Abstract
The accurate diagnosis of benign lesions in a cirrhotic liver is clearly important to avoid misdiagnosis of hepatocellular carcinoma (HCC) or other malignancies and to direct the patient toward the most appropriate management. In this chapter we review the appearance of the most common benign lesions encountered on CT and MR imaging of a cirrhotic liver. The lesions are divided into two main groups depending on the histological composition as non-hepatocellular lesions (i.e., cysts and peribiliary cysts, hemangiomas, pseudolesions) and hepatocellular lesions (i.e., nonmalignant cirrhosis-associated nodules, focal nodular hyperplasia like nodules, and hepatobiliary hypointense nodules without arterial phase hyperenhancement).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Non-hepatocellular Lesions
1.1 Peribiliary Cysts and Hepatic Cysts
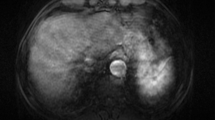
Peribiliary cysts result from dilatation of obstructed periductal glands and have a prevalence of 9% in cirrhotic patients (Baron et al. 1994; Bazerbachi et al. 2018). They are usually multiple and seen along the portal triads in the hilum and central part of liver (Elsayes et al. 2018; Ronot et al. 2017). Peribiliary cysts do not communicate with the bile ducts but may mimic biliary ductal dilatation (Baron et al. 1994). Their presence on both sides of portal vein without upstream biliary dilatation is key finding for a proper diagnosis (Ronot et al. 2017) (Fig. 1). When the diagnosis is unclear on contrast-enhanced CT or MR cholangiography, injection of a hepatobiliary contrast agent (i.e., Gd-EOB-DTPA or Gd-BOPTA) may be considered to differentiate the contrast-enhanced bile ducts from the unenhanced peribiliary cysts on hepatobiliary phase (Bazerbachi et al. 2018; Ronot et al. 2017). Size progression of peribiliary cysts has been reported with worsening of the liver disease and rarely results in biliary obstruction (Bazerbachi et al. 2018).
Axial T2-weighted MR image (a) in a 63-year-old man with cirrhosis and para-coronal thick slab MR cholangiography image (b) in a 78-year-old man with cirrhosis show multiple small cystic lesions (arrows in a, b) compatible with peribiliary cysts. The location of the cysts on both sides of the portal vein and the lack of upstream biliary ductal dilatation are key features for a proper diagnosis
Hepatic cysts in cirrhosis do not usually represent a diagnostic challenge as they maintain the same imaging appearance as in non-cirrhotic livers (Ronot et al. 2017). The lack of enhancement and the bright signal intensity on T2-weighted MR images are key features for a confident diagnosis.
1.2 Hemangiomas
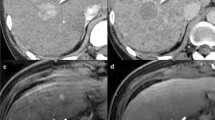
Hemangiomas are among the most common benign lesions, but are less frequently encountered in cirrhotic patients (Dodd et al. 1999; Duran et al. 2015). In the early stages of hepatic fibrosis hemangiomas show a classic appearance including peripheral nodular and progressive enhancement that remains isoattenuating/isointense to blood pool, as well as high signal intensity on T2-weighted MR images (Brancatelli et al. 2001) (Fig. 2). As fibrosis progresses, hemangiomas become smaller and may lack the typical imaging features, thus making it difficult to characterize radiologically (Brancatelli et al. 2001). Additionally, regressing hemangiomas may show capsular retraction further complicating the differential diagnosis with other lesions in cirrhosis. In such cases, other imaging features may help in the differential diagnosis with HCC, including the low signal intensity on diffusion weighted images and the evolution over time from prior studies. More recently, Duran et al. (Duran et al. 2015) found no significant difference in the rate of typical enhancement pattern on MR images for hemangiomas arising in a normal liver compared to those arising in a fibrotic or cirrhotic liver.
