Abstract
Paediatric hepatic tumours are relatively rare with malignant lesions being twice as frequent as benign neoplasms and are mostly metastases.
Hepatic tumors in children include lesions that are unique to the pediatric age group and others that are more common in adults.
Important considerations when evaluating a child with a liver tumor are the age of the patient, laboratory findings, and specific imaging features.
Imaging has a significant role in the evaluation of most paediatric liver tumours. Differentiating benign from malignant tumours is important as it significantly affects treatment decisions.
The current emphasis is on imaging features, which are helpful not only for the initial diagnosis, but also for pre- and post-treatment evaluation and follow-up.
The role of advanced imaging test such as magnetic resonance imaging, which allow for non-invasive assessment of liver tumors, is of utmost importance in pediatric patients, especially when repeated imaging tests are needed and radiation exposure should be avoided.
Knowledge of the imaging features of these tumors can help radiologists offer an appropriate differential diagnosis and management plan.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Primary hepatic neoplasms account for 1–4% of all children solid tumors with approximately two-thirds of the primary hepatic neoplasms being malignant (Yikilmaz et al. 2017)
Metastatic disease is the most common neoplasm involving the liver in children (Chung et al. 2010)
Most of the focal hepatic masses cause abdominal pain or palpable abdominal mass or distention. Imaging evaluation is necessary for characterizing and managing pediatric patients with suspected focal hepatic masses. Although abdominal radiograph may provide helpful imaging findings that can suggest the presence of focal hepatic masses such as hepatomegaly, ultrasound is the first imaging modality because of its low cost and availability, without lack of ionizing radiation (Adeyiga et al. 2012)
Contrast-enhanced US (CEUS) is currently emerging as a promising modality in detecting and characterizing liver tumors (Anupindi et al. 2017)
CT was often performed for lesion characterization, staging, and surgical planning for liver masses and now remains the gold standard for detecting lung metastasis in malignant hepatic neoplasms. Parameters like kV, mAs, and pitch can be adjusted for decreasing children’s exposure to radiation maintaining adequate diagnostic image quality. IV contrast agent is essential to characterizing tumor and vascular supply to both the tumor and the normal hepatic parenchyma.
Unlike adults, multiphase CT liver imaging should not be performed in pediatric patients to avoid excessive exposure to radiation. It is recommended a single portal-venous phase with a 50-second delay to initiate imaging after injection of IV contrast agent.
The diagnostic role of CT in characterization, staging, and surgical planning of hepatic tumors in the pediatric population has been reduced with recent advances in MR imaging.
MRI is becoming the modality of choice for the imaging of pediatric abdominal masses because of its very good multiplanar spacial resolution and excellent multiparametric tissue characterization without exposure to ionizing radiation. Newer and faster image sequences have also been developed for reducing motion artifact and for improving detailed visualization of vascular anatomy.
However, the disadvantages of MR imaging include longer imaging time and the frequent need for sedation or anesthesia in smaller children.
The imaging protocol employed should attempt to minimize scan time while maximizing image resolution (Rozell et al. 2014; Roebuck 2009).
An ideal MR imaging protocol should be performed with axial and coronal T2-weighted turbo spin-echo; axial in- and opposed-phase T1 gradient recalled echo, axial diffusion-weighted and axial/coronal post-contrast 3-dimensional fat-suppressed gradient recalled-echo sequences with arterial, portal venous, equilibrium and delayed post-contrast phases up to 20 min with the use of a hepatocyte-specific gadolinium-based contrast agent (such as gadoxetate disodium) (Shelmerdine et al. 2016; Chavhan et al. 2016; Mitchell and Vasanawala 2011; Meyers et al. 2011)
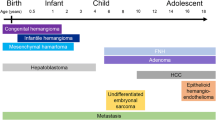
1 Benign Tumors
One-third of primary liver tumors are benign, which may be of mesenchymal or epithelial origin; the most common benign tumors are infantile hemangioma, mesenchymal hamartoma, focal nodular hyperplasia (FNH), nodular regenerative hyperplasia (NRH), and hepatocellular adenoma (Table 1) (Stocker 2001; Chung et al. 2010)
1.1 Infantile Hepatic Hemangioma
Infantile hepatic hemangiomas are the most common benign vascular tumor, which is composed of vascular endothelium, and are divided into three subtypes: focal, multifocal, and diffuse.
The congenital sub-type is well-formed at birth while infantile hemangiomas typically present 4–8 weeks after birth and continue to grow up to a year, followed by a slow phase of regression over 8–9 years and typically resolve by puberty. Clinicians and radiologists, to distinguish between infantile and congenital hemangiomas, have to know the timing of lesion presentation or GLUT-1 expression by tissue sampling (which is a sensitive and specific marker for infantile hemangiomas) (Masand 2018).
Solitary tumor size varies from 0.5 to 14 cm in maximum dimension while multifocal lesions are usually around 1 cm in diameter.
