Abstract
Proton-conducting oxides receive more and more attention in the current world because of its wide applications in renewable and sustainable devices. Among all these applications, the use of proton-conducting oxide for fuel cells is becoming quite a hot topic as it avoids the problems for traditional fuel cells using oxide electrolyte and also lowers the working temperature of fuel cells, making them possible for practical applications. With the framework of proton-conducting solid oxide fuel cells, the utilization of nanomaterials now playing an essential part in the whole community, and this chapter will briefly summarize the nanomaterials for protonic oxide fuel cells that are also known as proton-conducting solid oxide fuel cells (SOFCs).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
8.1 Proton Conduction in Oxides
Ionic conduction has been discovered in oxides long back, and for a long period, people can only find the migration of oxygen ions in the oxide at high temperatures, and there is no evidence suggesting proton could migrate in the oxide. However, Iwahara et al. [1] discovered that some oxides exhibit proton conductions under a wet atmosphere which opens a new door in the solid-state ionic’s field. Although the proton migration mechanism in oxides is not completely revealed, most of the evidence suggests that H2O incorporates into the lattice and fills the oxygen vacancies to form the proton defects [2]:
Compared with the traditional oxygen-ion conductors, proton-conducting oxides have smaller activation energies that are helpful for their low-temperature operations [3]. It should be noted that the low-temperature operation of SOFCs is important as it could extend the lifetime of the fuel cells and also provide the possibility for the quick start-up of fuel cell devices [4]. In addition, the fuel cells using proton-conducting electrolyte offer another advantage of producing H2O at the cathode side that will not dilute the fuels that always happens for the oxygen-ion conducting SOFCs, making the development of proton-conducting SOFCs becomes a hot topic in the community [5]. As a fuel cell device, it usually contains the components including electrolyte, anode, and cathode. In the following sections, we will briefly introduce the state-of-the-art as well as the recent development of nanomaterials for protonic SOFCs.
8.2 Proton-Conducting Electrolytes
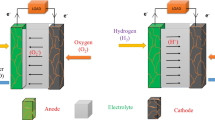
Proton conduction was firstly found in doped SrCeO3 materials [1] and then different oxides are reported to show proton conduction in the past four decades [6,7,8]. However, the most classical proton-conducting oxides are still the ABO3-based perovskite oxides, and they are usually used as electrolytes for proton-conducting SOFCs [9]. As the fuel cell electrolyte, it is desirable to have a low resistance which could avoid the high ohmic loss in the fuel cell operations. This requires high conductivity for the electrolyte materials. According to the classical “brick-layer model,” the solid-state electrolyte consisted of grain interiors (also called as “bulk”) and the grain boundaries, and protons have to pass through the grains and the grain boundaries for the transportation [10], as schemed in Fig. 8.1. It has been demonstrated that the resistance of the grain boundary is a few orders of magnitude of larger than that of the grains, suggesting the reduction of the grain boundary resistance is the key for the improvement of the overall electrolyte conductivity [10]. Generally, there are two routes to improve the grain boundary conductivity. One is to decrease the thickness of the grain boundary so that protons can pass through less distance with the high resistance grain boundaries. In this case, the specific grain boundary resistance could be decreased. The other approach is to increase grain size which decreases the volume of the grain boundaries. Although the specific grain boundary resistance is not changed with this strategy, the total grain boundary resistance is reduced because of the reduced grain boundary volume that allows the protons to meet fewer grain boundaries during their transportation. Nanotechnologies and nanomaterials play an important role in both strategies and let us introduce them in detail.
8.2.1 Reducing the Specific Grain Boundary Resistance
Comparing with the grain which has typically a size of a few micrometers, the grain boundary usually has a thickness of a few to tens of nanometers. Therefore, tailoring the thickness at the nanoscale is quite challenging in early studies but now some effective strategies have been used to reduce the specific grain boundary resistance by tailoring the grain boundaries. Y doped BaZrO3 (BZY) is one of the most investigated proton-conducting oxides in the field but its conductivity is highly hindered by its grain boundary resistance [11]. To overcome this drawback, Sun et al. [12] have used Li2O as the sintering aid to improve the sinterability of BZY. Different to other sintering aid-modified BZY materials that have large grains, the Li2O modified BZY pellet shows a small grain size of a few hundred nanometers. However, electrochemical measurements indicate that the conductivity of Li2O modified BZY shows high conductivity compared with other sintering aids-modified BZY. Further, material analysis reveals that there are two factors helping the conductivity for the Li2O modified BZY. One is that Li2O participates in the sintering procedure but it evaporates leaving the sample without Li element. In this case, the electrochemical properties of the sintered BZY electrolyte are free of the influence of the Li element. It has been demonstrated that although sintering aids could improve the sinterability of the samples, they impair the electrical properties, so the dense sample free of the sintering aid could maintain the high conductivity of BZY. Second, the microstructure analysis reveals that the formed BZY pellet has a very clear and thin grain boundary, as shown in Fig. 8.2. The thickness of the grain boundary is about 4 nm, which narrows the distance that protons must pass through for each grain boundary and thus improves the conductivity.
Microstructure of BZY electrolyte sintered with the addition of Li2O, showing clean and thin grain boundaries [12]. Reproduced by permission of the PCCP Owner Societies
One may think how about the complete removal of the grain boundaries; although this task is difficult for traditional protonic SOFCs, it becomes feasible in the filed thin-film SOFC. Pergolesi et al. [13] have fabricated a grain boundary-free BZY thin films by the pulsed laser deposition (PLD) method on the MgO substrate and found that the conductivity of the BZY film is much larger than that of the polycrystalline BZY pellet and the conductivity value is also higher than most of the oxygen-ion conductors, implying the advantage in electrochemistry by using proton conductors. Furthermore, the measured conductivity of the BZY films matches well with the theoretical bulk conductivity of BZY, suggesting the grain boundary is indeed removed and the total conductivity is equal to the bulk conductivity. This study demonstrates for the first time that the grain boundary of proton conductor could be removed from an experimental way, achieving high conductivity for the sample. It should be noted that this BZY thin film is fabricated on the MgO substrate that is not the configuration for fuel cell devices, so the fabrication of nanoscale electrolyte on the conventional NiO-based electrolyte is necessary to explore the possibility of using BZY thin films for fuel cell applications. Fabbri et al. have employed to PLD method to fabricate a thin BZY film on the NiO-based anode and tested in the fuel cell condition. However, the fuel cell performance of this fuel cell is not high, due to the use of the single-chamber mode. In addition, the BZY electrolyte film was fabricated on the NiO-based anode. Unlike the single-crystal MgO cathode used in the previous study, the NiO-based anode did not have a specific orientation that made the growth of single-crystal BZY film difficult. Therefore, although the thin-film BZY has been fabricated by the PLD method, the grain boundary still existed for this electrolyte and decreased the conductivity of the electrolyte and thus decreased the overall fuel cell performance [14]. Similarly, the BZY thin film was also fabricated on the sintered BCY electrolyte, forming the bilayer electrolyte structure [15]. The main purpose of this structure is to shield the unstable BCY electrolyte with the stable BZY thin film, making this electrolyte has better chemical stability. Although the conductivity of the bilayer electrolyte is obviously larger than that of the pure BZY electrolyte, the value is still apparently lower than that of the BCY electrolyte from the protective layer. In spite of the thin BZY electrolyte used, the polycrystalline BZY electrolyte still influences the overall conductivity of the electrolyte. Recently, Bae et al. [16] have tried to solve this problem by fabricating the BZY column-like crystalline electrolyte. As seen in Fig. 8.3, different from the conventional polycrystalline BZY electrolyte, the column-like grain allows the transportation of protons from the anode side to the cathode side without passing through the grain boundaries, facilitating the migration procedure and thus improves the electrolyte conductivity. Although this work and that work presented in [14] both use PLD fabricated thin-film BZY electrolyte, the current work shows much higher fuel cell performance, suggesting the removal of the grain boundary during the proton transport is essential for its high performance. However, it should be noted that all these works have to rely on the PLD method and PLD is an advanced physical deposition method that is more suitable for small-scale applications. The tailoring of the grain boundary at the nanoscale with the traditional processing method could be more significant for fuel cell applications.