Axial contrast-enhanced CT images in a 53-year-old-male with history of alcoholic cirrhosis show a small hypodense nodule with peripheral nodular enhancement (arrow) on the image obtained during the arterial phase (a). The nodule is isoattenuating on the image obtained during the portal venous phase (b). The diagnosis of hemangioma was pathologically proven at fine needle biopsy
The radiologists using Gd-EOB-DTPA-enhanced MR for the surveillance of HCC in cirrhotic patients should be aware of an important diagnostic pitfall related to the diagnosis of hemangiomas. Flash-filling hemangiomas are usually hypointense on hepatobiliary phase images and may appear hypointense also on the images acquired during the transitional phase (i.e., delay of at least 120 seconds post-contrast). The phenomenon, referred as “pseudo-washout,” is due to the rapid uptake of contrast agent in the surrounding liver after the extracellular phase and may result in misdiagnosis of HCC (Chernyak et al. 2019; Doo et al. 2009). This phenomenon emphasizes that washout for the diagnosis of HCC should only be assessed during the portal venous phase, when using Gd-EOB-DTPA as the contrast agent, in order to reduce the number of false-positive diagnoses (Joo et al. 2015).
1.3 Pseudolesions
Pseudolesions are observations only detected on imaging in absence of a true pathological lesion (Papadatos et al. 2018). Vascular pseudolesions are usually due to arterioportal shunting, as a consequence of the altered microcirculation in cirrhosis (American College of Radiology 2018). They classically appear as triangular-shaped, subcapsular, hyperenhancing areas visible only on the images obtained during the arterial phase (Fig. 3). When they appear round, the isodensity/isointensity to the background liver on all other phases and sequences and the lack of mass effect on vascular and biliary structures are clues to differentiate them from HCC (Murakami and Tsurusaki 2014). Other pseudolesions that can be encountered in cirrhosis include a hypertrophic pseudomass and confluent hepatic fibrosis (Papadatos et al. 2018; Elsayes et al. 2018). A hypertrophic pseudomass is a hypertrophic area of liver surrounded by fibrosis mimicking a mass on imaging (Fig. 4). A hypertrophic pseudomass can be differentiated from HCC or other malignancies on the basis of iso-enhancement to the background parenchyma and lack of mass effect on vessels or bile ducts. Confluent hepatic fibrosis (or focal parenchymal extinction) is a focal or regional area of marked increased fibrosis and parenchymal loss compared to the surrounding liver parenchyma (Wanless et al. 1995). On imaging, confluent hepatic fibrosis typically appears as wedge-shaped observations, peripherally located most often in segments 4, 5, and 8 and associated with liver surface retraction and delayed enhancement (Ohtomo et al. 1993) (Fig. 5). The morphology and the typical location as well as the progressive contraction at imaging follow-up are key features for a correct diagnosis (Elsayes et al. 2018).
Axial contrast-enhanced CT image obtained during the arterial phase (a) shows a triangular-shaped, subcapsular hyperenhancing “lesion” (arrow) lacking washout on the corresponding image obtained during the portal venous phase (b). The combination of imaging features and enhancement pattern is most compatible with a pseudolesion, such as arterioportal shunt in cirrhosis
Axial contrast-enhanced CT image obtained during the portal venous phase shows a hypertrophic caudate lobe (arrow) simulating a liver mass. The “lesion” iso-attenuation to the background liver parenchyma and the lack of mass effect on vessels (arrowhead) are key imaging features to exclude malignancy
Gross pathology specimen (a) demonstrating cirrhosis and confluent hepatic fibrosis (arrow). Axial T1-weighted MR images obtained during the delayed phase postinjection of Gd-BOPTA (b) demonstrate wedge-shaped area of delayed enhancement with capsular retraction (arrow) compatible with confluent hepatic fibrosis
2 Hepatocellular Lesions
2.1 Regenerative and Dysplastic Nodules
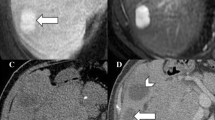
Cirrhosis is the endpoint of a long-standing fibrotic repair process in response to repetitive injuries (Papadatos et al. 2018). The key pathologic features of cirrhosis are bridging fibrosis surrounding regenerative nodules (RN), also called cirrhotic nodules. RNs are composed of nonneoplastic hepatocytes and are histologically and radiologically identical to the background liver. On contrast-enhanced cross-sectional imaging, RNs are not hypervascular and mostly appear isointense compared to the surrounding liver on all MR sequences, including the images obtained during the hepatobiliary phase (American College of Radiology 2018; Ronot et al. 2017) (Fig. 6). Dysplastic nodules are caused by clonal expansion of a certain cell lineage and are typically distinct from the background parenchyma. They may have a higher content of fat and iron compared to the surrounding liver which may result in intrinsic hyperintensity on T1-weighted imaging (American College of Radiology—LI-RADS 2018). Based on the presence and degree of atypia DNs are further classified into low-grade and high-grade subtypes (Ronot et al. 2017). Low-grade DNs are histologically benign although they have the potential to become neoplastic due to presence of molecularly aberrant cells (American College of Radiology—LI-RADS 2018). High-grade DNs are the last step in the hepatocarcinogenetic process (premalignant nodules) before early and progressed HCC. High-grade DNs show cellular and architectural atypia without stromal invasion or other malignant features (Choi et al. 2015). They may show changes in their blood supply resulting in imaging features commonly described in HCC, such as arterial phase hyperenhancement because of arterial neo-angiogenesis and hypointensity on the hepatobiliary phase after injection of Gd-EOB-DTPA because of reduced expression of the organic anion transporter (OATP), the peptide responsible for the intracellular accumulation of Gd-EOB-DTPA (Motosugi et al. 2015) (Fig. 7).
