Abstract
Sperm DNA fragmentation (SDF) is associated with male infertility, and it adversely affects reproductive outcomes. Both chromatin integrity and protamination status determine the extent of DNA damage. Oxidative stress due to increased levels of reactive oxygen species in the seminal fluid damages sperm DNA. Several tests have been introduced into the clinical laboratory settings to assess the sperm chromatin integrity and the extent of SDF. This chapter elucidates the molecular changes, specifically proteomic alterations, caused due to SDF. Moreover, the factors affecting sperm DNA integrity and the consequences of increased SDF are highlighted. It also focusses on the importance of SDF testing and its impact on reproductive outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Chromatin organization and protamination state determine the sperm DNA integrity.
-
Molecular changes due to sperm DNA damage are reflected as alterations in the sperm proteome.
-
SDF adversely affects the fertilization rate and embryo development.
-
SDF testing is highly recommended in men with idiopathic infertility.
Introduction
Worldwide concern regarding upsurge of male infertility contributing to almost 50% of the overall infertility cases urges specific research interventions to address its potential causes [1]. Considerable advent of assisted reproductive technology (ART) could hardly mitigate stillbirth complications [2]. Proper approach to ameliorate male fertility should not be compensated with ART. Management of male infertility, which in most of the cases remains idiopathic, can be effective once its diagnosis is feasible. In order to do so, the etiology of male infertility from every aspect should be considered and possible mechanisms be explored and conceptualized. In this regard, understanding the molecular and genetic processes associated with sperm functions is of prime importance. The significance of sperm DNA integrity in association with sperm function tests is regaining research priority, which has huge impact on reproductive outcomes. Sperm DNA fragmentation (SDF) owing to various exogenous and endogenous factors directly affects sperm functional and morphological characteristics, rendering them impotent in carrying out reproductive functions [3, 4]. SDF assays help in advancing clinical andrology by several steps by offering a potential diagnostic tool for male infertility. SDF testing, though not yet recommended for routine testing in the evaluation of infertile men, is being acknowledged in the American Urological Association (AUA) and European Association of Urology (EAU) guidelines [5, 6].
This chapter elucidates the etiology of SDF with its contribution to male infertility, the mechanisms by which environmental, lifestyle, and endogenous factors mediate SDF, and the contemporary SDF assessments in the diagnosis of male infertility.
Etiology of Sperm DNA Damage
Sperm structure is precisely made for the successful transmission of the haploid genome to the secondary oocyte. The success of fusion and delivery of DNA content is directly related to the compaction of genetic material in an extremely limited volume of the nucleus. Mammalian sperm chromatin differs from somatic cells in structure and composition, which maintains genetic integrity during transport of the paternal genome into oocyte [7]. Protamination is a unique process that replaces histones with positively charged protamines during the nuclear chromatin condensation process. Defect at any stage of this process may result in SDF during its transport and fertilization. Indeed, the extent of DNA damage/breaks varies from sperm to sperm even in fertile men [8]. Emerging evidences support the significance of chromatin organization during fertilization and embryo development [9,10,11]. However, in normal state, meiotic prophase passes through recombination checkpoint that restricts progression to meiotic division-I till the DNA is completely repaired or the incompetent impaired spermatocytes are removed [12]. Ligation of DNA breaks is crucial for both conserving the primary DNA integrity and reassembly of the DNA loop domain for the genome expression [13]. This reassembly includes delicate steps such as chromatin loosening through histone hyper-acetylation by endogenous nuclease activity and ligation of DNA breaks by topoisomerase II [14]. Usually chromatin packaging around the new protamine cores and restoration of DNA integrity are accomplished during epididymal transit [15]. However, the presence of endogenous nicks in spermatozoa after epididymal transit may indicate an improper chromatin packaging at spermiogenesis and an incomplete maturation process. The differential susceptibility of chromosomes to sperm DNA fragmentation is determined based on its association with either of the DNA packaging molecules such as histones or protamines [16].
Besides the defects in sperm chromatin compaction, numerous other intrinsic and extrinsic factors have been reported in the etiopathogenesis of SDF, including varicocele, infection, advanced male age, heat stress, lifestyle factors, environmental toxins, and ionizing/non-ionizing radiations [17, 18]. Most of these etiologies are mediated by reactive oxygen species (ROS) leading to elevated SDF [19]. Abortive apoptosis [20] and defective maturation [21] correlate with the role of intrinsic factors in testicular SDF. Moreover, evidence show more DNA fragmentation in epididymal and ejaculated sperm than testicular sperm, signifying the impact of extrinsic factors [22]. Presence of large amount of polyunsaturated fatty acids (PUFA) in the plasma membrane makes sperm susceptible to ROS-induced damage [23].