Reproduced with permission from [16]
Microstrure for the BZY thin-film electrolyte with columnar structure grain boundaries, facilitating the migration of protons.
The above strategies have been proposed to reduce the grain boundary thickness or eliminate the high resistance grain boundaries, but these methods did not aim to improve the conductivity of the specific grain boundary conductivity at a given thickness, while it is reasonable to assume that the conductivity of the grain boundary could be enhanced if the grain boundaries becomes conductive. Although the high resistance of grain boundary is recognized, the reason for this high resistance is still debating, and the most popular opinion is that the form of the space charge layer is the main reason for the high resistive grain boundaries [17, 18]. The formation of the space charge layer is due to the accumulation of positive charge of the core of the grain boundary that blocks the transportation of protons. Therefore, neutralizing the positive charge at the grain boundary core to suppress the space charge layer could be an effective way to improve the grain boundary conductivity. However, the grain boundary is only a few nm, while the thickness of the grain boundary core is only a few Å that makes the modification difficult. Recently, high-temperature treatments were found to be a possible way to tailor the properties of the grain boundary [19, 20]. After sintering the pellet by spark plasma sintering (SPS), the sample was further treatment at high temperatures that allowed the diffusion of the dopant to the grain boundary region. The SPS method was used here to guarantee the high densification of the pellet as well as almost no grain growth in further heat treatment. In this case, the difference in grain size (the grain boundary volume) contribution to the grain boundary conductivity is negligible. Due to the negative charge of the dopant \(({\text{M}}^{\prime}_{\text{Zr}} )\), the accumulation of the dopant at the grain boundary core could mitigate the blocking effect of the grain boundary. The microstructure analysis has confirmed that the dopant indeed diffuses into the grain boundary region, while the electrochemical studies indicate that the conductivity of the sample is dramatically improved compared with the sample without tailoring the grain boundary. This evidence suggests that tailoring the grain boundary at nanoscale to suppress the space charge layer effect is the key to improve the grain boundary properties.
Besides tailoring the grain boundary itself, the introduction of noble metal nanoparticles is reported to be an effective method to improve the grain boundary conductivity. Tong et al. [21] have reported the conductivity enhancement for the BZY electrolyte with the use of nano-ionic composite. They have used Pd metal nanoparticles as the dispersed phase and the Pd has little effect on the conductivity when it incorporates into the BZY lattice. After reducing in H2 atmosphere, Pd nanoparticles exsolve from the BZY lattice, forming the nanocomposite, and the exsolved Pd nanoparticles are found to accumulate at the BZY grain boundaries, as shown in Fig. 8.4. Although all the BZY-Pd nanoparticle composites show higher conductivity than that of BZY without exsolved Pd, the sample with the small Pd nanoparticles (5–10 nm) shows the best conductivity which is 2.7 times higher than that of the pure BZY sample, suggesting the smaller Pd particle size could better promote the conductivity of the whole sample.

Reproduced from [21] with permission from Elsevier B.V.
The use of Pd nanoparticles at the grain boundary of BZY could enhance the conductivity.
8.2.2 Reducing the Overall Grain Boundary Resistance
The other strategy to improve the electrolyte conductivity is to increase the grain size of the electrolyte and thus decreases the volume of the grain boundaries and thus reduces the overall grain boundary resistance. According to the ceramic sintering principle, the oxide powder with small particle size having high surface energy could be easily sintered and the better growth of grain size could be expected [22]. Proton-conducting oxides are traditionally synthesized by a solid-state reaction, in which metal oxides powders are mixed together and then co-fired at a high temperature to achieve the desired phase for the target proton-conducting oxides. The solid-state reaction method provides a simple and straightway to prepare proton-conducting oxides, but high calcination temperatures have to be used in this method to accelerate the inter-diffusion between the starting oxide materials, and the high calcination temperature inevitably leads to the formation of coarse powders [23]. It is recognized that the coarse powder is not good for the later material sintering procedure, and ultra-high sintering temperature has to be used to density the pellet and sometimes causes the material evaporation because of the too high sintering temperature used. In contrast, the nano-sized powder is more desirable for the electrolyte fabrication and wet chemical routes are used to prepare nano-sized proton-conducting oxides. It has been reported that the solid-state reaction prepared BZY powder has to be sintered at 1800–2200 °C, while the sintering temperature of 1600 °C is sufficient for sintering the nano-sized proton-conducting powders [23]. Yamazaki et al. [24] have used the sol–gel method to prepare nanometric BZY powder and the dense BZY electrolyte pellet could be obtained after sintering at 1600 °C. In addition, the grain size of the BZY pellet is around 1 µm that dramatically reduces the volume of the grain boundaries and thus leads to high total conductivity of 0.01 S cm−1 at 450 °C.
Similar results are also obtained by Fabbri et al., who use Pr and Y co-doped BaZrO3 (BZPY) as the electrolyte for protonic SOFCs [25]. The combustion method was employed to prepare nano-sized BZPY powders. The sinterability tests indicate that the incorporation of Pr into BZY can significantly promote the sinterability of the material, leading to not only the higher shrinkage but also the large grains. As a result, the BZYP pellet shows higher conductivity than that of the BZY pellet without using Pr, leading a conductivity value of 0.01 S cm−1 at 600 °C, without impairing the chemical stability. Furthermore, the introduction of 10 mol% Pr into BZY does not bring obvious electronic conduction at intermediate temperatures. Recent work reveals that even with a small addition of Pr (5 mol%) forming the nano-sized particles, the sinterability and conductivity could be significantly improved [26].
NiO also plays a similar role in the sinterability and conductivity improvements for BZY. Shafi et al. [27] use NiO as the dopant instead of external sintering aid for BZY. It is found that the doping concentration of NiO in BZY is less than 10 mol% and the sinterability of the material greatly improves with an only doping amount of 4 mol%. As a result, the electrolyte can be easily sintered at 1400 °C and the anode supported fuel cell generates a peak power density of 428 mW cm−2 at 700 °C which is one of the largest power outputs for BZY-based cells at the time reported.
