Kaur R, Kushwaha JP, Singh N (2019) Amoxicillin electro-catalytic oxidation using Ti/RuO2 anode: mechanism, oxidation products and degradation pathway. Electrochim Acta 296:856–866
Keenan CR, Sedlak DL (2008) Factors affecting the yield of oxidants from the reaction of nanonarticulate zero-valent iron and oxygen. Environ Sci Technol 42:1262
Kim Y., Kim, Y., Choi, K., Jung, J., Park, S., Kim, P.-G., Park, J., (2007) Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major Sulfonamides, and their potential ecological risks in Korea. Environ Int 33(3):370–375
Kondalkar, V. V, Mali, S.S., Mane, R.M., Dandge, P.B., Choudhury, S., Hong, C.K., Patil, P.S., Patil, S.R., Kim, J.H., Bhosale, P.N., (2014) Photoelectrocatalysis of cefotaxime using nanostructured TiO2 photoanode: identification of the degradation products and determination of the toxicity level. Ind Eng Chem Res 53(47):18152–18162
Kuhn AT (1971) Electrolytic decomposition of cyanides, phenols and Thiocyanates in effluent streams-a literature review. J Appl Chem Biotechnol 21(2):29–34
Kumbhar SS, Mahadik MA, Shinde SS, Rajpure KY, Bhosale CH (2015) Fabrication of ZnFe2O4 films and its application in photoelectrocatalytic degradation of salicylic acid. J Photochem Photobiol B Biol 142:118–123
Kümmerer K (2008) Pharmaceuticals in the environment: sources, fate, effects and risks, 3rd edn. Springer, Berlin
Kümmerer K, Henninger A (2003) Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin Microbiol Infect 9:1203–1214
Kümmerer K (2010) Pharmaceuticals in the environment. Annu Rev Environ Resour 35(1):57–75
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Elsevier Ltd
Lee H, Lee HJ, Sedlak DL, Lee C (2013) PH-dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide. Chemosphere, 92 (6): 652-658
Levine AD, Meyer MT, Kish G (2006) Evaluation of the persistence of micropollutants through pure-oxygen activated sludge nitrification and denitrification. Water Environ Res 78(11):2276–2285
Li, D., Jia, J., Zheng, T., Cheng, X., Yu, X., (2016a) Construction and characterization of visible light active Pd Nano-crystallite decorated and CNS-co-doped TiO2 nanosheet array photoelectrode for enhanced photocatalytic degradation of acetylsalicylic acid. Appl Catal B Environ 188:259–271
Li, G., Nie, X., Chen, J., Wong, P.K., An, T., Yamashita, H., Zhao, H., (2016b) Enhanced simultaneous PEC eradication of bacteria and antibiotics by facilely fabricated high-activity {001} facets TiO2 mounted onto TiO2 nanotubular photoanode. Water Res 101:597–605
Li X, Chen S, Angelidaki I, Zhang Y (2018) Bio-electro-Fenton processes for wastewater treatment: advances and prospects. Chem Eng J 354:492–506
Li X, Jin X, Zhao N, Angelidaki I, Zhang Y (2017) Novel bio-electro-Fenton technology for Azo dye wastewater treatment using microbial reverse-electrodialysis electrolysis cell. Bioresour Technol 228:322–329
Ling T, Huang B, Zhao M, Yan Q, Shen W (2016) Repeated oxidative degradation of methyl orange through bio-electro-Fenton in bioelectrochemical system (BES). Bioresour Technol 203:89
Liu L, Li R, Liu Y, Zhang J (2016) Simultaneous degradation of Ofloxacin and recovery of cu (II) by photoelectrocatalysis with highly ordered TiO2 nanotubes. J Hazard Mater 308:264–275
Liu N, Lu N, Yan S, Wang P, Xie Q (2019) Fabrication of G-C3N4/Ti3C2 composite and its visible-light Photocatalytic capability for ciprofloxacin degradation. Sep Purif Technol 211:782–789