The close relationship between ROS and SDF is also evident from the etiopathologies of all grades of clinical varicocele. The imbalance between ROS (produced by testicular hypoxia, scrotal hyperthermia, reflux of metabolites, and endocrine disruption) and protective antioxidant system was demonstrated by the higher level of ROS and lipid peroxidation products in infertile men with varicocele than infertile men without varicocele [24]. Moreover, treatment of varicocele is effective in decreasing both ROS [25] and SDF [26].
Thus, it is apparent that sperm functions and morphology are impaired via multifarious intrinsic and extrinsic factors. The abnormal spermatozoa together with these factors lead to increased ROS levels that afflict sperm DNA integrity and thereby results in infertility, impaired ART outcomes, and birth defects, as illustrated in Fig. 9.1.
Molecular Changes associated with Sperm DNA Fragmentation
Sperm DNA damage affects both the nuclear and mitochondrial genome, as well as the molecular machinery at the subcellular level [17, 27, 28]. SDF also causes alterations in the sperm ultrastructure, such as vacuolation in the nucleus, severe sperm morphological abnormalities including teratozoospermia [29]. These changes adversely affect normal sperm functions such as hyperactivation, capacitation, and acrosome reaction which are critical for the binding of spermatozoa with the oocyte during fertilization [30, 31]. Especially, the proteome of the sperm and seminal plasma are altered in the patients with high SDF [28, 32]. It has significant impact on sperm protein expression and molecular processes associated with triacylglycerol metabolism, energy production, protein folding, response to unfolded proteins, and cellular detoxification [28]. Also, the postgenomic pathways associated with sperm metabolism, function, and protection against oxidative stress get affected in spermatozoa with high DNA fragmentation [28]. Elevated SDF also disrupts spermatogenesis by altering the expression of prolactin-induced protein and its precursor protein (pPIP). Most of the proteins associated with DNA binding (such as sperm protein associated with nucleus in the X chromosome and histone proteins), oxidative stress, and mitochondrial function are differentially expressed [33].
Seminal plasma proteome also reflects the pathology associated with SDF, and these are modulated depending upon the extent of sperm DNA damage [32]. Intasqui et al. also reported that the postgenomic pathways are altered in the seminal plasma of normozoospermic men with low and high DNA fragmentation. Molecular pathways such as fatty acid binding and prostaglandin biosynthesis functions were reported to be enriched in DNA-damaged spermatozoa [34]. Cysteine-rich secretory protein LCCL domain-containing 1 (CRISPLD1), cysteine-rich secretory protein LCCL domain-containing 2 (CRISPLD2), and retinoic acid receptor responder protein 1 were proposed as biomarkers for low SDF, whereas proteasome subunit alpha type-5 protein was considered to be potential seminal biomarker for high SDF [34]. The molecular changes in the seminal plasma of smokers with high SDF were mainly related to decreased acrosome integrity and mitochondrial activity. Furthermore, the seminal plasma of patients with high SDF portrays activation of the pathways associated with positive regulation of prostaglandin secretion, protein kinase A signaling, cytokine mediated signaling, and acute inflammatory responses [35]. In infertile patients exhibiting high levels of ROS along with SDF, enzymes linked to DNA binding mechanism were altered in the seminal plasma [36].
Overall, the molecular protein signatures of both the spermatozoa and seminal plasma are altered in high SDF conditions. Differentially expressed proteins may serve as potential biomarkers in the sperm pathology with compromised DNA integrity.
Male Infertility Factors/Conditions Associated with SDF
Studies reporting the link between male factor infertility and SDF have diverse observations. Many studies have correlated SDF and male infertility with evidence of decreased sperm functions [37,38,39], while others have reported that high SDF can also be observed in sperm with normal motility and morphology [40,41,42]. An elevated level of SDF is also reported in men with abnormal semen parameters and normozoospermic partners of infertile couples [43]. However, SDF is a crucial factor to maintain male fertility and development of a healthy embryo. In a recent article by Agarwal et al., the role of female factors in the management of SDF for a better outcome in ART has been elucidated [44]. Authors discussed the complex interplay between the SDF and ovarian reserve on the clinical outcomes of ART; the presence of an intact oocyte repair machinery in good quality oocytes has a pivotal role in reproductive outcomes including SDF which serves as a safety check to avoid passage of defective genetic information to offspring [44]. However, several male infertility factors are associated with SDF.
