Abstract
Protein homeostasis (Proteostasis) is essential for correct and efficient protein function within the living cell. Among the critical components of the Proteostasis Network (PN) are molecular chaperones that serve widely in protein biogenesis under physiological conditions, and prevent protein misfolding and aggregation enhanced by conditions of cellular stress. For Alzheimer’s, Parkinson’s, Huntington’s diseases and ALS, multiple classes of molecular chaperones interact with the highly aggregation-prone proteins amyloid-β, tau, α-synuclein, huntingtin and SOD1 to influence the course of proteotoxicity associated with these neurodegenerative diseases. Accordingly, overexpression of molecular chaperones and induction of the heat shock response have been shown to be protective in a wide range of animal models of these diseases. In contrast, for cancer cells the upregulation of chaperones has the undesirable effect of promoting cellular survival and tumor growth by stabilizing mutant oncoproteins. In both situations, physiological levels of molecular chaperones eventually become functionally compromised by the persistence of misfolded substrates, leading to a decline in global protein homeostasis and the dysregulation of diverse cellular pathways. The phenomenon of chaperone competition may underlie the broad pathology observed in aging and neurodegenerative diseases, and restoration of physiological protein homeostasis may be a suitable therapeutic avenue for neurodegeneration as well as for cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Protein homeostasis is regulated by the proteostasis network (PN) to control protein synthesis, folding and macromolecular assembly, localization, and degradation, processes that are essential for all living cells and organisms. An imbalance in the PN enhances the properties of destabilized mutant proteins that take advantage of the capacity of molecular chaperones to escape unfolding and degradation, leading to malignant phenotypes in cancer (see other chapters in this collection). The opposite scenario of failure of protein homeostasis is associated with aging and a plethora of protein misfolding diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS). In this chapter, we discuss how interactions between molecular chaperones and neurodegenerative disease-associated substrates amyloid-β (Aβ), tau, α-synuclein, polyglutamine-expansion proteins and SOD1 are exploited in vivo to counteract toxic protein aggregation, whereas these same interactions can lead to the sequestration of molecular chaperones and the collapse of proteostasis.
The main classes of molecular chaperones are also known as heat shock proteins (Hsps) after the discovery in Drosophila that the expression of Hsps is induced by heat shock, a sudden increase in temperature. However, many of the genes encoding these chaperones are also constitutively expressed to ensure the proper balance of protein synthesis, folding, trafficking and translocation of a wide variety of proteins under physiological conditions. Under conditions of cellular stress such as heat shock, molecular chaperones such as Hsp70 and the J-domain protein Hdj-1 are titrated by misfolded client proteins from association with heat shock transcription factor 1 (HSF1) with which they interact under normal growth conditions (Zheng et al. 2016; Abravaya et al. 1992; Shi et al. 1998). Upon release from chaperones, HSF1 forms functional trimers that bind to heat shock elements in the promoters of genes encoding molecular chaperones and other components of the PN. This results in the release of the paused RNA polymerase II, post-translational modifications of HSF1 and rapid inducible transcription of the heat shock genes. The heat shock transcriptional response can attenuate either during prolonged exposure to heat shock stress as translation is arrested at the heat shock temperature, or upon return to ambient conditions, in both scenarios reducing the requirement for chaperones to prevent further misfolding (Abravaya et al. 1992; Shi et al. 1998; Krakowiak et al. 2018).
Among the most ubiquitous chaperones are the Hsp70 family that is essential for protein synthesis and folding of a wide range of client proteins, via a mechanism that involves cycles of substrate binding and release driven by ATP hydrolysis. Hsp70 and its constitutively expressed counterpart Hsc70 function in a molecular machine that involves co-chaperones including Hsp40 proteins, which recruit substrates and regulate ATP hydrolysis of Hsp70, and Hsp110 and BAG proteins, which serve as nucleotide exchange factors (NEFs) (Kampinga and Craig 2010; Kim et al. 2013). Depending on the nucleotide binding and co-chaperone interactions, Hsp70 can function to hold non-native clients in a folding competent state or direct folding to a functional state (Freeman et al. 1995; Freeman and Morimoto 1996). Furthermore, specific combinations of Hsp70, Hsp40 and Hsp110 proteins form disaggregase machineries that can resolve luciferase aggregates (Nillegoda et al. 2015) and α-synuclein fibrils (Gao et al. 2015). The Hsp70 chaperone system also interacts with Hsp90, which is another ATP-dependent chaperone. Hsp90 interacts with specific co-chaperones downstream of Hsp70 to aid the folding of a wide array of clients including kinases, phosphatases, transcription factors and other signalling molecules (Morán Luengo et al. 2019). The chaperonins of the Hsp60 family, corresponding to GroEL in bacteria and TRiC/CCT in eukaryotes, exist as multimeric assemblies that form a cage in which substrates are allowed to obtain their native fold dependent upon ATP hydrolysis. This machinery is critical to fold large filamentous proteins such as actin and tubulin (Hayer-Hartl et al. 2016; Gestaut et al. 2019). The small Hsps (sHsps) are ATP-independent chaperones and can function in holding denatured or non-native protein conformations to prevent their misfolding and aggregation (Treweek et al. 2015).
In this chapter we will discuss the detailed modes of interaction between these classes of molecular chaperones and aggregation-prone proteins and peptides as characterised in vitro, and summarize evidence that chaperone activity is beneficial to combat protein aggregation in in vivo models of neurodegenerative diseases. Under these disease conditions, molecular chaperones may eventually become overwhelmed by these misfolding-prone proteins, leading to impaired protein homeostasis of physiological processes in the cell. The concept of chaperone competition not only serves as an explanation for the collapse of protein homeostasis during aging and neurodegenerative diseases, but also provides opportunities for therapeutic intervention. These approaches are also relevant to cancer in which it has been shown that the downregulation of certain molecular chaperones can be exploited to induce chaperone competition, promoting aggregation or degradation of oncoproteins and halting further proliferation of tumor cells (see other chapters in this collection).
2 Molecular Chaperones and Protein Aggregation in Neurodegenerative Diseases
2.1 Amyloid-β
Aβ is the main component of extracellular plaques that accumulate in the brains of AD patients. Processing of the amyloid precursor protein (APP) generates multiple isoforms of the Aβ peptide of which the most abundant species, the 42-residue Aβ peptide (Aβ42) is more aggregation-prone than the 40-residue form. In vitro, the aggregation of Aβ42 is delayed by the intracellular chaperones Hsp70 and Hsp90 at sub-stoichiometric ratios of ~1:50 (Evans et al. 2006). This effect is dependent on ATP, and the potency of Hsp70 is increased by the co-chaperone Hsp40 (family member DNAJB1), which stimulates ATPase activity, suggesting that to prevent aggregation the chaperones employ the same catalytic cycle that aids protein folding (Evans et al. 2006). Suppression of Aβ42 aggregation can also be achieved by sub-stoichiometric levels of DNAJB6, a member of the Hsp40 family, and quantitative analysis has revealed that DNAJB6 blocks both primary and secondary nucleation (Fig. 4.1) by preferentially binding to oligomeric species (Månsson et al. 2014a). The protective effects of Hsp70 against Aβ-associated toxicity have also been observed in vivo in the fruit fly Drosophila melanogaster. Overexpression of Hsp70 effectively suppresses neurotoxicity associated with the extracellular deposition of Aβ42 in the fly disease model, irrespective of whether the chaperone is expressed intracellularly or targeted to the extracellular space (Fernandez-Funez et al. 2016; Martín-Peña et al. 2018). The beneficial effect of intracellular Hsp70 may occur by a general enhancement of global proteostasis, whereas in the extracellular space it likely depends on its ‘holding’ activity in the absence of ATP.