Gross pathology specimen (a) of a cirrhotic liver shows innumerable tan, regenerative nodules delineated by fibrous septa. On the axial T1-weighted MR images obtained during the arterial (b) and portal venous (c) phases after injection of Gd-BOPTA, the regenerative (cirrhotic) nodules are barely visible and only minimally hypoenhancing on the portal venous phase image
Axial MR images of a cirrhotic liver obtained during the arterial (a), portal venous (b), and hepatobiliary phase (c) following injection of Gd-EOB-DTPA show a small hepatobiliary hypointense nodule (arrow in c) without arterial phase hyperenhancement. Hematoxylin and eosin staining of the specimen (d) obtained at ultrasound-guided core biopsy of the lesion shows areas of increased cellularity with hepatocytes displaying uniform hyperchromatic nuclei with slightly increased nuclear/cytoplasmic ratio and focal irregular nuclear contour, suggesting a dysplastic nodule. The lesion was monitored with repeated MR imaging studies and developed arterial phase hyperenhancement (arrow in e) and portal venous washout (arrow in f) 5 years later while maintaining hypointensity on hepatobiliary phase (arrow in g). The microscopic evaluation of the surgical specimen post resection (h) demonstrates hypercellular proliferation of hepatoid appearing cells in a trabecular pattern with pseudo-acini, bile production, and Mallory hyaline compatible with hepatocellular carcinoma. Unpaired (so-called aberrant) arteries were also identified (black arrows in h, inlet) (Images d and h are courtesy of Marta I. Minervini, MD, Department of Pathology, University of Pittsburgh)
2.2 Focal Nodular Hyperplasia-Like Nodules
Focal nodular hyperplasia (FNH) is a benign hepatic lesion arising as a hyperplastic response to a congenital vascular malformation in an otherwise normal liver (Vilgrain et al. 1992). FNH-like lesions are microscopically, macroscopically, and immunohistochemically identical to FNH but occur in patients with liver diseases or hepatic vascular abnormalities (Yoneda et al. 2016; Vilgrain 2006). Although rare in cirrhosis, FNH-like nodules have been described more often when the etiology of liver disease is alcoholic with an incidence ranging from 3.4 to 8% (Quaglia et al. 2003; Libbrecht et al. 2006) (Fig. 8). In a cirrhotic background, these nodules likely arise from a hyperplastic hepatocellular response to a localized arterial over-inflow caused by focal angio-architectural anomalies (Nakashima et al. 2004). Histologically, FNH-like lesions may show a modest increase in cell density with irregular trabecular pattern, unpaired arteries, and diffuse capillarization of the sinusoids similar to well-differentiated HCC. In such cases, immunohistochemistry is helpful, demonstrating increased staining for HSP70 GPC3 and glutamine synthetase in FNH-like lesions (Choi et al. 2011). On contrast-enhanced cross-sectional imaging FNH-like lesions generally present with brisk arterial phase hyperenhancement, lack of washout on the extracellular phase images, and iso- to hyperintensity on the images acquired during the hepatobiliary phase postinjection of Gd-BOPTA or Gd-EOB-DTPA (Figs. 9 and 10). The signal intensity on hepatobiliary phase images is key for differentiating FNH-like lesions from HCC. Most HCCs are hypointense on hepatobiliary phase images, although 10–15% of HCC have been reported to be iso- to hyperintense given the overexpression of OATP (also referred as “paradoxical uptake”). The most common appearance of both FNH-like lesions and HCCs with contrast uptake on hepatobiliary phase images is diffuse homogeneous hyperintensity. Other patterns of signal distribution have also been described, which could help with the diagnosis. A mosaic pattern and a nodule-in-nodule appearance are typical of HCC while a doughnut pattern is more commonly seen in FNH-like nodule. Presence of washout, a low ADC value, and the absence of a central scar are other features helpful to differentiate HBP hyperintense HCC from FNH-like lesion (Kim et al. 2017; Kitao et al. 2018). Finally, longitudinal assessment of changes in lesion size is important because FNH-like lesions tend to show no change or decrease in size over time. Recently a new subset of FNH-like lesions has been described in alcoholic cirrhosis which shows strong immune-reactivity to serum amyloid A (SAA) with histologic features commonly seen in inflammatory adenomas including sinusoidal dilatation, ductular reaction, and inflammatory reaction (Yoneda et al. 2016). While FNH-like nodules are typically iso- to hyperintense on hepatobiliary phase images, SAA-positive nodules may appear hypointense (Yoneda et al. 2016; Sasaki et al. 2015).