Male Age
It has been reported that among the couples seeking treatment by ART, fathers are significantly older compared with those not needing ART (36.6 vs. 33.5 years) [45]. Men with age of 40 years or older are also found to be at higher risk of sperm DNA damage [46]. But some of the studies have reported no correlation between paternal age and SDF [47, 48]. However, most of the studies have reported that with increasing male age, the incidence ROS generation [46] and diploidy/aneuploidy increases in sperm [48, 49].
Diet, Lifestyle, and Modifiable Risk Factors
The correlation between oxidative DNA damage and the consumption of foods supplemented with antioxidant compounds, with better general and reproductive health, has been reported in diverse studies [50, 51]. Most of the reports indicate that increased intake of individual antioxidants or antioxidant-rich foods can reduce the basal level of sperm DNA damage [52, 53]. It is apparent that endogenous sperm DNA oxidation levels are modulated through diet or supplementation, but a number of variables such as type and dose of antioxidant, basal level of antioxidant plasma concentrations, and smoking or alcohol consumption can interfere with the effectiveness of the outcome. Smoking [54] and alcohol consumption [55] trigger SDF separately as well as in combination [56]. The mechanism of smoking or alcohol-mediated SDF is due to the excess generation of ROS that affects sperm quality and, ultimately, fertility potential of the spermatozoa. In chronic smokers, activation of the checkpoint kinase 1 (Chk1) facilitates S and G2 checkpoint arrest, in response to DNA damage. The expression of Chk1 is associated with SDF and apoptosis, the reduction of which may lead to decreased sperm repair and increased sperm apoptosis, with a subsequent effect on semen quality [54]. Reports regarding SDF and alcohol consumption suggest that during intrinsic apoptotic cascade, hydrogen peroxide released from the sperm mitochondria can induce SDF in the nucleus [57]. Much later in the apoptotic process, the sperm DNA begins to fragment [58].
Obesity
There has been an emerging concern over the past few decades on the impact of obesity on male fertility. Infertility has been linked to male overweight or obesity, and conventional semen parameter values alter in case of high body mass index (BMI) [59]. Male obesity is associated with an increased risk of sperm DNA damage and lower sperm motility and thus poor sperm quality [59]. Numerous human and animal studies have determined that a relationship between obesity and reduced sperm DNA integrity exists, despite the use of a variety of different methodologies to measure sperm DNA integrity [60]. Obesity induces OS and disrupts endocrine balance in men that brings about a negative impact on sperm DNA integrity [61].
Environmental Toxicants
Environmental and occupational exposure of heavy metals [62], pesticides [63], and other endocrine disrupting chemicals (EDCs) are involved in deteriorating the male reproductive health resulting in male infertility. Exposure to these EDCs also positively correlates with SDF [64]. Different agents that act on germ cells at various stages of development usually showed SDF when those germ cells arrive in the epididymis or in the ejaculate. Some of these treated samples were capable of successful in vitro fertilization but with frequent embryo failure. Extensive DNA fragmentation probably cannot be repaired by the oocyte, and the spontaneous abortion rate approximately doubles in men with more than 30% of sperm showing DNA fragmentation [65]. DNA fragmentation is an excellent marker for exposure to potential reproductive toxicants and a diagnostic tool for potential male infertility.
Chemo/Radiotherapy
In the last few decades, numerous reports have confirmed negative impact of ionizing and non-ionizing radiations on male infertility [18, 66]. Ionizing radiations from medical equipment and radiotherapy for cancer treatment positively correlate with SDF and declining sperm quality [18]. Cancer treatments are well known to adversely affect male fertility. Reduction of sperm count arises from the cytotoxic effects of chemo- or radiotherapy upon the spermatogenic epithelium [67]. Studies have also confirmed that radiotherapy in testicular germ cell tumors is associated with an increase in SDF compared to chemotherapy alone [68]. Non-ionizing radiations from cell phones, Wi-Fi, and other radioactive sources also have significant negative impacts on male fertility and sperm DNA integrity [66].