Schematic reaction of amyloid formation indicating where chaperones act to prevent protein misfolding in the case of amyloid-β, tau, α-synuclein, polyglutamine expansion proteins and/or SOD1. Inhibition of primary nucleation is inferred from the binding of chaperones to the monomeric proteins. Hsp90 alone and with its co-chaperone Aha1 has also been reported to promote aggregation in the case of tau
Another class of chaperone activities is represented by the Brichos domains of ProSP-C and Bri2 that inhibit Aβ42 aggregation in vitro. Whereas the Brichos domain of ProSP-C blocks secondary nucleation of Aβ42 by binding to the fibrillar surface (Cohen et al. 2015), the Brichos domain derived from Bri2 prevents elongation of Aβ42 in addition to secondary nucleation by binding both to the fibril surface and to fibril ends (Arosio et al. 2016) (Fig. 4.1). Brichos domains physiologically function in the extracellular space, and expression of Brichos domains from ProSP-C and from Bri2 has been shown to be protective in Drosophila Aβ42 models by improving motor function and lifespan (Hermansson et al. 2014; Poska et al. 2016). Overexpression of the Brichos domain of ProSP-C increases the levels of soluble versus insoluble Aβ42 and delays its deposition during aging of the flies, suggesting that this Brichos domain could act by preventing the formation of toxic oligomeric species (Hermansson et al. 2014). In further support, ProSP-C Brichos rescued the toxicity of a mixture of Aβ42 monomers and fibrils on mouse brain slices using an electrophysiology assay (Cohen et al. 2015).
A role for the TRiC/CCT chaperonin on Aβ42 phenotypes was demonstrated in a genetic screen of the chaperome in C. elegans expressing Aβ42 in the body wall muscle cells. Individual knock-down of each of the eight subunits of TRiC/CCT decreased the motility of the worms, and the screen also identified Hsc70, Hsp40, Hsp90 and its co-chaperones Cdc37 and Sti1 (Brehme et al. 2014). Many of the same chaperones were shown to be protective against toxicity in a C. elegans model expressing expanded polyglutamine, suggesting that this subset of chaperones may have a general beneficial effect on proteostasis, rather than or in addition to making direct interactions with Aβ42. The relevance of TRiC/CCT was furthermore underlined by several of its subunits being downregulated in the aging human brain and in patients with AD (Brehme et al. 2014).
sHsps, a class of ATP-independent chaperones, have also been linked to Aβ aggregation, as it was shown that expression of human Aβ in C. elegans body wall muscle cells induces Hsp16 (Link et al. 1999), the overexpression of which completely restores the paralysis phenotype of the Aβ worms (Fonte et al. 2008). This effect appears to result from a direct interaction that modulates aggregation, not only because Hsp16 co-localises with the deposits, but also because its overexpression reduces the amyloid plaque load in the worms, leaving total Aβ levels unaltered. In vitro, the sHsp αB-crystallin binds along the sides and at the ends of Aβ42 fibrils, suggesting it can inhibit secondary nucleation and elongation of the fibrils (Shammas et al. 2011).
An important aspect of chaperone biology is that not all chaperones are protective against protein aggregation. The extracellular chaperone clusterin, which is a risk factor for late-onset AD, was reported to have more complex effects in mouse models of Aβ aggregation, presumably because it shifts the clearance pathways of Aβ, thus complicating the interpretation of its potential anti-aggregation effect (DeMattos et al. 2002; Wojtas et al. 2017). Extracellular chaperones may also alter Aβ toxicity by modulating targets that interact with toxic Aβ species. Sti1, which is a co-chaperone of Hsp90, but observed to be secreted by astrocytes, was shown to bind to the PrPC receptor and thereby block its interaction with Aβ oligomers in vitro and in cell culture (Ostapchenko et al. 2013). Increased levels of Sti1 have been observed in aged AD mice, as well as in human AD patients compared to control brains, consistent with a protective mechanism (Ostapchenko et al. 2013).
2.2 Tau
Tau is a microtubule-associated protein that forms intracellular aggregates in AD brains, and in patients suffering from various types of frontotemporal dementia (FTD) and ALS, collectively known as Tauopathies. Both Hsp70 and Hsp90 chaperones have been shown to directly interact with tau in vitro, which may seem surprising given the disordered and highly hydrophilic nature of tau. However, the sequence motifs that have β-strand propensity, and are involved in the formation of cross-β fibrils, contain hydrophobic residues that mediate binding to the constitutively expressed Hsc70 chaperone (Mitul et al. 2008). Hsp70 also interacts with tau monomers and oligomers to inhibit its nucleation as well as elongation in in vitro aggregation assays (Kundel et al. 2018). In C. elegans models expressing human tau in mechanosensory neurons, co-expression of human Hsp70 has modest beneficial effects on restoration of the touch response (Miyasaka et al. 2005). In mice, overexpression of Hsp70 reduces endogenous tau levels in aged animals, and especially reduces the insoluble high-molecular weight species (Petrucelli et al. 2004). Similar results have been observed using small molecule inhibitors of the ATPase activity of Hsp70 that lead to reduced levels of total and phosphorylated tau in tau transgenic mice. This suggests that both overexpression and inhibition of the folding cycle of Hsp70 may converge to promote tau degradation by the ubiquitin-proteasome system (Jinwal et al. 2009). Both Hsc70/Hsp70 and Hsp90 are co-localized with tau tangles in a transgenic mouse model and in human AD brains; moreover upregulation of these chaperones suppresses the formation of tau aggregation in cellular models by partitioning tau into a productive folding pathway that restores tau binding to microtubules (Luo et al. 2007).
