Abstract
Patient survival depends on determining the cause of the underlying condition and treating the pathology, as well as supportive care until restoration of normal physiology. Resuscitation guidelines have long been focused on macrohemodynamic variables, such as cardiac output, as well as indirect measures of blood flow and tissue perfusion, such as lactate. In recent years, interest has been developing in the assessment and management of the microcirculation. The early goals of resuscitation in circulatory shock are to restore global blood flow, oxygen delivery, and organ perfusion pressure with the ultimate aim of improving microcirculatory perfusion and cellular oxygen metabolism. The aim of this chapter is to outline the principles of the microcirculation, microcirculatory dysfunction in sepsis, and therapies to improve microcirculatory function and tissue perfusion. Sepsis is characterized by macrocirculatory alterations such as relative hypovolemia, a decrease in vascular tone, myocardial depression, a heterogeneous pattern of blood flow in microcirculation, as well as the incapacity of cells to extract and adequately use oxygen. With newer and more sophisticated monitoring techniques, the microcirculation can be used to guide resuscitative efforts towards more targeted therapies. In addition to clinical examination and biochemical markers as methods of assessment, handheld vital microscopes allow direct visualization of capillaries and a number of indices and classifications have been developed which show good inter-observer reproducibility. Despite the advances in our understanding of microcirculatory dysfunction there remains a deficit in knowledge regarding how best to treat it.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
There are a multitude of reasons why critically ill patients may have reduced cardiac output and perfusion of tissues. Patient survival depends on determining the cause of disease and treating the pathology, as well as supportive care until restoration of normal physiology. Resuscitation guidelines have long been focused on macrohemodynamic variables , such as cardiac output, as well as indirect measures of blood flow and tissue perfusion, such as lactate. In recent years, interest has been developing in the assessment and management of the microcirculation. The microcirculation is the interface between the main oxygen consumers (parenchymal cells in the tissues) and the oxygen supplier (the circulatory system). It serves to deliver nutrients and remove metabolic products in order to support normal tissue function.
For aerobic metabolism to occur there must be a constant delivery of oxygen to cells. Gas exchange occurs in the pulmonary vasculature, oxygenated blood is then circulated systemically via the cardiovascular system, and oxygen diffuses into cells at the level of the microcirculation. It is then used to make cellular adenosine 5′-triphosphate (ATP) through the process of aerobic respiration, primarily through oxidative phosphorylation in the mitochondria.
The microcirculation is a network of small blood vessels (<100 μm diameter), which consists of arterioles, capillaries, and venules. It includes endothelial cells, smooth muscle cells (mostly in arterioles), red blood cells (RBCs), leukocytes and platelets [1]. The microcirculation is structured such that every cell has at least one capillary adjacent to it for the purpose of passive diffusion of oxygen from the vasculature into the cell.
The early goals of resuscitation in circulatory shock are to restore global blood flow, oxygen delivery, and organ perfusion pressure with the ultimate aim of improving microcirculatory perfusion and cellular oxygen metabolism. The aim of this chapter is to outline the principles of the microcirculation, microcirculatory dysfunction in sepsis, and therapies to improve microcirculatory function and tissue perfusion.
2 Microcirculatory Dysfunction in Sepsis
Sepsis is characterized by macrocirculatory alterations such as relative hypovolemia, a decrease in vascular tone, myocardial depression, and a heterogeneous pattern of blood flow in the microcirculation, as well as the incapacity of cells to extract and adequately use oxygen [1].
Sepsis results in derangements in the microcirculation, with these derangements being most marked in severely ill patients. Clinical and global hemodynamic parameters do not correlate well with microcirculatory perfusion and indeed these microcirculatory abnormalities may persist after the correction of systemic hemodynamic parameters, such as mean arterial pressure (MAP) [2].
The cardiovascular system circulates blood throughout the body but it is the microcirculation in particular that actively and passively regulates the distribution of RBCs and plasma throughout individual organs (Fig. 20.1). In animal models of sepsis, lipopolysaccharide (LPS)-induced microvascular heterogeneity and increased oxygen-diffusion distances affect the distribution of RBCs and oxygen flow within the heart, leading to tissue hypoxia [3]. Endothelial malfunction and rupture of the glycocalyx can lead to microthrombi, capillary leakage, leukocyte rolling, and rouleaux formation [4]. Microcirculatory alterations increase the diffusion distance for oxygen and, due to the heterogeneity of microcirculatory perfusion in sepsis, may promote the development of areas of tissue hypoxia in close proximity to well-oxygenated zones. Improvement of tissue perfusion and oxygenation should be considered the ultimate goal of any resuscitative efforts.