Multifocal lesions are small and uniform while large focal may present central hemorrhage, necrosis, fibrosis, and calcification. In diffuse disease, the liver is replaced by multiple large masses that cause mass effect on adjacent organs (Chung et al. 2010)
At postnatal US, infantile hemangioma appears as a well-demarcated mass, generally hypoechoic or of mixed echogenicity, relative to adjacent live, unlike adults hemangioma which is typically iperechoic (Keslar et al. 1993)
Large hemangiomas may be heterogeneous because of central hemorrhage, necrosis, or fibrosis.
On CT precontrast images infantile hemangioma presents as a well-defined hypoattenuating mass with calcifications in up to 50% of cases. After contrast injection, it shows intense peripheral nodular enhancement on arterial phase enhancement with a progressive centripetal fill-in on portal venous and delayed phase images, similar to adult hemangioma. Small multifocal tumors enhance intensely and uniformly, whereas large focal tumors enhance centripetally and may never completely enhance in the center (Kassarjian et al. 2004)
On MRI congenital or infantile hemangiomas present as focal mass lesions with hypointense T1-weighted and avidly hyperintense T2- weighted signal. On post-contrast sequences, they usually demonstrate a peripheral, discontinuous, and nodular pattern of enhancement on the arterial phase images. On delayed post-contrast images, these lesions continue to fill in and exhibit hypointense signal relative to the liver parenchyma (hepatobiliary phase) using a hepatocyte-specific contrast (Masand 2018; Dickie et al. 2009; Christison-Lagay et al. 2007) (Fig. 1).
4 months old female with hepatomegaly. US shows well-demarcated multiple hypoechoic nodular masses (a), without vascular signal on ECD (b). On CT (c, d, e) and on MRI (f, g, h) the liver is totally replaced by multiple hemangiomas with intense peripheral nodular enhancement on the arterial phase with a progressive centripetal fill-in on portal venous and delayed phase images. Control after propranolol and corticosteroid therapy shows complete regression of hemangiomas 1 year later (i)
1.2 Mesenchymal Hamartoma
Mesenchymal hamartomas of the liver are the second most common benign tumors in childhood, that occur typically before 3 years of age and are composed of disorganized bile ducts, immature fluid-filled mesenchymal tissue, and hepatocytes (Moore et al. 2009)
The appearance of mesenchymal hamartoma depends on its components, which range from a predominantly cystic mass with thin or thick septa to a predominantly solid (stromal or mesenchymal) mass with a few small cysts.
On US, the cystic portions of the mass are anechoic or nearly anechoic with thin or thick echogenic septa while the solid portions appear echogenic (Chung et al. 2010)
On CT mesenchymal hamartoma appears like a multilocular low-attenuation cystic mass with enhancing thick or thin septae and solid component; calcification is rare (Yikilmaz et al. 2017)
On MRI the most common multiseptated cystic mass presents low signal intensity on T1-weighted and high signal intensity on T2-weighted images. The intervening septations are of intermediate signal intensity and display enhancement on the post-contrast sequences. Mesenchymal hamartomas can be solid or have a mixed solid/cystic appearance. Solid mesenchymal hamartoma cannot be differentiated from hepatoblastoma on imaging alone and demonstrates heterogeneously hyperintense T2-weighted signal, hypointense T1-weighted signal, and heterogeneous post-contrast enhancement within the mass (Masand 2018).
Hepatoblastoma is generally distinguished from mesenchymal hamartoma by marked elevation of the serum AFP level and the solid appearance and finding of calcification.
Undifferentiated embryonal sarcoma (UES) is similar to mesenchymal hamartoma for imaging and pathologic features but it occurs in an older age group (6–10 years of age) and often presents hemorrhage and necrosis with a frankly malignant stroma (Chung et al. 2010)
1.3 Focal Nodular Hyperplasia (FNH)
FNH represents 2% of all primary hepatic tumors in children from birth to age 20 years with a prevalence in the pediatric population between the ages of 2 and 5 years (Stocker 2001; Meyers 2007).
It has been supposed that focal nodular hyperplasia develops within a congenital vascular malformation or occurs secondary to iatrogenic hepatic vascular damage such as after chemotherapy (Masand 2018).
On US most FNH appears hypoechoic; however, they may be isoechoic and hyperechoic. The identification of isoechoic tumors may be difficult and recognizing indirect signs of compression adjacent to the mass may be helpful for the diagnosis. A hypoechoic halo is present in 32% of cases (Bartolotta et al. 2004; Wu et al. 2012).
On CT scan, FNH has more specific characteristics and typically demonstrates uniform enhancement with IV contrast during the arterial phase. In the later phases, FNH becomes isoattenuating to the liver (Ma et al. 2015).
A stellate scar, when present, is typically hypoattenuating on early contrast-enhanced images and demonstrates enhancement on delayed images.