Studies on Hsp90 have shown that the site of Hsp90 binding on tau includes a broad region encompassing the hydrophobic motifs, generating an extended interaction surface held together by a combination of weak hydrophobic and electrostatic interactions (Karagöz et al. 2014). The levels of phosphorylated tau but not total tau levels in a transgenic mouse model are reduced when the ATPase activity of Hsp90 is inhibited by small molecules (Dickey et al. 2007). Hsp90 inhibition also leads to activation of the heat shock response and the subsequent increase in expression of chaperones including Hsp70, but drug treatment may have a stronger effect than only inducing the heat shock response. Reduction in phospho-tau levels upon the inhibition of Hsp90 may depend on increased activity of its co-chaperone CHIP, which mediates ubiquitination and proteasomal degradation (Dickey et al. 2007). Another small molecule inhibitor of Hsp90 also reduces levels of phosphorylated tau as well as total tau, while increasing Hsp70 levels in mouse models expressing wild-type tau or the FTD-associated tau mutant P301L (Luo et al. 2007). In these studies, Hsp90 interacts directly with the mutant but not wild-type tau, suggesting that the mechanisms promoting tau clearance may differ between the two models, and thus potentially between AD and other types of (familial) dementia (Luo et al. 2007).
Modulation of Hsp90 activity involves co-chaperones such as Aha1, and together these can promote tau aggregation both in vitro and in a tau transgenic mouse model. Moreover, a small molecule that blocks the Hsp90-Aha1 interaction reduces this effect, demonstrating the power of combining mechanistic insights from in vitro experiments with in vivo models in developing therapeutic avenues (Shelton et al. 2017). Co-chaperones may also affect protein aggregation independently, as noted above for Aβ. For tau, it has been shown that the Hsp40 protein DnaJA2 is a potent inhibitor of its aggregation in vitro (Mok et al. 2018). DnaJA2 binds to monomeric tau, and also reduces seeded aggregation in cells, suggesting that DnaJA2 may have an effect on multiple steps of the aggregation process. In AD patient neurons with tau pathology, DnaJA2 is highly abundant and is localised with aggregated tau, perhaps by being upregulated as a protective, but insufficient cellular response (Mok et al. 2018). In contrast, FKBP51, another co-chaperone of Hsp90 that co-localises with tau pathology in AD brains may have a role in promoting tau misfolding (Blair et al. 2013). Overexpression of FKBP51 in tau transgenic mice results in increased overall tau levels and neuronal loss, whereas the numbers of tau tangles are decreased. Consistent with these results, in vitro experiments have suggested that FKBP51 in complex with Hsp90 may especially increase the formation of oligomeric tau species (Blair et al. 2013).
A role for the sHsp Hsp27 in modulating tau was shown in transgenic mice overexpressing Hsp27 that reduced tau levels and ameliorated the defects in long-term potentiation. This effect was shown to depend on the oligomerisation of Hsp27, since a phosphorylation mutant of Hsp27 that cannot undergo this cycle did not affect tau pathology or other phenotypes of the mouse (Abisambra et al. 2010).
2.3 α-Synuclein
The 140-residue, largely disordered protein α-synuclein is found in intracellular inclusions termed Lewy Bodies, which form primarily in dopaminergic neurons of the substantia nigra in PD patients. Several Hsps have been identified as components of Lewy Bodies (Auluck et al. 2002; McLean et al. 2002; Outeiro et al. 2006), and sub-stoichiometric concentrations of Hsp70 are sufficient in vitro to suppress the formation of α-synuclein fibrils in the absence of ATP (Dedmon et al. 2005; Luk et al. 2008; Roodveldt et al. 2009; Aprile et al. 2017). This effect is dependent on the interaction of Hsp70 with the hydrophobic NAC region of α-synuclein, which is essential for fibril formation (Luk et al. 2008). Upon addition of ATP, Hsp70 delays fibril formation primarily by binding to the fibril ends, thus inhibiting elongation (Aprile et al. 2017). Furthermore, a complex of the constitutively expressed Hsc70 together with specific Hsp40 co-chaperones and a Hsp110 NEF can dissociate preformed α-synuclein fibrils (Gao et al. 2015). The effect of Hsp70 on α-synuclein aggregation in vivo is thus likely to depend on the relative levels of specific co-chaperones and ATP.
Similarly, in yeast expressing human α-synuclein, induction of Hsp70 expression by a brief heat shock is protective against α-synuclein-induced apoptosis and the generation of reactive oxygen species. Similar effects have been obtained by direct overexpression of the yeast Hsp70 orthologue Ssa3 or by inhibiting Hsp90 using geldanamycin, which also induces the heat shock response (Flower et al. 2005). On the other hand, in C. elegans, knock-down of Hsp70 does not affect α-synuclein inclusion formation in muscle cells, suggesting that this chaperone does not have a beneficial effect in this model system (Van Ham et al. 2008). However, knock-down of Hip, an Hsp70 co-chaperone, significantly increases the number of inclusions in this C. elegans model, suggesting that the Hsp70-Hip complex acts against inclusion formation (Roodveldt et al. 2009).
In a Drosophila model of α-synuclein, co-expression of human Hsp70 with wild-type α-synuclein or the familial mutants A53T or A30P in dopaminergic neurons restores locomotion and lifespan without affecting the number or size of Lewy Body-like inclusions (Auluck et al. 2002). This is further supported by geldanamycin treatment which induces the heat shock response and similarly protects against neurodegeneration (Auluck and Bonini 2002; Auluck et al. 2005), whereas LB-like pathology is not affected and the levels of insoluble α-synuclein are even increased (Auluck et al. 2005), suggesting that Hsp70 may reduce the presence of toxic oligomeric species.
Rodent models for expression of human α-synuclein have yielded inconsistent results. Overexpression of rat Hsp70 in a mouse model resulted in a strong decrease in both high molecular weight α-synuclein species, and Triton X-100 insoluble protein (Klucken et al. 2004), which contrasts with another study in which human Hsp70 and α-synuclein A53T were co-overexpressed and the levels of high molecular weight and insoluble α-synuclein were unaffected (Shimshek et al. 2010). Another study found beneficial effects from co-expressing Hsp70 with α-synuclein in the rat brain, showing a reduction in the number of dystrophic neurites which typically precede neurodegeneration (Moloney et al. 2014). The protective effects of Hsp70 may depend on the ratio of Hsp70, its co-chaperones and α-synuclein in these models as mentioned above, and potential differences in the binding affinities between Hsp70 from different species and wild-type and A53T α-synuclein could furthermore affect the outcome.
In addition to Hsp70 and Hsp40, the expression of Hsp27 is increased upon viral expression of α-synuclein in mouse brains (St Martin et al. 2007). Likewise, mice expressing α-synuclein A53T had increased levels of Hsp25 and αB-crystallin, Hsp25 being primarily increased in astrocytes rather than neurons. αB-crystallin inhibits in vitro aggregation of α-synuclein isolated from the mouse brain (Wang et al. 2008), which is consistent with another in vitro result that αB-crystallin interacts directly with α-synuclein fibrils to prevent fibril elongation from pre-formed seeds (Waudby et al. 2010). It has also been reported to interact with early intermediates in in vitro aggregation reactions (Rekas et al. 2007). In line with these findings, expression of αB-crystallin in the fly eye ameliorates the rough eye phenotype induced by α-synuclein expression (Tue et al. 2012).