Microcirculatory derangements in sepsis. Derangements are mainly characterized by a reduction in vessel density, alteration in flow, and a heterogeneous distribution of perfusion. Sepsis is associated with intermittently under-perfused capillaries in close proximity to well-perfused capillaries. This causes a decrease in capillary density and increase in heterogeneity of vessels
3 Hemodynamic Reconciliation in Physiology
Septic shock is a subset of sepsis in which oxygen delivery to cells is insufficient to maintain cellular activity and support organ function. It is associated with a greater risk of mortality than with sepsis alone. It can be clinically identified by a vasopressor requirement to maintain a MAP of ≥65 mmHg and serum lactate level <2 mmol/l (18 mg/dl) in the absence of hypovolemia [5]. For resuscitative measures to be effective there must be coherence between the macrocirculation and the microcirculation. That is to say, normalization of systemic variables must result in a parallel improvement in perfusion of the microcirculation, oxygenation of parenchymal cells, and restoration of normal cellular activity. In many shock states, tissues can remain hypoperfused even after MAP has been restored.
The potential pathophysiologic mechanisms behind this hemodynamic incoherence during or following resuscitation include unregulated inflammation, cytokine storm, reactive oxygen species, degradation and shedding of the endothelial glycocalyx, endothelial dysfunction and increased permeability, and mitochondrial dysfunction [6].
The persistence of microcirculatory hypoperfusion after restoration of systemic variables has been shown in numerous studies and is associated with worse outcomes. Ince suggested four different types of microcirculatory alterations underlying the loss of hemodynamic coherence [7]:
-
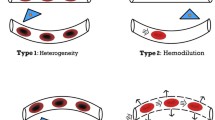
Type 1 (obstructive): heterogeneous microcirculatory flow in which there are obstructed capillaries next to capillaries with flowing RBCs. Persistence of this type of microcirculatory dysfunction in the presence of normal systemic hemodynamic variables has been associated with adverse outcomes [8]. Increased microcirculatory perfusion during resuscitation is associated with reduced organ failure in septic patients with comparable global hemodynamics [9].
-
Type 2 (hemodilution/anemic): reduced capillary density induced by hemodilution and anemia, in which dilution of blood causes a loss of RBC-filled capillaries and results in increased diffusion distances between oxygen-carrying RBCs and tissue cells.
-
Type 3 (hypoperfused): microcirculatory flow reduction caused by vasoconstriction or tamponade in which vasoconstriction of arterial vessels results in microcirculatory ischemia or raised venous pressures inducing microcirculatory tamponade.
-
Type 4 (distributive): tissue edema caused by capillary leak, which results in increased diffusion distances between the RBCs and tissue cells.
The second consensus on the assessment of the sublingual microcirculation was published in 2018 [10]. It introduced a classification system in order to better characterize microcirculatory alterations other than solely those associated with sepsis. The types of alterations include:
-
Type 1: complete stagnated capillaries (circulatory arrest, excessive use of vasopressors)
-
Type 2: reduction in the number of flowing capillaries (hemodilution)
-
Type 3: stopped-flow vessels are seen next to vessels with flowing cells (sepsis, hemorrhage, and hemodilution)
-
Type 4: hyperdynamic flow within capillaries (hemodilution, sepsis)
4 The Role of the Endothelium and Coagulation
The normal microcirculation maintains a network of perfused capillaries by autoregulation, which is a regulatory mechanism allowing microcirculatory flow to remain independent of changes in systemic blood pressure (Fig. 20.2). The main component of this autoregulated system is the endothelial cell [1].
The endothelial glycocalyx is a negatively charged, carbohydrate-rich layer, which lines the luminal surface of the vascular endothelium [11]. It consists of proteoglycans, glycoproteins bound with sialic acid, glycosaminoglycans (GAGs), and associated plasma proteins. Proteins such as albumin, fibrinogen, fibronectin, thrombomodulin, antithrombin III, superoxide dismutase, and cell-adhesion molecules all interact with GAGs [12].
