Abstract
Nanomaterials have become relevant in large-scale industrial applications and are used in many common devices. This may have negative effects in terms of health and environment. There is increasing concern about nanotoxicity in plants because they are not only a fundamental part of the ecology but also the basis of trophic systems. Nanotoxicology is the branch of toxicology responsible for describing the effects of nanomaterials on living organisms and the environmental consequences resulting from their use. Thorough studies are important, because nanomaterials have stronger toxicological effects than those reported for the same materials in bulk or in solution. The main toxicological factor observed in various organisms is oxidative stress. Many techniques have been used to evaluate the toxicity of nanomaterials in plants; however, currently, there is no consensus on the optimal method for evaluating toxicity in plants. A concern is that bioaccumulation of nanoparticles in plants can enter the food chain of animals and humans constituting a health problem. Therefore, extensive safety research projects and regulations are urgently needed. To understand some toxic effects that nanomaterials may have on plants, this chapter describes basic concepts of plant nanotoxicology, including some properties of nanomaterials, the anatomy and physiology of plants, as well as the methodologies so far existing to evaluate the toxicity of nanoparticles. The resistance and responses of plant cells to nanomaterials are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Verse
Verse Paracelsus (1493–1541): “dosis sola facit venenum.” “The dose alone makes the poison.”
The etymological roots of the term toxicology stem from the Greek words τοξικον (toxikon = poison) and λογια (logia = treatise or science). Toxicology, therefore, was defined as the field of science responsible for the study of poisons. Current research largely focuses on elucidating and describing the potential effects of different substances and materials considered harmful to living organisms. Other subfields of toxicology are related to nature, incidence, dose-response relationship, cellular uptake, severity (genetic, cellular, systemic, and immunogenic, among others), reversibility, and mechanisms of action of compounds and to response mechanisms in the organism (Burcham 2014; Murphy 1979). Conversely, the prefix nano (νανοζ), which originally meant dwarf, is currently used in the International System of Units to indicate a factor of 10−9.
Thus, nanotoxicology is defined as the branch of toxicology responsible for describing the effects of nanomaterials (or the nanometric scale) on living organisms, considering their physicochemical characteristics, dose-response relationship, cellular uptake, mechanisms of action, severity, and reversibility, among other variables.
2 Nanomaterials
To standardize terms in scientific and industrial contexts, at least two definitions of nanomaterials have been accepted for regulatory purposes. First, the definition adopted by the International Organization for Standardization/Technical Specifications (ISO/TS) 80004-1:2015 (ISO 2015) establishes that nanomaterials are those materials with at least one of their dimensions (internal, external, or surface) on the nanoscale (1–100 nm). In turn, the definition published by the European Commission in 2011 (2011/696/EU) considers a nanomaterial as “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm-100 nm” (European Union Commission Recommendation 2011).
Nanomaterials have gained considerable relevance in industrial fields as components of paints, cosmetics, rubber, additives, electronics, environmental remediation devices, tools, textiles, and sports equipment. In the biomedical field, nanomaterials are mainly used in drug release, imaging, implant coatings, and aseptic methods (Roszek et al. 2005); in agriculture, they are used for the controlled release of herbicides, pesticides, and fertilizers. Currently, nanomaterial engineering research has gained relevance (Caballero-Guzman and Nowack 2016; Charitidis et al. 2014; Joo and Zhao 2017). In all the aforementioned areas, properties such as electrical conductivity, high resistance, structure, electronic affinity, catalysis, photo-optical, and electronic characteristics, functionalization, biocompatibility, flexibility, and malleability are used for specific purposes, and many nanomaterials are produced on a large scale, which has increased their presence in the environment (Goswami 2017; Peralta-Videa et al. 2011).
One of the disadvantages of nanomaterials is their potential toxicological-environmental effects. It has been shown that, due to their physicochemical characteristics (size, surface area, shape, chemical composition), nanomaterials have stronger toxicological effects than those reported for the same materials in bulk or in solution (Joo and Zhao 2017; Stone et al. 2010). Most studied nanomaterials have shown dose-dependent toxicity, which is directly related to the availability of the nanomaterial in the environment (culture medium, air, water, soil, and food, among others), to the mechanism of cellular uptake or penetration, and, lastly, to the duration of contact with the nanomaterial (Buzea et al. 2007; Krug and Wick 2011; Navarro et al. 2008). The primary toxicological factor observed in various organisms, regardless of the nanomaterial, is oxidative stress, a process by which the production of reactive oxygen (ROS) and nitrogen species (RNS) is induced. Cellular damage, such as apoptosis, mitochondrial damage, plastid damage, autophagy, and genotoxic effects due to deoxyribonucleic acid (DNA) fragmentation and chromosomal abnormalities have also been reported. In tissues, the damage depends on the type and amount of the captured nanomaterial and the exposed tissue, causing histological changes, cell growth inhibition, carcinogenic and/or teratogenic effects, and even death (Buzea et al. 2007; Gerloff et al. 2017; Lewinski et al. 2008).
2.1 Classification of Nanomaterials
There is no single classification of nanomaterials. Depending on the authors and research areas, aspects such as the chemical basis (organic or inorganic) (Mageswari et al. 2016), origin (natural or artificial), and physicochemical characteristics (morphology, volume, surface area, surface charge, crystallinity) are considered for classification purposes. Finally, they can also be classified based on their dimensionality (0, 1, 2, and 3D) (Khan et al. 2019; Lewinski et al. 2008). This chapter will address a classification focused on their chemical basis, albeit mentioning other characteristics.
2.1.1 Carbon-Based Nanomaterials
Graphene materials, fullerenes, carbon nanotubes, and nanodiamonds, among others, are included in this group. Graphene is described as a two-dimensional (2D) sheet of sp2-bonded carbon atoms arranged in a flat hexagonal lattice structure, similar to a honeycomb (Allen et al. 2010). This arrangement can be modified on its surface, by oxidation-reduction reactions or by functionalization, which directly affects its electronic structure and therefore its physicochemical characteristics and interaction with other materials, including cells. Studies have shown that graphene toxicity is dose-dependent and that sheet size is a determining factor (Montagner et al. 2016; Sanchez et al. 2012). Fullerenes, in turn, consist of between 28 and 1500 carbon atoms arranged in box-like structures. Their structures can have up to 120 different symmetries, thanks to the arrangement of their atoms in pentagonal and hexagonal rings. The most symmetrical fullerene is the C60 fullerene, with a size of 0.7 nm and a spheroid shape similar to that of a soccer ball. As with other nanomaterials, C60 fullerenes have a large surface area and, despite their low reactivity, are susceptible to functionalization, which makes it possible to improve their chemical or biological activity (Isaacson et al. 2009). Carbon nanotubes (1D) have an elongated shape, similar to fiber. They can be single-walled carbon nanotubes (SWCNTs) or multiwalled carbon nanotubes (MWCNTs). As with other allotropes of carbon, they are susceptible to chemical modification, which determines their physical and chemical characteristics and is related to their fate and toxicity potential (Alshehri et al. 2016; Allegri et al. 2016). Nanodiamonds, conversely, are more stable than other carbon-based nanomaterials . To date, studies have reported that these allotropes have low toxicity (Schrand et al. 2007).
2.1.2 Inorganic-Based Nanomaterials
Inorganic-based nanomaterials include nanoparticles (0D) and thin films (2D). Both materials can have a metallic, ceramic, or semiconductor composition. Metallic nanomaterials contain zerovalent compounds such as iron, silver, and gold. They are mainly synthesized from metal salt precursors (Khan et al. 2019). Ceramic materials are nonmetallic inorganic solids produced by heat treatment; they can be found in amorphous, polycrystalline, dense, porous, or hollow shapes (Yamamoto et al. 2004). Semiconductors, in turn, have intermediate properties, that is, between metals and nonmetals. They usually consist of elements from groups II to VI or III to V (Lewinski et al. 2008; Owen and Brus 2017). Inorganic-based nanomaterials are currently produced by controlling synthetic methods for generating these materials with specific morphology, size, charge, coating, and optoelectrical properties. Biocompatibility or biocidal activity has been described for many nanomaterials. Thus, for example, among the toxic mechanisms against various pathogens (bacteria, fungi, and protozoa, among others) (Rodríguez-Torres et al. 2019; Vazquez-Muñoz et al. 2017) identified in many metal nanoparticles (Ag, Cu, and Zn, among others), the release of metal ions that interact with various cellular components has been reported (Krug and Wick 2011). Another significant deleterious effect reported for metallic, ceramic, and semiconductor nanoparticles is mechanical stress on cells or tissues due to the accumulation and/or morphology of the nanomaterial (Yamamoto et al. 2004). Unfortunately, cytotoxic effects are also observed in nonpathogenic organisms, in animal (including human) cells and tissues, and plants.