MR imaging is the modality of choice for characterizing FNH, which appears isointense to slightly hypointense on T1-weighted and T2-weighted MR images, and enhances homogeneously. After contrast injection, the lesion enhances strongly during the arterial phase and early portal venous phase, and becomes isointense to slightly hyperintense compared to the adjacent liver parenchyma during the late portal venous and delayed phase without a wash-out pattern. Enhancement during the hepatocyte phase using Gd-EOB-DTPA or Gd-BOPTA is a characteristic feature of FNH which allows differentiating FNH from other benign and malignant tumors of the liver (Yikilmaz et al. 2017)
In 80% of cases, there is a central scar that is typically hypointense on T1-weighted MR images and hyperintense on T2-weighted MR images. This scar enhances during the portal venous phase and delayed phases using extracellular contrast agents while it does not enhance during the hepatocyte phase using hepatocyte-specific contrast agents (Sutherland et al. 2014)
Fibrolamellar carcinoma may also demonstrate a collagenous central scar (unlike the vascular myxomatous scar of FNH) which is hypointense, rather than hyperintense, on T2-weighted images and does not enhance on delayed images (Fig. 2).
10 years old female with a previous history of neuroblastoma. MRI shows multiple hypointense polilobulated lesions on T2-weighted images (a) which strongly enhance during the arterial phase and early portal venous phase (b, c). CE persisting during the hepatobiliary phase (d) allows differentiating FNH from metastatic disease
1.4 Nodular Regenerative Hyperplasia
Nodular regenerative hyperplasia is a rare disorder consisting of a diffuse micronodular transformation of hepatic parenchyma without intervening fibrous septa (Adeyiga et al. 2012).
There is typically no underlying cirrhotic liver disease or fibrosis. It occurs in many clinical conditions such as lymphoproliferative disease, autoimmune disorders, collagen vascular disease, portal hypertension, biliary atresia, and Budd-Chiari syndrome (Trenschel et al. 2000).
At US, the nodules may be invisible or may manifest only as heterogeneous echotexture or distortion of normal architecture. If visible, the nodules are generally well-circumscribed, homogeneous, and hypoechoic but may be hyperechoic compared with normal liver (Casillas et al. 1997; Dachman et al. 1987; Clouet et al. 1999).
On CT, small hepatic nodules may not be detected. They usually appear hypodense without significant enhancement after contrast injection. Sometimes there may be a diffuse or peripheral rim-like enhancement.
On MRI, the visible is often homogeneous and slightly hyperintense on T1-weighted images and variable on T2-weighted images. On fat-suppressed T1-weighted images a decreased signal intensity due to intracellular fat may be observed. After intravenous injection of gadolinium contrast material, the nodules may enhance preferentially in the portal venous phase like normal liver parenchyma (Chung et al. 2010)
1.5 Hepatocellular Adenoma
Hepatic adenoma is a rare benign tumor in childhood, accounting for 2–4% of all liver tumors. It is much more common in adults with a typical presentation in healthy young women with a history of oral contraceptives (Yikilmaz et al. 2017)
In recent years, four distinct subtypes of hepatocellular adenomas have been classified by Bordeaux classification: inflammatory adenoma (40–50%), hepatocyte nuclear factor-1 alpha-mutated adenoma (30–40%), beta-catenin-activated adenoma (10–15%), and unclassified adenoma (10–25%) (Masand 2018; Van Aalten et al. 2011)
US findings of hepatic adenoma are nonspecific and depend on the presence of fat, hemorrhage, and necrosis. Lesions with a high lipid content or hemorrhage may be hyperechoic to the normal liver. On doppler imaging, hepatic adenoma may show internal vascularity (Adeyiga et al. 2012)
On CT hepatic adenoma is usually hypodense because of the fat content; areas of hemorrhage appear hyperdense. After iodinated contrast injection hepatocellular adenomas demonstrate preferentially hepatic arterial enhancement and appear isoattenuating in the portal venous and delayed phases.
On MRI hepatic adenoma appears isointense to slightly hyperintense on T1-weighted sequences and hyperintense on T2-weighted sequences. There is usually early arterial enhancement after intravenous administration of gadolinium which becomes isointense to the liver on portal venous and delayed phase images (especially in inflammatory subtype). The enhancement may not continue during the portal venous phase and delayed phase in hepatocyte nuclear factor 1 alpha mutated type (Pugmire and Towbin 2016).
2 Malignant Tumors
Metastatic disease is the most common neoplasm affecting children’s liver, especially from neuroblastoma, Wilms tumor, or lymphoma. Two-thirds of primary liver tumors in the pediatric population are malignant, and malignant primary hepatic tumors account for 1–2% of all childhood cancers (Chung et al. 2011).
The most common malignant tumors in decreasing order of frequency are hepatoblastoma, hepatocellular carcinoma (HCC), undifferentiated (embryonal) sarcoma (UES), angiosarcoma, and embryonal rhabdomyosarcoma. Epithelioid hemangioendothelioma (EHE) may also occur in adolescents (Table 1) (Ishak et al. 2001).
