Abstract
In the last decades, neuromodulation, especially deep brain stimulation (DBS), has become an important treatment option in many medical refractory neurological and psychiatric disorders. However, there are still many limitations of DBS especially in terms of efficacy, side effects, and efficiency. A main reason explaining these limitations is the traditional open-loop DBS design, which allows a constant level of stimulation that does not correspond with the fluctuating clinical need. One way to circumvent this limitation is to make DBS act in a responsive way based on the presence of pathological neural activity or other biomarkers. This form of stimulation is called adaptive DBS (aDBS) or closed-loop DBS. At present the only disorder in which aDBS is clinically applied is epilepsy. However, there is an emerging field working on aDBS in other neurological disorders, especially movement disorders, with promising results. In this chapter, an in-depth analysis of the current applications and barriers of aDBS in neurological and psychiatric diseases will be given. The chapter will start with principles of aDBS, followed by indications, possible biomarkers, and evidence for aDBS in a disease-specific way. Finally, future, more data-driven approaches for applying aDBS will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Deep brain stimulation
- Brain–computer interface
- Adaptive DBS
- Responsive
- Closed loop
- Movement disorders
- Neuromodulation
- Epilepsy
- Tourette’s syndrome
Introduction
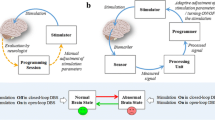
Deep brain stimulation (DBS) is a treatment for refractory neurological and psychiatric disorders that has been successfully clinically applied for over 30 years (see Chap. 1). Although many technical advances have been realized since its first clinical implementation, DBS is in the vast majority still provided in a virtually similar way; high-frequency stimulation of deep brain nuclei is provided in a constant, i.e., open-loop, way, and stimulation parameters are manually adjusted if stimulation is no longer effective and/or induces side effects (Fig. 5.1a) Although these adjustments can lead to clinical improvement (Moro et al. 2006), the potential of DBS in its current form is still limited.
Continuous versus adaptive DBS. (a) In continuous DBS, the clinical effect is evaluated after a certain time by a clinician and DBS settings will be manually adjusted. (b) In adaptive DBS, the clinical effect is continuously measured indirectly via a biomarker. This signal will be evaluated and adjustment of DBS settings will be performed automatically. Thin dotted arrows represent sporadic events, while bold arrows represent continuous events
In Parkinson’s disease (PD), motor symptoms typically only improve around 45% on the motor section of the unified PD rating scale (UPDRS) in the OFF dopaminergic state (Horn et al. 2017). One of the reasons for this limited effect is that the severity or presence of symptoms changes over time, and conventional DBS only provides one type of stimulation irrespective of symptom severity. In epilepsy, a typical paroxysmal disorder, for example, symptoms occur only in a very small fraction of time. In PD, symptoms fluctuate over shorter intervals due to dopaminergic status (i.e., ON or OFF) and over longer intervals due to disease progression. This volatility, which can be found in virtually every disorder, occurs over different time scales and has proven to be a useful biomarker to titrate DBS. This adaptive form of DBS is called adaptive DBS (aDBS) or responsive or closed-loop stimulation. For consistency reasons, the term aDBS will be used throughout this chapter.
General Concept of Adaptive DBS
Principles of aDBS
Adaptive DBS systems are based on a closed-loop system: the system measures and analyzes one or more variables reflecting the clinical state (i.e., symptom severity) of a patient and determines whether stimulation parameters need adjustment (Fig. 5.1b). Because aDBS stimulates in an “on-demand” manner (i.e., when symptoms are sufficiently present), aDBS is suggested to be more effective with less stimulation-induced side effects, increased battery longevity, and possibly less habituation.
One of the most important steps in developing aDBS systems is the choice of a suitable biomarker. Biomarkers should be able to accurately reflect the cardinal symptoms of a disorder. In order to do this, it is sometimes necessary to add additional implants or devices to obtain this biomarker-based feedback. The invasiveness of these additional implants or devices should be taken into consideration when choosing a biomarker as well as the computational requirements for real-time analysis of the biomarker (Fig. 5.2). In this chapter, possible biomarkers and their (dis)advantages for PD, essential tremor, epilepsy, dystonia, OCD and Tourette’s syndrome will be discussed. This restriction does not imply that aDBS cannot be applied in other disorders, but until now there is neither experimental nor clinical evidence for this.
Potential recording sites (numbers) and biomarkers (letters) for applying aDBS. (1) Intracranial recording sites including cortical and subcortical locations. (2) Muscular recording sites. (3) External recording sites. (4) Recording sites in DBS batteries. (a) Local field potentials, (b) electromyography, (c) accelerometer signals. (Derived, with permission, from Piña-Fuentes et al. Neurosurgical Focus 2018b)
Besides these various input options to aDBS systems, there are also multiple possible output options called stimulation algorithms. Based on the experimental findings so far, the most widely used algorithm to apply aDBS is the modulation of DBS stimulation amplitude (electric potential, Fig. 5.3). Amplitude modulation can be divided into a binary ON/OFF approach (Little et al. 2013), which only stimulates when the input signal exceeds a certain threshold, and a scalar approach, which adapts the stimulation in continuous steps (Rosa et al. 2017). Next to the amplitude modulation approach, there might also be a role for phase-dependent stimulation of clinical symptoms (e.g., tremor) or biomarkers with an oscillatory nature (Cagnan et al. 2017). With this phase-dependent stimulation, single DBS pulses are provided in critical oscillatory phases. Since there are no systematic studies assessing the effect of stimulation parameters (Bogdan et al. 2019), stimulation frequency and DBS pulse-width might also have potential in aDBS systems (Fasano and Lozano 2014).