2.1.3 Quantum Dots
Quantum dots are semiconductor materials formed from elements of groups II to VI or III to V or from carbon-based materials. They are crystalline nanometric structures with a diameter that is smaller than twice the Bohr radius of its exciton (electron hole-electron pair), thereby producing their quantum confinement. These materials have adjustable band gaps depending on the size of the material and on its crystalline structure, in addition to showing remarkable luminescent properties. Although quantum dots are usually better known, other 1D and 2D crystalline nanomaterials have similar characteristics (Frecker et al. 2016; Owen and Brus 2017; Valizadeh et al. 2012).
2.1.4 Organic-Based Nanomaterials (Biomaterials)
Organic-based nanomaterials (lipids, carbohydrates, and other biopolymers) have been primarily studied in biomedical applications, particularly in the development of nanomaterials for drug delivery. Lipid nanomaterials can be micelles, liposomes, solid lipids, and nanostructured lipid carriers, which are used in the pharmaceutical, cosmetic, and food industries, mainly for molecule encapsulation and delivery to tissues (Angelova et al. 2017; Barriga et al. 2019; Jobin and Netto 2019; Tapeinos et al. 2017). Polymeric nanoparticles are synthesized from cross-linked biopolymers. Alginate, chitosan, gelatin, hyaluronic acid, polylactic-co-glycolic acid (PLGA), polylactide (PLA), polycaprolactone (PCL), and polyanionic cellulose (PAC) are among the most studied polymeric nanoparticles (Kumari et al. 2010; Nitta and Numata 2013). Dendrimers are polymeric molecules with a regular size and geometry and with well-defined and controlled branching via the appropriate selection of materials for their synthesis, thereby defining properties such as size, porosity, cavity type, hydrophobicity, and hydrophilicity, among others (Kesharwani et al. 2018; Kim et al. 2018). With the development of genetic engineering, protein-based nanoparticles have been recently produced from the synthesis of self-assembling protein subunits, which makes it possible to control characteristics such as surface loading, encapsulation, and ligand release, among others (Heddle et al. 2017; Tarhini et al. 2017). To date, few cytotoxic effects of organic-based nanomaterials have been reported, although nanotoxicology remains a relatively young research field.
2.2 Nanomaterial Production
According to some authors, nanomaterials have been naturally produced through processes such as volcanism, hydrothermal systems (Navarro et al. 2008), soil erosion, and significant energy release events such as lightning or meteorite impact (Isaacson et al. 2009). Interestingly, the evolution of living beings on the planet generated the production of nanomaterials from cellular processes, such as the production of nanoparticles from bacteria (Faivre and Schüler 2008; Li et al. 2016) and fungi (Park et al. 2016). The formation of carbon nanomaterials as a result of the combustion of organic materials in forest fires has also been observed (Buzea et al. 2007). With mass production, nanomaterials have become a potential risk. Different sources of production include fossil fuel and agricultural waste burning, internal combustion engines, chemical-biological waste generation and disposal, and water treatment processes (Bour et al. 2015; Nowack 2017). Recently, material engineering has become highly relevant in large-scale industrial applications, as has research on the controlled production of nanomaterials with a specific shape and size, and with high purity, which are used in a large number of commonly used devices (Koivisto et al. 2017).
2.3 Transport, Distribution, and the Fate of Nanomaterials in the Environment
In nature, nanometric- and micrometric-scale materials are ubiquitously distributed. The wind is one of the most important mechanisms of natural transport of nanomaterials (Joo and Zhao 2017). Propagation through bodies of water not only by surface runoff and ocean currents but also by aerosol formation (also determined by local climatic factors) is another key dispersion mechanism (Gottschalk et al. 2011; Joo and Zhao 2017). In addition, anthropogenic activities, both industrial and agricultural, are an important transport route. Nanomaterials reach the air, bodies of water, the soil, streambeds, and the seabed naturally (due to atmospheric deposition, rain, and surface runoff, among other processes) or due to poor landfill management, via sewage sludge, or through its application in agricultural soils, among other activities (Wigger et al. 2015).
In the environment, nanomaterials may be exposed to various processes that affect their mobility patterns, bioavailability, fate, and toxicity (direct or indirect). Such processes involve homo-aggregation with nanomaterials of the same composition, hetero-aggregation with nanomaterials of different composition or with other molecules, changes in size, shape, surface charge, chemical stability, age, phototransformation, dissolution, interactions with other ions, and interactions and/or transformation with macromolecules and/or biopolymers contained in organic matter (Dwivedi et al. 2015; Goswami 2017).
3 Plants
To understand the toxic effects that naturally or artificially produced nanomaterials may have on plants, this chapter will provide an overview of the general morpho-anatomical and physiological characteristics of plants that affect the mechanisms of the interactions and the ease of uptake from the environment and discuss the resistance and responses of plant cells to nanomaterials. The plant kingdom is diverse; it includes nonvascular plants (bryophytes), seedless vascular plants (ferns, whisk ferns, horsetails, and lycophytes), and seed vascular plants, which are divided into two broad groups: gymnosperms (cycads, ginkgo, gnetophytes, and conifers) and angiosperms, which produce flowers and seeds (magnoliids and mono- and dicotyledonous plants) (Kaplan 2001).
Plant cells are delimited by a cell membrane (plasmalemma) that consists of phospholipids and proteins, which are coated with a semirigid cell wall with a similar architecture in all plants, composed of cellulose microfibrils, polysaccharides, lignin (only in vascular plants), structural proteins, and enzymes (peroxidases, pectin esterases, extensins, and expansins, among others). They may also contain phenolic compounds, gums, resins, silica, calcium carbonate, suberin, waxes, cutin, and Ca2+. The thickness can vary from a few hundred nanometers to a few micrometers. The composition of the cell wall and membrane varies depending on the species and even on the tissue (Khalil et al. 2010; Lee et al. 2011) (Fig. 3.1a). Adjacent cells are interconnected through plasmodesmata, that is, intercellular channels in which proteins and membranes subdivide cell walls into microscopic channels of 3–4 nm in diameter and that can reach just over 10 nm. Plasmodesmata are contact areas through which water and nutrients flow from or to the vascular system (Fig. 3.1b) (Knox and Benitez-Alfonso 2014; Sevilem et al. 2015). As in other eukaryotic organisms, the cellular cytoplasm has networks of microtubules and organelles, such as the endoplasmic reticulum, the Golgi complex, mitochondria, the cell nucleus, and nucleic acids. Additionally, plant cells have chloroplasts, with a large number of photosynthetic pigments (chlorophyll a and b) (Staehelin 2003); amyloplasts, the function of which is to store starches and other reserve substances; and chromoplasts, which contain pigments that act as both photosynthetic pigments and antioxidants, such as α- and β-carotenes, lycopene, cryptoxanthins, lutein, lycopene, and anthocyanins (water-soluble flavonoids) (Vershinin 1999). Vacuoles or tonoplasts are organelles that occupy a large part of the cell volume. They contain water, sugars, other organic and inorganic solutes, and pigments (Hall et al. 1984).
3.1 Nonvascular Plants
Bryophytes (liverworts, hornworts, and mosses) (Fig. 3.2a–c) are plants that did not develop a vascular system, which is why they are known as poikilohydric plants (dependent on a layer of water on their surface to maintain their hydration). Most bryophytes can only develop in aquatic habitats, although many species are tolerant to dehydration (Zechmeister et al. 2003). Their dependence on water has been a consequence of selection pressure; therefore, these plants form simple, small, and thin tissues, and their cells lack lignin (Roberts et al. 2012). During their life cycle, they develop spores in specialized structures or sporophytes. Once bryophytes germinate, their sporophytes produce structures termed gametophores, which have elongated cells that fix the plant and absorb water from soil termed rhizoids. The thallus is the undifferentiated photosynthetic tissue that grows above the substrate and consists of epidermal and subdermal cells, thin-walled parenchymal cells, and conductive cells. The leaves, when present, usually have a thick cell wall, except in the midribs and margins, which have multiple layers of differentiated cells. Gametophytes exhibit structures that are responsible for producing gametes (sexual reproduction cells) (Fig. 3.2a–c). Bryophytes are highly sensitive to contamination; therefore, they are considered good indicators (Sheffield and Rowntree 2009; Zechmeister et al. 2003).
3.2 Vascular Plants
Vascular plants have specialized, complex tissues, including meristematic, dermal, basal, vascular, and root tissues. These plants consist of different cell lines with distinct characteristics (Fig. 3.3).
Diagram of the main structures of a plant. Above the substrate, growth zones (shoot apical meristem) and axillary buds will give rise to branches. The stem shows lenticels, branches, and leaves, whereas the leaves have stomata. The vascular cylinder runs internally. Under the substrate, the root cap, the mucigel sheath, and the formation of lateral roots and root hairs are shown
3.2.1 Tissues
The dermal tissue or epidermis is formed by a layer of cells located on the surface of the plant. It is usually covered with a waxy (lipophilic) cuticle, which protects the plants from water loss and pathogen attacks. It has cuticular pores, the diameter of which ranges from approximately 2 to 2.4 nm (Eichert and Goldbach 2008). The epidermis also has specialized cells (guard cells) that respond to variations in the external and internal environments by changing shape, opening, or closing pores or stomata (intercellular gaps up to 100 nm or greater) (Eichert and Goldbach 2008), depending on the stimulus. They are checkpoints through which water vapor, oxygen, and carbon dioxide travel. Some plant species have structures that are specialized for gas exchange termed lenticels; they lack a cuticle, and their calculated pore size is larger than 100 nm (Lendzian 2006). Trichomes are structures with defensive functions in plants that accumulate heavy metals collected from the air (Fig. 3.4) (Lavid et al. 2001; Psaras et al. 2000).