Infections and Testicular Trauma
As discussed above, multiple pathological factors acting at both intratesticular and post-testicular levels may contribute to sperm DNA damage. Bacteriospermia is one of the pathological conditions that manifests as acute or chronic inflammation and increases leukocyte infiltration in the genital tract resulting in higher ROS production [69]. Patients with leukocytospermia, Chlamydia and Mycoplasma infections, testicular cancer, and varicocele have also reported to have more SDF caused by excessive production of ROS [26, 70, 71]. However, SDF in patients with Chlamydia and Mycoplasma infections were reported to decrease after a course of antibiotics [70].
Techniques Used for SDF Assessment
A variety of assays are used to assess sperm DNA damage. These are classified as direct and indirect tests, which either measure the maturity and integrity of sperm chromatin or DNA fragmentation (Table 9.1). Most commonly used SDF tests are sperm chromatin structure assay (SCSA), terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), sperm chromatin dispersion (SCD), and the Comet assay. A cross-sectional survey across 19 countries by Majzoub et al. showed that 30.6% of SDF measurements are done using TUNEL and SCSA, 20.4% and 6.1% using SCD and single-cell gel electrophoresis (Comet), respectively [72]. The test results of each assay are different and are not interchangeable.
Sperm Chromatin Maturity Testing
Aniline Blue Staining (AB)
Immature spermatozoa contain lysine-rich histones, and mature spermatozoa have arginine and cysteine abundant protamines. AB is an acidic dye that reacts with the lysine and stains the immature spermatozoa blue, whereas matured spermatozoa remain unstained. Stained spermatozoa are visualized under simple bright field microscope. The integrity of the sperm chromatin is assessed based on the intensity of the stain [73].
Chromomycin A3 (CMA3)
Protamination state of the spermatozoa determines its chromatin integrity status. The lesser the protamine content, the poorer the DNA packaging and the higher the sperm DNA damage. CMA3 binds to the sperm DNA deficient of protamine and stains light yellow [74]. The intensity of color is high in sperm with increased protamination [75]. Fertilization rate in ICSI were reported to be significantly lower with DNA damage of >30% in semen samples determined by CMA3 assay [76].
Sperm DNA Fragmentation Testing
Sperm Chromatin Structure Assay (SCSA)
SCSA is an indirect SDF test and used to detect breaks in the single-stranded DNA (ssDNA) of sperm. Acridine orange (AO) dye binds with the ssDNA and emits red fluorescence, whereas AO bound to double-stranded DNA emits green fluorescence, and the signals are captured using a flow cytometer [77]. SCSA can be done on both fresh and frozen sperm, and a clinical reference value for DNA fragmentation index (DFI) of 30% was established for SCSA [78, 79].
Sperm Chromatin Dispersion (SCD) Test
SCD is also known as halo assay and was first introduced by Fernández et al. [80, 81]. The sperm cells embedded into the low-melting agarose-coated slides produce halos/chromatin dispersion when denatured with acid solution. Slides are stained with DAPI (4′,6-diamidino-2-phenylindole) and visualized under fluorescent microscope to differentiate the fragmented (small halos/non-dispersed) form from the highly condensed chromatin (large/distinct halos). This test is performed on both neat and washed sperm, and the size of the halos is directly proportional to the DNA damage [82].
Comet Assay/Single-Cell Gel Electrophoresis (SCGE)
In this technique, DNA from the lysed sperm is subjected to agarose gel electrophoresis. The intact DNA remains inside the head of the sperm, whereas the fragmented DNA migrates and appears as a tail [83]. Fluorescent dye SYBR Green I is used for staining, and the fragmented DNA is visualized under fluorescent microscope. The length of the tail (fragmented DNA) is an indicator of the extent of DNA damage. SCGE assay is performed on fresh semen samples, and it requires a minimum of 5000 spermatozoa. Thus, the SDF can be assessed easily in oligozoospermic samples using comet assay [84].
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL)
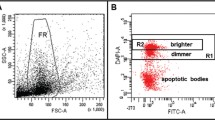
TUNEL assay identifies both the single- and double-strand DNA breaks in the spermatozoa from neat, washed, and cryopreserved semen samples. It is becoming a popular technique and gaining clinical importance among the other available assays used to measure SDF, for its rapid and easy procedure. DNA breaks are labeled with 2′-deoxyuridine 5′-triphosphates (dUTPs) coupled with fluorescein isothiocyanate (FITC). Incorporation of the dUTPs at 3′hydroxyl (OH) break ends of ssDNA and dsDNA is carried out by template-independent DNA polymerase known as terminal deoxynucleotidyl transferase (TdT). Further, propidium iodide (PI) is used as a counter dye to stain the nucleus. Fluorescence signals emitted are directly proportional to the DNA breaks and can be determined either by fluorescence microscope or flow cytometer [85, 86]. Detection of DNA breaks using flow cytometer is highly sensitive and most accurate technique with high reproducibility [87].
