Abstract
The majority of pure magnesium produced worldwide is made by using silicothermic method (Pidgeon process). However, the Pidgeon process suffers poor efficiency, low industrial concentration, and intermittent production. The main reason is that Pidgeon process requires to keep the reaction chamber in a vacuum state during the entire reduction process. By analyzing the thermodynamic reaction principle, we reveal that it is the low magnesium partial pressure instead of vacuum that is necessary for the silicothermic process. Based on this understanding, we develop a new technique that can produce pure magnesium under the atmospheric pressure by using silicothermic method. Flowing argon is used to carry away the magnesium vapor around the reactants, which reduces the local magnesium partial pressure. The magnesium production efficiency reaches 82.36% that is comparable to the widely used Pidgeon method, and high purity of magnesium with 99.97 wt% after melting is produced directly. The industrialized application of this exciting technique is expected to help the silicothermic to realize high efficiency, automated, and continuous production while at the same time completely change the poor production conditions, and reduce energy consumption and pollution.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
As the lightest structural metal, magnesium has promising applications in energy intensive sectors such as automobile and aerospace industry. China produces most of primary magnesium. In 2018, the amounts of primary magnesium produced by China were about 800,000 tons, which accounts for about 80% of the world’s total production [1]. As the dominant process of primary magnesium production in China, Pidgeon process suffers several technical drawbacks, such as high labor intensity, low production efficiency, high energy consumption, and serious environmental pollution [2]. One of the most important origins of these problems is the fact that Pidgeon process requires vacuum, which makes the continuous and automated production very difficult. Once the terminal application markets of magnesium-based materials fully open, which will require a large amount of primary magnesium, such backward process will hamper the development of entire magnesium industry. Therefore, a new magnesium production process that can achieve continuous, automated and low pollution is pressingly needed. Recently, some efforts have been made to modify the reduction tank [3,4,5] and reducing agent [6,7,8,9,10,11] to improve the thermal reduction process. However, problems such as low efficiency, low automation, and high pollution are not fully solved because these modified processes still require vacuum. The method for the preparation of magnesium under inert gas proposed by J.R. Wynnyckyj et al. is of great value in achieving efficient, automated, and continuous production [12,13,14,15,16]. However, this method has not been able to develop into an industrial production process for magnesium. Moreover, only limited number of studies attempted to investigate the kinetics of the reaction of individual pellet [17,18,19]. The current work mainly targets on the development of this new magnesium production process under atmospheric pressure.
Analyses of Reaction Principle Under Atmospheric Pressure
To reduce magnesium from magnesium oxide by silicon under atmospheric pressure, the temperature needs to exceed 2373 ℃ [20]. Such high temperature will dramatically increase the cost of heating devices and the materials of reduction tank. By introducing calcium oxide into the slag, the reaction temperature decreases to 1750 ℃. Some efforts have to be made to produce primary magnesium under the atmospheric pressure at such applicable temperature [21,22,23,24,25,26,27]. However, such a method still suffers ultra-high energy consumption and cost. Most of these efforts stopped after China built large number of primary magnesium plants using the low cost Pidgeon process.
As for the formation of magnesium oxide with magnesium vapor, the standard Gibbs free energy change (\( \Delta G^{\theta } \)) of the reaction between magnesium and oxygen is related to the pressure of vapor [20],
where \( \Delta G_{\text{Real}}^{\theta } \) is the real standard Gibbs free energy change (\( \Delta G^{\theta } \)), and R is the gas constant equal to 8.314 \( {\text{J}}/\left( {{\text{mol}} \cdot {\text{K}}} \right) \). T is the absolute temperature.
\( \Delta G_{\text{Real}}^{\theta } \) is larger than \( \Delta G^{\theta } \) when magnesium partial pressure (\( P_{\text{Mg}} \)) is lower than standard atmosphere (\( P^{\theta } \)). That is to say, by reducing the magnesium partial pressure, the temperature of reaction can decrease effectively. That is why in vacuum the Pidgeon process can be proceeded even the temperature is 1200 ℃, which is much lower than the 2373 ℃ mentioned above [28, 29]. Figure 1 shows the schematic diagram of magnesium partial pressure (\( P_{\text{Mg}} \)) around the surface of reactants reduced by vacuum and flowing argon. \( P_{\text{Mg}} \) is reduced in vacuum mainly through the pressure gradient between reactants zone and condensation zone (the condensation zone is the place where magnesium crown forms, and this place is close to the vacuum pump). Under flowing argon, magnesium atoms generated from pellets collide with argon atoms and move to condensation zone together with the flowing argon atoms. In the latter case, the \( P_{\text{Mg}} \) around reactants can also be significantly decreased.