Diagram of leaf tissues. External structures such as the cuticle and the epidermis are shown, in addition to trichomes associated with the upper epidermis. Stomata are located in the lower epidermis. Photosynthetic (palisade and spongy mesophyll) parenchyma cells and different components of the vascular cylinder are also shown
Meristems are clusters of undifferentiated, thin-wall (<100 nm in thickness) stem cells (Galway 2006), which are responsible for generating cell populations that differentiate into (dermal, vascular, and growth) tissues formed at different stages of maturation. Meristems are located at growth sites in root and stem apices and in vascular cambium and cork cambium tissues (plants with secondary growth). The basal tissue mainly consists of parenchymal cells, which typically have a cell wall that varies in thickness, albeit thin, and in morphology. They are the most abundant cells in plants and are part of the mesophilic or photosynthetic (leaves and stems), epidermal, cortical (a region located between the vascular bundles and epidermis), and medullary (center of the stem) tissues and of the vascular system. Parenchyma cells specialized in storage are found in bulbs and tubers, seeds (endosperm), and cotyledons (Gibson 2012; Morris et al. 2016). In aquatic plants, these cells have a tissue termed the aerenchyma, which is characterized by intercellular spaces containing air (a specialized tissue for plant buoyancy) (Smirnoff and Crawford 1983). They can also be part of the glandular, secretory, and trichome systems. Collenchyma cells are also part of the basal tissue, small, and elongated, and their primary cell walls have different thicknesses and proportions of cellulose, hemicellulose, and pectin. These components allow them to be more elastic than other cells. They provide support to leaves and stems and are usually found near the surface of the cortex and around vascular junctions in petiolate leaves and stems (Leroux 2012). Sclerenchyma cells (fibers and sclereids) are short cells with a thick, densely lignified, and rigid secondary cell wall. They provide mechanical support to the plant. They are found next to vascular ducts and in leaf veins and margins (Fig. 3.5a) (Calvin 1967).
The evolution of lignified structures in the form of tubules, which transport water and nutrients as components of the xylem and phloem in vascular systems, has contributed to the diversification of plants with roots, stems, and leaves. The xylem is the system responsible for transporting water and dissolved minerals, consisting of tracheids and vessel elements (Fig. 3.5b). Both structures are generated by living cells, which die at maturity, leaving their thick, lignified, and interconnected cell walls behind. Collectively, they form conductive tubules, the cell walls of which usually have thin areas termed pits, with an average pore diameter ranging from 5 to 420 nm in angiosperms and from 10 nm to 200 μm in gymnosperms (Fig. 3.5c) (Carlquist and Schneider 2002; Jansen et al. 2009). Liquid flows through these pits inward from the tissues or outward to the tissues. Tubule architectures and pore sizes vary between species (Choat et al. 2008). Tracheids are aligned side by side, whereas vessel elements are aligned end to end; therefore, substances in the latter are transported vertically (Luo et al. 2019). Conversely, the phloem, which functions in the transport of sugars and solutes, consists of tubular filtration elements (sieve elements) formed by living cells that are interconnected through lateral and terminal openings in their cell walls (sieve plates). Sieve elements are surrounded by specialized parenchymal cells, termed companion cells, which are responsible for transporting sugars to conductive tubes through plasmodesmata (Fig. 3.5b) (White 2012). In conifers and primitive vascular plants, sieve cells are found in the phloem. In vascular plants, the xylem and phloem are arranged in long, continuous strands, forming vascular bundles with arrangement patterns that are genetically determined and that differ significantly among monocots, dicots, and other groups (Figs. 3.5c, d) (White 2012).
Roots usually grow below the surface; their function is to absorb water and minerals, to store nutrients, and to fix the plant to the soil. They have an apical meristem with a root cap, which is responsible for protecting the apex against mechanical damage and against the action of heavy metals present in the soil. In this region of the plant, cells secrete mucilage and exudates, composed of polysaccharides, organic acids, alcohols, secondary metabolites, antimicrobial proteins, and extracellular DNA (Driouich et al. 2013), thereby varying the microenvironmental conditions near the root tissue and promoting nutrient availability through their dissolution by changing the pH and substrate moisture (Baetz and Martinoia 2014). Morphologically, from outside to inside, the layers of root cells are arranged from the epidermis (protection), through the cortex (storage), to the endodermis formed by one or several layers of suberin-coated cells, known as Casparian strips , which function as a hydrophobic barrier (Chen et al. 2011; Lynch 1995). The pericycle consists of parenchyma cells that surround the vascular cylinder (xylem, phloem). In each organism, the root growth pattern is genetically determined. Many mono- and dicotyledonous plants have a primary root, secondary roots, and absorbing root hairs (lateral extensions), and some may also develop adventitious or aerial root systems (Longstreth and Borkhsenious 2000) (Fig. 3.6a–c). Water is transported from the roots through two pathways, the symplastic and apoplastic pathways; in both, water is absorbed through root hairs. In the symplastic pathway, water and minerals are transported from cell to cell through the cytoplasm between plasmodesmata. In the apoplastic pathway, water moves through extracellular spaces located between the plasmalemma and cell walls, albeit only up to the endodermis, wherein Casparian strips force water to enter the cells to reach the vascular cylinders (Fig. 3.6d) (Sevilem et al. 2013).
Diagram of the tissues that form the root and vascular cylinder. (a) General representation of a root. (b) Arrangement of vascular tissues and bundles in a dicotyledonous plant. (c) Distribution of vascular tissues and bundles in a monocotyledonous plant. (d) Water and nutrient transport pathways. In the apoplastic pathway, water is transported between the interstices separating the membranes and cell walls, only until reaching the endodermis, which has cells coated with suberin (hydrophobic). In the symplastic pathway, transport occurs via the cytoplasm, through plasmodesmata
3.2.2 Seeds
Seeds contain a mature embryo, food reserves, and a coat or testa (Fig. 3.7a–c). Their formation begins with fertilization of the egg cell by sperm nuclei (pollen). The union gives rise to the zygote and the primary endosperm cell. The zygote initiates a series of mitotic divisions until the formation of a mature embryo with structures such as the root apex, shoot apex (epicotyl) with the first true leaves (plumule), and one or two seed leaves or cotyledons in mono- and dicotyledonous plants, respectively (Beeckman et al. 2000; Jones and Rost 1989). Dicotyledonous embryos usually absorb nutrients (starch, proteins, sugars, and lipids) from the endosperm, storing them in cotyledons (Maroder et al. 2003). In monocotyledonous plants, the embryo has no contact with the endosperm until the seed germinates and digestive enzymes are activated (Fincher 1989). The seeds are found in the fruits, the primary function of which is protection. In some cases, the fruits contribute to dispersion. They can be dehiscent, fleshy, simple, aggregate, or multiple.
3.2.3 Germination
Germination refers to the embryo development process after the latency period, which occurs when environmental conditions (temperature, humidity, amount of oxygen, light, and nutrients) are adequate. It begins with the movement of water molecules inside the seed (imbibition) by attracting the hydrophobic groups of proteins in the endosperm or in cotyledons, which swells the seed and ruptures the testa. This process increases the amount of oxygen, the temperature, and access to light hours, thus initiating the growth process, first at the root apex or radicle and then at the stem apex or epicotyl (Rajjou et al. 2012). When the seed coat or testa no longer covers the radicle, the cotyledon or cotyledons in most seeds show an increased number of photosynthetic chloroplasts, fulfilling the feeding function until the true leaves develop (Yan et al. 2014).
3.3 Associations with Microorganisms
Mycorrhizae are a mutualistic association between a plant species (pteridophytes, gymnosperms, and angiosperms) and a fungal species (Basidiomycota, Ascomycota, and Glomeromycota) (Martin et al. 2016; Tedersoo et al. 2010). Although they are not a plant tissue, fungi and their associations with the plant are an important element in the absorption of water and nutrients that are difficult to obtain, such as phosphates, zinc, and molybdenum, among others. The mycelium covers root surfaces and redirects them toward nutritionally rich areas, allowing the plant to take advantage of resources and grow, especially in poor soils. There are two types of plant-fungus interactions. In ectomycorrhiza, the hyphae do not penetrate the root cells but instead are located in the intercellular space (Martin et al. 2016); in endomycorrhiza, the hyphae penetrate root cells, wherein vesicles and arbuscules are formed for exchange between the fungus and the host plant (Luginbuehl and Oldroyd 2017). In turn, some plant species may also form a symbiosis with bacteria in root nodules. This association begins when nitrogen-fixing symbiotic bacteria enter roots through root hairs and subsequently infect cortical cells, wherein the bacteria reproduce and induce genetic-morphological changes. These changes lead to the formation of root nodules or tumor growths, consisting of the infected cells and plant tissue, thus enabling the plant to obtain the nitrogen necessary for its metabolic functions (Fig. 3.3) (Frank et al. 2017; Santoyo et al. 2016).