Apart from the use of wet chemical routes, the utilization of proper calcination method is recently found to be feasible for further reducing the initial particle size of the powder that helps the electrolyte densification and grain growth in the later sintering procedure. Microwave sintering is a sintering method that has been employed in ceramic sintering and it offers the advantage of fast sintering, eco-friendly, and low sintering temperatures [28]. Although the phrase “sintering” usually refers to the heat treatment to densify the material, the basic working principle of microwave sintering makes it also feasible for the calcination of the powder, in spite it has not been used for proton-conducting oxides before. However, a recent study indicates that the use of microwave sintering to calcine the BaZr0.1Ce0.7Y0.2O3−δ (BCZY) proton-conducting oxide powder could lower the phase formation temperature and also reduce the dwell time, which leads to small particle size for the initial powder [29]. It has been reported that the BCZY powder forms a pure phase at 900 °C with a dwell time of 1 h when the microwave sintering method was used. In contrast, the BCZY powder calcined in the conventional condition needs a thermal treatment condition of 1000 °C and 5 h for getting a pure phase. Both the calcination temperature and the dwell time are larger than those used in the microwave condition. As a result, the particle size of the microwave sintering method prepared BCZY powder is about 25 nm, while the corresponding value for the conventionally calcined BCZY powder is 100 nm, as shown in Fig. 8.5. The small initial powder size for the microwave method prepared BCZY allows the densification of the BCZY electrolyte membrane at 1300 °C. Although the electrolyte membrane with the conventionally calcined BCZY powder appears to be dense after firing at 1300 °C, the grain size of this electrolyte is much smaller than that of the electrolyte with the microwave prepared BCZY powder. This is because the small initial powder with small particle size could be better sintered in the later sintering step, leading to improved grain growth of the electrolyte. The large grain is desirable for the electrolyte which could reduce the volume of grain boundaries and thus improve the fuel cell performance. As a result, the single cell with the BCZY electrolyte started from the microwave prepared powder generated a peak power density of 791 mW cm−2, which is at the high level for protonic SOFCs. All these evidences suggest that nanomaterials used in the electrolyte materials are beneficial for the overall fuel cell performance of protonic fuel cells.

Reproduced from [29] with permission from Elsevier B.V.
The particle of BCZY material prepared under different thermal treatment conductions, showing the microwave method could reduce the initial size of the BCZY powder.
8.3 Anode Nanomaterials for Protonic SOFCs
In fuel cells, the anode should be catalytic active for H2 that splits H2 into protons. Then protons pass through the electrolyte to react with O2 at the cathode side. For SOFCs, the anode is not only used as the electrode for catalytic purposes but also serves as the support for the complete cell. Therefore, the anode is normally thicker than the electrolyte layer and the cathode layer to guarantee the mechanical strength for the complete cell. Up to now, the most used anode is the NiO-based anode in which NiO powder is mixed with the electrolyte powder to assure first the thermo-compatibility with the electrolyte and second improved the triple-phase boundaries (TPBs). In protonic SOFCs, the anode is usually the composite anode consisted of NiO and proton-conducting oxides. As the anode research is relatively mature and there are no many other materials that could compete with the NiO-based anode now, the research on anode for protonic SOFCs is much less than that for electrolytes and cathodes. However, it is that the use of nanomaterials in the anode could be beneficial for the anode performance as well as for the cell fabrications. The anode powder is traditionally prepared by the mechanical mixing method, in which NiO powder and the proton-conducting oxide powder were mixed by ball milling. Although this method is straightforward and cost-effective, the mixing of NiO and proton-conducting oxide might not be homogenous. In addition, the size of the NiO is relatively large. Some attempts have been carried out to prepare anode powder in wet chemical routes that could make the powder in a nanometric scale with homogenous distribution of two phases. Bi et al. [30] have used the NiO-BZY composite anode prepared by the combustion method for protonic SOFCs. In their study, the preparation of the NiO-BZY anode powder by the wet chemical routes offered a small particle size as well as a homogenous distribution of NiO and BZY. As a result, the nano-sized NiO-BZY anode powder showed better performance than that of the NiO-BZY anode powder with the same composition prepared by the traditional solid-state reaction method. In addition to the improved electrochemical performance, the nano-sized anode powder showed better sinterability that allowed the densification of the deposited electrolyte during the co-sintering procedure. It is known that the BZY suffers from its very poor sinterability and the sintering for BZY is difficulty, usually requiring high temperatures. However, the high sintering temperature is not feasible for use in the fuel cell fabrication as the BZY electrolyte is usually co-sintered with the electrode, and the high sintering temperature could lead to severe interfacial reactions. It is found that the BZY electrolyte cannot reach dense if the electrolyte film is deposited on the traditional mechanically mixed BZY-NiO anode, even after sintering at 1400 °C. However, the situation was changed by employing the wet chemical route prepared BZY-NiO anode. As the wet chemical route prepared BZY-NiO anode powder is nano-sized powder, it shows better sinterability than that of mechanically mixed BZY-NiO anode powder, leading to a higher shrinkage. During the cell fabrication procedure, the electrolyte and anode are co-sintered and shrink together. The high shrinkage of the anode could promote the deposited BZY film, allowing the densification of the electrolyte without deliberately adding the sintering aids [31]. Similar work was also carried out for NiO-BCY anode for protonic SOFCs, and the composite NiO-BCY also showed excellent electrochemical performance [32].
However, it is also noted that the anode nanomaterials have to be prepared by wet chemical routes, which are more complicated and less straightforward than the traditional mechanical mixing routes. The mass production of this kind of powder is challenging and its application in the practical application seems limited. Therefore, the use of these nano-sized anode materials as the functional layer instead of the whole anode could be more proper for practical applications. Bi et al. [33] have investigated the effect of the anode functional layer on the performance of protonic SOFCs. In this study, the nano-sized anode functional layer power was prepared by a combustion method and then fabricated on the anode substrate as a transition layer for the cell. One can see that the use of the anode functional layer significantly improves the microstructure for the cell. Obvious cracks appear for the cell without the anode functional layer, which is probably due to the inhomogeneous distribution of proton-conducting phase and NiO phase as well as the coarse powder used. When the anode functional layer was used, the interface of the anode/electrolyte, where the electrochemical reaction happens, becomes the interface of the anode functional layer and the electrolyte. The nano-sized anode functional layer extends the TPBs compared with the anode due to its small particle size. Furthermore, the anode functional layer makes an improved contact between the anode part with the electrolyte layer by eliminating macro-pores at the interface. As a result, both ohmic resistance and polarization resistance of the cell with the anode functional layer have been greatly reduced, leading to higher fuel cell performance compared with the traditional cell without the anode functional layer. It is also true that many high-performance protonic SOFCs now tend to use an anode functional layer to improve the electrolyte/anode interfacial contact and thus to enhance the fuel cell performance [34].