2.1 Hepatoblastoma
Hepatoblastoma is the most common malignant primary hepatic tumor in childhood. In many cases, it occurs in the first 2 years of life and causes a rapidly growing abdominal mass, hepatomegaly, pain, anorexia, and weight loss (Yikilmaz et al. 2017).
It usually occurs sporadically, but it may be associated with Beckwith-Wiedemann syndrome, hemihypertrophy, and familial adenomatous polyposis coli (McCarville and Roebuck 2012; Czauderna et al. 2014).
Serum alpha-fetoprotein levels are elevated in up to 90% of children with hepatoblastoma.
Metastatic disease most frequently involves the lungs, with pulmonary metastases seen in 10–20% of cases.
Histologically, hepatoblastoma is classified into the epithelial type and the mixed epithelial and mesenchymal type. Epithelial hepatoblastomas typically demonstrate a homogeneous appearance, while mixed epithelial and mesenchymal tumors appear more heterogeneous.
The appropriate initial diagnostic imaging in a patient with palpable abdominal mass is abdominal ultrasound (US). On US hepatoblastoma appears well-defined, lobulated, heterogeneous, and mildly echogenic masses. Calcifications, hemorrhage, and necrosis may be detected (Baheti et al. 2018)
On CT hepatoblastoma appears as a sharply circumscribed mass, slightly hypoattenuating on unenhanced and contrast-enhanced images. Epithelial hepatoblastomas are more homogeneous than a mixed tumor. About one-half of hepatoblastomas appear lobulated or septated, especially on contrast-enhanced images. After contrast injection hepatoblastoma enhances slightly, less than the adjacent liver. In the arterial phase, it may present peripherical or septal enhancement (Chung et al. 2011)
On MRI hepatoblastoma can be unifocal or multifocal and typically appears hypointense on T1-weighted images, heterogeneously hyperintense on T2-weighted images, with variable characteristics based on the degree of bleeding and necrosis. After gadolinium injection, it enhances heterogeneously with possible areas of early washout from arteriovenous shunting. On the diffusion-weighted sequence (DWI), hepatoblastoma shows intense diffusion restriction (Masand 2018) (Figs. 3 and 4).
Hepatoblastoma in a 5 years old male who presented with an abdominal mass, abdominal pain, and weight loss. US shows a well-defined solid mild echogenic mass (a) with a poor vascular spot on color-doppler (b). On CT the mass appears slightly hypoattenuating on unenhanced (c) and contrast-enhanced images (d, e), with areas of necrosis on venous phase (e). On MRI it appears iso-slightly iperintense on T2 coronal image (f) with a mild restriction on DWI (g) and shows poor contrast enhancement after gadolinium injection (h). Surgery specimen confirmed the diagnosis of hepatoblastoma (i)
The International Childhood Liver Tumor Strategy Group (SIOPEL) designed the Pretreatment Assessment of Tumor Extension (PRETEXT) system for staging and risk stratification in liver tumors, particularly hepatoblastoma and HCC.
The PRETEXT system is made of two components: the PRETEXT group and the annotation factors. The PRETEXT group describes the extent of a tumor within the liver and is based on determining the number of contiguous tumor-free liver sections. The annotation factors help to describe associated features such as vascular involvement (either portal vein or hepatic vein/inferior vena cava), extrahepatic disease, multifocality, tumor rupture, and metastatic disease (to both the lungs and lymph nodes) (Towbin et al. 2018)
CT is the gold standard for evaluating pulmonary metastatic disease in children. MRI of the lungs is not yet considered sufficiently sensitive to identify small pulmonary nodules, the detection of which would impact the outcome. 18F-FDG PET/CT has no role in hepatoblastoma staging, although it.
might be useful in select cases of suspected relapse when AFP levels are elevated but no site of disease is revealed by conventional imaging (Voss 2018; Cistaro et al. 2013)
The main differential diagnosis includes infantile hemangioendothelioma, which occurs almost exclusively in children under 1 year of age and may also contain calcifications, but more fine and granular, with a high enhancement, more than adjacent liver, and mesenchymal hamartoma of the liver that manifests in the same age group as hepatoblastoma but presents normal serum AFP levels and is usually predominantly cystic (Chung et al. 2011) (Fig. 5).