3.4 Plant Stress Response Mechanisms
Plants are continually exposed to stressors, such as variations in water availability (water stress), drastic changes in temperature, intense radiation, nutrient deficiencies (macronutrients, N, P, K, Ca, Mg, and S; micronutrients, Fe, Zn, Mn, Cu, B, and Mo, among others) (Lynch et al. 2012), overexposure to heavy metals or minerals, and pathogen infections (bacteria, fungi, worms), among others (Zhu 2016). Such factors are capable of inducing changes in physiological and genetic responses, either transient or permanent, at a local or systemic level, altering the standard conditions of metabolic functioning and growth. The main plant response mechanism includes the generation of reactive oxygen species (ROS), which involves the partial reduction of molecular oxygen (O2) to other molecules, such as oxygen singlet (1O2), superoxide anion (O2−), hydrogen peroxide (H2O2), or hydroxyl radicals (OH−). ROS production remains at baseline levels, thanks to antioxidant systems such as catalase (CAT), peroxidase (PER), peroxiredoxin (PRX), glutathione peroxidase (GPX), superoxide dismutase (SOD), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDAR) enzymes, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)-like (respiratory burst oxidase homologs – RBOHs) proteins, among others; in addition, regulatory mechanisms of iron storage and uptake are also involved in maintaining ROS at baseline levels (Mittler 2017). Chloroplasts are the organelles that produce the greatest amount of ROS. ROS are also produced in mitochondria, peroxisomes, and other cellular compartments with proteins and molecules with reduction-oxidation (REDOX) reaction potential (Zhu 2016). ROS are considered signaling molecules that regulate development and differentiation near the baseline state (Noctor et al. 2018). Biotic and abiotic stress signals and interactions with microorganisms increase or decrease the presence of ROS, which can induce cytostatic or even cytotoxic effects on the plant. Exacerbated ROS production in cells can trigger chain reactions that are harmful to constitutive organic molecules. For example, oxygen radicals induce lipid peroxidation (Ayala et al. 2014; Cherchi et al. 2011; Nair and Chung 2014) and can promote enzymatic inactivation and mutations or even degradation of nucleic acids. Hypermethylation and gene expression changes are other mechanisms of plant cell defense against stress (Ghosh et al. 2019).
4 Plant Nanotoxicology
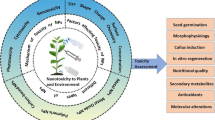
There is increasing concern about nanotoxicity in plants because they are not only a fundamental part of the ecology and ecological balance of the planet but also the basis of trophic systems. Both positive and negative effects of nanomaterials on plants have been described. Such effects vary with plant morphology, physiology, species, and the plant’s uptake capacity at different stages of maturation and with the presence of mycorrhizae or root nodules. The effects of a nanomaterial are determined by its chemical composition, morphology, size, structure, solubility, concentration, area, and surface charge. In turn, the composition of the environment (organic matter, types of grain, pH, and humidity) and climatic conditions also help to strengthen or mitigate these effects. Characteristics such as the size, charge, and concentration of nanomaterials have been reported as the main factors underlying the uptake and distribution in plant tissues (Fig. 3.8).
4.1 Uptake of Nanomaterials in Plant Tissues
As mentioned above, nanomaterials are distributed by air, water, and land. Their mobility and toxicity vary with physicochemical factors of the particles and with their interaction with environmental components. Plants are very diverse, have colonized terrestrial and aquatic environments, and exhibit abilities to take up, resist, and respond to the presence of nanomaterials that depend on complex interactions. The uptake of nanomaterials present in the soil occurs through the root system, in which the first key interaction is with symbiotic associations. Mycorrhizal and bacterial associations can function as remediation mechanisms for toxicity induced by nanomaterials present in the soil through their retention in hyphae and nodules, thereby preventing their accumulation in roots or their translocation to other tissues and mitigating their toxic effects on host plants. Feng et al. (2013) measured the effects of silver (Ag NPs) and iron monoxide nanoparticles (FeO NPs) on mycorrhizal fungi associated with white clover (Trifolium repens). Simultaneously, they measured the effects of NPs on plants with mycorrhizae and in control plants without mycorrhizae. Their results revealed a decrease in fungal biomass. They also observed reduced nutrient uptake in control plants than in those with mycorrhizae.
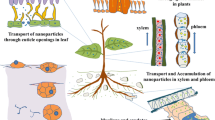
Wang et al. (2016) also described adverse effects on maize plants and on mycorrhizae exposed to high concentrations of zinc oxide (ZnO) nanoparticles. They observed that plants with arbuscular mycorrhizae achieved better alleviation due to the lower bioavailability of nanoparticles in the medium and therefore lower accumulation in plants. Judy et al. (2015) showed that silver sulfide (Ag2S) nanoparticles were less toxic to tomato plants (Lycopersicum esculentum ) with mycorrhizae than to those without, despite the decrease in fungal mass. In addition to the decrease in phytotoxicity in tomato plants (L. esculentum) with mycorrhizae exposed to Ag NPs, Noori et al. (2017) observed dose-dependent changes in both mycelial length and the expression of genes encoding membrane proteins (aquaporin channels, plasma membrane intrinsic protein, tonoplast membrane intrinsic channel, and potassium channels). These phenomena may have been involved in nanoparticle uptake, which was lower in plants with mycorrhizae. Associations with bacteria could have a similar effect because studies have shown that plants with root nodules exhibit some tolerance to the toxic effects of heavy metals. However, studies aimed at assessing the effect of nanomaterials have reported no results for control plants without root nodules (Cherchi et al. 2011; Guo and Chi 2014); therefore, no experimental evidence is currently available for tolerance mechanisms resulting from these associations (Tian et al. 2019). A key protective barrier, which decreases or favors the uptake of nanomaterials present in the soil, is the mucilage layer secreted by the root cap. Various researchers, such as Ma et al. (2011), have reported that the mucilage content of some plant species is able to acidify the soil, promoting the partial dissolution of some types of nanoparticles, whereas organic ligands associated with the root are able to take up metal ions. These authors showed that organic acids secreted by the cucumber plant (Cucumis sativus L.) modify the shape and size of the nanomaterials used (LaO3 NPs), altering their mobility and ability to translocate into tissues. In a study of cucumber plants and 7 and 25 nm cerium nanoparticles, Zhang et al. (2011) observed that only a fraction of the 7 nm cerium NPs was able to enter the vascular system of the plant. The remaining nanoparticles were recovered in root washings, suggesting that the secreted mucilage layer functioned as a trap. The charge of the nanomaterials favors or decreases their ability to interact with plant tissues and therefore their uptake. Some studies have shown that positively charged nanoparticles are translocated at a higher rate than negatively charged materials and quantum dots. Wang et al. (2014) observed that CdSe/CdZn quantum dots coated with cationic polymers are more easily taken up by Eastern cottonwood trees (Populus deltoides) than QDs coated with anionic polymers. A study by Al-Salim et al. (2011) showed that quantum dots are translocated into study plant tissues of ryegrass (Lolium perenne), onion (Allium cepa), and chrysanthemum (Chrysanthemum sp.), possibly due to their physicochemical characteristics, again demonstrating that the charge of the particle and their interactions with plant fluids determine the mobility of QDs. Conversely, the entry of nanomaterials has been suggested to be favored in areas associated with growth, such as areas of rapid mitotic division in root meristems or in thin areas of nutrient uptake in root hairs (Fellows et al. 2003; Lv et al. 2015; Zhang et al. 2011). After entering the plant cells, the materials can move through apoplastic and/or symplastic pathways and reach the vascular ducts (Larue et al. 2012; Lv et al. 2015). The uptake of nanomaterials in shoots (stems and leaves) is associated with the number of nanomaterials present in the air (aerosols) and with their physicochemical characteristics (hydrophobicity or hydrophilicity), allowing them to interact with organic molecules of the plant and thereby cross biological barriers such as the cuticle or induce their passage through stomata to reach the vascular system (Uzu et al. 2010). Although the mechanism of nanomaterial penetration in seeds is in turn not well known, they may be able to enter through intercellular spaces between parenchymal cells during the imbibition process, crossing the cell membrane in the endosperm (Thuesombat et al. 2014).
4.2 Toxic Effects of Nanomaterials on Plants
Several studies have shown that in plants, nanomaterials are taken up by various tissues, both roots and shoots. These nanomaterials can produce dose-dependent abiotic stresses; that is, most nanomaterials cause cytotoxicity when they reach critical concentrations, and an imbalance in ROS production is induced. Furthermore, depending on the cellular compartment and on the generated physiological response mechanisms, the condition may be local or systemic (affecting morphology, physiology, metabolism, and genetics). Some authors have suggested that the reduction in the photosynthetic capacity of plants is likely associated with lipid peroxidation in chloroplast membranes, which is associated with exacerbated ROS production in these organelles. Damage to photosystems directly results in decreased biomass (Dewez et al. 2018). In turn, changes in the numbers of carotene pigments and in phenols and flavonoids have been observed in plants exposed to high concentrations of silver nanoparticles (Gupta et al. 2018; Nair and Chung 2014). Dose-dependent genetic expression changes (upstream or downstream) have also been observed, especially in genes associated with ROS responses and those encoding cationic transporters associated with uptake (Taylor et al. 2014). Tables 3.1, 3.2, 3.3, 3.4, 3.5, and 3.6 show some damage-response examples that have been reported for vascular and nonvascular plants.