8.4 Cathode Nanomaterials for Protonic SOFCs
Compared with the relatively few researches on anode for protonic SOFCs, the research on cathode materials for protonic SOFCs is now becoming the major research topic in the field. Different materials and microstructures are proposed, aiming to improve cathode performance. For protonic SOFCs in the early years, people just take the cathode materials that work well in oxygen-ion SOFCs for protonic SOFCs [35]. Although these cathodes can generate reasonable fuel cell performance, it is not a rational way. In oxygen-ion SOFCs, oxygen ions and electrons participate the cathode reactions, and the electronic conduction in the cathode is usually a few orders of magnitude larger than that of the oxygen-ion conduction. Therefore, the design and discovery of cathode materials with high oxygen-ion conduction are critical for cathodes for oxygen-ion SOFCs [36]. However, the cathode reaction for protonic SOFCs is different from that of oxygen-ion SOFCs, making the design of the cathode different. Peng et al. [37] have investigated the cathode process for protonic SOFCs and found that both oxygen-ion migration and proton migration are involved at triple-phase boundaries (TPBs). Therefore, the development of cathode materials with both oxygen-ion conduction and proton conduction is essential for high-performance protonic SOFCs. Although most traditional cathode materials have oxygen-ion conduction and electronic conduction, the proton conduction is not revealed before and the search of cathode materials with proton conduction is a hot topic in the community but also quite challenging. Figure 8.6 shows the scheme for the cathode reaction for protonic SOFCs using oxygen-ion and electron mixed conductor as the cathode; one can see that the TPBs are restricted at the cathode and electrolyte interface, which limits the reaction active area and thus restricts the cathode performance. In order to improve the cathode performance, one of the most feasible approaches is to couple the cathode material with the electrolyte material, which could extend the TPBs to the connections where electrolyte and cathode meet. In this case, the TPBs are extended compared with the single-phase cathode. This method is widely used in the past decade and even now for protonic SOFCs and the use of nanomaterials is critical for the enhancement of cathode performance as nanomaterials could enlarge the TPBs compared with the large size cathodes. In the past two decades, many studies have been carried out to find the suitability of the cathode for protonic SOFCs and most of the studies use the nano-sized composite cathode [38]. Usually, wet chemical routes are employed to prepare the cathode nanomaterials as well as the proton-conducting electrolyte materials, and then, these two nanomaterials are mixed together to form the composite cathode [39, 40]. Many different cathode materials were evaluated for protonic SOFCs and they generally produced decent fuel cell performance [9]. In order to further maximize the TPBs, the impregnation method was employed to coat cathode nanoparticles on the electrolyte backbone, which allows a further improvement in TPBs and thus higher electrochemical performance [41]. This method receives more attention these days due to its demonstrated suitability of improving the fuel cell performance for protonic SOFCs. Da’as et al. [42] have used the impregnated Sr-doped LaMnO3 nanoparticles as the cathode for BZY-based fuel cells and the performance is impressive. In their study, LSM precursor was deposited on the BZY backbone forming the LSM-BZY composite cathode by an ink-jet printing method (Fig. 8.7). LSM is the first-generation cathode for SOFCs and it is regarded not appropriate for intermediate temperature SOFCs due to its pure electronic conducting feature [43]. It is true that most LSM-based SOFCs have to work at high temperatures (above 700 °C) and the performance of LSM cathode for BZY-based fuel cells is reported to be low [44]. However, in the study of Da’as et al., the peak power density of the BZY cell using the LSM nanoparticles reaches 200 mW cm−2 at 600 °C. The power output value is one order of magnitude larger than that of the same cell using the mechanically mixed LSM cathode, suggesting the extension of the TPBs by tailoring the particle size of LSM to nanoscale is the key for the much-improved fuel cell performance. This study somehow turnovers the traditional thinking that LSM is not proper to use at intermediate temperatures, extending the application of the LSM material. It also should be highlighted that although impregnation method for preparing cathode nanoparticles is widely reported in the community, this study presents an interesting strategy of using ink-jet printing for impregnation [42]. Different from the traditional manual impregnation method that confronts difficulties in controllability and reproducibility, the impregnation with ink-jet printing technique allows the fabrication of nanostructured cathodes in a controllable manner in which the whole impregnation process is carried by the machine. In this case, the cathode fabrication is independent of the people once the parameters for the ink-jet printer are fixed. Furthermore, the traditional impregnation method is only suitable for small-scale applications and the impregnation, and firing procedure has to be repeated several times until the required loading amount of LSM has been achieved. In the ink-jet printing procedure, only one step of impregnation and firing procedure is used, saving much time and effort. Also, the ink-jet printing can precisely control the volume of the drops, and the use of very tiny drops in the impregnation procedure is feasible. In this case, the backbone could be coated with a large number of tiny drops that could guarantee the homogeneity of cathodes. Although the performance of the fuel cell with the impregnated LSM cathode has been dramatically improved with the traditional study, the power output is still not at the top level of the reported protonic SOFCs which is mainly due to the pure electronic conduction nature of LSM. By using LSM nanoparticles covering the BZY backbone, indeed the TPBs are extended and the performance is enhanced. However, it is also noticed that the oxygen-ion can only transport via the LSM surface while the transportation of oxygen-ion through the bulk is unlikely for LSM. Therefore, it is reasonable to assume that the fuel cell performance could be further improved by using the oxygen-ion and electron mixed conductors. Bi et al. [45] have attempted to use the impregnated Sm0.5Sr0.5CoO3(SSC) cathode for BZY-based protonic SOFCs. Compared with the almost pure electronic conductor LSM, SSC is a well-known mixed conductor that allows the diffusion of oxygen ions and the cathode reaction activity could be much improved with the use of SSC cathode. By using the SSC nanostructured cathode, the BZY cell generates a high peak power density of 602 mW cm−2 at 600 °C and this value is one of the largest ever reported for BZY cells and it is also at the high level for protonic SOFCs (Fig. 8.8). To further demonstrate the feasibility of using nanostructured mixed conductors for protonic SOFCs, the impregnated PrBaCo2O5+x(PBCO) material is also used for BZY-based cells. It is interesting to find that the PBCO without obtaining the pure phase shows better performance than that of the cell with pure phase PBCO cathode. Unlike the SSC material that can form the pure phase at 800 °C, the formation of the PBCO phase requires a high firing temperature of 1200 °C. However, with the increase of the calcination temperature, the size of cathode powder inevitably increases. Although the calcination temperature of 1200 °C could allow the formation of pure phase PBCO impregnated particles, such a high firing temperature also leads to the loss of the nanostructure for the cathode. In contrast, the PBCO precursor fired at 800°C cannot achieve the pure PBCO double perovskite phase but form the BaCoO3 and PrCoO3-based intermediates, while the nanostructured of the cathode is maintained. The BZY cell with the 800 °C fired PBCO cathode shows a record-high performance of 650 mW cm−2 at 600 °C. This study suggests that the formation of the nanostructured for the cathode sometimes is more important than the formation of the pure phase of the target cathode material, provided the intermediates still have the catalytic activity for protonic SOFCs.

Reproduced from [60] with permission from The Royal Society of Chemistry
Scheme for the cathode reaction using traditional oxygen-ion and electron mixed conducting cathodes.

Reproduced from [42] with permission from Science China Press and Springer-Verlag GmbH Germany, part of Springer Nature
Scheme for the preparation of nanoparticles cathodes for proton-conducting SOFCs with the impregnation method integrated with the ink-jet printing technique.

Reproduced from [45] with permission from WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim
BZY cells with impregnated SSC as the cathode: a surface and b cross-sectional view.