Hsp90 can inhibit α-synuclein aggregation in vitro in seeded aggregation assays of α-synuclein A53T. This activity is ATP-independent, and relies on the interaction of Hsp90 with oligomeric α-synuclein species (Daturpalli et al. 2013). The yeast disaggregase Hsp104 can inhibit α-synuclein aggregation and remodel pre-formed α-synuclein fibrils in vitro, and overexpressing it together with the A30P α-synuclein mutant in rats reduces inclusion formation and neuronal loss (Lo Bianco et al. 2008).
2.4 Polyglutamine Expansions
Nine human neurodegenerative diseases are associated with genetic expansions leading to the production of different proteins with expanded polyglutamine (polyQ) tracts. Irrespective of the protein, disease symptoms occur beyond a pathogenic threshold of ~35–40 glutamine residues, accompanied by the formation of cytoplasmic and nuclear inclusions in neuronal tissue (Lieberman et al. 2019). The onset of pathology and disease is correlated with the length of the polyQ tract, with longer polyQ lengths having increasing aggregation propensity in vitro and in cellular models. HD is the most prevalent of these polyQ disorders, and is related to an expansion within the huntingtin gene HTT. In particular, a fragment of the huntingtin protein corresponding to the first exon of the gene in which the polyQ expansion is located is found to accumulate in disease, and this fragment is sufficient to drive neurodegeneration and inclusion formation in mouse models (Mangiarini et al. 1996; Scherzinger et al. 1997).
In the nematode worm C. elegans, expression of a construct comprising 40 glutamine residues (Q40) with a YFP-tag for visualisation in the body wall muscle cells is sufficient for protein aggregation and formation of toxic immobile inclusions (Morley et al. 2002). Expression of 35 residues (Q35) also leads to aggregation and toxicity, but later in adulthood, whereas shorter polyQ lengths of 19 or 24 glutamine residues remain diffuse. These polyQ lines were used for a genome-wide genetic screen to identify genetic modifiers of protein aggregation which identified components of the proteostasis network for transcription and splicing, translation, folding, transport and degradation including the chaperones Hsp70, Hsp40 and subunits of TRiC/CCT (Nollen et al. 2004). The mechanism by which TRiC/CCT inhibits polyQ aggregation has been further explored in vitro, and it was shown to interact with the tips of mutant huntingtin fibrils and to encapsulate smaller oligomers (Shahmoradian et al. 2013).
Other genetic screens in yeast and Drosophila have additionally identified multiple Hsps, including Hsp70, Hsp40, Hsp90 and sHsps, as well as Hsp104, the yeast disaggregase (Krobitsch and Lindquist 2000; Willingham et al. 2003; Kazemi-Esfarjani and Benzer 2000; Giorgini et al. 2005; Zhang et al. 2010; Jimenez-Sanchez et al. 2015). Overexpression of Hsp70 and Hsp40 ameliorates multiple phenotypes in Drosophila and mouse polyQ disease models, typically without altering the numbers of mature protein aggregates (Warrick et al. 1999; Chan et al. 2000; Cummings et al. 2001; Hay et al. 2004; Labbadia et al. 2012). Consistent with these findings, Hsp70 has been shown to associate with huntingtin oligomers in vitro, but not with monomers or detergent-insoluble inclusions, and it is able to prevent further aggregation together with Hsp40 and in the presence of ATP (Lotz et al. 2010).
More mechanistic studies on the activities of Hsp70, Hsp40 and Hsp110 against huntingtin exon 1 aggregation performed in cell culture have revealed that DNAJB6 and DNAJB8 members of the Hsp40 family are highly effective (Hageman et al. 2010). Subsequently, DNAJB6 was shown to inhibit protein aggregation in an HD mouse model, delaying the onset of symptoms and extending lifespan (Kakkar et al. 2016). In vitro, DNAJB6 inhibits the primary nucleation of polyQ peptides which depends on a serine-threonine rich region on its surface (Kakkar et al. 2016). This activity does not, however, depend on the presence of Hsp70 or ATP (Månsson et al. 2014b), providing an interesting example of independent chaperone activity of Hsp40 proteins.
2.5 SOD1
Point mutations in superoxide dismutase 1 (SOD1) are one of the causes of familial forms and sporadic cases of ALS (Cook and Petrucelli 2019). In contrast to the proteins discussed above that are largely disordered, SOD1 is a well folded soluble globular protein that binds copper and zinc ions and is stabilized by a disulfide bond. Disease-associated mutations are thought to destabilize the native state of SOD1, rendering it more prone to aggregation (Lindberg et al. 2005; Prudencio et al. 2009).
In a genome-wide RNAi screen on a C. elegans strain expressing SOD1 with G85R mutation throughout the neurons, the majority of hits belonged to the category of protein quality control, including the regulator of the heat shock response HSF1, several chaperones and components of the degradation machinery (Wang et al. 2009). Induction of the heat shock response is protective against SOD1 G93A aggregation and toxicity in mice, as demonstrated by the overexpression of SIRT1, which has HSF1 amongst its substrates. The beneficial effect in this case was found to be limited by the expression levels of inducible Hsp70, which were not sufficient to restore the phenotype of mice with higher levels of SOD1 (Watanabe et al. 2014).
A role for the Hsp70/Hsp40/Hsp110 machinery in aggregate disassembly as described above for α-synuclein has also been suggested for SOD1. Overexpression of Hsp110 in mice expressing YFP-tagged SOD1 G85R in motorneurons extends their lifespan, and a reduction of aggregates has been observed in a subset of animals (Nagy et al. 2016). Addition of YFP-SOD1 G85R to isolated squid axoplasm inhibits axonal transport, and supplementing Hsc70, but more so Hsp110, was found to suppress these defects (Song et al. 2013). Likewise, overexpression of the Hsp40 family member DNAJB2 reduces aggregation of SOD1 G93A in late stages of disease progression in mice, and improves motorneuron survival and muscle strength. DNAJB2 has been found to associate with the SOD1 aggregates, providing evidence for a direct interaction which was suggested to reduce aggregate formation by promoting ubiquitination (Novoselov et al. 2013).
sHsps have also been proposed to modulate SOD1 aggregation and toxicity. Hsp25 and αB-crystallin co-elute with insoluble SOD1 mutant protein from mice (Wang et al. 2003), and in in vitro studies using a brain homogenate, αB-crystallin inhibits aggregation (Wang et al. 2005a). This is further supported by experiments with recombinant Hsp27 and αB-crystallin that inhibit SOD1 G93A aggregation by interfering with aggregate growth, rather than the primary nucleation step (Yerbury et al. 2013). Mutations in Hsp27 have been identified in sporadic cases of ALS, which may be related to the inability to prevent SOD1 aggregation (Capponi et al. 2016).