5 Nanotoxicological Evaluation Techniques in Plants
Many techniques have been used to evaluate the toxicity of nanomaterials in plants. These techniques mainly depend on studies of the nanomaterial traced within the tissues, the plant species, and the tissue type. To determine the phytotoxicity of a nanomaterial, physicochemical characterization of the nanomaterial should be performed to collect data on morphology, size, charge, surface area, and the presence of functional groups, among other properties. To evaluate morphology and size, images are usually acquired using the following high-resolution techniques: transmission electron microscopy, scanning electron microscopy, scanning transmission electron microscopy, and atomic force microscopy. Spectroscopic techniques such as Raman spectroscopy, Fourier transform infrared spectroscopy, ultraviolet-visible spectroscopy, and X-ray diffraction provide data on the chemical composition, electronic properties, and crystalline structure. Particle size is measured by dynamic light scattering. The hydrodynamic radius is determined by measuring the Z-potential (Peralta-Videa et al. 2011). Inductively coupled plasma optical emission spectroscopy (ICP-OES) , inductively coupled plasma mass spectrometry (ICP-MS) , and single-particle inductively coupled plasma mass spectrometry (SP-ICP-MS) are used to quantify metal nanomaterials in suspension (Bao et al. 2016; Larue et al. 2012). Some of these techniques have been useful for collecting data on nanomaterials in tissues.
Currently, there is no consensus on the optimal method for evaluating toxicity in plants, which has led to various sample preparation techniques and study parameters. Growth effects are determined by measuring root elongation and development (Lahiani et al. 2015), growth from the base of the plant to its highest point, stem diameter, number of secondary shoots, shoot length, and frond changes (Hu et al. 2013; Lee et al. 2010). Germination times, percentages, and seed viability have also been evaluated (Vannini et al. 2014). Parameters that have been useful in determining the REDOX potential by evaluating ROS production in plant tissues are the activity of antioxidant enzymes, such as catalase, ascorbic acid peroxidase, superoxide dismutase, and peroxidase, and the reduced/oxidized glutathione ratio (Dimkpa et al. 2013; Hu et al. 2013). Dye tests are also used to quantify the REDOX potential, including Alamar blue (Ong et al. 2014), nitro-tetrazolium blue (Speranza et al. 2013), and 2′,7′-dichlorodihydrofluorescein diacetate assays (Yan and Chen 2019). Conversely, X-ray absorption spectroscopy (XAS) has been very useful for locating nanomaterials in different tissue sections. Micro-X-ray fluorescence analysis (μ-XRF) and micro-X-ray absorption near-edge spectroscopy (μ-XANES) have been used to highlight the location and type of nanomaterials and to track fluorescence or measure radioactivity (Hernandez-Viezcas et al. 2013; López-Moreno et al. 2010; Lv et al. 2015; Zhang et al. 2011). Extraction with organic solvents, quantification of chlorophyll and other pigments, and relative quantification of the plant biomass (wet and dry weight) have also been used as nanotoxicity evaluation parameters (Dewez et al. 2018). Finally, some studies have utilized genomic analysis (RT-PCR, endpoint PCR, qPCR, random amplification of polymorphic DNA (RAPD), and DNA microarrays) (Hu et al. 2017; Taylor et al. 2014). Analyses of DNA methylation patterns, proteomics (Vannini et al. 2014; Mustafa et al. 2015), and metabolomics have also been performed to highlight changes. Techniques such as terminal deoxynucleotidyl transferase dUTP nick end labeling (Kumar et al. 2017), the Comet (also known as single-cell gel electrophoresis) (Cvjetko et al. 2018), and Allium (Liman et al. 2019) assays have been very useful in evaluating the genotoxicity of nanomaterials.
6 Conclusions
Nanotechnology is a discipline that has acquired great relevance in many areas of research (physics, chemistry, biology, engineering, electronics, medicine, etc.), as well as in industrial and pharmaceutical development. A perspective of nanotechnology is the expansion of promising applications, such as antimicrobial bandages, drug carriers, catalysts, scratch-resistant coatings, self-cleaning glasses, semiconductors, and UV-protected garments. However, as with many technological developments, nanotechnology may have both positive and negative effects in terms of health and the environment.
In the last decade, the number of nanomaterials included in objects and devices of common use has increased. However, the possible environmental consequences, resulting from the massive use of nanomaterials, have not been thoroughly addressed. Materials at the nanoscale behave differently than they do in their bulk form and may be a risk to living organisms at different organization levels. Nanotoxicology is a relatively new research area which addresses this issue by studying the uptake, accumulation, chemical interaction, and biological effects of nanomaterials. Novel methodologies are being developed to characterize the nanomaterials present in the environment and to better understand their interaction with cells and tissues, in order to determine if they constitute a threat. Soil microorganisms in symbiotic association with plants are the first link damaged by an increase of nanomaterials in the environment, sometimes considerably reducing the growth of plants. When nanomaterials reach plant tissues by penetrating through their openings in roots, stems, and leaves, they may induce stress mechanisms that could achieve toxic levels in tissues, causing deterioration of plants, altering whole crops or even ecosystems. Furthermore, due to a bioaccumulation phenomenon in plants, nanomaterials can enter the food chain of animals and humans constituting a health problem. Therefore, extensive safety research projects and regulations on the use of nanomaterials are still needed. In this chapter, basic concepts of plant nanotoxicology were described, including some properties of nanomaterials, and the anatomy and physiology of plants, as well as the methodologies so far existing to evaluate the toxicity of nanoparticles.
Developments using nanomaterial engineering for specific purposes should be accompanied by the corresponding toxicological studies and by studies assessing the potential environmental damage.
References
Aleksandrowicz-Trzcińska M, Bederska-Błaszczyk M, Szaniawski A, Olchowik J, Studnicki M (2019) The effects of copper and silver nanoparticles on container-grown scots pine (Pinus sylvestris L.) and pedunculate oak (Quercus robur L.) seedlings. Forests 10:269. https://doi.org/10.3390/f10030269
Allegri M, Perivoliotis DK, Bianchi MG, Chiu M, Pagliaro A, Koklioti MA, Trompeta AA, Bergamaschi E, Bussolati O, Charitidis CA (2016) Toxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomeration. Toxicol Rep 3:230–243. https://doi.org/10.1016/j.toxrep.2016.01.011
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110:132–145. https://doi.org/10.1021/cr900070d
Al-Salim N, Barraclough E, Burgess E, Clothier B, Deurer M, Green S, Malone L, Weir G (2011) Quantum dot transport in soil, plants, and insects. Sci Total Environ 409:3237–3248. https://doi.org/10.1016/j.scitotenv.2011.05.017
Alshehri R, Ilyas MA, Hasan A, Arnaout A, Ahmed F, Memic A (2016) Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J Med Chem 59:8149–8167. https://doi.org/10.1021/acs.jmedchem.5b01770
Angelova A, Garamus VM, Angelov B, Tian Z, Li Y, Zou A (2017) Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv Colloid Interfac 249:331–345. https://doi.org/10.1016/j.cis.2017.04.006
Aoyagi H, Ugwu CU (2011) Fullerene fine particles adhere to pollen grains and affect their autofluorescence and germination. Nanotechnol Sci Appl 4:67–71. https://doi.org/10.2147/NSA.S14263
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med Cell Longev 2014:360438. https://doi.org/10.1155/2014/360438
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98. https://doi.org/10.1016/j.tplants.2013.11.006
Bao D, Oh ZG, Chen Z (2016) Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis. Front Plant Sci 1(7):32. https://doi.org/10.3389/fpls.2016.00032
Barriga HMG, Holme MN, Stevens MM (2019) Cubosomes: the next generation of smart lipid nanoparticles? Angew Chem Int Edit 58:2958–2978. https://doi.org/10.1002/anie.201804067