However, it should be noted that although nanomaterials were used in these studies, the extension of TPBs is still limited at the interface of the proton-conducting phase (normally the electrolyte material in the composite cathode) and electron-oxygen mixed conducting phase. The use of impregnated nanoparticles as cathode for protonic SOFCs just further improves the TPB area compared with the mechanically mixed two-phase composite cathode and it is a microstructure optimization without using the new materials. According to the reaction mechanism of protonic SOFC cathodes, the ideal cathode for protonic SOFCs should have the proton, oxygen-ion, and electron conductions which could extend the reaction active area to the whole electrode surface instead of only the connection between the cathode phase and the proton-conducting electrolyte phase, as schemed in Fig. 8.9. Although the advantage of using materials with proton, oxygen-ion, and electron conductions is evidently recognized, the development of materials with proton, oxygen-ion, and electron conductions is quite challenging. Unlike the oxygen–electron mixed conducting materials that are widely available [36], few materials are reported to have proton, oxygen-ion, and electron conductions and the situation is changed only in recent years.

Reproduced from [60] with permission from The Royal Society of Chemistry
Scheme for the use of triple-conducting material (proton, oxygen-ion, and electron) as the cathode for proton-conducting SOFCs, extending the reaction active area to the whole cathode surface.
The mixed proton-electron conducting materials were firstly designed by tailoring the traditional proton conductor with proper dopants. BaCeO3, which is a well-known proton conductor, is doped with Bi-element, allowing the material to show both protonic and electron conduction. Ba(Ce1−xBix)O3 (x = 0.0–0.5) are reported to show both electronic conduction and protonic conduction [46], although the total conductivity of these materials is relatively low, reaching only about 0.1 S cm−1 at 600 °C. The following work has demonstrated that the use of BaCe0.5Bi0.5O3 material as cathode could allow reasonable performance for protonic SOFCs, reaching 321 mW cm−2 at 700 °C with a polarization of 0.28 Ω cm2 at the same temperature [47]. This power output value is at the high level for protonic SOFCs at the time reported and the polarization resistance is similar to many composite cathodes in the literature. It should be noted that only single-phase BaCe0.5Bi0.5O3 material was used as the cathode instead of the conventional composite cathode, implying protonic conduction in this material. Otherwise, the polarization resistance could be huge due to the absence of proton conduction in the cathode [48]. These studies suggest that the modification of existing proton conductors could be a feasible route to design cathode materials with proton and electron mixed conductivity. Following this idea, other materials have been designed and prepared. Compared with the mild electronic conduction potential of Bi-doping materials, the modification with transition metal oxides could be a more straightforward way due to the valence change of the metal elements. Similar to BaCe0.5Bi0.5O3 material, Fe was used as a dopant to modify the BeCeO3 proton conductor forming the Fe doped BaCeO3 materials as cathodes for protonic SOFCs [49]. Different phase structures have been obtained with the different Fe-doping level and the BaCe0.5Fe0.5O3 material shows the best electrochemical performance. It is also noted that doped BaCeO3 is a well-known proton conductor but also gets criticized for its poor chemical stability. Therefore, the modification of stable proton conductors could be a more rational way of designing the cathodes with proton condition. Rao et al. [50] have used Co-doped BaZrO3 as the cathode materials for protonic SOFCs. In their research, they have demonstrated the BaZrO3 material with 40 mol% Co-doping shows superior performance than that of Sm0.5Sr0.5CoO3-based composite cathode, even though Sm0.5Sr0.5CoO3 is regarded as one of the most promising cathode materials for intermediate temperature SOFCs, implying the proton conduction in the material. A similar approach has been used to dope Pr in BaZrO3, finding that the high concentration of Pr-doping allows the appearance of electronic conduction for BaZrO3. Moreover, the ionic conduction is still maintained for these materials, making them attractive as cathodes for protonic SOFCs [51]. However, it should be noted that the Pr-doping strategy does not introduce sufficient electronic conduction to make Pr-doped BaZrO3 to be used independently, and they have to be used together with other electronic conductors as the composite cathode. Even in this case, the 30 mol% Pr-doped BaZrO3 used in the cathode allows an encouraging electrochemical performance [52], making the use of proton-electron mixed conductor as one of the most interesting directions for developing cathode materials for protonic SOFCs.
It should be mentioned that the direct measurement of proton conduction in the cathode material is still quite challenging. The direct measurement of the conductivity of the material can only give the value of the total conductivity, including electronic conductivity, oxygen-ion conductivity, and protonic conductivity. In a mixed conductor, such as the cathode material, the electronic conduction is usually a few orders of magnitude larger than that of the ionic (both oxygen-ion and proton) conductivity, which makes the value of the total conductivity almost equals to that of the electronic conduction. The oxygen-ion conductivity in the mixed conductor could be further separated by using some strategies, such as oxygen permeation tests or concentration cell tests in oxygen-containing atmospheres. However, such strategies cannot be applied for the separation of the protonic conductivity from the total conductivity. The cathode material tends to decomposes in the reducing atmosphere, so the tests, such as hydrogen permeation tests or hydrogen concentration cell tests, are not feasible. As a result, it is difficult to use conventional experimental methods to measure the specific proton conduction in the cathodes. In order to overcome this technical difficulty, theoretical approaches have been employed to investigate the proton mobility in the cathode materials from an atomic point of view.
The density functional theory (DFT) method has been recently employed in the field of protonic SOFCs to calculate the proton migration ability in the cathode, which could avoid the technical problems that appeared in experimental approaches [53, 54]. Wang et al. [55] have evaluated the performance of Sr3Fe2O7−δ as the cathode for protonic SOFCs. Although Sr3Fe2O7−δ shows similar oxygen-ion conduction with the traditional oxygen–electron mixed conductor, its application in protonic SOFCs shows superior performance suggesting the possible proton migration in this material. DFT method is employed to investigate the proton migration energies in this material, suggesting feasibility for proton migration in this material. In addition, the Sr3Fe2O7−δ shows a decreased performance when coupling it with proton-conducting oxide as the composite cathode, indicating the occupancy of the Sr3Fe2O7−δ by proton-conducting oxide impairs the fuel cell performance. Generally, the composite cathode using a certain amount of proton-conducting oxide shows improved electrochemical performance than that of the single-phase cathode, and this study shows an opposite trend which suggests the different electrochemical reaction for the Sr3Fe2O7−δ cathode.