The endothelial glycocalyx controls capillary permeability and acts as a barrier [13]. Its negative charge prevents negatively charged proteins such as albumin from passing into the extravascular space, which in turn prevents fluid from passing into the extravascular space. It serves as a barrier to adhesion of leukocytes to the endothelium and indeed shedding of the glycocalyx during activation of endothelial cells may be an essential part of the inflammatory response [14]. It may also serve as a mechanosensor and mediate the release of nitric oxide in response to shear stress [15]. The permeability of the vascular endothelium is of critical importance in the regulation of fluid homeostasis between the intravascular space and the interstitium and in the regulation of physiological functions of organs [16].
During sepsis, the glycocalyx is degraded by enzymes such as metalloproteinases, heparanase and hyaluronidase, which are activated as part of the inflammatory response [12]. These enzymes are activated in inflammatory states by reactive oxygen species, tumor necrosis factor (TNF)-alpha, and interleukin 1-beta (IL-1β) [17]. Studies have found a significant reduction in the thickness of the glycocalyx in sepsis [18]. Observational studies have found an association between the levels of markers of endothelial damage and the severity of sepsis [19]. This degradation of the glycocalyx leads to vascular hyper-permeability, unregulated vasodilation, microvessel thrombosis, and augmented leukocyte adhesion [12].
Inflammatory-mediated degradation of the glycocalyx may lead to specific organ dysfunction in sepsis, such as acute respiratory distress syndrome (ARDS), acute renal failure, and hepatic dysfunction. Inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-10, have been implicated in the degradation of the glycocalyx [18].
5 Assessment of the Microcirculation
5.1 Microcirculatory Targets
Macrocirculatory parameters such as MAP are poor predictors of cardiac output. The sympathetic response to stress and neural adaptation tends to initially maintain the MAP in the face of decreasing flow [20]. It is thus a poor indicator in the early assessment of shock. Indeed systemic hemodynamic variables do not indicate the onset of shock but rather indicate the onset of cardiovascular decompensation. Measures of microvascular flow enable the earlier detection of shock (Fig. 20.3).
5.2 Clinical Assessment
5.2.1 Capillary Refill Time
The capillary refill time (CRT) is defined as the time required for a distal capillary bed (i.e., the nail bed) to regain its color after pressure has been applied to cause blanching [21]. Historically the upper limit of normal was quoted to be 2 s; however more recent studies have suggested the upper limit of normal for capillary refill time to be 3.5–4.5 s [22, 23]. It does however vary with factors such as age and temperature, and is subject to inter-observer variability.
Hernandez et al. reported that CRT and central-to-toe temperature difference (Tc-toe) were more significant predictors of successful resuscitation than normalization of metabolic parameters such as central venous oxygen saturation (ScvO2) and central-venous-arterial CO2 difference (P[cv-a]CO2 gap). In their study, the presence of normal values of both CRT and Tc-toe at 6 h was independently associated with successful resuscitation [24]. This suggests that noninvasive measures of peripheral perfusion such as CRT and Tc-toe may be used as surrogates for more invasive techniques in the assessment of peripheral perfusion.
Patients with persistently abnormal peripheral perfusion as measured by CRT following initial resuscitation have been shown to have a higher likelihood of developing complications such as organ failure and decreased survival [25].
5.2.2 Skin Mottling
Skin mottling is defined as a bluish skin discoloration that typically manifests near the elbows or knees and has a distinct patchy pattern. It is the result of heterogeneic small vessel vasoconstriction and is thought to reflect abnormal skin perfusion [21]. The mottling scoring system (from 0 to 5), based on mottling area extension from the knees to the periphery, is a simple and reliable tool used for the assessment of peripheral perfusion (Fig. 20.4). Higher skin mottling scores have been found to be predictive of mortality in patients admitted to the intensive care unit (ICU) [26].
5.3 Biochemical Markers
Biochemical markers are used to indicate global perfusion in both the intensive care and the nonintensive care setting. Mixed venous oxygen saturation (SvO2), ScvO2, plasma lactate, and P[cv-a]CO2 gap are commonly used measurements for this purpose.
5.3.1 SvO2 and ScvO2
Using the SvO2 , the Fick equation can be used to calculate the cardiac output [27]:
where Hb is the hemoglobin concentration, SaO2 the arterial oxygen saturation, and VO2 whole-body O2 consumption.
When the SaO2, VO2, and hemoglobin remain constant, decreasing values of cardiac output result in decreases in SvO2 secondary to increases in the oxygen extraction rate. Normal values for SvO2 range from 70% to 75%. The measurement of SvO2 requires a pulmonary arterial catheter; however, ScvO2 can be obtained from a central venous catheter and although oxygen levels vary slightly it can be used as a surrogate.