3 Chaperone Competition as a Basis for Proteostasis Collapse in Protein Misfolding Diseases
3.1 The Chaperone Competition Hypothesis
The interaction of molecular chaperones with intermediate states of highly aggregation-prone disease-related proteins is a finely tuned process, in which chaperones can either direct and maintain functional client interactions for cellular health or result in protein aggregation and proteome mismanagement in aging and stress. This imbalance can be enhanced by increased protein expression, coding mutations, posttranslational modifications or alterations in the composition and functional properties of the proteostasis network to shift the balance towards aggregation and proteotoxicity. While misfolded and aggregated proteins have been directly linked to cellular toxicity (Bucciantini et al. 2002; Baglioni et al. 2006; Marsh et al. 2000; Fath et al. 2002), the diverse protein misfolding diseases have very complex pathologies likely resulting from multiple molecular defects. The chaperone competition hypothesis provides an explanation why aggregation of a disease-associated protein can interfere with multiple cellular pathways (Yu et al. 2014; Yu et al. 2019). The multifaceted roles of chaperone networks raise the possibility that competition between misfolding proteins and endogenous clients for limited chaperone resources will have consequences on protein functionality. The higher localized concentration of misfolded proteins in aggregates results in a spatial redistribution of chaperones and other components of the proteostasis network. Kinetically, this will have negative effects on many chaperone-regulated processes resulting in multiple pathological symptoms, exacerbating disease progression. Chaperone sequestration initiated by intracellular accumulation of misfolded and aggregated proteins is common to all protein misfolding diseases, supporting the hypothesis that the loss of chaperone function upon protein aggregation can accelerate cellular toxicity.
3.2 Chaperone Sequestration During Protein Misfolding
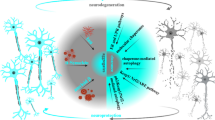
Multiple families of chaperones and co-chaperones form extensive protein-protein interaction networks to assist in the folding of diverse clients. Chaperone-client interactions are transient in nature to allow reversible engagement with multiple substrates including nascent polypeptides, unfolded and misfolded proteins and folding intermediates (Kim et al. 2013; Hiller 2019; Koldewey et al. 2017). Compared with on-pathway substrates for Hsc70, misfolded species are more likely to expose hydrophobic regions to allow Hsc70 to bind with higher apparent avidity (Kundel et al. 2018; Pemberton et al. 2011). Consequently, Hsc70 is preferentially occupied by aberrantly folded protein substrates in stressed cells or upon expression of metastable proteins. When protein aggregates form after stable interaction of misfolded proteins, Hsc70 as well as other interacting partners become sequestered into the aggregates (Fig. 4.2).
Aggregate-driven chaperone competition explains the pathological complexity associated with disease-associated aggregation-prone proteins. Shown is a model depicting chaperone competition between protein aggregates and the protein folding and vesicular trafficking arms of the proteostasis network. Under normal conditions (left), Hsc70 is at sufficiently high levels to mediate CME as well as basal protein client folding. Under disease conditions where protein aggregates have accumulated and the Hsc70 relocalizes to aggregates, both protein folding and CME are inhibited (right). This can be reversed by increasing the levels of Hsc70 by small molecule activation of HSF1 to restore chaperone function
Histochemical and biochemical studies have revealed that Hsc70 and other chaperones colocalize with a variety of protein inclusions in multiple cell and animal models of disease-associated protein aggregation and in patient-obtained brain tissues. Proteomic analysis of laser dissected amyloid plaques (Liao et al. 2004) and tau tangles (Wang et al. 2005b) from AD patient brains show that these inclusions sequester molecular chaperones, and other proteins that may be conformationally challenged. Hsc70 and the proteasome also co-localize with intracellular Aβ aggregates in a cellular model (Bückig et al. 2002). A human cell system for seeding-dependent tau aggregation has shown that the chaperones Hsc70/Hsp70, Hsp90 and J-domain co-chaperones are sequestered by tau aggregates (Yu et al. 2019). Likewise in PD, immunohistochemistry and proteomics have identified major chaperones including Hsc70, Hsp90, Hsp40 and Hsp27 as constituents of filamentous Lewy bodies, co-localizing with α-synuclein (Uryu et al. 2006; Leverenz et al. 2007).
In the context of HD, Hsc70 binds specifically to the N-terminal flanking region of huntingtin exon 1. Using a conditional human cell system and immunofluorescence, the chaperones BiP/GRP78, Hsp70, Hsp40, proteasome subunits and other aggregation-prone proteins were shown to colocalize with the perinuclear inclusions of huntingtin exon 1 with an expanded polyQ (Waelter et al. 2001). Proteomic profiling of HD inclusions revealed widespread sequestration of proteins into the mutant huntingtin inclusion bodies (Hosp et al. 2017). Similarly, chaperones colocalize with ataxin 1 and ataxin 3 polyQ protein inclusions (Cummings et al. 1998; Chai et al. 1999). For ALS, mutant SOD1 is a substrate of interactions with Hsc70/Hsp70, and mice expressing mutant SOD1 form inclusions containing ubiquitin, the proteasome and Hsc70 (Zetterström et al. 2011). Hsc70-positive inclusions have also been detected in sporadic ALS cases (Watanabe et al. 2001). Chaperone association has also been detected in cells expressing an artificial aggregation-prone β-sheet protein (Olzscha et al. 2011). Collectively, these observations provide strong evidence for sequestration of key components of the proteostasis network such as molecular chaperones and constituents of the protein degradation machinery as a unifying feature of protein misfolding diseases. The delicate balance between the levels of available molecular chaperones and client proteins is further underscored by the fact that many types of cancer cells depend on elevated levels of chaperones for their survival, accommodating for the increased demand by destabilized and misfolding-prone oncoproteins (see other chapters in this collection).
3.3 Functional Consequence of Chaperone Sequestration
As described above, many cellular processes are affected by the sequestration of chaperones by protein aggregation. The functional consequences of chaperone competition were determined by measuring multiple Hsc70-mediated cellular processes (Yu et al. 2014, 2019). Clathrin-mediated endocytosis (CME), the main entry route of biological molecules into the cell (Kirchhausen et al. 2014) involves the self-assembly of trimeric clathrin units on the plasma membrane to cause membrane curvature and the formation of coated pits. The released vesicles rapidly lose their clathrin coats in a process catalyzed by Hsc70 together with the co-chaperone auxilin in order to fuse with intracellular endosomes (Massol et al. 2006). Reducing cellular levels of Hsc70 by siRNA inhibits CME, indicating the requirement of Hsc70 for the assembly and disassembly of clathrin-coated vesicles (Yu et al. 2014).
The kinetic correlation between cytosolic Hsc70 concentration and CME therefore provides a highly sensitive functional readout of chaperone competition in the presence of protein aggregation. By monitoring CME in prostate cancer PC-3 cells expressing different aggregation-prone proteins including polyglutamine, huntingtin, ataxin1 and SOD1, a reduction of CME due to the sequestration of Hsc70 by aggregates was observed (Yu et al. 2014). The sensitivity of CME to Hsc70 depletion suggests that chaperone abundance is rate-limiting in cells expressing aggregation-prone proteins. Moreover, suppression of CME by protein aggregation could be reversed by conditionally increasing Hsc70. These effects were also observed in neuronal cells showing that protein aggregation causes dysregulated internalization of the AMPA receptor, a neuron-specific CME cargo.