To introduce proton conduction in the cathode materials, the evaluation of hydration ability is another rational approach to screen the possible cathodes with proton and electron conduction. Poetzsch et al. [56] have measured the proton uptake of Ba0.5Sr0.5Fe0.8Zn0.2O3−δ from the hydration ability tests, suggesting Ba0.5Sr0.5Fe0.8Zn0.2O3−δ is a mixed conductor with protonic conduction and the hydration ability is the key for the protonic conduction. Based on this concept, some new materials have been proposed for protonic SOFCs, aiming to improve the hydration ability and thus the proton migration ability. Ba0.5Sr0.5Co0.8Fe0.2O3−δ, which is a high-performance cathode for oxygen-ion SOFCs [57], does not show similar high performance in protonic SOFCs [58]. It is well known that Ba0.5Sr0.5Co0.8Fe0.2O3−δ has good oxygen-ion migration ability and the lack of sufficient proton mobility could be the reason for the low performance for Ba0.5Sr0.5Co0.8Fe0.2O3−δ in protonic SOFCs. To enhance the proton mobility, one straightforward strategy could be the improvement of hydration ability in the material. Therefore, K was used as the dopant for Ba0.5Sr0.5Co0.8Fe0.2O3−δ and K partially replaced Ba in the material, aiming to enhance the hydration ability of the material [59]. As K has stronger basicity than that of Ba, the K-doped Ba0.5Sr0.5Co0.8Fe0.2O3−δ material could possess higher hydration ability than that of the K-free sample. The hydration ability tests indicate the Ba0.4K0.1Sr0.5Co0.8Fe0.2O3−δ has higher hydration ability than that of Ba0.5Sr0.5Co0.8Fe0.2O3−δ material. The improved hydration is also demonstrated from theoretical point of view, in which Ba0.4K0.1Sr0.5Co0.8Fe0.2O3−δ has lower hydration energy than that of Ba0.5Sr0.5Co0.8Fe0.2O3−δ. In addition, the proton mobility investigation carried out by DFT calculations also suggests that the K-doping strategy changes the proton hopping and rotation energies, leading to an easier proton migration in Ba0.4K0.1Sr0.5Co0.8Fe0.2O3−δ. As a result, the protonic SOFC using the Ba0.4K0.1Sr0.5Co0.8Fe0.2O3−δ cathode shows an encouraging fuel cell performance of 1275 mW cm−2 at 700 °C.
Sn and Bi co-doping strategy also shows a similar impact on the cathode. Xia et al. [60] have used Sn and Bi as the co-dopant for traditional BaFeO3-based cathode materials. The material shows higher hydration ability with an increased Bi-doping level until 30 mol%. After that doping concentration, the material cannot maintain a single phase. Although the doping of Bi could reduce the total conductivity of the sample, the conductivity value is still higher than 1 S cm−1 at the SOFC testing temperature range, making it suitable as the electrode for fuel cells. Moreover, the oxygen-ion and proton migration ability improve with the Bi-doping, allowing the triple-conduction in the target material. As a result, the cell using the BaFe0.5Sn0.2Bi0.3O3 cathode generates a high peak power density of 1277 mW cm−2 at 700 °C, which is the highest ever reported for protonic SOFCs using cobalt-free cathodes.
Besides the design of new materials as cathodes for protonic SOFCs, interfacial engineering is demonstrated to be important for cell performance. Choi et al. have used PrBa0.5Sr0.5Co1.5Fe0.5O5+δ as the cathode for protonic SOFCs [61]. Due to the proton and electron conduction nature of PrBa0.5Sr0.5Co1.5Fe0.5O5+δ, the cell exhibits high fuel cell performance reaching 800 mW cm−2 at 600 °C. Further, tailoring the cathode/electrolyte interface with a thin layer of dense PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (around 100 nm) could boost the fuel cell performance, leading to a peak power density of 1098 mW cm−2 at the same testing temperature. The fuel cell performance is record-high for protonic SOFCs until now. The addition of the thin PrBa0.5Sr0.5Co1.5Fe0.5O5+δ layer could improve the contact at the cathode/electrolyte interface, reducing the ohmic resistance of the cell and thus improving the overall fuel cell performance. In addition, the improvement in fuel cell performance by adding the dense PrBa0.5Sr0.5Co1.5Fe0.5O5+δ layer also demonstrates the protonic conduction in the PrBa0.5Sr0.5Co1.5Fe0.5O5+δ. If the material has no proton conduction, the dense layer could block the migration of proton from the anode to the cathode side and then dramatically reduces the fuel cell performance. All the above evidence suggests that the development of new materials with proton conduction and the optimization of microstructure are now two hot directions for the cathode designs for protonic SOFCs
8.5 Conclusions
The discovery of proton conduction in some oxides opens a new door in the solid-state ionic field and many devices based on proton-conducting oxides have been developed. Among all these devices, protonic SOFCs become a hot topic recently due to their unique advantages over the traditional SOFCs. Nanomaterials now play more and more important roles in protonic SOFCs, including the utilization of nanomaterials in electrolyte, anode, and cathode. The electrolyte powder is preferred to be restricted at the nanoscale that will be beneficial for later sintering procedures to produce high-quality electrolyte membranes. Different methods have been employed to produce nano-electrolyte powders and wet chemical routes are more favorable than the traditional solid-state reaction methods. In addition, the microwave method has been recently used for the synthesis of proton-conducting oxide powders. It is found that the microwave sintering method could allow the formation of pure phase at relatively low temperatures with a shorter dwell time compared with the traditional calcination method. As a result, the size of the electrolyte powder could be further reduced. Although microwave sintering is not widely used in the community of protonic SOFCs, its advantages over traditional sintering make it appealing not only in nano-sized powder preparation but also in electrolyte membrane preparations. The low preparation temperature not only reduces the particle size for the initial powders but also depresses metal element evaporations during the co-sintering procedure, leading to high membrane conductivity and thus high fuel cell performance. Also, the tailoring of the grain boundary core at sub-nanoscale has been demonstrated to improve the specific grain boundary conductivity, although now the high-temperature thermal treatment method is the only way to tailor the grain boundary core. The development of proper strategies to design the core of grain boundary could be interesting but also challenging for the improvement of proton-conducting electrolyte materials.
Anode materials are less studied due to the maturity of NiO-based anode. NiO-based anodes are widely used in protonic SOFCs due to their good catalytic activity and chemical compatibility. Rather than the investigations on the materials as anode for protonic SOFCs, the optimization of the microstructure of the anode is critical for the overall fuel cell performance. The use of the composite nanomaterials as the anode functional layer is found to be beneficial for the cell performance. The nano-sized composite anode provides a more active reaction area compared with the traditional mechanically mixed anode. In addition, the anode functional layer serves as a transition layer which improves the interfacial contacts between the anode and the electrolyte layer, reducing the overall ohmic resistance and thus improving the fuel cell performance.
Among all the key components for protonic SOFCs, cathodes are mostly investigated. Nanomaterials are widely used in this aspect, including the fabrication of nano-sized cathode particles and the design of novel cathode materials with protonic conduction. The use of the impregnation method could deposit cathode nanoparticles on the electrolyte backbone, allowing the extension of TPBs compared with the traditional mechanically mixed composite cathode. Moreover, one recent study demonstrates that the maintenance of nanoparticles for the cathode is somehow more important than the formation of targeting structure, provided the intermediates still have sufficient catalytic activities. Due to the different reaction mechanisms of cathodes for protonic SOFCs compared with that for traditional oxygen-ion conducting SOFCs, the introduction of proton conduction is the key to the development of high-performance cathodes for protonic SOFCs. Different strategies have been proposed, aiming to enhance the proton migration ability and also the hydration ability of the materials, and it is believed that this area will be still the hot topic for protonic SOFCs in the following years.