Methods
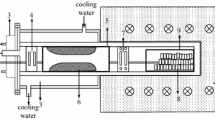
The raw materials used in our experiments, including calcined dolomite, ferrosilicon, and calcium fluoride, are provided by Fugu JingFu Coal Chemical Co. LTD (Yulin, Shaanxi Province, China). The chemical component in weight is 49.85% CaO, 30.67% MgO, 12.63% Si, 4.19% Fe, and 2.5% CaF2. The experiment of reduction is conducted in a vertical tube furnace with 700 mm heating zone and 500 mm uniform temperature zone, which can achieve temperature up to 1500 ℃. The experimental setup is shown in Fig. 2a. A series of sealable graphite tubes with different length are placed inside the corundum tube. The flowing rates of argon are controlled by the flowmeter and reducing valve. Argon is heated to 1200 ℃ in the preheating zone and blew through the surface of reactants. High purity argon (99.99%) is used in all experiments. The air in the tube is pumped before experiment, and the pumping is stopped when heating started. The purity of collected pure magnesium is analyzed by arc spark optical emission spectroscopy (OES, GNR, S5).
One typical result is shown in Fig. 2b. Most of the produced magnesium vapor condensed on the condenser. The condensed products grow mainly in the form of dendrites, which shows fresh and reflective surface without obvious impurities and oxidation. A magnesium ingot was melt using these dendritic magnesium in an induction furnace with a high purity graphite crucible (>99.99%) under argon atmosphere. The surface of the magnesium ingot was polished by the lathe. The final product is shown in Fig. 2c.
Surprisingly, the magnesium produced by this method is very pure. Table 1 compares the purity and main impurities of industrial crude magnesium produced under vacuum and the ingot produced in this work. It can be seen that magnesium with higher purity of 99.97 wt% can be directly produced under atmospheric pressure. The contents of main impurities in our study like Ca, Mn, Si, Al, and Fe are much lower than that in industrial crude magnesium. Therefore, the production method developed in this work is expected to produce high purity magnesium.
We effectively achieved the heating of large flow rate of argon. By reacting at 1200 ℃ for 2 h, we achieved the reduction efficiency of 82.36%, which is comparable to the reduction efficiency in industry that uses Pidgeon method under vacuum. We also found that increasing argon flow rate could effectively enhance the reduction efficiency.
This new method for the production of pure magnesium using carrier gas is expected to solve the problems in conventional vacuum-based approach. Although such process will incur some additional costs due to the use of argon, it is estimated that the industrialization of such process will reduce the overall cost by about 15–20% because this process can achieve large-scale and continuous production, and the argon can be recycled.
Summary
Magnesium partial pressure is the key factor in silicothermic process and can be effectively decreased by vacuum or flowing argon. By conducting the silicothermic reduction under flowing argon at atmospheric pressure, we achieved the production efficiency of 82.36%, which is comparable to industrial efficiency under vacuum condition. And high purity of magnesium with 99.97 wt% was produced by the method developed in this work.
References
E.L. Bray, Magnesium metal U.S. Geological Survey, 2019 Minerals Yearbook, 2019.
M. Halmann, A. Frei, A. Steinfeld, Magnesium Production by the Pidgeon Process Involving Dolomite Calcination and MgO Silicothermic Reduction: Thermodynamic and Environmental Analyses, Industrial & Engineering Chemistry Research 47(7) (2008) 2146–2154.
C. Zhang, C. Wang, S. Zhang, L. Guo, Experimental and Numerical Studies on a One-Step Method for the Production of Mg in the Silicothermic Reduction Process, Industrial & Engineering Chemistry Research 54(36) (2015) 8883–8892.
J. Deng, X. Wang, Experimental Research on the Inner Thermal Magnesium Reduction Process, Materials Reports 28(24) (2014) 81–179.
Z. Fan, X. Wang, Development of new shaft semi-continuous magnesium smelting furnace with internal electro-thermal process, China Nonferrous Metallurgy 4 (2012) 3.
R. Winand, M.v. Gysel, A. Fontana, L. Segers, Production of magnesium by vacuum carbothermic reduction of calcined dolomite, (1990).
L.H. Prentice, M.W. Nagle, T.R.D. Barton, S. Tassios, B.T. Kuan, P.J. Witt, K.K. Constanti-Carey, Carbothermal Production Of Magnesium: Csiro’s Magsonic™ Process, Magnesium Technology 2012 (2012).