5 years old male who presented with an abdominal palpable mass. US shows a polilobulated ipoechoic solid mass (a) with high peri- and intralesional vascular signal (b). On contrast-enhanced CT, it appears slightly ipodense with some intralesional vessels and small necrosis foci (c, d). MRI demonstrates a slightly hyperintense mass on T2-weighted images (e), with intense diffusion restriction on DWI images (f). On multiphase images, it appears slightly hypointense to the adjacent liver (g, h) and remarkably hypointense on epatobiliary phase (i). This lesion was first considered by radiologists as a hepatoblastoma but on surgical treatment, pathology revealed an inflammatory pseudotumor-like follicular dendritic cell sarcoma (FDC), a very rare low-grade sarcoma
2.2 Hepatocellular Carcinoma (HCC)
HCC is the most common primary hepatic malignancy in adolescence and the second most common primary pediatric malignancy of the liver. Pediatric HCC differs from adult-type because preexisting liver disease is seen in only 30–50% of pediatric patients (Shelmerdine et al. 2016)
Serum alpha-fetoprotein levels are elevated in 55–65% of the cases. Affected children usually present with an abdominal mass, constitutional symptoms, and abdominal pain (Murawski et al. 2016)
The most predisposing factors are biliary atresia, cholestatic syndromes, hemochromatosis, hereditary tyrosinemia, glycogen storage disorders, Wilson’s disease, and hepatitis B infection.
On US, HCC is variable: smaller HCCs are predominantly hypoechoic to normal liver, although they may be isoechoic or hyperechoic while larger lesions may be more heterogeneous with hyperechoic areas representing fat or acute hemorrhage and anechoic areas due to necrosis or old hemorrhage. Infiltrative HCC may appear as a diffuse disruption of the normal liver echotexture (Helmberger et al. 1999)
On CT, the mass is usually hypodense or isodense with well-defined or ill-defined margins and calcifications in 40% of HCC. After contrast injection, HCC presents early arterial enhancement with rapid wash-out. In delayed phases, it can present a peripherical capsule. CT is the gold standard for identifying vascular invasion and metastatic disease. Enlarged metastatic lymph nodes are seen at the porta hepatis in more than 50% of children’s HCC. The most common metastatic disease spreads to the lungs, mediastinum, skeleton, and brain. Segmental liver involvement, vascular invasion, and distant metastatic spread are determinants of upfront resectability and are evaluated in PRETEXT, a radiologic staging system for primary hepatic malignancies of childhood (Yikilmaz et al. 2017; Jha et al. 2009)
On MRI, hepatocellular carcinoma is heterogeneously hyperintense on the T2-weighted sequences, hypointense on T1-weighted sequences and presents intense diffusion restriction on the diffusion-weighted sequence. On dynamic phases, HCC enhances heterogeneously on the arterial phase and shows washout on the portal venous phase because of arteriovenous shunting. Hemorrhagic areas present bright signals on the pre-contrast T1-weighted sequences while calcification or hemosiderin manifests as focal areas of susceptibility on the gradient recalled echo sequences. Tumor thrombus in the portal or hepatic veins enhances on the post-contrast T1-weighted images (Masand 2018).
2.3 Fibrolamellar Carcinoma
Fibrolamellar carcinoma is a rare variant of HCC, which primarily affects adolescents and young adults that occurs without predisposing factors such as cirrhosis or viral hepatitis.
The tumor appears as a large, circumscribed, and nonencapsulated mass in 80–90% of cases (Levy 2002).
On US fibrolamellar carcinoma is heterogeneous, associated calcification may be seen as hyperechoic foci with posterior acoustic shadowing. A central scar appears as a hyperechoic focus (Yikilmaz et al. 2017)
On CT fibrolamellar carcinoma presents well-defined lobulated margins, it’s hypoattenuating relative to the adjacent liver, frequently with a central scar with or without calcifications. After contrast administration, the tumor is hyperattenuating relative to the adjacent liver in the arterial phase with variable attenuation in the portal venous phase.
On MRI, the lesion is usually isointense or hypointense on T1-weighted MR images and hyperintense on T2-weighted MR images, and on dynamic phases, it shows avid contrast enhancement during the arterial phase with variable enhancement during the portal venous phase. The central scar is usually hypointense on both T1-weighted and T2-weighted MR without enhancement on dynamic phases not enhance unlike the scar of FNH (Smith et al. 2008)
2.4 Undifferentiated Embryonal Sarcoma (UES)
Undifferentiated embryonal sarcoma (UES) is the third most common hepatic malignancy of mesenchymal origin, accounting for 6% of all pediatric liver tumors and 10–15% of all malignant pediatric liver tumors and typically occurs in children 6–10 years of age. Serum AFP level is normal in UES.
On US, it appears as a large heterogeneous solid mass (>10 cm at diagnosis). While UES appears solid on US, paradoxically, it shows cystic aspect on CT and MR imaging because of its myxoid stroma which is a similar imaging feature of myxoid sarcoma and synovial sarcoma (Yikilmaz et al. 2017)
On CT, UES shows water attenuation (because of myxoid stroma) with soft tissue components, especially on the periphery of forming septa. A dense pseudocapsule with peripherical enhancement or hemorrhagic foci may be observed. After intravenous administration of iodinated contrast material, predominantly peripheral enhancement is noted on delayed images (Buetow et al. 1997)
On MRI, undifferentiated embryonal sarcoma appears as a large mixed solid and multicystic mass with central necrosis and areas of hemorrhage that present hyperintense T1-weighted signal. The cystic components have hypointense T1-weighted and hyperintense T2-weighted signal while the solid components show iso- to hypointense T1-weighted and heterogeneously hyperintense T2-weighted signal intensity. A T1- and T2-weighted hypointense pseudocapsule may be seen around the tumor. Post-contrast sequences demonstrate heterogeneous enhancement, usually peripherally and within the solid components of the mass (Masand 2018).