References
H. Iwahara, T. Esaka, H. Uchida, N. Maeda, Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen-production. Solid State Ionics 3–4, 359–363 (1981)
T. Norby, M. Wideroe, R. Glockner, Y. Larring, Hydrogen in oxides. Dalton Trans. 19, 3012–3018 (2004)
K.D. Kreuer, Proton-conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003)
E.D. Wachsman, K.T. Lee, Lowering the temperature of solid oxide fuel cells. Science 334(6058), 935–939 (2011)
H.K. Dai, H.N. Kou, H.Q. Wang, L. Bi, Electrochemical performance of protonic ceramic fuel cells with stable BaZrO3-based electrolyte: a mini-review. Electrochem. Commun. 96, 11–15 (2018)
H. Iwahara, H. Uchida, K. Ono, K. Ogaki, Proton conduction in sintered oxides based on BaCeO3. J. Electrochem. Soc. 135(2), 529–533 (1988)
R. Haugsrud, T. Norby, Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nat. Mater. 5(3), 193–196 (2006)
E. Fabbri, D. Pergolesi, E. Traversa, Materials challenges toward proton-conducting oxide fuel cells: a critical review. Chem. Soc. Rev. 39(11), 4355–4369 (2010)
E. Fabbri, L. Bi, D. Pergolesi, E. Traversa, Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton-conducting electrolytes. Adv. Mater. 24(2), 195–208 (2012)
S.M. Haile, G. Staneff, K.H. Ryu, Non-stoichiometry, grain boundary transport and chemical stability of proton conducting perovskites. J. Mater. Sci. 36(5), 1149–1160 (2001)
A. D’Epifanio, E. Fabbri, E. Di Bartolomeo, S. Licoccia, E. Traversa, Design of BaZr0.8Y0.2O3 protonic conductor to improve the electrochemical performance in intermediate temperature solid oxide fuel cells (IT-SOFCs). Fuel Cells 8(1), 69–76 (2008)
Z.Q. Sun, E. Fabbri, L. Bi, E. Traversa, Lowering grain boundary resistance of BaZr0.8Y0.2O3 with LiNO3 sintering-aid improves proton conductivity for fuel cell operation. Phys. Chem. Chem. Phys. 13(17), 7692–7700 (2011)
D. Pergolesi, E. Fabbri, A. D’Epifanio, E. Di Bartolomeo, A. Tebano, S. Sanna, S. Licoccia, G. Balestrino, E. Traversa, High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 9(10), 846–852 (2010)
D. Pergolesi, E. Fabbri, E. Traversa, Chemically stable anode-supported solid oxide fuel cells based on Y-doped barium zirconate thin films having improved performance. Electrochem. Commun. 12(7), 977–980 (2010)
E. Fabbri, D. Pergolesi, A. D’Epifanio, E. Di Bartolomeo, G. Balestrino, S. Licoccia, E. Traversa, Design and fabrication of a chemically-stable proton conductor bilayer electrolyte for intermediate temperature solid oxide fuel cells (IT-SOFCs). Energy Environ. Sci. 1(3), 355–359 (2008)
K. Bae, D.Y. Jang, H.J. Choi, D. Kim, J. Hong, B.K. Kim, J.H. Lee, J.W. Son, J.H. Shim, Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 8, 14553 (2017)
E.E. Helgee, A. Lindman, G. Wahnstrom, Origin of space charge in grain boundaries of proton-conducting BaZrO3. Fuel Cells 13(1), 19–28 (2013)
M. Shirpour, R. Merkle, J. Maier, Space charge depletion in grain boundaries of BaZrO3 proton conductors. Solid State Ionics 225, 304–307 (2012)
M. Shirpour, B. Rahmati, W. Sigle, P.A. van Aken, R. Merkle, J. Maier, Dopant segregation and space charge effects in proton-conducting BaZrO3 perovskites. J. Phys. Chem. C 116(3), 2453–2461 (2012)
M. Shirpour, R. Merkle, J. Maier, Evidence for space charge effects in Y-doped BaZrO3 from reduction experiments. Solid State Ionics 216, 1–5 (2012)
J.H. Tong, A. Subramaniyan, H. Guthrey, D. Clark, B.P. Gorman, R. O’Hayre, Electrical conductivities of nano ionic composite based on yttrium-doped barium zirconate and palladium metal. Solid State Ionics 211, 26–33 (2012)
V. Esposito, E. Traversa, Design of electroceramics for solid oxides fuel cell applications: playing with ceria. J. Am. Ceram. Soc. 91(4), 1037–1051 (2008)
L. Bi, E. Traversa, Synthesis strategies for improving the performance of doped-BaZrO3 materials in solid oxide fuel cell applications. J. Mater. Res. 29(1), 1–15 (2014)
Y. Yamazaki, R. Hernandez-Sanchez, S.M. Haile, High total proton conductivity in large-grained yttrium-doped barium zirconate. Chem. Mater. 21(13), 2755–2762 (2009)
E. Fabbri, L. Bi, H. Tanaka, D. Pergolesi, E. Traversa, Chemically stable Pr and Y co-doped barium zirconate electrolytes with high proton conductivity for intermediate-temperature solid oxide fuel cells. Adv. Funct. Mater. 21(1), 158–166 (2011)
H.L. Dai, Proton conducting solid oxide fuel cells with chemically stable BaZr0.75Y0.2Pr0.05O3 electrolyte. Ceramics International 43(9), 7362–7365 (2017)
S.P. Shafi, L. Bi, S. Boulfrad, E. Traversa, Y and Ni co-doped BaZrO3 as a proton-conducting solid oxide fuel cell electrolyte exhibiting superior power performance. J. Electrochem. Soc. 162(14), F1498–F1503 (2015)
C.K. Ng, S. Ramesh, C.Y. Tan, A. Muchtar, M.R. Somalu, Microwave sintering of ceria-doped scandia stabilized zirconia as electrolyte for solid oxide fuel cell. Int. J. Hydrogen Energy 41(32), 14184–14190 (2016)
B. Wang, X.H. Liu, L. Bi, X.S. Zhao, Fabrication of high-performance proton-conducting electrolytes from microwave prepared ultrafine powders for solid oxide fuel cells. J. Power Sources 412, 664–669 (2019)
L. Bi, E. Fabbri, Z.Q. Sun, E. Traversa, BaZr0.8Y0.2O3-NiO composite anodic powders for proton-conducting SOFCs prepared by a combustion method. J. Electrochem. Soc. 158(7), B797–B803 (2011)
L. Bi, E. Fabbri, Z.Q. Sun, E. Traversa, Sinteractive anodic powders improve densification and electrochemical properties of BaZr0.8Y0.2O3 electrolyte films for anode-supported solid oxide fuel cells. Energy Environ. Sci. 4(4), 1352–1357 (2011)
L. Chevallier, M. Zunic, V. Esposito, E. Di Bartolomeo, E. Traversa, A wet-chemical route for the preparation of Ni-BaCe0.9Y0.1O3 cermet anodes for IT-SOFCs. Solid State Ionics 180(9–10), 715–720 (2009)
L. Bi, E. Fabbri, E. Traversa, Effect of anode functional layer on the performance of proton-conducting solid oxide fuel cells (SOFCs). Electrochem. Commun. 16(1), 37–40 (2012)
