Abstract
The scope of this work is designated to make use of multiple micro- and nano-additives to examine their effect collectively on fresh and hardened state of cement. Two sizes of volcanic ash, VA (6 and 17 μm), were incorporated alternatively at a constant ratio of 5%, whereas aluminosilicate was introduced via metakaolin and synthetic aluminosilicate nanoparticles. The incorporation of metakaolin and aluminosilicate nanocomposites serves for the purpose of varying the ratio of micro- to nano-aluminosilicates. Aluminosilicate composites were tailored via sol–gel method by using aramid polymer as a support, with varying precursor ratio to acquire three compositions with Si:Al ratios of 3:7, 5:5, and 7:3. BET analysis revealed that higher pore volume and surface area were associated with higher Si:Al ratio. Raman spectroscopy provided an insight into the pozzolanic rate of cement hydration as influenced by the composition of aluminosilicate nanoparticles. Micro- and nano-aluminosilicates displayed higher uniformity in pore structure as revealed by BET microstructural analysis. Nanoindentation results showed higher modulus and hardness values due to the reduction in the particle size of the volcanic ash. The combination of pozzolanic volcanic ash additives (6 μm VA) and aluminosilicate nanoparticles helped in the involvement of Portlandite to form additional calcium-aluminum-silicate-hydrate (C-A-S-H) phase leading to enhanced strength development.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Background
Volcanic ash is one of the most common pozzolanic cement additives that provides enhanced strength and durability properties [1]. Additionally, SiO2 and Al2O3 nanoparticles increase pozzolanic reactivity when added in small fractions to the Portland cement-based systems [2, 3]. Enhancement in compressive strength was observed when 2% of OPC was replaced with a combination of SiO2 and Al2O3 nanoparticles [4, 5], whereas other study showed an increase in tensile and flexural strength with a replacement ratio of up to 3% [6]. In the work performed by Oltulu et al. [7], a replacement ratio of 1.25% containing equal amounts of Al2O3 and SiO2 nanoparticles showed an optimum increase in compressive strength among all other single replacements except for 1.25% nano-SiO2, where these findings are corroborated by the minimal pore volume studied by water permeability [6, 7]. The binary composite incorporation in concrete also revealed a higher resistance toward shear, bending, and abrasion [5].
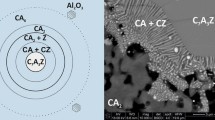
Metakaolin is another common supplementary cementitious material used for various cementing applications as it can be effectively used to control the rheological properties of modern-day cements. Metakaolin is a natural aluminosilicate, and it enhances the cementitious binder by producing aluminum-based crystalline gels, commonly known as the calcium-aluminum-silicate-hydrate (C-A-S-H) gels [8]. Similarly, zeolites are among the most aluminosilicates-rich pozzolanas with highly ordered system, also studied for their effect on concrete durability and performance. Investigations on the effect of zeolites on concrete showed that optimum replacement ratios are up to 10% [9]. Although metakaolin, zeolite, nano-SiO2/nano-Al2O3 binary mix, and aluminosilicate NPs all acquire SiO2 and Al2O3 species, they differ in their molecular structural arrangement that goes down to the atomistic level (see Fig. 1).
This work harnesses the chemical and physical combination of silica and alumina using volcanic ash, metakaolin, and aluminosilicate nanoparticles while examining the effect of aluminosilicate at the micro- and nanoscale.
2 Experimental Procedure
2.1 Materials and Methods
The aluminosilicates were prepared using the procedural protocol originated by Al-Omani et al. [10] for recovering titania nanoparticles except that the temperature used for recovering the aluminosilicate nanoparticles was 550 °C rather than 450 °C. Three compositions of aluminosilicate NPs were prepared in which precursor amounts (TEOS and aluminum ethoxide) were varied alternatively to obtain composites with Si:Al ratios of 3:7, 5:5, and 7:3 abbreviated as (3SA), (5SA), and (7SA), respectively. The cement paste specimens were prepared with the compositions shown in Fig. 2 with the corresponding test performed, where volcanic ash was obtained from the Super Burkani factory in Jeddah-Medina Province, Saudi Arabia, and metakaolin from Advanced Cement Technologies, St. Blaine WA, USA. All cement paste mixtures were hydrated with a w/c ratio of 0.5 and studied by Raman during their freshly hydrated state and by nanoindentation and BET using water/nitrogen analysis at their hardened state (w/c = 0.4).