2.5 Epithelioid Hemangioendothelioma
Epithelioid hemangioendothelioma is an epithelial liver tumor classified under the category of malignant vascular tumors in the latest ISSVA classification (ISSVA 2014).
Epithelioid hemangioendothelioma occurs almost exclusively in young adults and appears, on imaging, as multiple discrete nodules ranging from 0.5 cm to 12 cm, or as confluent coalescent masses. Peripheral lesions may cause retraction of the liver capsule in up to 69% of the cases (Yikilmaz et al. 2017)
On US, epithelioid hemangioendothelioma may appear as individual nodules, confluent nodules, or diffusely heterogeneous echotexture of the liver. Nodules usually appear hypoechoic because of central myxoid stroma, but they may appear hyperechoic with or without a hypoechoic rim or isocheoic with a hypoechoic halo (Makhlouf et al. 1999; Miller et al. 1992; Buetow et al. 1994; Lyburn et al. 2003)
On CT, epithelioid hemangioendothelioma presents central hypodensity without contrast enhancement because of the presence of myxoid and hyalinized stroma or necrosis. It shows peripheral enhancement during the arterial phase with possible progressive centripetal enhancement during the portal venous and delayed phases; residual non-enhancing areas may persist (Yikilmaz et al. 2017)
ON MRI epithelioid hemangioendothelioma appears hypointense to the liver on T1-weighted images, with a possible more hypointense central portion, and heterogeneously hyperintense on T2-weighted images with a more hyperintense central portion. After contrast administration, there is a typical peripheral enhancement, although very large lesions may show more heterogeneous enhancement (Chung et al. 2011)
Fluorine 18 (18F) fluorodeoxyglucose positron emission tomography (PET) demonstrates moderate to intense uptake in the tumors and may demonstrate the involvement of adjacent lymph nodes and extrahepatic sites (Nguyen 2004).
2.6 Angiosarcoma
Angiosarcoma is a high-grade malignant tumor of the liver that is derived from endothelial cells, which occurs rarely in children. The mean presenting age is 3 years and the prognosis is poor.
Angiosarcoma may present multiple nodules or a large dominant mass or a mixture of a dominant mass and multiple nodules and, least commonly, diffuse micronodular infiltration of the liver (Koyama et al. 2002)
On US, angiosarcoma may appear as multiple nodules, a large mass, or both or diffuse heterogeneous echotexture of the entire liver. The echogenicity of the nodules depends on hemorrhagic or necrotic change.
On CT, lesions are usually hypoattenuating relative to the liver on arterial and venous phase images with foci of early heterogeneous, occasionally central enhancement or ring enhancement. On delayed images, persistent enhancement is observed without centripetal fill-in.
On MRI, angiosarcoma presents as a large mass hypointense on T1-weighted images and heterogeneously hyperintense on T2-weighted images and shows disorganized enhancement secondary to random vessel distribution on arterial phase imaging. On dynamic it presents markedly heterogeneous enhancement, which is progressive on the delayed phase, without central fill-in (Chung et al. 2011)
18F fluorodeoxyglucose PET/CT may demonstrate marked uptake in the liver tumors and can be helpful in localizing extrahepatic disease (Maeda et al. 2007)
2.7 Embryonal Rhabdomyosarcoma
Embryonal rhabdomyosarcoma is the only malignant tumor that arises in the biliary tree in children. It typically presents under the age of 5 years of age with jaundice, abdominal pain, nausea, vomiting, and fever.
The tumor usually involves major extrahepatic bile ducts but may originate in or grow into intrahepatic biliary ducts and invade the liver and causes biliary duct dilatation.
Biliary rhabdomyosarcoma typically shows an intraductal growth pattern and appears on US as a hypoechoic intraductal mass causing biliary duct dilatation (Kirli et al. 2012)
On CT, rhabdomyosarcoma appears as a heterogeneous mass filling the biliary tree with a grape-like or branching pattern, which shows variable enhancement after contrast administration (Yikilmaz et al. 2017)
On MRI, rhabdomyosarcoma presents T1 hypointense and T2 hyperintense signal or predominantly fluid-intensity signal and appears as a mass in the common duct or biliary radicals or a heterogeneous intrahepatic mass with large fluid- intensity areas. On CPRM it may appear as a partially cystic lesion in the common bile duct and as a mass adjacent to the duct that causes mural irregularity (Spunt et al. 2000; Kitagawa and Aida 2007; Lopez-Terrada and Finegold 2014; Nemade et al. 2007).