Bayramzadeh V, Ghadiri M, Davoodi MH (2019) Effects of silver nanoparticle exposure on germination and early growth of Pinus sylvestris and Alnus subcordata. Sains Malaysiana 48:937–944. https://doi.org/10.17576/jsm-2019-4805-02
Beeckman T, De Rycke R, Viane R, Inzé D (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113:139–148. https://doi.org/10.1007/pl00013924
Bour A, Mouchet F, Silvestre J, Gauthier L, Pinelli E (2015) Environmentally relevant approaches to assess nanoparticles ecotoxicity: a review. J Hazar Mat 283:764–777. https://doi.org/10.1016/j.jhazmat.2014.10.021
Burcham PC (2014) The emergence of modern toxicology. In: An introduction to toxicology. Springer, London, pp 1–27. https://doi.org/10.1007/978-1-4471-5553-9_1
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17–MR71. https://doi.org/10.1116/1.2815690
Caballero-Guzman A, Nowack B (2016) A critical review of engineered nanomaterial release data: are current data useful for material flow modeling? Environ Pollut 213:502–517. https://doi.org/10.1016/j.envpol.2016.02.028
Calvin CL (1967) The vascular tissues and development of sclerenchyma in the stem of the mistletoe, Phoradendron flavescens. Bot Gaz 128:35–59. https://doi.org/10.1086/336379
Canivet L, Dubot P, Garçon G, Denayer FO (2015) Effects of engineered iron nanoparticles on the bryophyte, Physcomitrella patens (Hedw.) Bruch & Schimp, after foliar exposure. Ecotoxicol Environ Saf 113:499–505. https://doi.org/10.1016/j.ecoenv.2014.12.035
Carlquist S, Schneider EL (2002) The tracheid–vessel element transition in angiosperms involves multiple independent features: cladistic consequences. Am J Bot 89:185–195. https://doi.org/10.3732/ajb.89.2.185
Charitidis CA, Georgiou P, Koklioti MA, Trompeta AF, Markakis V (2014) Manufacturing nanomaterials: from research to industry. Manuf Rev 1:11. https://doi.org/10.1051/mfreview/2014009
Chen T, Cai X, Wu X, Karahara I, Schreiber L, Lin J (2011) Casparian strip development and its potential function in salt tolerance. Plant Signal Behav 6:1499–1502. https://doi.org/10.4161/psb.6.10.17054
Cherchi C, Chernenko T, Diem M, Gu AZ (2011) Impact of nano titanium dioxide exposure on cellular structure of Anabaena variabilis and evidence of internalization. Environ Toxicol Chem 30:861–869. https://doi.org/10.1002/etc.445
Choat B, Cobb AR, Jansen S (2008) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177:608–626. https://doi.org/10.1111/j.1469-8137.2007.02317.x
Cvjetko P, Zovko M, Štefanić PP, Biba R, Tkalec M, Domijan AM, Vrček IV, Letofsky-Papst I, Šikić S, Balen B (2018) Phytotoxic effects of silver nanoparticles in tobacco plants. Environ Sci Pollut R 25:5590–5602. https://doi.org/10.1007/s11356-017-0928-8
Dewez D, Goltsev V, Kalaji HM, Oukarroum A (2018) Inhibitory effects of silver nanoparticles on photosystem II performance in Lemna gibba probed by chlorophyll fluorescence. Curr Plant Biol 16:15–21. https://doi.org/10.1016/j.cpb.2018.11.006
Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ (2013) Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol 47:1082–1090. https://doi.org/10.1021/es302973y
Driouich A, Follet-Gueye ML, Vicré-Gibouin M, Hawes M (2013) Root border cells and secretions as critical elements in plant host defense. Curr Opin Plant Biol 16:489–495. https://doi.org/10.1016/j.pbi.2013.06.010
Dwivedi AD, Dubey SP, Sillanpää M, Kwon YN, Lee C, Varma RS (2015) Fate of engineered nanoparticles: implications in the environment. Coord Chem Rev 287:64–78. https://doi.org/10.1016/j.ccr.2014.12.014
Eichert T, Goldbach HE (2008) Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces – further evidence for a stomatal pathway. Physiol Plant 132:491–502. https://doi.org/10.1111/j.1399-3054.2007.01023.x
European Union Commission Recommendation (2011) On the definition of nanomaterial (EEA relevance) (2011/696/EU). Official Journal. Retrieved from http://data.europa.eu/eli/reco/2011/696/oj
Faivre D, Schüler D (2008) Magnetotactic bacteria and magnetosomes. Chem Rev 108:4875–4898. https://doi.org/10.1021/cr078258w
Fellows RJ, Wang Z, Ainsworth CC (2003) Europium uptake and partitioning in oat (Avena sativa) roots as studied by laser-induced fluorescence spectroscopy and confocal microscopy profiling technique. Environ Sci Technol 37:5247–5253. https://doi.org/10.1021/es0343609
Feng Y, Cui X, He S, Dong G, Chen M, Wang J, Lin X (2013) The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ Sci Technol 47:9496–9504. https://doi.org/10.1021/es402109n
Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40:305–346. https://doi.org/10.1146/annurev.pp.40.060189.001513
Frank AC, Saldierna Guzmán JP, Shay JE (2017) Transmission of bacterial endophytes. Microorganisms 5(4):70. https://doi.org/10.3390/microorganisms5040070
Frecker T, Bailey D, Arzeta-Ferrer X, McBride J, Rosenthal SJ (2016) Review-quantum dots and their application in lighting, displays, and biology. ECS J Solid State Sci 5:R3019–R3031. https://doi.org/10.1149/2.0031601jss
Galway ME (2006) Root hair cell walls: filling in the framework. Special Issue on Plant Cell Biology. Can J Bot 84:613–621. https://doi.org/10.1139/b06-006
Gerloff K, Landesmann B, Worth A, Munn S, Palosaari T, Whelan M (2017) The adverse outcome pathway approach in nanotoxicology. Computat Toxicol 1:3–11. https://doi.org/10.1016/j.comtox.2016.07.001
Ghosh I, Sadhu A, Moriyasu Y, Bandyopadhyay M, Mukherjee A (2019) Manganese oxide nanoparticles induce genotoxicity and DNA hypomethylation in the moss Physcomitrella patens. Mutat Res Gen Toxicol Environ 842:146–157. https://doi.org/10.1016/j.mrgentox.2018.12.006
Gibson LJ (2012) The hierarchical structure and mechanics of plant materials. J Royal Soc Interface 9:2749–2766. https://doi.org/10.1098/rsif.2012.0341
Goswami L (2017) Engineered nano particles: nature, behavior, and effect on the environment. J Environ Manag 196:297–315. https://doi.org/10.1016/j.jenvman.2017.01.011
Gottschalk F, Ort C, Scholz RW, Nowack B (2011) Engineered nanomaterials in rivers-exposure scenarios for Switzerland at high spatial and temporal resolution. Environ Pollut 159:3439–3445. https://doi.org/10.1016/j.envpol.2011.08.023
Guo J, Chi J (2014) Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 375:205–214. https://doi.org/10.1007/s11104-013-1952-1
Gupta SD, Agarwal A, Pradhan S (2018) Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotox Environ Safe 161:624–633. https://doi.org/10.1016/j.ecoenv.2018.06.023
Hall JL, Flowers TJ, Roberts RM (1984) Plant cell structure and metabolism, 2nd edn. Longman Group, Harlow, Essex. pp 480
Heddle JG, Chakraborti S, Iwasaki K (2017) Natural and artificial protein cages: design, structure and therapeutic applications. Curr Opin Struct Biol 43:148–155. https://doi.org/10.1016/j.sbi.2017.03.007
Hernandez-Viezcas JA, Hiram Castillo M, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL (2013) In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7:1415–1423. https://doi.org/10.1021/nn305196q
Hu C, Liu Y, Li X, Li M (2013) Biochemical responses of duckweed (Spirodela polyrhiza) to zinc oxide nanoparticles. Arch Environ Contam Toxicol 64:643–651. https://doi.org/10.1007/s00244-012-9859-z
Hu C, Xu Liu LX, Zhao Y (2014) Evaluation of growth and biochemical indicators of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ Sci Pollut R 21:732–739. https://doi.org/10.1007/s11356-013-1970-9
Hu J, Guo H, Li J, Gan Q, Wang Y, Xing B (2017) Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ Pollut 221:199–208. https://doi.org/10.1016/j.envpol.2016.11.064
International Organization for Standardization (2015) Nanotechnologies –Vocabulary-Part 1: Core terms. Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-1:ed-2:v1:en
Isaacson CW, Kleber M, Field JA (2009) Quantitative analysis of fullerene nanomaterials in environmental systems: a critical review. Environ Sci Technol 43:6463–6474. https://doi.org/10.1021/es900692e
Jansen S, Choat B, Pletsers (2009) Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot 96:409–419. https://doi.org/10.3732/ajb.0800248
Jobin J, Netto G (2019) Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J Cosmet Dermatol-US 18:315–321. https://doi.org/10.1111/jocd.12504
Jones TJ, Rost TL (1989) Histochemistry and ultrastructure of rice (Oryza sativa) zygotic embryogenesis. Am J Bot 76:504–520. https://doi.org/10.2307/2444345
Joo SH, Zhao D (2017) Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: a review. J Hazard Mater 322:29–47. https://doi.org/10.1016/j.jhazmat.2016.02.068