We have established TUNEL protocol for the measurement of SDF using Accuri C6 benchtop flow cytometer for clinical laboratories [88]. Initially, a reference value of 19.25% was established to differentiate healthy donors from infertile men [89]. Recently, benchtop flow cytometer was used to measure SDF in large cohort size of infertile patients (n = 261) and compared with proven fertile donors. The assay had a high positive predictive value (91.4%) and specificity (91.6%) with a reference value of 16.8% [90]. Apart from standardizing the threshold values for SDF, our center had also compared the SDF results for the same samples determined using Accuri C6 benchtop flow cytometer from another reference laboratory at Basel, Switzerland. The interlaboratory variation was significantly less, and both the centers had a high correlation of r = 0.94 [91]. Based on the reports of the several conducted experiments, a standardized, simple, and easy protocol had been proposed for SDF testing using TUNEL technique in clinical laboratories [85, 88, 89, 91].
SDF Testing for Male Infertility
Damage in the paternal genome is one of the leading causes of fertilization failure. SDF testing is an emerging and advanced tool for evaluation of male infertility. The clinical practice guideline (CPG) proposed by Agarwal et al. provides an evidence-based recommendations for the clinical utility of SDF testing in infertile men [86]. SDF testing for patients with clinical varicocele and borderline semen parameters can help the physicians for selecting these patients to restore impairments caused by varicocele and achieve better fertility outcome [86]. Additional SDF testing of ejaculated sperm in oligozoospermic patients and men with high SDF can be benefited by the use of testicular sperm for ART procedures [22, 92, 93]. Also, SDF testing is considered as a predictive tool to assess the outcomes of natural pregnancy and ART. Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis revealed that CPG can be implemented in the daily routine practice for the integration of SDF testing to increase the outcome of ART [94]. Table 9.2 describes the effect of SDF on outcome of natural pregnancy and other IVF techniques.
Conclusion
In this chapter, we have provided a concise explanation of the underlying mechanisms of SDF in context to its induction via multiple factors and association of the same with male infertility. We suggest that potential diagnosis of male infertility can be achieved through assessment of SDF to bring about effective management approach to male infertility leading to satisfactory rates of successful pregnancy outcomes.
Review Criteria
Extensive literature search was performed on search engines such as PubMed, Medline, Cochrane, Google Scholar, and ScienceDirect databases. Information from the studies published for the past five decades until August 2018 were extracted. The literature search was limited only for the articles written in English language. “Sperm DNA damage and fragmentation” and “male infertility” were the main key terms used for conducting literature search. Other keywords used to retrieve relevant articles were “SDF and proteomics and metabolomics,” “SDF assay,” and “SDF and TUNEL.” Book chapters and data published in scientific meetings relevant to sperm DNA damage were also included in this review.
References
Fleming S, Green S, Hall J, Hunter A. Analysis and alleviation of male infertility. Microsc Anal. 1995;45:35–7.
Neri Q, Tanaka N, Wang A, Katagiri Y, Takeuchi T, Rosenwaks Z, et al. Intracytoplasmic sperm injection. Minerva Ginecol. 2004;56:189–96.
Al Omrani B, Al Eisa N, Javed M, Al Ghedan M, Al Matrafi H, Al Sufyan H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod Biol Endocrinol. 2018;16(1):49.
González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13(11):14026–52.
Jarrow J, Sigman M, Kolettis PN, Lipshultz LR, McClure RD, et al. Optimal evaluation of the infertile male. AUA best practice statement reviewed and validity confirmed. 2011.
Male infertility. EAU guidelines [Internet]. 2017 [cited September, 2018]. Available from: https://uroweb.org/guideline/male-infertility/.
Conwell CC, Vilfan ID, Hud NV. Controlling the size of nanoscale toroidal DNA condensates with static curvature and ionic strength. Proc Natl Acad Sci. 2003;100(16):9296–301.
Simon L, Aston K, Emery B, Hotaling J, Carrell D. Sperm DNA damage output parameters measured by the alkaline comet assay and their importance. Andrologia. 2017;49(2):e12608.