Liu X, Zhou Y, Zhang J, Luo L, Yang Y, Huang H, Peng H, Tang L, Mu Y (2018) Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: mechanism study and research gaps. Chem Eng J 347:379–397
Malpass GRP, Miwa DW, Machado SAS, Motheo AJ (2008) Decolourisation of real textile waste using electrochemical techniques: effect of electrode composition. J Hazard Mater 156(1–3):170–177
Malpass GRP, Miwa DW, Miwa ACP, Machado SAS, Motheo AJ (2009) Study of photo-assisted electrochemical degradation of carbaryl at dimensionally stable anodes (DSA). J Hazard Mater 167(1–3):224–229
Malpass GRP, Miwa DW, Gomes L, Azevedo EB, Vilela WFD, Fukunaga MT, Guimarães JR, Bertazzoli R, Machado S a S, Motheo a J (2010) Photo-assisted electrochemical degradation of the commercial herbicide atrazine. Water Sci Technol 62(12):2729–2736
Mansour D, Fourcade F, Huguet S, Soutrel I, Bellakha Nl, Dachraoui M, Hauchard D, Amrane A (2014) Improvement of the activated sludge treatment by its combination with electro Fenton for the mineralization of Sulfamethazine. Int Biodeterior Biodegradation 88:29–36
Mansour D, Fourcade F, Soutrel I, Hauchard D, Bellakhal N, Amrane A (2015) Relevance of a combined process coupling electro-Fenton and biological treatment for the remediation of sulfamethazine solutions – application to an industrial pharmaceutical effluent. Comptes Rendus Chimie 18(1):39–44
Martínez-Huitle CA, Brillas E (2008) Electrochemical alternatives for drinking water disinfection. Angew Chem Int Ed Engl 47:1998–2005
Martínez-Huitle CA, Rodrigo MA, Sirés I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev 115(24):13362–13407
Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N (2003) Trimethoprimesulfamethoxazole revisited. Arch Intern Med 163(4):402–410
Meyer A, Sarcinelli P, Moreira J (1999) Are some Brazilian population groups subject to endocrine disrupters? Cad Saude Publica 15(4):845–850
Mora-Gomez J, Ortega E, Mestre S, Pérez-Herranz V, García-Gabaldón M (2019) Electrochemical degradation of norfloxacin using BDD and new Sb-doped SnO2 ceramic anodes in an electrochemical reactor in the presence and absence of a cation-exchange membrane. Sep Purif Technol 208:68–75
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2015) Degradation of trimethoprim antibiotic by UVA Photoelectro-Fenton process mediated by Fe (III) – carboxylate complexes. Appl Catal B Environ 162:34–44
Mousset E, Trellu C, Oturan N, Rodrigo MA, Oturan MA (2017) Soil Remediation by Electro-Fenton Process. pp. 399–423. https://doi.org/10.1007/698_2017_38
Murillo-Sierra JC, Ruiz-Ruiz E, Hinojosa-Reyes L, Guzmán-Mar JL, Machuca-Martinez F, Hernández-Ramirez A (2018) Sulfamethoxazole mineralization by solar photo electro-Fenton process in a pilot plant. Catal Today 313:175–181
Neppolian B, Ashokkumar M, Tudela I, González-Garcia J (2012) Hybrid Sonochemical treatment of contaminated wastewater: sonophotochemical and sonoelectrochemical approaches. Part I: description of the techniques. In: Advances in water treatment and pollution prevention. Springer, pp 267–302
Nidheesh PV, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15
Nilsson A, Ronlán A, Parker VD (1973) Anodic oxidation of phenolic compounds. Part III. Anodic hydroxylation of phenols. A simple general synthesis of 4-alkyl-4-hydroxycyclo-hexa-2,5-dienones from 4-alkylphenols. J Chem Soc Perkin Trans 1:2337–2345
Olvera-Vargas H, Cocerva T, Oturan N, Buisson D, Oturan MA (2016a) Bioelectro-Fenton: a sustainable integrated process for removal of organic pollutants from water: application to mineralization of Metoprolol. J Hazard Mater 319:13–23