Judy JD, Kirby JK, Creamer C, McLaughlin MJ, Fiebiger C, Wright C, Cavagnaro TR, Bertsch PM (2015) Effects of silver sulfide nanomaterials on mycorrhizal colonization of tomato plants and soil microbial communities in biosolid-amended soil. Environ Pollut 206:256–263. https://doi.org/10.1016/j.envpol.2015.07.002
Kaplan DR (2001) The science of plant morphology: definition, history, and role in modern biology. Am J Bot 88:1711–1141. https://doi.org/10.2307/3558347
Kesharwani P, Gothwal A, Iyer AK, Jain K, Chourasia MK, Gupta U (2018) Dendrimer nanohybrid carrier systems: An expanding horizon for targeted drug and gene delivery. Drug Discov Today 23:300–314. https://doi.org/10.1016/j.drudis.2017.06.009
Khalil HPSA, Yusra AFI, Bhat AH, Jawaid M (2010) Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Ind Crop Prod 31:113–121. https://doi.org/10.1016/j.indcrop.2009.09.008
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Kim Y, Park EJ, Na DH (2018) Recent progress in dendrimer-based nanomedicine development. Arch Pharm Res 41:571–582. https://doi.org/10.1007/s12272-018-1008-4
Knox JP, Benitez-Alfonso Y (2014) Roles and regulation of plant cell walls surrounding plasmodesmata. Curr Opin Plant Biol 22:93–100. https://doi.org/10.1016/j.pbi.2014.09.009
Koivisto AJ, Østerskov Jensen AC, Kling KI, Nørgaard A, Brinch A, Christensen F, Jensen KA (2017) Quantitative material releases from products and articles containing manufactured nanomaterials: towards a release library. Nano Impact 5:119–132. https://doi.org/10.1016/j.impact.2017.02.001
Krug HF, Wick P (2011) Nanotoxicology: an interdisciplinary challenge. Angew Chem Int Ed 50:1260–1278. https://doi.org/10.1002/anie.201001037
Kumar V, Sharma N, Maitra SS (2017) In vitro and in vivo toxicity assessment of nanoparticles. Int Nano Lett 7:243–256. https://doi.org/10.1007/s40089-017-0221-3
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles-based drug delivery systems. Colloid Surface B 75:1–18. https://doi.org/10.1016/j.colsurfb.2009.09.001
Lahiani MH, Chen J, Irin F, Puretzky AA, Green MJ, Khodakovskaya MV (2015) Interaction of carbon nanohorns with plants: uptake and biological effects. Carbon 81:607–619. https://doi.org/10.1016/j.carbon.2014.09.095
Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank AM, Brisset F, Carriere M (2012) Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ 431:197–208. https://doi.org/10.1016/j.scitotenv.2012.04.073
Lavid N, Barkay Z, Tel-Or E (2001) Accumulation of heavy metals in epidermal glands of the waterlily (Nymphaeaceae). Planta 212:313–322. https://doi.org/10.1007/s004250000399
Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez JP (2010) Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem 29:669–675. https://doi.org/10.1002/etc.58
Lee KJD, Marcus SE, Knox JP (2011) Cell wall biology: perspectives from cell wall imaging. Mol Plant 4:212–219. https://doi.org/10.1093/mp/ssq075
Lendzian KJ (2006) Survival strategies of plants during secondary growth: barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J Exp Bot 57:2535–2546. https://doi.org/10.1093/jxb/erl014
Leroux O (2012) Collenchyma: a versatile mechanical tissue with dynamic cell walls. Ann Bot 110:1083–1098. https://doi.org/10.1093/aob/mcs186
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49. https://doi.org/10.1002/smll.200700595
Li J, Li Q, Ma X, Tian B, Li T, Yu J, Dai S, Weng Y, Hua Y (2016) Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int J Nanomedicine 11:5931–5944. https://doi.org/10.2147/IJN.S119618
Liang L, Tang H, Deng Z, Liu Y, Chen X, Wang H (2018) Ag nanoparticles inhibit the growth of the bryophyte, Physcomitrella patens. Ecotoxicol Environ Saf 164:739–748. https://doi.org/10.1016/j.ecoenv.2018.08.021
Liman R, Acikbas Y, Ciğerci IH (2019) Cytotoxicity and genotoxicity of cerium oxide micro and nanoparticles by Allium and Comet tests. Ecotoxicol Environ Saf 168:408–414. https://doi.org/10.1016/j.ecoenv.2018.10.088
Lin X, Chen L, Hu X, Feng S, Huang L, Quan G, Wei X, Yang ST (2017) Toxicity of graphene oxide to white moss Leucobryum glaucum. RSC Adv 7:50287–50293. https://doi.org/10.1039/C7RA10096E
Longstreth DJ, Borkhsenious ON (2000) Root cell ultrastructure in developing aerenchyma tissue of three Wetland species. Ann Bot 86:641–646. https://doi.org/10.1006/anbo.2000.1151
López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2010) X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem 58:3689–3693. https://doi.org/10.1021/jf904472e
Luginbuehl LH, Oldroyd GE (2017) Understanding the arbuscule at the heart of endomycorrhizal symbioses in Plants. Curr Biol 27:R952–R963. https://doi.org/10.1016/j.cub.2017.06.042
Luo J, Lian C, Liu R, Zhang S, Yang F, Fei B (2019) Comparison of metaxylem vessels and pits in four sympodial bamboo species. Sci Rep 9:10876. https://doi.org/10.1038/s41598-019-47419-7
Lv J, Zhang S, Luo L, Zhang J, Yang K, Christie P (2015) Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ Sci Nano 2:68–77. https://doi.org/10.1039/C4EN00064A
Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109:7–13. https://doi.org/10.1104/pp.109.1.7
Lynch J, Marschner P, Rengel Z (2012) Effect of internal and external factors on root growth and development. In: Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, pp 331–346. https://doi.org/10.1016/B978-0-12-384905-2.00013-3
Ma Y, He X, Zhang P, Zhang Z, Guo Z, Tai R, Xu Z, Zhang L, Ding Y, Zhao Y, Chai Z (2011) Phytotoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumis sativus). Nanotoxicol 5:743–753. https://doi.org/10.3109/17435390.2010.545487
Mageswari A, Srinivasan R, Subramanian P, Ramesh N, Gothandam KM (2016) Nanomaterials: Classification, biological synthesis and characterization. In: Nanoscience in food and agriculture, vol 3. Springer, Cham, pp 31–71. https://doi.org/10.1007/978-3-319-48009-1_2
Maroder H, Prego I, Maldonado S (2003) Histochemical and ultrastructural studies on Salix alba and S. matsudana seeds. Trees 17:193–199. https://doi.org/10.1007/s00468-002-0221-3
Martin F, Kohler A, Murat C, Veneault-Fourrey C, Hibbett DS (2016) Unearthing the roots of ectomycorrhizal symbioses. Nat Rev Microbiol 14:760–773. https://doi.org/10.1038/nrmicro.2016.149
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Montagner ASB, Tenori E, Bidussi M, Alshatwi AA, Tretiach M, Prato M, Syrgiannis Z (2016) Ecotoxicological effects of graphene-based materials. 2D Mat 4:012001. https://doi.org/10.1088/2053-1583/4/1/012001
Morris H, Plavcová L, Cvecko P, Fichtler E, Gillingham M, Martínez-Cabrera H, McGlinn DJ, Wheeler E, Zheng J, Ziemińska K, Jansen S (2016) A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytol 209:1553–1565. https://doi.org/10.1111/nph.13737
Murphy SD (1979) Some concepts in toxicology. EHP 32:261–266. https://doi.org/10.1289/ehp.7932261
Mustafa G, Sakata K, Hossain Z, Komatsu S (2015) Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J Proteome 122:100–118. https://doi.org/10.1016/j.jprot.2015.03.030
Nair PMG, Chung IM (2014) Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 112:105–113. https://doi.org/10.1016/j.chemosphere.2014.03.056
Nair R, Mohamed MS, Gao W, Maekawa T, Yoshida Y, Ajayan PM, Kumar DS (2012) Effect of carbon nanomaterials on the germination and growth of rice plants. J Nanosci Nanotech 12:2212–2220. https://doi.org/10.1166/jnn.2012.5775
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, MiaoAJ QA, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386. https://doi.org/10.1007/s10646-008-0214-0
Navarro DA, Bisson MA, Aga DS (2012) Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J Hazard Mat 211:427–435. https://doi.org/10.1016/j.jhazmat.2011.12.012
Nitta S, Numata K (2013) Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci 14:1629–1654. https://doi.org/10.3390/ijms14011629
Noctor G, Reichheld JP, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Sem Cell Develop Biol 80:3–12. https://doi.org/10.1016/j.semcdb.2017.07.013
Noori A, White JC, Newman LA (2017) Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure. J Nanopart Res 19:66. https://doi.org/10.1007/s11051-016-3650-4
Nowack B (2017) Evaluation of environmental exposure models for engineered nanomaterials in a regulatory context. NanoImpact 8:38–47. https://doi.org/10.1016/j.impact.2017.06.005
Ong KJ, MacCormack TJ, Clark RJ, Ede JD, Ortega VA, Felix LC, Dang MK, Ma G, Fenniri H, Veinot JGC, Goss GG (2014) Widespread nanoparticle-assay interference: implications for nanotoxicity testing. PLoS One 9:e90650. https://doi.org/10.1371/journal.pone.0090650