5.3.2 Lactate
Lactate is perhaps the most commonly used biochemical indicator of resuscitation in sepsis. It is the most easily measurable and interpretable and can be done rapidly with point-of-care testing. In contrast to SvO2 and ScvO2 it does not require central venous access.
Lactate is a product of anaerobic metabolism and thus indicates inadequate oxygen delivery to or metabolism by the tissues. It is included in the most recent definition of septic shock. The combination of a lactate level >2 mmol/l and vasopressor requirement to maintain MAP >65 mmHg in the absence of hypovolemia has been associated with mortality of >40% [5]. Lactate clearance as a target of resuscitation has been shown to be non-inferior to using ScvO2 monitoring [28].
5.3.3 Central-Venous-Arterial CO2 Difference
The P[cv-a]CO2 gap can be used as an adjunct in the assessment of the macro- and microcirculation. Using a central venous catheter, the ScvO2 can be measured and used as a surrogate for global tissue hypoxia, and the P[cv-a]CO2 gap can be calculated and used as a surrogate for the cardiac index. Persistence of a PCO2 gap >0.8 kPa (6 mmHg) after 24 h of treatment has been associated with a higher mortality [29].
CO2 is 20 times more soluble than O2 and thus as a result is a more sensitive marker of hypoperfusion. Where there is a barrier to O2 diffusion as a result of hypoperfused or nonfunctional capillaries, CO2 will still flow into the venous system. In the context of a patient with a ScvO2 >70% and persistently elevated lactate, the PCO2 gap may be elevated reflecting a barrier to O2 diffusion at the level of the microcirculation.
5.4 Peripheral Perfusion Index
The peripheral perfusion index is based on the analysis of the pulse oximetry signal. It is the ratio between the pulsatile blood flow and non-pulsatile static blood flow in peripheral tissues. It is a noninvasive method that uses the ubiquitous pulse oximeter for its calculation. In a 2002 study by Lima et al. [30], a cutoff of <1.4 was suggested to predict poor peripheral perfusion and a peripheral perfusion index <0.2 predicted ICU mortality in septic patients following resuscitation, with accuracy similar to lactate [31].
5.5 Handheld Vital Microscopy
Handheld vital microscopes were introduced clinically in the 1990s and investigations of their use have played a key role in understanding the microcirculation in sepsis. They allow single RBCs to be visualized in the capillaries. The third and most recent generation of handheld vital microscopes is based on a mode of dark-field microscopy called incident dark-field (IDF) imaging. This device gives improved resolution and an increased number of capillaries can be seen in comparison to the previous generation, which was based on sidestream dark-field (SDF) imaging [32].
The most common anatomical location imaged is the sublingual circulation. Microcirculatory abnormalities visualized include increased heterogeneity of perfused vessels, proportion of perfused vessels, total density of small microvessels, and microvascular flow index (MFI) [27]. Currently the clinical relevance of microvascular alterations is expressed in terms of proportion of perfused vessels and MFI [10].
The MFI is used to characterize the velocity of microcirculatory perfusion. The screen is divided into four quadrants. The average flow of RBCs is then calculated and described as absent (0), intermittent (1), sluggish (2), or normal (3) [33]. An MFI <2.6 in combination with tachycardia >90 beats per minute (bpm) has been shown to be an independent risk factor for increased in-hospital mortality [34].
Handheld vital microscopes can also be used to measure the perfused boundary region of the microcirculation, which is calculated as the dimensions of the permeable part of the glycocalyx allowing penetration of circulating RBCs. This provides an index of glycocalyx damage [4]. The perfused boundary region has been shown to be increased in critically ill patients compared with healthy controls reflecting decreased thickness of the endothelial glycocalyx [35]. The perfused boundary region has been shown to be reproducible and have good inter-observer reliability, which makes it a promising tool for guiding resuscitation and decision-making in sepsis.
Monitoring of the microcirculation during resuscitation can help to verify whether interventions have been successful in restoring perfusion and oxygenation of the tissues and in restoring hemodynamic coherence.