The observations of chaperone competition extend beyond Hsc70, as other chaperones, including Hsp90, Hsp40 and Hsp27, are also sequestered in tau inclusions. Single-cell analysis of protein folding and CME in a cell model of tau aggregation reveals that both chaperone-dependent activities are impaired by tau aggregation (Yu et al. 2019). These observations are further supported by the finding in yeast that sequestration of Sis1p, a low-abundant Hsp40 homolog chaperone, by polyQ aggregates interferes with nuclear degradation of misfolded proteins and leads to the formation of cytosolic inclusions (Park et al. 2013). Consequently, the decline in chaperone-dependent arms of the proteostasis network will have profound negative effects on the protein quality control capacity of the cell. Besides cytosolic chaperones, various species of misfolded proteins and aggregates interact with and sequester components of the protein degradation machinery. This sequestration will further compromise the protein quality control capacity (Thibaudeau et al. 2018; Guo et al. 2018; Yang et al. 2015; Holmberg et al. 2004).
3.4 Restoration of Chaperone Homeostasis as a Therapeutic Strategy
The functional dissection of chaperone competition will lead to a better understanding of the early events of protein aggregation, and may uncover novel strategies to intervene at early stages of protein misfolding diseases. All Hsc70-regulated processes will be negatively affected by a subcellular redistribution of Hsc70 among its clients, resulting in a decline in multiple chaperone-dependent processes leading to multiple pathological symptoms that exacerbate disease progression. Consequently, restoring Hsc70 homeostasis could be an effective strategy to battle age-related protein conformational diseases. The appearance of a misfolded conformational state of tau associated with CME failure can be detected before the appearance of mature tau inclusions and the stable sequestration of Hsc70 (Yu et al. 2019). The timing of these cellular events therefore suggests that the inhibition of CME is an early cellular event of tauopathy and precedes failure of other cellular processes such as chaperone-dependent protein folding. Moreover, both CME and protein folding can be restored by small molecule regulators of HSF1 resulting in expression of cytosolic chaperones (Yu et al. 2019), suggesting that enhancing chaperone expression could have beneficial consequences on chaperone function and cellular health without reversing tau aggregation. Similarly, overexpressing protein sequestrated by polyQ aggregates has been shown to rescue loss of function phenotypes and relieve polyQ dependent toxicity (Park et al. 2013).
4 Outlook
Further studies will be required to bridge the gap between the kinetic studies of protein aggregation and their modulation by molecular chaperones in vitro and in vivo in healthy and disease states. Cellular probes capable of detecting aggregating protein species, especially in the early and oligomeric states, may allow real-time monitoring of chaperone engagement of disease-associated substrates. Furthermore, molecular chaperones do not operate in isolation and the coordination of chaperone networks to maintain all arms of the proteostasis network needs to be further characterized for each of the aggregation-prone proteins in disease-relevant tissues and cell types. The systems approach for proteostasis in neurodegenerative diseases likely will differ from cancer, although both classes of diseases are related by the consequence of aging on the robustness of cell stress responses and the functional capacity of the proteostasis network. The use of primary patient derived cells and live cell-based approaches to measure the cellular state of proteostasis during disease progression and in the context of aging will provide a basis to screen for small molecules that restore cellular proteostasis. Targeting the restoration of proteostasis, in particular chaperone homeostasis, can potentially serve as a major therapeutic avenue to treat many forms of protein misfolding disorders ranging from neurodegenerative diseases to cancer.
References
Abisambra JF et al (2010) Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal Plasticity Deficits in Tau Transgenic Mice. J Neurosci 30:15374 LP–15315382
Abravaya K, Myers MP, Murphy SP, Morimoto RI (1992) The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 6:1153–1164
Aprile FA et al (2017) Inhibition of α-Synuclein Fibril Elongation by Hsp70 Is Governed by a Kinetic Binding Competition between α-Synuclein Species. Biochemistry 56:1177–1180. https://doi.org/10.1021/acs.biochem.6b01178
Arosio P et al (2016) Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat Commun 7:10948
Auluck PK, Bonini NM (2002) Pharmacological prevention of Parkinson disease in Drosophila. Nat Med 8:1185–1186
Auluck PK, Chan HYE, Trojanowski JQ, Lee VMY, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 295:865–868
Auluck PK, Meulener MC, Bonini NM (2005) Mechanisms of suppression of α-synuclein neurotoxicity by geldanamycin in Drosophila. J Biol Chem 280:2873–2878
Baglioni S et al (2006) Prefibrillar amyloid aggregates could be generic toxins in higher organisms. J Neurosci 26:8160–8167
Blair LJ et al (2013) Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J Clin Invest 123:4158–4169
Brehme M et al (2014) A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep 9:1–16
Bucciantini M et al (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511
Bückig A, Tikkanen R, Herzog V, Schmitz A (2002) Cytosolic and nuclear aggregation of the amyloid β-peptide following its expression in the endoplasmic reticulum. Histochem Cell Biol 118:353–360
Capponi S et al (2016) Molecular chaperones in the pathogenesis of amyotrophic lateral sclerosis: the role of HSPB1. Hum Mutat 37:1202–1208
Chai Y, Koppenhafer SL, Bonini NM, Paulson HL (1999) Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine disease. J Neurosci 19:10338–10347
Chan HYE, Warrick JM, Gray-Board GL, Paulson HL, Bonini NM (2000) Mechanisms of chaperone suppression of polyglutamine disease: selectivity, synergy and modulation of protein solubility in Drosophila. Hum Mol Genet 9:2811–2820
Cohen SIA et al (2015) A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat Struct Mol Biol 22:207–213
Cook C, Petrucelli L (2019) Genetic convergence brings clarity to the enigmatic red line in ALS. Neuron 101:1057–1069
Cummings CJ et al (1998) Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet 19:148–154
Cummings CJ et al (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 10:1511–1518
Daturpalli S, Waudby CA, Meehan S, Jackson SE (2013) Hsp90 inhibits α-synuclein aggregation by interacting with soluble oligomers. J Mol Biol 425:4614–4628
Dedmon MM, Christodoulou J, Wilson MR, Dobson CM (2005) Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem 280:14733–14740
DeMattos RB et al (2002) Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 99:10843–10848
Dickey CA et al (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117:648–658
Evans CG, Wisén S, Gestwicki JE (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1-42) aggregation in vitro. J Biol Chem 281:33182–33191