Olvera-Vargas H, Oturan N, Buisson D, Oturan MA (2016b) A coupled bio-EF process for mineralization of the pharmaceuticals furosemide and ranitidine: feasibility assessment. Chemosphere 155:606–613
Olvera-Vargas H, Trellu C, Oturan N, Oturan MA (2017) Bio-electro-Fenton: a new combined process – principles and applications. In: Electro-Fenton Process. Springer, pp 29–56
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44(23):2577–2641
Oturan N, Oturan MA (2018) Electro-Fenton process: background, new developments, and applications. In: Electrochemical water and wastewater treatment. Elsevier, pp 193–221
Panizza M (2010) Importance of electrode material in the electrochemical treatment of wastewater contaiining organic pollutants. In: Electrochemistry for the environment. Springer, New York, p 25
Panizza M, Cerisola G (2003) Electrochemical oxidation of 2-naphthol with in situ electro- generated active chlorine. Electrochim Acta 48:1515–1519
Panizza M, Cerisola G (2001) Removal of organic pollutants from industrial wastewater by Electrogenerated Fenton’s reagent. Water Res 35(16):3987–3992
Pelegrini R, Bertazzoli R (2002) Descoloração e Degradação de Poluentes OrgâNicos Em Soluções Aquosas Através Do Processo Fotoeletroquímico. Química Nova 25:477
Pelegrini R, Peralta-Zamora P, de Andrade AR, Reyes J, Durán N (1999) Electrochemically assisted photocatalytic degradation of reactive dyes. Appl Catal B Environ 22(2):83–90
Peng X, Wang Z, Kuang W, Tan J, Li K (2006) A preliminary study on the occurrence and behavior of sulfonamides, ofloxacin and chloramphenicol antimicrobials in wastewaters of two sewage treatment plants in Guangzhou, China. Sci Total Environ 371(1–3):314–322
Peralta-Hernández JM, Meas-Vong Y, Rodríguez FJ, Chapman TW, Maldonado MI, Godínez LA (2006) In situ electrochemical and photo-electrochemical generation of the Fenton reagent: a potentially important new water treatment technology. Water Res 40:1754–1762
Petrovic M, Barceló D (2006) Application of liquid chromatography/quadrupole time-of-flight mass spectrometry (LC-QqTOF-MS) in the environmental analysis. J Mass Spectrom 41:1259–1267
Pignatello JJ (1992) Dark and photoassisted Iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ Sci Technol 26(5):944–951
Pillai SC, McGuinness NB, Byrne C, Han C, Lalley J, Nadagouda M, Falaras P, Kontos AG, Gracia-Pinilla MA, O’Shea K, Mangalaraja RV, Christophoridis C, Triantis T, Hiskia A, Dionysiou DD (2017) Photocatalysis as an effective advanced oxidation process. In: Advanced oxidation processes for water treatment: fundamentals and applications, vol 16, pp 333–381
Pollet B (2012) Power ultrasound in electrochemistry: from versatile laboratory tool to engineering solution. John Wiley & Sons
Rahman MF, Yanful EK, Jasim SY (2009) Occurrences of endocrine disrupting compounds and pharmaceuticals in the aquatic environment and their removal from drinking water: challenges in the context of the developing world. Desalination 248:578–585
Rajeshwar K, Ibanez JG (1997) Environmental electrochemistry: fundamentals and applications in pollution sensors and Abatemen. Academic press, San Diego, CA
Rajkumar D, Kim JG (2006) Oxidation of various reactive dyes with in situ electro-generated active chlorine for textile dyeing industry wastewater treatment. J Hazard Mater 136(2):203–212