6 How We Can Modify the Microcirculation in Sepsis
Microvascular perfusion is directly related to the driving pressure (difference between pressure at the entry and exit site of the capillary) and the radius of the vessel (to the fourth power) and inversely related to blood viscosity. Intravenous fluids are a mainstay of supportive care in sepsis and may help to increase microvascular perfusion by increasing the driving pressure, decreasing the blood viscosity, or affecting the interaction between circulating cells and the endothelium [36].
Volume expansion with fluid has been shown to improve microcirculatory perfusion in the early (<24 h) but not in the late (>48 h) phase following the diagnosis of severe sepsis [36]. It is not conclusive yet whether colloids or crystalloids are superior in improving the microcirculation.
Assessment of microcirculatory variables such as MFI may be helpful in determining the need for fluid therapy. A study by Pranskunas et al. [37] examined macrohemodynamic variables, such as oliguria, MAP, ScvO2 and hyperlactatemia, and MFI, as a tool to select ICU patients eligible for fluid therapy. In patients with an MFI of <2.6, fluid therapy resulted in a significant increase in median MFI as well as a reduction in the median number of clinical signs of impaired organ perfusion. In contrast, in patients with an MFI >2.6 at baseline, fluid challenge did not increase the median MFI nor did it result in a reduction in the number of clinical signs of impaired organ perfusion. This suggests that in patients with a normal or near-normal MFI, causes other than microcirculatory flow were to blame for persistent organ dysfunction. Since none of the macrohemodynamic variables were able to discriminate an MFI <2.6, direct assessment of microcirculatory flow may help guide decisions regarding administration of fluids [37].
A study by Hanson et al. [38] looked at liberal fluid resuscitation in severe falciparum malaria. Severity of disease in terms of lactic acidosis correlated with the degree of erythrocyte sequestration as visualized by orthogonal polarized spectroscopy (OPS) rather than hypovolemia. Liberal administration of fluid did little to improve erythrocyte sequestration; however, it did increase the risk of potentially lethal complications of fluid overload and increased capillary permeability. In addition to suggesting against liberal fluid administration in severe falciparum malaria the results also suggest that direct visualization and assessment of the type of microcirculatory dysfunction may guide decisions about whether administration of fluid may be futile and lead to complications rather than resolution.
Further to that, hypervolemia has also been associated with microcirculatory dysfunction and increased degradation of the glycocalyx in sepsis. In a study in patients undergoing on- and off-pump coronary artery bypass surgery, increased levels of atrial natriuretic peptide (ANP) preceded elevation of inflammatory cytokines and shedding of the glycocalyx [39]. Chappell et al. also observed increased levels of ANP and shedding of the glycocalyx in response to volume loading and hypervolemia [40]. ANP has been shown to independently induce shedding of the endothelial glycocalyx in pig hearts as evidenced by increased levels of syndecan-1 and histologically visible degradation of the glycocalyx on electron microscopy. It in turn led to increased vascular permeability [41].
In a study of patients with severe sepsis, high levels of syndecan-1 were associated with the risk of intubation following large-volume fluid resuscitation compared to patients with low levels of syndecan-1. This may reflect the increased vascular permeability following degradation of the glycocalyx leading to increased risk of respiratory failure [42].
Apart from its use as a colloid, albumin may have other beneficial effects on the microcirculation. Sphingosine-1-phosphate (S1P) is an important plasma phospholipid for the maintenance of vascular permeability [43]. S1P suppresses the activity of metalloproteinase, which in turn protects against the loss of syndecan-1 and degradation of the glycocalyx [44]. RBCs are an important source of S1P and albumin may attenuate the degradation of the glycocalyx by acting as a carrier of S1P from RBCs to the endothelium [45].
Transfusion of RBCs has been shown to improve the microcirculation independently of the macrocirculation and the hemoglobin level in hemorrhagic shock patients [46]. A systematic review by Nielsen et al. failed to find a benefit of RBC transfusion in most critically ill patients. However, they identified a number of studies that found that patients with abnormalities in tissue oxygenation or microcirculatory indices do demonstrate improvement following transfusion [47].