X. Xu, H.Q. Wang, J.M. Ma, W.Y. Liu, X.F. Wang, M. Fronzi, L. Bi, Impressive performance of proton-conducting solid oxide fuel cells using a first-generation cathode with tailored cations. J. Mater. Chem. A 7(32), 18792–18798 (2019)
E. Fabbri, L. Bi, J.L.M. Rupp, D. Pergolesi, E. Traversa, Electrode tailoring improves the intermediate temperature performance of solid oxide fuel cells based on a Y and Pr co-doped barium zirconate proton conducting electrolyte. RSC Adv. 1(7), 1183–1186 (2011)
J.A. Kilner, M. Burriel, Materials for intermediate-temperature solid-oxide fuel cells. Annu. Rev. Mater. Res. 44, 365–393 (2014)
R.R. Peng, T.Z. Wu, W. Liu, X.Q. Liu, G.Y. Meng, Cathode processes and materials for solid oxide fuel cells with proton conductors as electrolytes. J. Mater. Chem. 20(30), 6218–6225 (2010)
Y. Chen, S. Yoo, K. Pei, D.C. Chen, L. Zhang, B. deGlee, R. Murphy, B.T. Zhao, Y.X. Zhang, Y. Chen, M.L. Liu, An in situ formed, dual-phase cathode with a highly active catalyst coating for protonic ceramic fuel cells. Adv. Funct. Mater. 28(5), 1704907 (2018)
W. He, R.H. Yuan, F.F. Dong, X.L. Wu, M. Ni, High performance of protonic solid oxide fuel cell with BaCo0.7Fe0.22Sc0.08O3 electrode. Int. J. Hydrogen Energy 42(39), 25021–25025 (2017)
N.A. Danilov, A.P. Tarutin, J.G. Lyagaeva, E.Y. Pikalova, A.A. Murashkina, D.A. Medvedev, M.V. Patrakeev, A.K. Demin, Affinity of YBaCo4O7-based layered cobaltites with protonic conductors of cerate-zirconate family. Ceram. Int. 43(17), 15418–15423 (2017)
S.P. Jiang, Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: advances and challenges. Int. J. Hydrogen Energy 37(1), 449–470 (2012)
E.H. Da’as, L. Bi, S. Boulfrad, E. Traversa, Nanostructuring the electronic conducting La0.8Sr0.2MnO3 cathode for high-performance in proton-conducting solid oxide fuel cells below 600 °C. Sci. China Mater. 61(1), 57–64 (2018)
S.P. Jiang, Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: a review. J. Mater. Sci. 43(21), 6799–6833 (2008)
C. Peng, J. Melnik, J.X. Li, J.L. Luo, A.R. Sanger, K.T. Chuang, ZnO-doped BaZr0.85Y0.15O3-proton-conducting electrolytes: Characterization and fabrication of thin films. J. Power Sources 190(2), 447–452 (2009)
L. Bi, S.P. Shafi, E.H. Da’as, E. Traversa, Tailoring the cathode-electrolyte interface with nanoparticles for boosting the solid oxide fuel cell performance of chemically stable proton-conducting electrolytes. Small 14(32), 1801231 (2018)
Z. Hui, P. Michele, Preparation, chemical stability, and electrical properties of Ba(Ce1−xBix)O3 (x = 0.0−0.5). J. Mater. Chem. 12(12), 3787–3791 (2002)
Z.T. Tao, L. Bi, L.T. Yan, W.P. Sun, Z.W. Zhu, R.R. Peng, W. Liu, A novel single phase cathode material for a proton-conducting SOFC. Electrochem. Commun. 11(3), 688–690 (2009)
E. Fabbri, S. Licoccia, E. Traversa, E.D. Wachsman, Composite cathodes for proton conducting electrolytes. Fuel Cells 9(2), 128–138 (2009)
Z.T. Tao, L. Bi, Z.W. Zhu, W. Liu, Novel cobalt-free cathode materials BaCexFe1−xO3-delta for proton-conducting solid oxide fuel cells. J. Power Sources 194(2), 801–804 (2009)
Y.Y. Rao, S.H. Zhong, F. He, Z.B. Wang, R.R. Peng, Y.L. Lu, Cobalt-doped BaZrO3: a single phase air electrode material for reversible solid oxide cells. Int. J. Hydrogen Energy 37(17), 12522–12527 (2012)
E. Fabbri, I. Markus, L. Bi, D. Pergolesi, E. Traversa, Tailoring mixed proton-electronic conductivity of BaZrO3 by Y and Pr co-doping for cathode application in protonic SOFCs. Solid State Ionics 202(1), 30–35 (2011)
E. Fabbri, L. Bi, D. Pergolesi, E. Traversa, High-performance composite cathodes with tailored mixed conductivity for intermediate temperature solid oxide fuel cells using proton conducting electrolytes. Energy Environ. Sci. 4(12), 4984–4993 (2011)
A.B. Munoz-Garcia, M. Tuccillo, M. Pavone, Computational design of cobalt-free mixed proton-electron conductors for solid oxide electrochemical cells. J. Mater. Chem. A 5(23), 11825–11833 (2017)
A.B. Munoz-Garcia, M. Pavone, First-principles design of new electrodes for proton-conducting solid-oxide electrochemical cells: a-site doped Sr2Fe1.5Mo0.5O6 perovskite. Chem. Mater. 28(2), 490–500 (2016)
Z.Q. Wang, W.Q. Yang, S.P. Shafi, L. Bi, Z.B. Wang, R.R. Peng, C.R. Xia, W. Liu, Y.L. Lu, A high performance cathode for proton conducting solid oxide fuel cells. J. Mater. Chem. A 3(16), 8405–8412 (2015)
D. Poetzsch, R. Merkle, J. Maier, Proton uptake in the H+-SOFC cathode material Ba0.5Sr0.5Fe0.8Zn0.2O3: transition from hydration to hydrogenation with increasing oxygen partial pressure. Faraday Discuss. 182, 129–143 (2015)
W. Zhou, R. Ran, Z.P. Shao, Progress in understanding and development of Ba0.5Sr0.5Co0.8Fe0.2O3-based cathodes for intermediate-temperature solid-oxide fuel cells: a review. J. Power Sources 192(2), 231–246 (2009)
Y.M. Guo, Y. Lin, R. Ran, Z.P. Shao, Zirconium doping effect on the performance of proton-conducting BaZryCe0.8−yY0.2O3 (0.0 ≤ y ≤ 0.8) for fuel cell applications. J. Power Sources 193(2), 400–407 (2009)
X. Xu, H.Q. Wang, M. Fronzi, X.F. Wang, L. Bi, E. Traversa, Tailoring cations in a perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J. Mater. Chem. A 7(36), 20624–20632 (2019)
Y.P. Xia, Z.Z. Jin, H.Q. Wang, Z. Gong, H.L. Lv, R.R. Peng, W. Liu, L. Bi, A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance. J. Mater. Chem. A 7(27), 16136–16148 (2019)
S. Choi, C.J. Kucharczyk, Y.G. Liang, X.H. Zhang, I. Takeuchi, H.I. Ji, S.M. Haile, Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3(3), 202–210 (2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Xu, X., Bi, L. (2020). Proton-Responsive Nanomaterials for Fuel Cells. In: Sun, Z., Liao, T. (eds) Responsive Nanomaterials for Sustainable Applications. Springer Series in Materials Science, vol 297. Springer, Cham. https://doi.org/10.1007/978-3-030-39994-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-39994-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39993-1
Online ISBN: 978-3-030-39994-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)