Rajkumar D, Song BJ, Kim JG (2007) Electrochemical degradation of reactive blue 19 in chloride medium for the treatment of textile dyeing wastewater with identification of intermediate compounds. Dyes Pigments 72(1):1–7
Reemtsma T, Jekel M (2006) Organic pollutants in the water cycle: properties, occurrence, analysis and environmental relevance of polar compounds. Wiley-VCH
Ren Y-Z, Wu ZL, Franke M, Braeutigam P, Ondruschka B, Comeskey DJ, King PM (2013) Sonoelectrochemical degradation of phenol in aqueous solutions. Ultrason Sonochem 20(2):715–721
Ren Y-Z, Franke M, Anschuetz F, Ondruschka B, Ignaszak A, Braeutigam P (2014) Sonoelectrochemical degradation of triclosan in water. Ultrason Sonochem 21:2020–2025
Ren Q, Yin C, Chen Z, Cheng M, Ren Y, Xie X, Li Y, Zhao X, Xu L, Yang H, Li W (2019) Efficient sonoelectrochemical decomposition of chlorpyrifos in aqueous solution. Microchem J 145:146–153
Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini G-E (2005) Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect 113(6):716–720
Robert D, Malato S (2002) Solar photocatalysis: a clean process for water detoxification. Sci Total Environ 291:85–97
Rodrigo MA, Oturan N, Oturan MA (2014) Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev 114(17):8720–8745
Rozendal RA, Leone E, Keller J, Rabaey K (2009) Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem Commun 11:1752
Sáez C, Rodrigo MA, Cañizares P (2008) Electrosynthesis of ferrates with diamond anodes. AICHE J 54(6):1600–1607
Salazar C, Ridruejo C, Brillas E, Yáñez J, Mansilla HD, Sirés I (2017) Abatement of the fluorinated antidepressant fluoxetine (Prozac) and its reaction by-products by electrochemical advanced methods. Appl Catal B Environ 203:189–198
Sankara Narayanan TSN, Magesh G, Rajendran N (2003) Degradation of O-chlorophenol from aqueous solution by electro-Fenton process. Fresenius Environ Bull 12(7):776–780
Serna-Galvis EA, Montoya-Rodríguez D, Isaza-Pineda L, Ibáñez M, Hernández F, Moncayo-Lasso A, Torres-Palma RA (2019) Sonochemical degradation of antibiotics from representative classes-considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason Sonochem 50:157–165
Serrano K, Michaud PA, Comninellis C, Savall A (2002) Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim Acta 48(4):431–436
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res 21(14):8336–8367
Skold O (2001) Resistance to trimethoprim and sulfonamides. Vet Res 32(3–4):261–273
Socha A, Chrzescijanska E, Kusmierek E (2005) Electrochemical and Photoelectrochemical treatment of 1-Aminonaphthalene-3,6-disulphonic acid. Dyes Pigments 67:71–75
Socha A, Sochocka E, Podsiadły R, Sokołowska J (2006) Electrochemical and photoelectrochemical degradation of direct dyes. Color Technol 122(4):207–212
Socha A, Sochocka E, Podsiadły R, Sokołowska J (2007) Electrochemical and Photoelectrochemical treatment of C. I. Acid Violet 1. Dyes Pigments 73:390–393
Su Y-f, Wang G-B, Kuo DTF, Chang M-l, Shih Y-h (2016) Photoelectrocatalytic degradation of the antibiotic sulfamethoxazole using TiO2/Ti photoanode. Appl Catal B Environ 186:184–192
Suárez S, Carballa M, Omil F, Lema JM (2008) How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters. Rev Environ Sci Biotechnol 7:125–138
Sun J, Lu H, Du L, Lin H, Li H (2011) Anodic oxidation of anthraquinone dye alizarin red S at Ti/BDD electrodes. Appl Surf Sci 257(15):6667–6671
Sun Y, Pignatello JJ (1993) Photochemical reactions involved in the total mineralization of 2, 4-D by iron (3+)/hydrogen peroxide/UV. Environ Sci Technol 27(2):304–310