Simon L, Murphy K, Shamsi M, Liu L, Emery B, Aston K, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29(11):2402–12.
Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2009;16(1):30–6.
Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod. 2006;75(3):442–51.
Page AW, Orr-Weaver TL. Stopping and starting the meiotic cell cycle. Curr Opin Genet Dev. 1997;7(1):23–31.
Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl. 2006;8(1):11–29.
Laberge R-M, Boissonneault G. On the nature and origin of DNA strand breaks in elongating spermatids. Biol Reprod. 2005;73(2):289–96.
Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22(1):45–53.
González-Rojo S, Fernández-Díez C, Guerra SM, Robles V, Herraez MP. Differential gene susceptibility to sperm DNA damage: analysis of developmental key genes in trout. PLoS One. 2014;9(12):e114161.
Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31(3):309–19.
Ahmad G, Agarwal A. Ionizing radiation and male fertility. In: Male infertility: Springer, New Delhi; 2017. p. 185–96.
Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg H-R, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83(3):635–42.
Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4(1):31–7.
Sakkas D, Manicardi G, Grace Bianchi P, Bizzaro D, Bianchi U. Relationship between the presence of endogenous nicks and sperm chromatin packaging in maturing and fertilizing mouse spermatozoa. Biol Reprod. 1995;52(5):1149–55.
Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104(6):1398–405.
John Aitken R, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41(1):183–97.
Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi K, Fujisawa M. The assessment of oxidative stress in infertile patients with varicocele. BJU Int. 2008;101(12):1547–52.
Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10(1):26–37.
Wang Y-J, Zhang R-Q, Lin Y-J, Zhang R-G, Zhang W-L. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25(3):307–14.
Intasqui P, Camargo M, Del Giudice PT, Spaine DM, Carvalho VM, Cardozo KHM, et al. Sperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasma. BJU Int. 2013;112(6):835–43.
Intasqui P, Camargo M, Del Giudice PT, Spaine DM, Carvalho VM, Cardozo KHM, et al. Unraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentation. J Assist Reprod Genet. 2013;30(9):1187–202.
Skowronek F, Casanova G, Alciaturi J, Capurro A, Cantu L, Montes JM, et al. DNA sperm damage correlates with nuclear ultrastructural sperm defects in teratozoospermic men. Andrologia. 2012;44(1):59–65.
Puga Molina LC, Luque GM, Balestrini PA, Marín-Briggiler CI, Romarowski A, Buffone MG. Molecular basis of human sperm capacitation. Front Cell Dev Biol. 2018;6:72.
Guraya SS. Cellular and molecular biology of capacitation and acrosome reaction in spermatozoa. Int Rev Cytol. 2000;199:1–64.
Intasqui P, Camargo M, Del Giudice PT, Spaine DM, Carvalho VM, Cardozo KH, et al. Sperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasma. BJU Int. 2013;112(6):835–43.
Behrouzi B, Kenigsberg S, Alladin N, Swanson S, Zicherman J, Hong S-H, et al. Evaluation of potential protein biomarkers in patients with high sperm DNA damage. Syst Biol Reprod Med. 2013;59(3):153–63.
Intasqui P, Camargo M, Antoniassi MP, Cedenho AP, Carvalho VM, Cardozo KHM, et al. Association between the seminal plasma proteome and sperm functional traits. Fertil Steril. 2016;105(3):617–28.
Antoniassi MP, Intasqui P, Camargo M, Zylbersztejn DS, Carvalho VM, Cardozo KH, et al. Analysis of the functional aspects and seminal plasma proteomic profile of sperm from smokers. BJU Int. 2016;118(5):814–22.
Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, Willard B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013;11:85.
Cho C-L, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: a systematic review. Arab J Urol. 2018;16(1):21–34.
Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79:1597–605.
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–43.
Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi P, Shoukir Y, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod. 1998;13(suppl_4):11–9.
Avendaño C, Franchi A, Taylor S, Morshedi M, Bocca S, Oehninger S. Fragmentation of DNA in morphologically normal human spermatozoa. Fertil Steril. 2009;91(4):1077–84.
Avendaño C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94(2):549–57.
Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78(2):313–8.
Agarwal A, Cho C-L, Majzoub A, Esteves SC. The role of female factors in the management of sperm DNA fragmentation. Transl Androl Urol. 2017;6(Suppl 4):S488.