Fath T, Eidenmüller J, Brandt R (2002) Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer’s disease. J Neurosci 22:9733 LP–9739741
Fernandez-Funez P et al (2016) Holdase activity of secreted Hsp70 masks amyloid-β42 neurotoxicity in Drosophila. Proc Natl Acad Sci U S A 113(35):E5212–E5221. https://doi.org/10.1073/pnas.1608045113
Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN (2005) Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson’s disease. J Mol Biol 351:1081–1100
Fonte V et al (2008) Suppression of in vivo beta-amyloid peptide toxicity by overexpression of the HSP-16.2 small chaperone protein. J Biol Chem 283:784–791
Freeman BC, Morimoto RI (1996) The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J 15:2969–2979
Freeman BC, Myers MP, Schumacher R, Morimoto RI (1995) Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J 14:2281–2292
Gao X et al (2015) Human Hsp70 disaggregase reverses Parkinson’s-linked α-synuclein amyloid fibrils. Mol Cell 59:781–793
Gestaut D, Limatola A, Joachimiak L, Frydman J (2019) The ATP-powered gymnastics of TRiC/CCT: an asymmetric protein folding machine with a symmetric origin story. Curr Opin Struct Biol 55:50–58
Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ (2005) A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet 37:526–531
Guo Q et al (2018) In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell 172:696–705
Hageman J et al (2010) A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell 37:355–369
Hay DG et al (2004) Progressive decrease in chaperone protein levels in a mouse model of Huntington’s disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet 13:1389–1405
Hayer-Hartl M, Bracher A, Hartl FU (2016) The GroEL–GroES chaperonin machine: a nano-cage for protein folding. Trends Biochem Sci 41:62–76
Hermansson E et al (2014) The chaperone domain BRICHOS prevents CNS toxicity of amyloid-β peptide in Drosophila melanogaster. Dis Model Mech 7:659–665
Hiller S (2019) Chaperone-bound clients: the importance of being dynamic. Trends Biochem Sci 44:517–527
Holmberg CI, Staniszewski KE, Mensah KN, Matouschek A, Morimoto RI (2004) Inefficient degradation of truncated polyglutamine proteins by the proteasome. EMBO J 23:4307–4318
Hosp F et al (2017) Spatiotemporal proteomic profiling of Huntington’s disease inclusions reveals widespread loss of protein function. Cell Rep 21:2291–2303
Jimenez-Sanchez M et al (2015) siRNA screen identifies QPCT as a druggable target for Huntington’s disease. Nat Chem Biol 11:347
Jinwal UK et al (2009) Chemical manipulation of Hsp70 ATPase activity regulates tau stability. J Neurosci 29:12079–12088
Kakkar V et al (2016) The S/T-Rich motif in the DNAJB6 chaperone delays polyglutamine aggregation and the onset of disease in a mouse model. Mol Cell 62:272–283
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592
Karagöz GE et al (2014) Hsp90-tau complex reveals molecular basis for specificity in chaperone action. Cell 156:963–974
Kazemi-Esfarjani P, Benzer S (2000) Genetic suppression of polyglutamine toxicity in Drosophila. Science 287:1837–1840
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Ulrich Hartl F (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355
Kirchhausen T, Owen D, Harrison SC (2014) Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol 6:a016725
Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ (2004) Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem 279:25497–25502
Koldewey P, Horowitz S, Bardwell JCA (2017) Chaperone-client interactions: non-specificity engenders multifunctionality. J Biol Chem 292:12010–12017
Krakowiak J et al (2018) Hsf1 and Hsp70 constitute a two-component feedback loop that regulates the yeast heat shock response. Elife 7:e31668
Krobitsch S, Lindquist S (2000) Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci U S A 97:1589–1594
Kundel F et al (2018) Hsp70 inhibits the nucleation and elongation of tau and sequesters tau aggregates with high affinity. ACS Chem Biol 13:636–646
Labbadia J et al (2012) Suppression of protein aggregation by chaperone modification of high molecular weight complexes. Brain 135:1180–1196
Leverenz JB et al (2007) Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol 17:139–145
Liao L et al (2004) Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem 279:37061–37068
Lieberman AP, Shakkottai VG, Albin RL (2019) Polyglutamine repeats in neurodegenerative diseases. Annu Rev Pathol Mech Dis 14:1–27
Lindberg MJ, Byström R, Boknäs N, Andersen PM, Oliveberg M (2005) Systematically perturbed folding patterns of amyotrophic lateral sclerosis (ALS)-associated SOD1 mutants. Proc Natl Acad Sci U S A 102:9754 LP–9759759
Link CD, Cypser JR, Johnson CJ, Johnson TE (1999) Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones 4:235–242
Lo Bianco C et al (2008) Hsp104 antagonizes a-synuclein aggregation and reduces dopaminergic degeneration in a rat model of Parkinson disease. J Clin Invest 118:3087–3097
Lotz GP et al (2010) Hsp70 and Hsp40 functionally interact with soluble mutant huntingtin oligomers in a classic ATP-dependent reaction cycle. J Biol Chem 285:38183–38193
Luk KC, Mills IP, Trojanowski JQ, Lee VMY (2008) Interactions between Hsp70 and the hydrophobic core of alpha-synuclein inhibit fibril assembly. Biochemistry 47:12614–12625
Luo W et al (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci U S A 104:9511–9516
Mangiarini L et al (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506
Månsson C et al (2014a) Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Aβ42) aggregates leads to sub-stoichiometric inhibition of amyloid formation. J Biol Chem 289:31066–31076
Månsson C et al (2014b) DNAJB6 is a peptide-binding chaperone which can suppress amyloid fibrillation of polyglutamine peptides at substoichiometric molar ratios. Cell Stress Chaperones 19:227–239
Marsh JL et al (2000) Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet 9:13–25
Martín-Peña A, Rincón-Limas DE, Fernandez-Fúnez P (2018) Engineered Hsp70 chaperones prevent Aβ42-induced memory impairments in a Drosophila model of Alzheimer’s disease. Sci Rep 8:1–14
Massol RH, Boll W, Griffin AM, Kirchhausen T (2006) A burst of auxilin recruitment determines the onset of clathrin-coated vesicle uncoating. Proc Natl Acad Sci U S A 103:10265–10270
McLean PJ et al (2002) TorsinA and heat shock proteins act as molecular chaperones: suppression of α-synuclein aggregation. J Neurochem 83:846–854
Mitul S, Jeff K, Gloria L (2008) Two motifs within the tau microtubule-binding domain mediate its association with the hsc70 molecular chaperone. J Neurosci Res 86:2763–2773
Miyasaka T et al (2005) Progressive neurodegeneration in C. elegans model of tauopathy. Neurobiol Dis 20:372–383
Mok SA et al (2018) Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat Struct Mol Biol 25:384–393
Moloney TC et al (2014) Heat shock protein 70 reduces α-synuclein-induced predegenerative neuronal dystrophy in the α-synuclein viral gene transfer rat model of Parkinson’s disease. CNS Neurosci Ther 20:50–58
Morán Luengo T, Mayer MP, Rüdiger SGD (2019) The Hsp70–Hsp90 chaperone cascade in protein folding. Trends Cell Biol 29:164–177
Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99:10417–10422