Axial contrast-enhanced CT images in a 57-year-old male with history of alcoholic cirrhosis show a 2 cm lesion with homogeneous enhancement on the image obtained during the arterial phase (arrow in a) and lack of washout on the image obtained during the portal venous phase (b). A FNH-like nodule was pathologically proven at resection
Axial MR images obtained prior to (a, b) and following (c–e) the administration of Gd-EOB-DTPA in a 64-year-old male with history of cirrhosis. The image obtained during the hepatobiliary phase (e) shows multiple small hyperintense lesions (arrow) which appear iso-intense on pre-contrast T1- (a) and T2-weighted (b) and post-contrast images obtained during the arterial (c) and portal venous (d) phases. The pathological analysis of the specimen obtained via ultrasound-guided biopsy of the lesion reveals changes similar to those seen in FNH lesions. The immunohistochemistry for glutamine synthetase (f) shows increased diffuse positive hepatocyte uptake without a definite maplike pattern. The CD34 staining (g) demonstrates diffuse capillarization of the hepatic sinusoids (Images (f, g) are courtesy of Marta I. Minervini, MD, Department of Pathology, University of Pittsburgh)
Surveillance ultrasound (a) in a cirrhotic patient shows an ill-defined hypoechoic nodule prompting further evaluation with contrast-enhanced MR. Axial (b) Gd-EOB-DTPA-enhanced MR image obtained during the hepatobiliary phase shows several hyperintense lesions with central hypointense scar suggesting FNH-like lesions. At follow-up MR imaging studies (not shown), the lesions remained stable in size (Case courtesy of Dr. Jessica Yang, MD, Concord Repatriation General Hospital, Sydney, Australia)
2.3 Hepatobiliary Hypointense Nodules Without Arterial Phase Hyperenhancement
This is a separate group of hepatocellular nodules encountered in cirrhotic patients after injection of hepatobiliary contrast agent (Motosugi et al. 2015; Motosugi et al. 2018). Although discussed in this chapter, pathologically these lesions may represent either early HCC or high-grade DNs (Golfieri et al. 2011). The key element to differentiate early HCCs from dysplastic nodules on histologic examination is the presence of stromal invasion, a feature that is not possible to discern on imaging (American College of Radiology 2018; Motosugi et al. 2015). Early HCC is a well-differentiated tumor usually described as “vaguely nodular” (The international Consensus Group for Hepatocellular neoplasia 2009). On imaging, early HCCs typically lack the hallmark arterial phase hyperenhancement of progressed HCCs, given the combination of reduced number of portal triads and a not-yet-fully-developed arterial neo-angiogenesis. These lesions are typically hypovascular during the dynamic contrast-enhanced CT or Gd-enhanced MRI study and have reduced expression of OATP, thus resulting in hypointensity on hepatobiliary phase compared to the surrounding liver parenchyma after injection of Gd-EOB-DTPA. Hepatobiliary hypointense nodules without arterial phase hyperenhancement should be carefully evaluated because of the increased risk of progression to typical HCC (Motosugi et al. 2015). A recent meta-analysis showed that 18.3, 25.2, and 30.3% of these nodules develop arterial phase hyperenhancement at 1, 2, and 3 years, respectively (Fig. 7). In contrast, only less than 1% of hypovascular nodules isointense on hepatobiliary phase images develop arterial hyperenhancement on subsequent imaging (Suh et al. 2017) (Fig. 11). The risk of progression to arterial hyperenhancement is associated with the initial nodule size (i.e., higher risk for nodules larger than 9 mm), the presence of intralesional fat, and the high signal intensity on T2-weighted and diffusion-weighted images (Suh et al. 2017). Moreover, the presence of these nodules increases the risk of developing cancer elsewhere in the liver and the risk of recurrence of HCC after resection (Komatsu et al. 2014; Toyoda et al. 2013). The management of hepatobiliary hypointense nodules without arterial phase hyperenhancement is still controversial – options include (1) further evaluation with another imaging modality to assess arterial phase hyperenhancement (e.g., contrast-enhanced ultrasound), (2) imaging follow-up, and (3) biopsy.