Engel W, Sancken U, Laccone F. Paternal age from a genetic point of view. J Reproduktionsmed Endokrinol. 2004;1:263–7.
Alshahrani S, Agarwal A, Assidi M, Abuzenadah AM, Durairajanayagam D, Ayaz A, et al. Infertile men older than 40 years are at higher risk of sperm DNA damage. Reprod Biol Endocrinol. 2014;12(1):103.
Winkle T, Rosenbusch B, Gagsteiger F, Paiss T, Zoller N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J Assist Reprod Genet. 2009;26(1):41–6.
Brahem S, Mehdi M, Elghezal H, Saad A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J Assist Reprod Genet. 2011;28(5):425–32.
Luetjens C, Rolf C, Gassner P, Werny J, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod. 2002;17(7):1826–32.
Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74(6):297–312.
Agarwal A, Sengupta P, Durairajanayagam D. Role of L-carnitine in female infertility. Reprod Biol Endocrinol. 2018;16(1):5.
Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed. 2016;14(12):729.
Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26(3):349–53.
Cui X, Jing X, Wu X, Wang Z, Li Q. Potential effect of smoking on semen quality through DNA damage and the downregulation of Chk1 in sperm. Mol Med Rep. 2016;14(1):753–61.
Akang EN, Oremosu AA, Osinubi AA, James AB, Biose IJ, Dike SI, et al. Alcohol-induced male infertility: is sperm DNA fragmentation a causative? J Exp Clin Anatomy. 2017;16(1):53.
Anifandis G, Bounartzi T, Messini C, Dafopoulos K, Sotiriou S, Messinis I. The impact of cigarette smoking and alcohol consumption on sperm parameters and sperm DNA fragmentation (SDF) measured by Halosperm®. Arch Gynecol Obstet. 2014;290(4):777–82.
De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, Hedges A, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009;81(3):517–24.
Mitchell L, De Iuliis G, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2011;34(1):2–13.
Dupont C, Faure C, Sermondade N, Boubaya M, Eustache F, Clément P, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013;15(5):622.
Bakos H, Mitchell M, Setchell B, Lane M. The effect of paternal diet‐induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34(5pt1):402–10.
Palmer NO, Bakos HW, Fullston T, Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012;2(4):253–63.
Sengupta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36(3):353–68.
Sengupta P, Banerjee R. Environmental toxins: alarming impacts of pesticides on male fertility. Hum Exp Toxicol. 2014;33(10):1017–39.
Jeng HA. Exposure to endocrine disrupting chemicals and male reproductive health. Front Public Health. 2014;2:55.
Evenson DP, Wixon R. Environmental toxicants cause sperm DNA fragmentation as detected by the Sperm Chromatin Structure Assay (SCSA®). Toxicol Appl Pharmacol. 2005;207(2):532–7.
Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89(1):124–8.
Morris ID. Sperm DNA damage and cancer treatment 1. Int J Androl. 2002;25(5):255–61.
Smit M, Van Casteren N, Wildhagen M, Romijn J, Dohle G. Sperm DNA integrity in cancer patients before and after cytotoxic treatment. Hum Reprod. 2010;25(8):1877–83.
Ochsendorf F. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update. 1999;5(5):399–420.
Gallegos G, Ramos B, Santiso R, Goyanes V, Gosálvez J, Fernández JL. Sperm DNA fragmentation in infertile men with genitourinary infection by chlamydia trachomatis and mycoplasma. Fertil Steril. 2008;90(2):328–34.
Erenpreiss J, Hlevicka S, Zalkalns J, Erenpreisa J. Effect of leukocytospermia on sperm DNA integrity: a negative effect in abnormal semen samples. J Androl. 2002;23(5):717–23.
Majzoub A, Agarwal A, Cho CL, Esteves SC. Sperm DNA fragmentation testing: a cross sectional survey on current practices of fertility specialists. Transl Androl Urol. 2017;6(Suppl 4):S710–9.
AUGER J, MESBAH M, HUBER C, DADOUNE JP. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl. 1990;13(6):452–62.
Manicardi GC, Bizzaro D, Basic SD. Clinical aspects of sperm chromomycin A3 assay. In: Zini A, Agarwal A, editors. Sperm chromatin: biological and clinical applications in male infertility and assisted reproduction. New York: Springer New York; 2011. p. 171–9.
Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility1. Biol Reprod. 1995;52(4):864–7.
Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi PG, Shoukir Y, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod. 1998;13(suppl_4):11–9.