Nagy M, Fenton WA, Li D, Furtak K, Horwich AL (2016) Extended survival of misfolded G85R SOD1-linked ALS mice by transgenic expression of chaperone Hsp110. Proc Natl Acad Sci U S A 113:5424–5428
Nillegoda NB et al (2015) Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524:247–251. https://doi.org/10.1038/nature14884
Nollen EAA et al (2004) Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc Natl Acad Sci U S A 101:6403–6408
Novoselov SS et al (2013) Molecular chaperone mediated late-stage neuroprotection in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. PLoS One 8:e73944
Olzscha H et al (2011) Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144:67–78
Ostapchenko VG et al (2013) The prion protein ligand, stress-inducible phosphoprotein 1, regulates amyloid-β oligomer toxicity. J Neurosci 33:16552–16564
Outeiro TF et al (2006) Small heat shock proteins protect against alpha-synuclein-induced toxicity and aggregation. Biochem Biophys Res Commun 351:631–638
Park S-H et al (2013) PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell 154:134–145
Pemberton S et al (2011) Hsc70 protein interaction with soluble and fibrillar alpha-synuclein. J Biol Chem 286:34690–34699
Petrucelli L et al (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13:703–714
Poska H et al (2016) Dementia-related Bri2 BRICHOS is a versatile molecular chaperone that efficiently inhibits Aβ 42 toxicity in Drosophila. Biochem J 473:3683–3704
Prudencio M, Hart PJ, Borchelt DR, Andersen PM (2009) Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease. Hum Mol Genet 18:3217–3226
Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA (2007) Monitoring the prevention of amyloid fibril formation by α-crystallin. FEBS J 274:6290–6304
Roodveldt C et al (2009) Chaperone proteostasis in Parkinson’s disease: stabilization of the Hsp70/alpha-synuclein complex by Hip. EMBO J 28:3758–3770
Scherzinger E et al (1997) Huntingtin encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90:549–558
Shahmoradian SH et al (2013) TRiC’s tricks inhibit huntingtin aggregation. Elife 2:e00710
Shammas SL et al (2011) Binding of the molecular chaperone αB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys J 101:1681–1689
Shelton LB et al (2017) Hsp90 activator Aha1 drives production of pathological tau aggregates. Proc Natl Acad Sci U S A 114:201707039
Shi Y, Mosser DD, Morimoto RI (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev 12:654–666
Shimshek DR, Mueller M, Wiessner C, Schweizer T, van der Putten PH (2010) The HSP70 molecular chaperone is not beneficial in a mouse model of α-synucleinopathy. PLoS One 5:1–7
Song Y et al (2013) Molecular chaperone Hsp110 rescues a vesicle transport defect produced by an ALS-associated mutant SOD1 protein in squid axoplasm. Proc Natl Acad Sci U S A 110:5428 LP–5425433
St Martin JL et al (2007) Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J Neurochem 100:1449–1457
Thibaudeau TA, Anderson RT, Smith DM (2018) A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun 9:1097
Treweek TM, Meehan S, Ecroyd H, Carver JA (2015) Small heat-shock proteins: important players in regulating cellular proteostasis. Cell Mol Life Sci 72:429–451
Tue NT, Shimaji K, Tanaka N, Yamaguchi M (2012) Effect of αB-crystallin on protein aggregation in Drosophila. J Biomed Biotechnol 2012:1–7
Uryu K et al (2006) Convergence of heat shock protein 90 with ubiquitin in filamentous α-synuclein inclusions of α-synucleinopathies. Am J Pathol 168:947–961
Van Ham TJ et al (2008) C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet 4:e1000027
Waelter S et al (2001) Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell 12:1393–1407
Wang J et al (2003) Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet 12:2753–2764
Wang J et al (2005a) Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: αB-crystallin modulates aggregation. Hum Mol Genet 14:2335–2347
Wang Q et al (2005b) Proteomic analysis of neurofibrillary tangles in Alzheimer disease identifies GAPDH as a detergent-insoluble paired helical filament tau binding protein. FASEB J 19:869–871
Wang J, Martin E, Gonzales V, Borchelt DR, Lee MK (2008) Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases. Neurobiol Aging 29:586–597
Wang J et al (2009) An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet 5:e1000350
Warrick JM et al (1999) Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 23:425–428
Watanabe M et al (2001) Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis 8:933–941
Watanabe S et al (2014) SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol Brain 7:62
Waudby CA et al (2010) The interaction of alphaB-crystallin with mature alpha-synuclein amyloid fibrils inhibits their elongation. Biophys J 98:843–851
Willingham S, Outeiro TF, Devit MJ, Lindquist SL, Muchowski PJ (2003) Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science 302:1769–1773
Wojtas AM et al (2017) Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc Natl Acad Sci U S A 114:E6962–E6971
Yang H et al (2015) Aggregation of polyglutamine-expanded ataxin 7 protein specifically sequesters ubiquitin-specific protease 22 and deteriorates its deubiquitinating function in the Spt-Ada-Gcn5-Acetyltransferase (SAGA) complex. J Biol Chem 290:21996–22004
Yerbury JJ et al (2013) The small heat shock proteins αb-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones 18:251–257
Yu A et al (2014) Protein aggregation can inhibit clathrin-mediated endocytosis by chaperone competition. Proc Natl Acad Sci U S A 111:E1481–E1490
Yu A et al (2019) Tau protein aggregates inhibit the protein-folding and vesicular trafficking arms of the cellular proteostasis network. J Biol Chem 294:7917–7930. https://doi.org/10.1074/jbc.RA119.007527
Zetterström P, Graffmo KS, Andersen PM, Brännström T, Marklund SL (2011) Proteins that bind to misfolded mutant superoxide dismutase-1 in spinal cords from transgenic amyotrophic lateral sclerosis (ALS) model mice. J Biol Chem 286:20130–20136
Zhang S, Binari R, Zhou R, Perrimon N (2010) A genomewide RNA interference screen for modifiers of aggregates formation by mutant Huntingtin in Drosophila. Genetics 184:1165 LP–1161179
Zheng X et al (2016) Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. Elife 5:1–26
Acknowledgements
We thank Christopher M. Dobson for critical reading of part of the manuscript. This work was supported by National Institutes of Health (National Institute on Aging), the Daniel F. and Ada L. Rice Foundation to RIM and research grants from Eli Lilly & Co Ltd, Lilly Research Centre and the Tau Consortium of the Rainwater Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sinnige, T., Yu, A., Morimoto, R.I. (2020). Challenging Proteostasis: Role of the Chaperone Network to Control Aggregation-Prone Proteins in Human Disease. In: Mendillo, M.L., Pincus, D., Scherz-Shouval, R. (eds) HSF1 and Molecular Chaperones in Biology and Cancer. Advances in Experimental Medicine and Biology, vol 1243. Springer, Cham. https://doi.org/10.1007/978-3-030-40204-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-40204-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40203-7
Online ISBN: 978-3-030-40204-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)