Axial MR images of a cirrhotic liver obtained during the portal venous (a) and hepatobiliary (b) phases following injection of Gd-EOB-DTPA show a small hypoenhancing lesion (arrow in a), which is isointense compared to the surrounding liver on hepatobiliary phase. The lesion was monitored with MRI for years. In an MR imaging study obtained 7 years later, the lesion shows long-term size stability and hypovascular appearance on portal venous phase (c). Arterial phase images (not showed) also demonstrate lack of arterialization after 7 years
3 Summary
Benign hepatic lesions may have imaging features overlapping with those of malignant lesions. Also, they frequently have atypical imaging features due to alteration in liver perfusion and architectural distortion in setting of cirrhosis. As such, radiologists have to be familiar with them in order to provide correct diagnosis and optimal care.
References
American College of Radiology. Liver imaging reporting and data system. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed July 2018
Baron RL, Campbell WL, Dodd GD 3rd (1994) Peribiliary cysts associated with severe liver disease: imaging-pathologic correlation. AJR Am J Roentgenol 3:631–636
Bazerbachi F, Haffar S, Sugihara T et al (2018) Peribiliary cysts: a systematic review and proposal of a classification framework. BMJ Open Gastroenterol 5(1):e000204
Brancatelli G, Federle MP, Blachar A, Grazioli L (2001) Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology 219(1):69–74
Chernyak V, Fowler KJ, Heiken JP, Sirlin CB (2019) Use of gadoxetate disodium in patients with chronic liver disease and its implications for liver imaging reporting and data system (LI-RADS). J Magn Reson Imaging 49(5):1236–1252
Choi JY, Lee HC, Yim JH et al (2011) Focal nodular hyperplasia or focal nodular hyperplasia-like lesions of the liver: a special emphasis on diagnosis. J Gastroenterol Hepatol 26(6):1004–1009
Choi BI, Lee JM, Kim TK et al (2015) Diagnosing borderline hepatic nodules in hepatocarcinogenesis: imaging performance. AJR Am J Roentgenol 205(1):10–21
Dodd GD 3rd, Baron RL, Oliver JH 3rd, Federle MP (1999) Spectrum of imaging findings of the liver in end-stage cirrhosis: Part II, focal abnormalities. AJR Am J Roentgenol 173(5):1185–1192
Doo KW, Lee CH, Choi JW et al (2009) “Pseudo washout” sign in high-flow hepatic hemangioma on gadoxetic acid contrast-enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol 193(6):W490–W496
Duran R, Ronot M, Di Renzo S et al (2015) Is magnetic resonance imaging of hepatic hemangioma any different in liver fibrosis and cirrhosis compared to normal liver? Eur J Radiol 84(5):816–822
Elsayes KM, Chernyak V, Morshid AI et al (2018) Spectrum of pitfalls, pseudolesions, and potential misdiagnoses in cirrhosis. AJR Am J Roentgenol 211(1):87–96
Golfieri R, Renzulli M, Lucidi V et al (2011) Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol J 6:1233–1242
Joo I, Lee JM, Lee DH et al (2015) Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol 25(10):2859–2868
Kim JW, Lee CH, Kim SB et al (2017) Washout appearance in Gd-EOB-DTPA-enhanced MR imaging: a differentiating feature between hepatocellular carcinoma with paradoxical uptake on the hepatobiliary phase and focal nodular hyperplasia-like nodules. J Magn Reson Imaging 45(6):1599–1608
Kitao A, Matsui O, Yoneda N et al (2018) Differentiation between hepatocellular carcinoma showing hyperintensity on the hepatobiliary phase of gadoxetic acid-enhanced MRI and focal nodular hyperplasia by CT and MRI. AJR Am J Roentgenol 21(2):347–357