2.2 Characterization of Aluminosilicate NPs
According to our XPS data, the high Si 2p binding energy is more characteristic for SiO2 which was observed for 7SA at 103.09 eV. This energy shift decreases in response to increasing the aluminum content and reaches a value of 102.70 eV, which is more characteristic to aluminosilicates and related to Si-O-Al bond [10]. TEM micrographs revealed more crystallization with decreasing Si:Al ratio (see Fig. 3).
HR TEM micrographs for the synthesized nanoparticles corroborate the previous findings. Crystallization of alumina becomes easier at lower temperature leading to the reduction in the particle size. From this standpoint, the amorphous layer surrounding the agglomerated portion was observed from HR TEM images, as shown in Fig. 4, and may belong to SiO2 surrounding some crystalline phases of Al2O3.
Surface areas obtained by N2 adsorption test of the three aluminosilicate NPs are in gradual increase with increasing SiO2 over Al2O3 content, and all composites showed higher surface area than metakaolin (see Table 1).
3 Discussion
3.1 In Situ Raman Study
In situ Raman spectroscopy measurements are effective in tracking the extent of pozzolanic reaction by the evolution of Raman band at ~360 cm−1 designated for Ca(OH)2 or by the broad bands in the region 600–700 cm−1 designated to deformation of Ca-O polyhedral [11, 12]. The combinations shown in Fig. 1 were recorded for their spectra at 1 h interval for 12 h, then after 24, 36, and 48 h (see Fig. 5). Control (17 μm VA) sample developed aluminate phases such as monosulfoaluminate, gypsum, and aragonite, whereas insoluble anhydrite was more evident for control (6 μm VA) which proves the effect of VA particle size on variations of sulfate phases and carbonation events. Additionally, incorporation of aluminosilicate NPs affected the resultant sulfate phases. For instance, 2-7SA (17 μm VA) revealed formation of ettringite, whereas 2-7SA (6 μm VA) showed evolution of insoluble anhydrite [13]. Although bands of ettringite and insoluble anhydrite are similar, ettringite can be distinguished from insoluble anhydrite from the strong signals at 980–1002 cm−1. Spectra of 2-5SA samples with both sizes of volcanic ash are quite similar and revealed the sulfate phase in the form of ettringite. Aragonite is a carbonate phase that has evolved upon decreasing both Si:Al ratio and volcanic ash particle size, as it is the case for 2-3SA containing 6 μm VA. Additionally, the volcanic ash particle size resulted in varying the sulfate phase evolved from 2-3SA (see Table 2 for summary of band assignment and phases evolved for all samples).
3.2 BET Microstructural Analysis
N2 molecules are not accessible to all pores, mainly excluding ones below 2–4 nm in radii, whereas H2O vapor acquires a smaller molecule size and a dipole that facilitates its penetration to interlayer spaces [19]. On the light of this understanding, we used the ratio of total pore volume found from both adsorbates (N2 and H2O) to infer how much volume is accessible to nitrogen compared to water vapor and therefore to estimate the total large to capillary pores and correlate them to compressive strength (see Fig. 6). It is expected that the lower \( {V}_{{\mathrm{N}}_2} \)/\( {V}_{{\mathrm{H}}_2\mathrm{O}} \) is accompanied with higher compressive strength and vice versa for \( {V}_{{\mathrm{H}}_2\mathrm{O}} \)− \( {\mathrm{V}}_{{\mathrm{N}}_2} \). The lowest value of \( {V}_{{\mathrm{N}}_2} \)/\( {V}_{{\mathrm{H}}_2\mathrm{O}} \)was found to be for 2-7SA (6 VA), followed by 2-5SA (6 VA), and then control (6 VA). The same trend goes for \( {V}_{{\mathrm{H}}_2\mathrm{O}} \)− \( {V}_{{\mathrm{N}}_2} \) value; highest value was found for 2-7SA (6 VA), followed by 2-5SA (6 VA), then control (6VA), 2-3SA (6 VA) and 2-3SA (17 VA) where the latter three are in proximity .
3.3 Nanoindentation Test
A typical load–displacement curve (also called P-H curve) for 2-3SA (6 μm VA) is shown in Fig. 7. Irregular curves which might have resulted from voids were discarded. The curve is initiated by increasing the load constantly followed by constant holding (where material suffers creeping), then constant unloading. The applied loading is associated with permanent plastic deformation and temporary elastic deformation, where the latter is recovered as indenter starts unloading [20]. The initial slope of elastic unloading allows inferring two mechanical properties: modulus (M) and hardness (H) [21, 22]. It was suggested by Doerner and Nix that the unloading stiffness can be obtained from a linear fit of the upper one-third of the unloading curve [23]. However, the hardness calculated by this method can be different than that when directly determining the size of residual hardness impression. Oliver et al. [21] described a simple power law relation to avoid the deviation resulting from (1) difference in stiffness between first and last unloading and (2) significant variation in the stiffness resulting from final unloading which depends on how much of the curve is used to fit [21]. However, the analysis must be complemented with SEM for assessing homogenous C-S-H regions .