3 Conclusions
Imaging should aim to clarify the presence of a lesion, the likelihood of malignancy and potential for complete surgical resection.
US is the initial investigation modality of choice. Both CT and MRI may be used for evaluating the extent of the tumor for further evaluation, to acquire additional information for differential diagnoses and to diagnose metastases to lung, lymph nodes or bone. CT requires less or no anesthesia due to faster scan times.
MRI has the advantage over CT of the absence of radiation exposure and the usage of mixed hepatocyte specific/extracellular contrast agents allows for better lesion characterisation and location, particularly with respect to the biliary system.
References
Adeyiga AO, Lee EY, Eisenberg RL (2012) Focal hepatic masses in pediatric patients. AJR Am J Roentgenol 199(4):W422–W440
Anupindi SA, Biko DM, Ntoulia A et al (2017) Contrast-enhanced US assessment of focal liver lesions in children. Radiographics 37(6):1632–1647
Baheti AD, Chapman T, Rudzinski E et al (2018) Diagnosis, histopathologic correlation and management of hepatoblastoma: what the radiologist needs to know. Clin Imaging 52:273–279
Bartolotta TV, Midiri M, Scialpi M et al (2004) Focal nodular hyperplasia in normal and fatty liver: a qualitative and quantitative evaluation with contrast-enhanced ultrasound. Eur Radiol 14:583–591
Buetow PC, Buck JL, Ros PR (1994) Malignant vascular tumors of the liver: radiologic pathologic correlation. Radiographics 14(1):153–166
Buetow PC, Buck JL, Pantongrag-Brown L et al (1997) Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology 203(3):779–783
Casillas C, Martí-Bonmatí L, Galant J (1997) Pseudotumoral presentation of nodular regenerative hyperplasia of the liver: imaging in five patients including MR imaging. Eur Radiol 7(5):654–658
Chavhan GB, Shelmerdine S, Jhaveri K et al (2016) Liver MR imaging in children: current concepts and technique. Radiographics 36:1517–1532
Christison-Lagay ER, Burrows PE, Alomari A et al (2007) Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg 42:62–67
Chung EM, Cube R, Lewis RB et al (2010) From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics 30(3):801–826
Chung EM, Lattin GE Jr, Cube R (2011) From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics 31(2):483–507
Cistaro A, Treglia G, Pagano M et al (2013) A comparison between (18F)F-FDG PET/CT imaging and biological and radiological findings in restaging of hepatoblastoma patients. Biomed Res Int 2013:709037
Clouet M, Boulay I, Boudiaf M et al (1999) Imaging features of nodular regenerative hyperplasia of the liver mimicking hepatic metastases. Abdom Imaging 24(3):258–261
Czauderna P, Lopez-Terrada D, Hiyama E et al (2014) Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 26:19–28
Dachman AH, Ros PR, Goodman ZD et al (1987) Nodular regenerative hyperplasia of the liver: clinical and radiologic observations. AJR Am J Roentgenol 148(4):717–722
Dickie B, Dasgupta R, Nair R et al (2009) Spectrum of hepatic hemangiomas: management and outcome. J Pediatr Surg 44:125–133
Helmberger TK, Ros PR, Mergo PJ et al (1999) Pediatric liver neoplasms: a radiologic- pathologic correlation. Eur Radiol 9(7):1339–1347
Ishak KG, Goodman ZD, Stocker JT (2001) Tumors of the liver and intrahepatic bile ducts. AFIP Atlas of Tumor Pathology, Third Series, Fascicle 31
ISSVA (International Society for the Study of Vascular Anomalies classification of vascular anomalies) (2014)
Jha P, Chawla SC, Tavri S et al (2009) Pediatric liver tumors–a pictorial review. Eur Radiol 19:209–219
Kassarjian A, Zurakowski D, Dubois J et al (2004) Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. AJR Am J Roentgenol 182(3):785–795
Keslar PJ, Buck JL, Selby DM (1993) Infantile hemangioendothelioma of the liver revisited. Radiographics 13(3):657–670
Kirli EA, Parlak E, Oguz B et al (2012) Rhabdomyosarcoma of the common bile duct: an unusual cause of obstructive jaundice in a child. Turk J Pediatr 54:654–657
Kitagawa N, Aida N (2007) Biliary rhabdomyosarcoma. Pediatr Radiol 37(10):1059
Koyama T, Fletcher JG, Johnson CD et al (2002) Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology 222(3):667–673
Levy AD (2002) Malignant liver tumors. Clin Liver Dis 6(1):147–164
Lopez-Terrada D, Finegold MJ (2014) Tumors of the liver. In: Suchy FJ, Sokol RJ (eds) Liver disease in children, 4th edn. Cambridge University Press, Cambridge, pp 728–729