Sopaj F, Rodrigo MA, Oturan N, Podvorica FI, Pinson J, Oturan MA (2015) Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem Eng J 262:286–294
Tantis I, Bousiakou L, Frontistis Z, Mantzavinos D, Konstantinou I, Antonopoulou M, Karikas G-A, Lianos P (2015) Photocatalytic and Photoelectrocatalytic degradation of the drug omeprazole on nanocrystalline Titania films in alkaline media: effect of applied electrical Bias on degradation and transformation products. J Hazard Mater 294:57–63
Tarr AM (2003) Chemical degradation methods for wastes and pollutants environmental and industrial applications. Marcel Dekker, New York
Thiam A, Sirés I, Brillas E (2015) Treatment of a mixture of food color additives (E122, E124 and E129) in different water matrices by UVA and solar photoelectro-Fenton. Water Res 81:178–187
Trivedi P, Vasudevan D (2007) Spectroscopic investigation of ciprofloxacin speciation at the goethite-water Interface. Environ Sci Technol 41:3153–3158
Tran N, Drogui P, Brar SK, De Coninck A (2017) Synergistic effects of ultrasounds in the sonoelectrochemical oxidation of pharmaceutical carbamazepine pollutant. Ultrason Sonochem 34:380–388
U.S. Department of Defense (DoD) (2006) Emerging contaminants
Vogler A (1993) O. Horváth, KL Stevenson: charge transfer photochemistry of coordination compounds, VCH, New York, Weinheim, 1993, ISBN 3–527–89564-7, 380 Pages, Price: DM 238.00. Berichte der Bunsengesellschaft für physikalische Chemie 97(9):1166
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31:1796
Wang Q, Jin T, Hu Z, Zhou L, Zhou M (2013) TiO2-NTs/SnO2-Sb anode for efficient electrocatalytic degradation of organic pollutants: effect of TiO2-NTs architecture. Sep Purif Technol 102:180–186
Wang XQ, Liu CP, Yuan Y, Li F b (2014) Arsenite oxidation and removal driven by a bio-electro-Fenton process under neutral PH conditions. J Hazard Mater 275:200
Wensing M, Uhde E, Salthammer T (2005) Plastics additives in the indoor environment – flame retardants and plasticizers. Sci Total Environ 339(1–3):19–40
Xia Y, Dai Q (2018) Electrochemical degradation of antibiotic levofloxacin by PbO 2 electrode: kinetics, energy demands and reaction pathways. Chemosphere 205:215–222
Xie YB, Li XZ (2006) Interactive oxidation of Photoelectrocatalysis and electro-Fenton for Azo dye degradation using TiO2–Ti mesh and reticulated vitreous carbon electrodes. Mater Chem Phys 95(1):39–50
Xu N, Zhang Y, Tao H, Zhou S, Zeng Y (2013) Bio-electro-Fenton system for enhanced Estrogens degradation. Bioresour Technol 138:136
Xu, Nan, Shungui Zhou, Yong Yuan, Huan Qin, Yu Zheng, and Canwei Shu. (2011) Coupling of anodic biooxidation and Cathodic bioelectro-Fenton for enhanced swine wastewater treatment. Bioresource technology 102:7777
Yang, Bo, Jiane Zuo, Peng Li, Kaijun Wang, Xin Yu, and Mengyu Zhang.(2016) Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem Eng J 287:30–37
Yang S, Cha J, Carlson K (2005) Simultaneous extraction and analysis of 11 tetracycline and Sulfonamide antibiotics in influent and effluent domestic wastewater by solid-phase extraction and liquid chromatography-electrospray ionization Tandemmass spectrometry. J Chromatogr A 1097(1–2):40–53
Yong XY, Gu DY, Wu YD, Yan ZY, Zhou J, Wu XY, Wei P, Jia HH, Zheng T, Yong YC (2017) Bio-Electron-Fenton (BEF) process driven by microbial fuel cells for Triphenyltin chloride (TPTC) degradation. J Hazard Mater 324:178