Owen J, Brus L (2017) Chemical synthesis and luminescence applications of colloidal semiconductor quantum dots. J Am Chem Soc 139:10939–10943. https://doi.org/10.1021/jacs.7b05267
Park TJ, Lee KG, Lee S (2016) Advances in microbial biosynthesis of metal nanoparticles. App Microbiol Biotechnol 100:521–534. https://doi.org/10.1007/s00253-015-6904-7
Peralta-Videa JR, Zhao L, Lopez-Moreno ML, de la Rosa G, Hong J, Gardea-Torresdey JL (2011) Nanomaterials and the environment: a review for the biennium 2008–2010. J Hazard Mat 186:1–15. https://doi.org/10.1016/j.jhazmat.2010.11.020
Psaras GK, Constantinidis TH, Cotsopoulos B, Manetas Y (2000) Relative abundance of nickel in the leaf epidermis of eight hyperaccumulators: evidence that the metal is excluded from both guard cells and trichomes. Ann Bot 86:73–78. https://doi.org/10.1006/anbo.2000.1161
Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D (2012) Seed germination and vigor. Annu Rev Plant Biol 63:507–533. https://doi.org/10.1146/annurev-arplant-042811-105550
Roberts A, Roberts E, Haigler C (2012) Moss cell walls: structure and biosynthesis. Front Plant Sci 3:166. https://doi.org/10.3389/fpls.2012.00166
Rodríguez-Torres MP, Acosta-Torres LS, Díaz-Torres LA, Hernández Padrón G, García-Contreras R, Millán-Chiu BE (2019) Artemisia absinthium-based silver nanoparticles antifungal evaluation against three Candida species. Mat Res Express 6:085408. https://doi.org/10.1088/2053-1591/ab1fba
Roszek B, De Jong WH, Geertsma RE (2005) Nanotechnology in medical applications: state-of-the-art in materials and devices. In: Rijksinstituut voor Volksgezondheid en Milieu. RIVM, pp 17–41. http://hdl.handle.net/10029/7265
Sanchez VC, Jachak A, Hurt RH, Kane AB (2012) Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol 25:15–34. https://doi.org/10.1021/tx200339h
Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda MC, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Schrand AM, Huang H, Carlson C, Schlager JJ, Ōsawa E, Hussain SM, Dai L (2007) Are diamond nanoparticles cytotoxic? J Phys Chem B 111:2–7. https://doi.org/10.1021/jp066387v
Sevilem I, Miyashima S, Helariutta Y (2013) Cell-to-cell communication via plasmodesmata in vascular plants. Cell Adhes Migr 7:27–32. https://doi.org/10.4161/cam.22126
Sevilem I, Yadav SR, Helariutta Y (2015) Plasmodesmata: channels for intercellular signaling during plant growth and development. In: Plasmodesmata. Humana Press, New York, pp 3–24. https://doi.org/10.1007/978-1-4939-1523-1_1
Sheffield L, Rowntree J (2009) Bryophyte biology, 2nd ed. Ann Bot 104(1):vi. https://doi.org/10.1093/aob/mcp109
Smirnoff N, Crawford RMM (1983) Variation in the structure and response to flooding of root aerenchyma in some wetland plants. Ann Bot 51:237–249. https://doi.org/10.1093/oxfordjournals.aob.a086462
Speranza A, Crinelli R, Scoccianti V, Taddei AR, Iacobucci M, Bhattacharya P, Ke PC (2013) In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ Pollut 179:258–267. https://doi.org/10.1016/j.envpol.2013.04.021
Staehelin LA (2003) Chloroplast structure: from chlorophyll granules to supra-molecular architecture of thylakoid membranes. Photosynth Res 76:185–196. https://doi.org/10.1023/a:1024994525586
Stone V, Nowack B, Baun A, van den Brink N, von der Kammer F, Dusinska M, Handy R, Hankin S, Hassellöv M, Joner E, Fernandes TF (2010) Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico-chemical characterization. Sci Total Environ 408:1745–1754. https://doi.org/10.1016/j.scitotenv.2009.10.035
Tan XM, Lin C, Fugetsu B (2009) Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 47:3479–3487. https://doi.org/10.1016/j.carbon.2009.08.018
Tapeinos C, Battaglini M, Ciofani G (2017) Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release 264:306–332. https://doi.org/10.1016/j.jconrel.2017.08.033
Tarhini M, Greige-Gerges H, Elaissari A (2017) Protein-based nanoparticles: from preparation to encapsulation of active molecules. Int J Pharm 522:172–197. https://doi.org/10.1016/j.ijpharm.2017.01.067
Taylor AF, Rylott EL, Anderson CW, Bruce NC (2014) Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS One 9:e93793. https://doi.org/10.1371/journal.pone.0093793
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263. https://doi.org/10.1007/s00572-009-0274-x
Thuesombat P, Hannongbua S, Akasit S, Chadchawan S (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf 104:302–309. https://doi.org/10.1016/j.ecoenv.2014.03.022
Tian H, Kah M, Kariman K (2019) Are nanoparticles a threat to mycorrhizal and rhizobial symbioses? A critical review. Front Microbiol 10:1660. https://doi.org/10.3389/fmicb.2019.01660
Tiwari M, Sharma NC, Fleischmann P, Burbage J, Venkatachalam P, Sahi SV (2017) Nanotitania exposure causes alterations in physiological, nutritional and stress responses in tomato (Solanum lycopersicum). Front Plant Sci 8:633. https://doi.org/10.3389/fpls.2017.00633
Uzu G, Sobanska S, Sarret G, Muñoz M, Dumat C (2010) Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol 44:1036–1042. https://doi.org/10.1021/es902190u
Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, Akbarzadeh A, Davaran S (2012) Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett 7:480–480. https://doi.org/10.1186/1556-276X-7-480
Vannini C, Domingo G, Onelli E, De Mattia F, Bruni I, Marsoni M, Bracale M (2014) Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol 171:1142–1148. https://doi.org/10.1016/j.jplph.2014.05.002
Vazquez-Muñoz R, Borrego B, Juárez-Moreno K, García-García M, Mota Morales JD, Bogdanchikova N, Huerta-Saquero A (2017) Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett 276:11–20. https://doi.org/10.1016/j.toxlet.2017.05.007
Vershinin A (1999) Biological functions of carotenoids-diversity and evolution. Bio Factors 10:99–104. https://doi.org/10.1002/biof.5520100203
Wang F, Liu X, Shi Z, Tong R, Adams CA, Shi X (2016) Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants – a soil microcosm experiment. Chemosphere 147:88–97. https://doi.org/10.1016/j.chemosphere.2015.12.076
Wang J, Yang Y, Zhu H, Braam J, Jl S, Alvarez PJJ (2014) Uptake, translocation, and transformation of quantum dots with cationic versus anionic coatings by populus deltoides × nigra cuttings. Environ Sci Technol 48:6754–6762. https://doi.org/10.1021/es501425r
White PJ (2012) Long-distance transport in the xylem and phloem. In: Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, pp 49–70. https://doi.org/10.1016/B978-0-12-384905-2.00003-0
Wigger H, Hackmann S, Zimmermann T, Köser J, Thöming J, von Gleich A (2015) Influences of use activities and waste management on environmental releases of engineered nanomaterials. Sci Total Environ 535:160–171. https://doi.org/10.1016/j.scitotenv.2015.02.042
Yamamoto A, Honma R, Sumita M, Hanawa T (2004) Cytotoxicity evaluation of ceramic particles of different sizes and shapes. J Biomed Mat Res A 68A:244–256. https://doi.org/10.1002/jbm.a.20020
Yan A, Chen Z (2019) Impacts of silver nanoparticles on plants: a focus on the phytotoxicity and underlying mechanism. Int J Mol Sci 20:1003. https://doi.org/10.3390/ijms20051003
Yan D, Duermeyer L, Leoveanu C, Nambara E (2014) The functions of the endosperm during seed germination. Plant Cell Physiol 55:1521–1533. https://doi.org/10.1093/pcp/pcu089
Yue L, Chen F, Yu K, Xiao Z, Yu X, Wang Z, Xing B (2019) Early development of apoplastic barriers and molecular mechanisms in juvenile maize roots in response to La2O3 nanoparticles. Sci Total Environ 653:675–683. https://doi.org/10.1016/j.scitotenv.2018.10.320
Zechmeister HG, Grodzińska K, Szarek-Łukaszewska G (2003) Bryophytes. In: Trace metals and other contaminants in the environment. Elsevier, pp 329–375. https://doi.org/10.1016/S0927-5215(03)80140-6
Zhang Z, He X, Zhang H, Ma Y, Zhang P, Ding Y, Zhao Y (2011) Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 3:816–822. https://doi.org/10.1039/C1MT00049G
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Millán-Chiu, B.E., del Pilar Rodriguez-Torres, M., Loske, A.M. (2020). Nanotoxicology in Plants. In: Patra, J., Fraceto, L., Das, G., Campos, E. (eds) Green Nanoparticles. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-39246-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-39246-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39245-1
Online ISBN: 978-3-030-39246-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)