Lyburn ID, Torreggiani WC, Harris AC et al (2003) Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. AJR Am J Roentgenol 180(5):1359–1364
Ma IT, Rojas Y, Masand PM et al (2015) Focal nodular hyperplasia in children: an institutional experience with review of the literature. J Pediatr Surg 50(3):382–387
Maeda T, Tateishi U, Hasegawa T et al (2007) Primary hepatic angiosarcoma on coregistered FDG PET and CT images. AJR Am J Roentgenol 188(6):1615–1617
Makhlouf HR, Ishak KG, Goodman ZD (1999) Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer 85(3):562–582
Masand PM (2018) Magnetic resonance imaging features of common focal liver lesions in children. Pediatr Radiol 48(9):1234–1244
McCarville MB, Roebuck DJ (2012) Diagnosis and staging of hepatoblastoma: imaging aspects. Pediatr Blood Cancer 59:793–799
Meyers RL (2007) Tumors of the liver in children. Surg Oncol 16(3):195–203
Meyers AB, Towbin AJ, Serai S et al (2011) Characterization of pediatric liver lesions with gadoxetate disodium. Pediatr Radiol 41:1183–1197
Miller WJ, Dodd GD 3rd, Federle MP et al (1992) Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol 159(1):53–57
Mitchell CL, Vasanawala SS (2011) An approach to pediatric liver MRI. AJR Am J Roentgenol 196:W519–W526
Moore M, Anupindi SA, Mattei P et al (2009) Mesenchymal cystic hamartoma of the liver: MR imaging with pathologic correlation. J Radiol Case Rep 3:22–26
Murawski M, Weeda VB, Maibach R et al (2016) Hepatocellular carcinoma in children: does modified platinum and doxorubicin-based chemotherapy increase tumor resectability and change outcome: lessons learned from the SIOPEL 2 and 3 studies. J Clin Oncol 34:1050–1056
Nemade B, Talapatra K, Shet T et al (2007) Embryonal rhabdomyosarcomaof the biliary tree mimicking a choledochal cyst. J Cancer Res Ther 3(1):40–42
Nguyen BD (2004) Epithelioid hemangioendothelioma of the liver with F-18 FDG PET imaging. Clin Nucl Med 29(12):828–830
Pugmire BS, Towbin AJ (2016) Magnetic resonance imaging of primary pediatric liver tumors. Pediatr Radiol 46:764–777
Roebuck DJ (2009) Assessment of malignant liver tumors in children. Cancer Imaging 9 (Special No A):S98–S103
Rozell JM, Catanzano P, Polansky SM et al (2014) Primary liver tumors in pediatric patients: proper imaging technique for diagnosis and staging. Semin Ultrasound CT MR 35(4):382–393
Shelmerdine SC, Roebuck DJ, Towbin AJ et al (2016) MRI of paediatric liver tumours: how we review and report. Cancer Imaging 16:21
Smith MT, Blatt ER, Jedlicka P et al (2008) Best cases from the AFIP: fibrolamellar hepatocellular carcinoma. Radiographics 28:609–613
Spunt SL, Lobe TE, Pappo AS et al (2000) Aggressive surgery is unwarranted for biliary tract rhabdomyosarcoma. J Pediatr Surg 35(2):309–316
Stocker JT (2001) Hepatic tumors in children. Clin Liver Dis 5(1):259–281
Sutherland T, Seale M, Yap K (2014) Part 1: MRI features of focal nodular hyperplasia with an emphasis on hepatobiliary contrast agents. J Med Imaging Radiat Oncol 58:50–55
Towbin AJ, Meyers RL, Woodley H (2018) 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric hepatic international tumour trial (PHITT). Pediatr Radiol 48(4):536–554
Trenschel GM, Schubert A, Dries V et al (2000) Nodular regenerative hyperplasia of the liver: case report of a 13-year-old girl and review of the literature. Pediatr Radiol 30:64–68
Van Aalten SM, Thomeer MG, Terkivatan T et al (2011) Hepatocellular adenomas: correlation of MR imaging with pathologic subtype classification. Radiology 261:172–181
Voss SD (2018) Staging and following common pediatric malignancies: MRI versus CT versus functional imaging. Pediatr Radiol 48:1324–1336
Wu S, Tu R, Liu G et al (2012) The frequency and clinical significance of the halo sign in focal nodular hyperplasia of the liver. Med Ultrason 14:278–282
Yikilmaz A, George M, Lee EY (2017) Pediatric Hepatobiliary neoplasms: An Overview and Update. Radiol Clin North Am 55(4):741–766
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Masselli, G., Guida, M., Ceccanti, S., Cozzi, D. (2021). Hepatic Tumoral Pathology: The Pediatric Liver. In: Quaia, E. (eds) Imaging of the Liver and Intra-hepatic Biliary Tract. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-39021-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-39021-1_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39020-4
Online ISBN: 978-3-030-39021-1
eBook Packages: MedicineMedicine (R0)