Hardness and modulus obtained from averaged mapped areas showed that all combinations with 6 μm volcanic ash inclusion were associated with higher hardness than their counterparts containing 17 μm volcanic ash (see Fig. 8). The overall trend of hardness is in agreement with that found in BET microstructural analysis which significantly corroborates our findings. It can be found from modulus values that all blends, except for 2-7SA (17 VA) and 2-3SA (6 VA), fall within the same region found for high-stiffness C-S-H (25–35 GPa) and for calcium hydroxide (35–45 GPa) [24]. The incorporation of metakaolin and aluminosilicate NPs both revealed a significant enhancement in overall hardness and modulus, except for 2-7SA (17 VA), which showed very little improvement. The represented hardness and modulus values for 2-7SA (6 VA) and 2-5SA (6 VA) were even greater than those reported in previous studies [25, 26]. The increased hardness and modulus of control samples over plain OPC can be attributed to C-A-S-H phases formed upon incorporating metakaolin. However, aluminosilicate NPs revealed only enhanced performance (for 17 μm VA) over metakaolin when Si:Al ratio was the smallest. This anomalous behavior can be ascribed to leached silica resulting from fast consumption of portlandite by 7SA and 5SA resulting in lower strength. Contrarily, the strength was enhanced for all 6 μm VA samples over their corresponding control samples with the opposite trend, which could be due to higher association degree of fine VA in the reactivity at early stage. Further investigation by compression test is highly recommended.
4 Conclusion
In this work, aluminosilicate NPs were successfully prepared using aramid support and examined for their effect on cement performance. Lower Si:Al ratio resulted in higher crystallization event and slower reactivity. The aluminosilicate species greatly influenced the early hydration of cement according to Raman spectra, where the composition of aluminosilicate NPs and volcanic ash particle size influenced the evolution of sulfate-based phases. The formation of aluminum-based phases by mico- and nano-aluminosilicates corresponded to higher pore structure uniformity. Yet, the choice of composition of aluminosilicate NPs and their supporting additives is highly critical for cement strength. Fast-reacting aluminosilicate NPs were found to be best associated with fine volcanic ash to minimize silica leaching events and engender optimal strength enhancement.
References
Kupwade-Patil K, Al-Aibani AF, Abdulsalam MF, Mao C, Bumajdad A, Palkovic SD, Büyüköztürk O (2016) Microstructure of cement paste with natural pozzolanic volcanic ash and Portland cement at different stages of curing. Constr Build Mater 113:423–441
Kupwade-Patil K, Cardenas H (2013) Electrokinetic nanoparticle treatment for corrosion remediation on simulated reinforced bridge deck. J Nanopart Res 15:1–16
Jamsheer AF, Kupwade-Patil K, Büyüköztürk O, Bumajdad A (2018) Analysis of engineered cement paste using silica nanoparticles and metakaolin using 29Si NMR, water adsorption and synchrotron X-ray diffraction. Constr Build Mater 180:698–709
Silva JV, Ismael R, Carmo RNF, Lourenço C, Soldado E, Costa H, Júlio E (2016) Influence of nano-SiO2 and nano-Al2O3 additions on the shear strength and the bending moment capacity of RC beams. Constr Build Mater 123:35–46
Ismael R, Silva JV, Carmo RNF, Soldado E, Lourenço C, Costa H, Júlio E (2016) Influence of nano-SiO2 and nano-Al2O3 additions on steel-to-concrete bonding. Constr Build Mater 125:1080–1092
Rashad AM (2013) A synopsis about the effect of nano-Al2O3, nano-Fe2O3, nano-Fe3O4 and nano-clay on some properties of cementitious materials – a short guide for civil engineer. Mater Des (1980–2015) 52:143–157
Oltulu M, Şahin R (2013) Effect of nano-SiO2, nano-Al2O3 and nano-Fe2O3 powders on compressive strengths and capillary water absorption of cement mortar containing fly ash: a comparative study. Energ Buildings 58:292–301