Venous vasodilators have been theorized to improve microcirculatory flow by dilating postcapillary venules, thereby increasing capillary flow, and decreasing transcapillary pressure and extravasation into the tissues. In vasoplegic shock, the macrocirculation is vasodilated whilst the microcirculation is vasoconstricted [7]. Studies on the effect of nitroglycerin on the microcirculation in septic shock have yielded conflicting results. Spronk et al. found that it did improve microcirculation [48], whilst Boerma et al. in a larger randomized controlled trial found that it did not improve the microcirculation compared with the placebo group [49]. In this study, both the placebo group and the nitroglycerin group achieved significant improvements in the microcirculation [49]. The median MFI of the nitroglycerin group improved from 1.67 to 2.71, and the placebo group went from 1.42 to 2.71. It may be that other resuscitative efforts were sufficient to improve the MFI to values approaching normal. Nitroglycerin may however be of use in septic shock if its use is decided based on the type of microcirculatory dysfunction and the resuscitation status of the patient, and it is titrated in combination with technologies that can monitor, directly assess, and quantitatively analyze the microcirculation.
Increasing the arterial blood pressure with increasing doses of vasopressors has not been found to improve MFI , proportion of perfused vessels, vessel density, or heterogeneity index [50, 51]. There may be a dissociation between increases in arterial pressure produced by vasopressor agents and improvement in microvascular perfusion and delivery of vital substrates. This is probably due to shunting between postcapillary venules and precapillary arterioles and due to a sustained increase in SvO2 (as commonly happens in the initial stages of septic shock).
Finally, in critically ill non-bleeding patients, transfusion of fresh frozen plasma (FFP) resulted in a decrease in the levels of syndecan-1 and factor VIII, suggesting an improvement in the endothelium. This was accompanied by, and perhaps a result of, an increase in ADAMTS13 levels and a decrease in von Willebrand factor levels [52].
7 Conclusion
The microcirculation is the vital interface at which oxygen is delivered to cells. Without a functioning microcirculation the restoration of aerobic metabolism is impossible. Increased understanding of microcirculatory dysfunction helps to explain the persistence of tissue hypoxia despite restoration of normal macrohemodynamic parameters.
The importance of the microcirculation in critical illness is becoming increasingly clear. With newer and more sophisticated monitoring techniques, the microcirculation can be used to guide resuscitative efforts towards more targeted therapies. In addition to clinical examination and biochemical markers as methods of assessment, handheld vital microscopes allow direct visualization of capillaries and a number of indices and classifications have been developed that show good inter-observer reproducibility. As these devices become more widely available they may become routinely used in the management of sepsis and other conditions that affect systemic hemodynamics.
Despite the advances in our understanding of microcirculatory dysfunction there remains a deficit in knowledge regarding how best to treat it. For example, both hypo- and hypervolemia seem to negatively affect the microcirculation and the endothelial glycocalyx, and the type of fluid that is best for restoring perfusion and maintaining endothelial integrity remains to be established. As more clinical studies adopt handheld vital microscopy and investigate treatments based on microcirculatory status we may see that the management of hemodynamics in sepsis becomes more nuanced and individualized.
References
Lipińska-Gediga M. Sepsis and septic shock – is a microcirculation a main player? Anaesthesiol Intensive Ther. 2016;48:261–5.
Spanos A, Jhanji S, Vivian-Smith A, Harris T, Pearse RM. Early microvascular changes in sepsis and severe sepsis. Shock. 2010;33:387–91.
Bateman RM, Tokunaga C, Kareco T, Dorscheid DR, Walley KR. Myocardial hypoxia-inducible HIF-1α, VEGF, and GLUT1 gene expression is associated with microvascular and ICAM-1 heterogeneity during endotoxemia. Am J Physiol Heart Circ Physiol. 2007;293:H448–56.
Donati A, Domizi R, Damiani E, Adrario E, Pelaia P, Ince C. From macrohemodynamic to the microcirculation. Crit Care Res Pract. 2013;2013:892710.
Weis S, Dickmann P, Pletz MW, Coldewey SM, Gerlach H, Bauer M. Eine neue Definition führt zu neuen Konzepten. Dtsch Arztebl Int. 2017;114:801–10.
Kanoore Edul VS, Ince C, Dubin A. What is microcirculatory shock? Curr Opin Crit Care. 2015;21:245–52.
Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19:S8.
De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41:791–9.
Trzeciak S, McCoy JV, Dellinger RP, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–7.
Ince C, Boerma EC, Cecconi M, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018;44:281–99.
Reitsma S, Slaaf DW, Vink H, Van Zandvoort MAMJ, Oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–59.
Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16.
Salmon AHJ, Ferguson JK, Burford JL, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–50.
Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–91.
Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40:828–39.
Steinberg BE, Goldenberg NM, Lee WL. Do viral infections mimic bacterial sepsis? The role of microvascular permeability: a review of mechanisms and methods. Antivir Res. 2012;93:2–15.