Evenson DP. The Sperm Chromatin Structure Assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56–75.
Evenson DP, LARSON KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43.
Evenson DP. Sperm Chromatin Structure Assay (SCSA®): 30 years of experience with the SCSA®. In: Sperm chromatin: Springer, New York, NY; 2011. p. 125–49.
Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84(4):833–42.
Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24(1):59–66.
Pratap H, Hottigoudar SY, Nichanahalli KS, Chand P. Assessment of sperm deoxyribose nucleic acid fragmentation using sperm chromatin dispersion assay. J Pharmacol Pharmacother. 2017;8(2):45–9.
Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123(1):291–8.
Singh NP, Danner DB, Tice RR, McCoy MT, Collins GD, Schneider EL. Abundant alkali-sensitive sites in DNA of human and mouse sperm. Exp Cell Res. 1989;184(2):461–70.
Sharma R, Masaki J, Agarwal A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol Biol 2013;927:121–36.
Gupta S, Sharma R, Agarwal A. Inter‐and intra‐laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Curr Protoc Toxicol. 2017;74(1):16.1. 1-.1. 22.
Mahfouz RZ, Said TM, Agarwal A. The diagnostic and therapeutic applications of flow cytometry in male infertility. Arch Med Sci Spec Issues. 2009;2009(1):108.
Sharma R, Cakar Z, Agarwal A. TUNEL assay by benchtop flow cytometer in clinical laboratories. In: A Clinician’s guide to sperm DNA and chromatin damage: Springer, Cham; 2018. p. 103–18.
Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, Agarwal A. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76(6):1380–6.
Sharma R, Ahmad G, Esteves SC, Agarwal A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet. 2016;33(2):291–300.
Ribeiro S, Sharma R, Gupta S, Cakar Z, De Geyter C, Agarwal A. Inter‐and intra‐laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology. 2017;5(3):477–85.
Moskovtsev SI, Jarvi K, Mullen JBM, Cadesky KI, Hannam T, Lo KC. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93(4):1142–6.
Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20(1):226–30.
Esteves SC, Agarwal A, Cho C-L, Majzoub A. A Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis on the clinical utility of sperm DNA fragmentation testing in specific male infertility scenarios. Transl Androl Urol. 2017;6(Suppl 4):S734.
Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G, et al. Sperm chromatin damage impairs human fertility. Fertil Steril. 2000;73(1):43–50.
Muriel L, Meseguer M, Fernández JL, Alvarez J, Remohí J, Pellicer A, et al. Value of the sperm chromatin dispersion test in predicting pregnancy outcome in intrauterine insemination: a blind prospective study. Hum Reprod. 2005;21(3):738–44.
Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17(12):3122–8.
Rilcheva VS, Ayvazova NP, Ilieva LO, Ivanova SP, Konova EI. Sperm DNA integrity test and assisted reproductive technology (art) outcome. J Biomed Clin Res. 2016;9(1):21–9.
Cissen M, van Wely M, Scholten I, Mansell S, de Bruin JP, Mol BW, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0165125.
Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SE. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod. 2010;25(7):1594–608.
Morris ID. Sperm DNA damage and cancer treatment. Int J Androl. 2002;25(5):255–61.
Virro M, Evenson D. Sperm chromatin structure assay (SCSA®) related to blastocyst rate, pregnancy rate, and spontaneous abortion in IVF and ICSI cycles. Fertil Steril. 2003;79:16.
Mohammad HN-E, Mohammad S, Shahnaz R, Maryam A, Shahla R, Fariba M, et al. Effect of sperm DNA damage and sperm protamine deficiency on fertilization and embryo development post-ICSI. Reprod Biomed Online. 2005;11(2):198–205.
Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online. 2013;26(1):68–78.
Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015;30(2):120–7.
Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27(10):2908–17.
Panner Selvam MK, Agarwal A. A systemic review on sperm DNA fragmentation in male factor infertility: laboratory assessment. Arab J Urol. 2018;16(1):65–76.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Panner Selvam, M.K., Sengupta, P., Agarwal, A. (2020). Sperm DNA Fragmentation and Male Infertility. In: Arafa, M., Elbardisi, H., Majzoub, A., Agarwal, A. (eds) Genetics of Male Infertility. Springer, Cham. https://doi.org/10.1007/978-3-030-37972-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-37972-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37971-1
Online ISBN: 978-3-030-37972-8
eBook Packages: MedicineMedicine (R0)