Komatsu N, Motosugi U, Maekawa S et al (2014) Hepatocellular carcinoma risk assessment using gadoxetic acid-enhanced hepatocyte phase magnetic resonance imaging. Hepatol Res 44(13):1339–1346
Libbrecht L, Cassiman D, Verslype C et al (2006) Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am J Gastroenterol 101(10):2341–2346
Motosugi U, Bannas P, Sano K, Reeder SB (2015) Hepatobiliary MR contrast agents in hypovascular hepatocellular carcinoma. J Magn Reson Imaging 41(2):251–265
Motosugi U, Murakami T, Lee JM et al (2018) LI-RADS HBA Working Group. Recommendation for terminology: nodules without arterial phase hyperenhancement and with hepatobiliary phase hypointensity in chronic liver disease. J Magn Reson Imaging 5:1169–1171
Murakami T, Tsurusaki M (2014) Hypervascular benign and malignant liver tumors that require differentiation from hepatocellular carcinoma: key points of imaging diagnosis. Liver Cancer 3(2):85–96
Nakashima O, Kurogi M, Yamaguchi R et al (2004) Unique hypervascular nodules in alcoholic liver cirrhosis: identical to focal nodular hyperplasia-like nodules? J Hepatol 41(6):992–998
Ohtomo K, Baron RL, Dodd GD 3rd et al (1993) Confluent hepatic fibrosis in advanced cirrhosis: appearance at CT. Radiology 188(1):31–35
Papadatos D, Fowler KJ, Kielar AZ et al (2018) Cirrhosis and LI-RADS. Abdom Radiol (NY) 43(1):26–40
Quaglia A, Tibballs J, Grasso A et al (2003) Focal nodular hyperplasia-like areas in cirrhosis. Histopathology 42(1):14–21
Ronot M, Dioguardi Burgio M, Purcell Y et al (2017) Focal lesions in cirrhosis: not always HCC. Eur J Radiol 93:157–168. https://doi.org/10.1016/j.ejrad.2017.05.040
Sasaki M, Yoneda N, Sawai Y et al (2015) Clinicopathological characteristics of serum amyloid A-positive hepatocellular neoplasms/nodules arising in alcoholic cirrhosis. Histopathology 66(6):836–845
Suh CH, Kim KW, Pyo J et al (2017) Hypervascular transformation of hypovascular hypointense nodules in the hepatobiliary phase of gadoxetic acid-enhanced MRI: a systematic review and meta-analysis. AJR Am J Roentgenol 209(4):781–789
The International Consensus Group for Hepatocellular Neoplasia (2009) Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49(2):658–664
Toyoda H, Kumada T, Tada T et al (2013) Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol 58(6):1174–1180
Vilgrain V, Fléjou JF, Arrivé L et al (1992) Focal nodular hyperplasia of the liver: MR imaging and pathologic correlation in 37 patients. Radiology 184(3):699–703
Vilgrain V (2006) Focal nodular hyperplasia. Eur J Radiol 2:236–245
Wanless IR, Wong F, Blendis LM (1995) Focal nodular hyperplasia of the liver: MR imaging and pathologic correlation in 37 patients. Radiology. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology 5:1238–1247
Yoneda N, Matsui O, Kitao A et al (2016) Benign hepatocellular nodules: hepatobiliary phase of gadoxetic acid-enhanced MR imaging based on molecular background. Radiographics 36(7):2010–2027
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Catania, R., Borhani, A.A., Furlan, A. (2021). Benign Lesions in Cirrhosis. In: Quaia, E. (eds) Imaging of the Liver and Intra-hepatic Biliary Tract. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-39021-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-39021-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39020-4
Online ISBN: 978-3-030-39021-1
eBook Packages: MedicineMedicine (R0)