Becker BF, Jacob M, Leipert S, Salmon AHJ, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80:389–402.
Wiesinger A, Peters W, Chappell D, et al. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One. 2013;8:e80905.
Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem. 2016;49:768–76.
Wo CC, Shoemaker WC, Appel PL, Bishop MH, Kram HB, Hardin E. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med. 1993;21:218–23.
Lima A, Bakker J. Clinical assessment of peripheral circulation. Curr Opin Crit Care. 2015;21:226–31.
Schriger DL, Baraff L. Defining normal capillary refill: variation with age, sex, and temperature. Ann Emerg Med. 1988;17:932–5.
Anderson B, Kelly AM, Kerr D, Clooney M, Jolley D. Impact of patient and environmental factors on capillary refill time in adults. Am J Emerg Med. 2008;26:62–5.
Hernandez G, Pedreros C, Veas E, Bruhn A, Romero C, Rovegno M, et al. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation. A clinical-physiologic study. J Crit Care. 2012;27:283–8.
Lima A, Jansen TC, Van Bommel J, Ince C, Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37:934–8.
Dumas G, Lavillegrand JR, Joffre J, et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit Care. 2019;23:211.
Huber W, Zanner R, Schneider G, Schmid R, Lahmer T. Assessment of regional perfusion and organ function: less and non-invasive techniques. Front Med. 2019;6:50.
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–46.
Van Beest PA, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial pCO2 difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013;39:1034–9.
Lima AP, Beelen P, Bakker J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med. 2002;17:1210–13.
He HW, Liu DW, Long Y, Wang XT. The peripheral perfusion index and transcutaneous oxygen challenge test are predictive of mortality in septic patients after resuscitation. Crit Care. 2013;17:R116.
Hutchings S, Watts S, Kirkman E. The Cytocam video microscope. A new method for visualising the microcirculation using Incident Dark Field technology. Clin Hemorheol Microcirc. 2016;62:261–71.
Pozo MO, Kanoore Edul VS, Ince C, Dubin A. Comparison of different methods for the calculation of the microvascular flow index. Crit Care Res Pract. 2012;2012:102483.
Vellinga NAR, Boerma EC, Koopmans M, et al. International study on microcirculatory shock occurrence in acutely ill patients. Crit Care Med. 2015;43:48–56.
Rovas A, Seidel LM, Vink H, et al. Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit Care. 2019;23:260.
Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36:949–55.
Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39:612–9.
Hanson JP, Lam SWK, Mohanty S, Alam S, Pattnaik R, Mahanta KC, et al. Fluid resuscitation of adults with severe falciparum malaria: effects on acid-base status, renal function, and extravascular lung water. Crit Care Med. 2013;41:972–81.
Bruegger D, Schwartz L, Chappell D, Jacob M, Rehm M, Vogeser M, et al. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on-and off-pump coronary artery bypass surgery. Basic Res Cardiol. 2011;106:1111–21.
Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18:1–8.
Bruegger D, Jacob M, Rehm M, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of Guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–9.
Puskarich MA, Cornelius DC, Tharp J, Nandi U, Jones AE. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J Crit Care. 2016;36:125–9.
Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, et al. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–72.
Zeng Y, Adamson RH, Curry FRE, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363–72.
Adamson RH, Clark JF, Radeva M, Kheirolomoom A, Ferrara KW, Curry FE. Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol. 2014;306:H1011–7.
Tanaka S, Escudier E, Hamada S, et al. Effect of RBC transfusion on sublingual microcirculation in hemorrhagic shock patients: a pilot study. Crit Care Med. 2017;45:e154–60.
Nielsen ND, Martin-Loeches I, Wentowski C. The effects of red blood cell transfusion on tissue oxygenation and the microcirculation in the intensive care unit: a systematic review. Transfus Med Rev. 2017;31:205–22.
Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–6.
Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38:93–100.
Jhanji S, Stirling S, Patel N, Hinds CJ, Pearse RM. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med. 2009;37:1961–6.
Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13:R92.
Straat M, Müller MCA, Meijers JCM, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19:163.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cantan, B., Martín-Loeches, I. (2020). Microcirculation in Patients with Sepsis: From Physiology to Interventions. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2020. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-37323-8_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-37323-8_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37322-1
Online ISBN: 978-3-030-37323-8
eBook Packages: MedicineMedicine (R0)