Yousef MI, Salem MH, Ibrahim HZ, Helmi S, Seehy MA, Bertheussen K (1995) Toxic effects of carbofuran and glyphosate on semen characteristics in rabbits. J Environ Sci Health B. 30:513–534
Yuan R, Ramjaun SN, Wang Z, Liu J (2011) Effects of chloride ion on degradation of acid Orange 7 by Sulfate radical-based advanced oxidation process: implications for formation of chlorinated aromatic compounds. J Hazard Mater 196:173–179
Zainal Z, Lee CY, Hussein MZ, Kassim A, Yusof NA (2005) Electrochemical-assisted Photodegradation of dye on TiO2 thin films: investigation on the effect of operational parameters. J Hazard Mater 118:197–203
Zanoni MVB, Sene JJ, Anderson MA (2003) Photoelectrocatalytic degradation of Remazol brilliant Orange 3R on titanium dioxide thin-film electrodes. J Photochem Photobiol A Chem 157(1):55–63
Zanoni MVB, Sene JJ, Selcuk H, Anderson MA (2004) Photoelectrocatalytic production of active chlorine on Nanocrystalline titanium dioxide thin-film electrodes. Environ Sci Technol 38(11):3203–3208
Zhang H, Fei C, Zhang D, Tang F (2007) Degradation of 4-Nitrophenol in aqueous medium by electro-Fenton method. J Hazard Mater 145(1–2):227–232
Zhang, Yanyu, Aimin Wang, Xiujun Tian, Zhenjun Wen, Hanjiao Lv, Desheng Li, and Jiuyi Li. (2016) Efficient mineralization of the antibiotic trimethoprim by solar assisted Photoelectro-Fenton process driven by a photovoltaic cell. J Hazard Mater 318:319–328
Zhang Y, Wang Y, Angelidaki I (2015) Alternate switching between microbial fuel cell and microbial electrolysis cell operation as a new method to control H2O2 level in bioelectro-Fenton system. J Power Sources 291:108–116
Zhao, Xu, Jiuhui Qu, Huijuan Liu, Zhimin Qiang, Ruiping Liu, and Chengzhi Hu. (2009) Photoelectrochemical degradation of anti-inflammatory pharmaceuticals at Bi2MoO6 – boron-doped diamond hybrid electrode under visible light irradiation. Appl Catal B Environ 91(1–2):539–545
Zhou M, Oturan MA, Sirés I (eds) (2018) Electro-Fenton Process. Springer Singapore, Singapore
Zhu X, Tian J, Liu R, Chen L (2011) Optimization of Fenton and electro-Fenton oxidation of biologically treated coking wastewater using response surface methodology. Sep Purif Technol 81(3):444–450
Zhu X, Ni J (2009) Simultaneous processes of electricity generation and P-Nitrophenol degradation in a microbial fuel cell. Electrochem Commun 11(2):274–277
Zuo Y, Hoigne J (1992) Formation of hydrogen peroxide and depletion of oxalic acid in atmospheric water by photolysis of Iron (III)-Oxalato complexes. Environ Sci Technol 26(5):1014–1022
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hussain, S., Ullah Khan, S., Gul, S. (2020). Electrochemical Treatment of Antibiotics in Wastewater. In: Hashmi, M. (eds) Antibiotics and Antimicrobial Resistance Genes. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-40422-2_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-40422-2_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40421-5
Online ISBN: 978-3-030-40422-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)


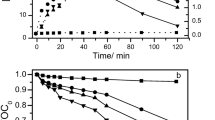
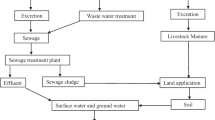






 0.04 M NaCl,
0.04 M NaCl,  0.08 M NaCl,
0.08 M NaCl,  0.1 M NaCl) concentration on (a) degradation; (b) TOC removal of SMX (condition: pH = 3, temperature = 25 °C, initial concentration = 200 mg L−1; current density = 40 mA cm−2
0.1 M NaCl) concentration on (a) degradation; (b) TOC removal of SMX (condition: pH = 3, temperature = 25 °C, initial concentration = 200 mg L−1; current density = 40 mA cm−2 