D. Xia, Y. Shang, A new style magnesium reduction process deoxidizing by liquid calcium, Light Metal 2 (2008) 3.
J. Long, Y. Wang, N. Feng, Magnesium Production by Aluminothermic Reduction in Vacuum, Journal of Vacuum Science and Technology 32(4) (2013) 6.
W. HU, N. Feng, Y. Di, J. Peng, Study on vacuum thermal reduction of calcined dolomite with Al-Si-Fe alloy as reductant, Vacuum 47(5) (2010) 3.
H. Pei, B. Xu, Y. Li, B. Yang, Q. Yu, Study on the thermal decomposition behavior of magnesite in carbothermic reduction extraction process for magnesium in vacuum, Light Metals (1) (2010) 5.
J.R. Wynnyckyj, E. Tackie, G. Chen, The Problem of Limited Recoveries in the Pidgeon Process for Magnesium Production, Canadian Metallurgical Quarterly 30(3) (1991) 139–143.
J.R. Wynnyckyj, Production of magnesium metal, Canada, 1985.
S.K. Barua, J.R. Wynnyckyj, Kinetics of the Silicothermic Reduction of Calcined Dolomite in Flowing Hydrogen, Canadian Metallurgical Quarterly 20(3) (1981) 295–306.
S.K. Barua, Silicothermic Reduction of Calcined Dolomite in Flowing Gases: Study of Physical Parameters of briquettes and Reaction Kinetics, 1979.
J.R. Wynnyckyj, E.N. Tackie, Bench-scale investigation of magnesium winning, Light Metals (Cham) (Light Metals (Warrendale, Pa.)), Springer International Publ., Minerals, Metals & Materials Society, 1988, pp. 807–815.
W. Wulandari, G.A. Brooks, M.A. Rhamdhani, B.J. Monaghan, Kinetic analysis of silicothermic process under flowing argon atmosphere, Canadian Metallurgical Quarterly 53(1) (2013) 17–25.
W. Wulandari, G. Brooks, A. Rhamdhani, B.J. Monaghan, Kinetics of silicothermic reduction of calcined dolomite in flowing argon atmosphere, Faculty of Engineering - Papers, 2010.
K.A.E. Barawy, I.M. Morsi, M.B. Morsi and S.R. Abdel-Gawad, Silicothermic Reduction of Dolomite Ore under Inert Atmosphere, Canadian Metallurgical Quarterly 41, No. 1 (2002) 15–28.
O. Kubaschewski, E.L. Evans, C.B. Alcock, Metallurgical thermochemistry, Pergamon Press 1967.
C. Faure, J. Marchal, Magnesium by the Magnetherm Process, 1964.
M. Abdellatif, Pilot Plant Demonstration Of The Mintek Thermal Magnesium Process, (2006).
M. Abdellatif, Mintek Thermal Magnesium Process (MTMP): Theoretical and Operational Aspects, 2006.
M. Abdellatif, Refining Testwork on Crude Magnesium Produced in the Mintek Thermal Magnesium Process, 2006.
R.T. Jones, T.R. Curr, Pyrometallurgy at Mintek, Southern African Pyrometallurgy (2006).
A. Schoukens, M. Abdellatif, M. Freeman, Technological Breakthrough of the Mintek Thermal Magnesium Process, 2006.
M. Abdellatif, Mintek Thermal Magnesium Process: Status and Prospective, 2008.
L.M. Pidgeon, J. M. Toguri, High-temperature studies of metallurgical processes Part I. The Thermal reduction of Magnesium Oxide with Silicon, Canadian Journal of Chemistry 39 (1961).
L.M. Pidgeon, J. M. Toguri, High-Temperature Studies of Metallurgical Processes Part II. The Thermal Reduction of Calcined Dolomite With Silicon, Canadian Journal of Chemistry 40 (1962).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2017YFB0702001), National Natural Science Foundation of China (Nos. 51601141), China Postdoctoral Science Foundation (2016M600788), and funding from the Science and Technology Departments of Shaanxi and Xi’an, China (Nos. 2016KTZDGY-04-03, 2016KTZDGY-04-04 and 201805064ZD15CG48). We acknowledge Wei-Yin Zhang (Fugu JingFu Coal Chemical Co. LTD) for providing the raw materials. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Liu, F., Yang, B., Liu, BY., Li, J., Chang, ZM., Shan, ZW. (2020). Producing Pure Magnesium Through Silicothermic Under the Atmospheric Pressure. In: Jordon, J., Miller, V., Joshi, V., Neelameggham, N. (eds) Magnesium Technology 2020. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-36647-6_46
Download citation
DOI: https://doi.org/10.1007/978-3-030-36647-6_46
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36646-9
Online ISBN: 978-3-030-36647-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)