Siddique R, Klaus J (2009) Influence of metakaolin on the properties of mortar and concrete: a review. Appl Clay Sci 43:392–400
Valipour M, Pargar F, Shekarchi M, Khani S (2013) Comparing a natural pozzolan, zeolite, to metakaolin and silica fume in terms of their effect on the durability characteristics of concrete: a laboratory study. Constr Build Mater 41:879–888
Al-Omani SJ, Bumajdad A, Al Sagheer FA, Zaki MI (2012) Surface and related bulk properties of titania nanoparticles recovered from aramid–titania hybrid films: a novel attempt. Mater Res Bull 47:3308–3316
Garbev K, Stemmermann P, Black L, Breen C, Yarwood J, Gasharova B (2007) Structural features of C–S–H(I) and its carbonation in air—a Raman spectroscopic study. Part I: Fresh phases. J Am Ceram Soc 90:900–907
Black L, Breen C, Yarwood J, Garbev K, Stemmermann P, Gasharova B (2007) Structural features of C–S–H(I) and its carbonation in air—a Raman spectroscopic study. Part II: Carbonated phases. J Am Ceram Soc 90:908–917
Black L (2009) Raman spectroscopy of cementitious materials. Spectrosc Inorg Organometall Compounds 40:72–127
Renaudin G, Segni R, Mentel D, Nedelec J-M, Leroux F, Taviot-Gueho C (2007) A Raman study of the sulfated cement hydrates: Ettringite and monosulfoaluminate. J Adv Concr Technol 5:299–312
Black L, Breen C, Yarwood J, Deng CS, Phipps J, Maitland G (2006) Hydration of tricalcium aluminate (C3A) in the presence and absence of gypsum-studied by Raman spectroscopy and X-ray diffraction. J Mater Chem 16:1263–1272
Bensted J (1976) Uses of Raman spectroscopy in cement chemistry. J Am Ceram Soc 59:140–143
Kirkpatrick RJ, Yarger JL, McMillan PF, Ping Y, Cong X (1997) Raman spectroscopy of C-S-H, tobermorite, and jennite. Adv Cem Based Mater 5:93–99
Ibáñez J, Artús L, Cuscó R, López Á, Menéndez E, Andrade MC (2007) Hydration and carbonation of monoclinic C2S and C3S studied by Raman spectroscopy. J Raman Spectrosc 38:61–67
Rouquerol J, Rouquerol F, Llewellyn P, Maurin G, Sing KSW (2013) Adsorption by powders and porous solids: principles, methodology and applications. Elsevier Science, Amsterdam
Zhu W, Hughes JJ, Bicanic N, Pearce CJ (2007) Nanoindentation mapping of mechanical properties of cement paste and natural rocks. Mater Charact 58:1189–1198
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7:1564–1583
Palkovic SD, Kupwade-Patil K, Yip S, Büyüköztürk O (2018) Random field finite element models with cohesive-frictional interactions of a hardened cement paste microstructure. J Mech Phys Solids 119:349–368
Doerner MF, Nix WD (1986) A method for interpreting the data from depth-sensing indentation instruments. J Mater Res 1:601–609
Constantinides G, Ulm FJ, Van Vliet K (2003) On the use of nanoindentation for cementitious materials. Mater Struct 36:191–196
Hou P, Kawashima S, Kong D, Corr DJ, Qian J, Shah SP (2013) Modification effects of colloidal nanoSiO2 on cement hydration and its gel property. Compos Part B 45:440–448
Zyganitidis I, Stefanidou M, Kalfagiannis N, Logothetidis S (2011) Nanomechanical characterization of cement-based pastes enriched with SiO2 nanoparticles. Mater Sci Eng B 176:1580–1584
Acknowledgments
The authors gratefully acknowledge the College of Graduate Studies (CGS), Kuwait University, Research Sector Project Unit (RSPU): Project No. (GS 01/01), Nanoscopy Center, Nanotechnology Lab Project No. GE01/07, and Dr. Shamsun Nahar for their support and funds.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Masmoudi, R., Bumajdad, A., Kupwade-Patil, K. (2020). Effect of Aluminosilicate Nanoparticles on Cement Blends Containing Volcanic Ash and Metakaolin. In: Bumajdad, A., Bouhamra, W., Alsayegh, O., Kamal, H., Alhajraf, S. (eds) Gulf Conference on Sustainable Built Environment. Springer, Cham. https://doi.org/10.1007/978-3-030-39734-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-39734-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39733-3
Online ISBN: 978-3-030-39734-0
eBook Packages: EnergyEnergy (R0)