Example of an aDBS algorithm. In the upper trace, a local field potential (LFP) of the globus pallidus of a patient with dystonia is depicted (unfiltered LFP). Below, the filtered LFP around the peak frequency in the low frequency range (4–12 Hz) is depicted (filtered LFP). In the third trace, the smoothed amplitude of this peak frequency is visual, including the stimulation threshold (red line) (amplitude envelope). In the bottom trace, the stimulation trigger is visualized showing at which moments DBS is switched on. Note that this is at the moment that the amplitude envelope exceeds the stimulation threshold. (Derived, with permission, from Piña-Fuentes et al. Neurobiology of Disease 2018a)
Clinical and Technical Implementation of aDBS
The field of aDBS has its history in control systems. Although control, i.e., closed loop, systems go back to antiquity, Maxwell described a landmark paper in 1868 titled “on governors” in which more formal analyses of closed-loop systems including the phenomenon of self-oscillations were described (Maxwell 1868). The first experimental evidence about the application of aDBS dates back to the 1960 and 1970s during which Delgado experimented with his “stimoceiver” that was able to apply aDBS based on the presence of “spindle” activity in the amygdala of a chimpanzee (Horgan 2005) or motion detectors (Delgado et al. 1976). In medicine, however, the implementation of (implanted) closed-loop technology in humans was not present before 1980. In this year, Mirowski et al. (1980) published about the first implanted and automated defibrillator for malignant ventricular arrhythmias. Despite this progress in cardiology, it was not before 2004 that closed-loop stimulation was first applied in epilepsy (Kossoff et al. 2004).
There are several reasons for the delay between the development of responsive stimulation in cardiology and neurology. First, the amplitudes of recorded pathological signals are of a different magnitude (mV vs. μV) requiring more amplification and more device resources. Furthermore, the complexity of pathological signals differs between epilepsy and arrhythmias, which requires more computations and also more device resources. Finally, the central nervous system is surrounded by a skull and highly functionally segregated which makes it more difficult to achieve optimal spatial targeting. These issues become more pronounced when proceeding towards aDBS in non-paroxysmal diseases like PD or tremor. When thinking of moving from continuous stimulation towards adaptive stimulation from an engineering point of view, the first thing that comes to mind is the sampling rate and frequency for detecting symptoms or biomarkers to see which hardware is required and which amount of energy is required for the extra closed-loop features. In the beginning of the aDBS era (Anderson et al. 2008), these energy consumption issues were more limiting as today, since rechargeable internalized pacemakers (IPGs) had in the meantime become commercially available and implanted on a regular basis.
aDBS in Movement Disorders
Parkinson’s Disease
PD is a neurodegenerative disorder with progressive motor and non-motor symptoms. Apart from the change of the nature and severity of motor symptoms over longer time scales, motor symptoms also fluctuate on shorter time scales. The most well-known fluctuation of PD symptoms over time is the change from the “ON” to the “OFF” dopaminergic state and vice versa. In the beginning of the disease, the ON state is achieved relatively easy and is maintained throughout the entire day with low doses of dopaminergic medication. However, after 5 years, the majority of patients experience motor complications (Hauser et al. 2006). These motor complications can be either (un)predictable fluctuations of motor benefit (such as “no-on,” “delayed-on,” or “wearing-OFF”) or the occurrence of involuntary movements (dyskinesias). Both phenomena can be debilitating and represent the typical indication for DBS besides refractory tremor and perhaps impulse control disorders in PD. In theory, DBS continuously alleviates motor symptoms due to the continuous application of electrical current. However, PD patients with DBS still require dopaminergic medication. One important reason for this is that DBS electrodes only stimulate the motor portion of the subthalamic nucleus (STN) and not the associative and limbic partition, which can lead to non-motor symptoms such as apathy (Martinez-Fernandez et al. 2016) in the absence of dopaminergic medication. This combination of therapies implicates that there is still a fluctuation in symptoms albeit the OFF state is severe. Nevertheless, the full potential of DBS is limited since the application of more current leads to a greater chance of the occurrence of dyskinesias, other side effects like stimulation-induced side effects, and sometimes even paradoxical bradykinesia (Chen et al. 2006). Besides these fluctuations based on the amount of dopaminergic medication, other factors also contribute to the severity of motor symptoms. The most important of these are disease progression , stress, diurnal rhythms, intercurrent diseases, and fatigue.
In summary, symptom severity changes over time in PD, whereas DBS is currently provided in an open-loop way. When DBS would be applied only when necessary in a closed-loop way, DBS could act more efficacious, with less stimulation-induced side effects, more efficient and with fewer (in-hospital) parameters adjustments. In order to apply aDBS, symptom severity needs to be recorded with a certain interval. At present, there are two approaches to do this, which are applied in experimental clinical settings. The first is by means of recording neural activity in the central nervous system, and the other is to record movements by using movement sensors. Another approach (i.e., subcortical neurochemical recording), has solely been investigated in preclinical research (Lee et al. 2017). Due to the current limitations of this approach, the need for additional subcortical sensors and the absence of clinical evidence so far, we will not further discuss this approach (Graupe et al. 2014). In the following sections, we will discuss the neurophysiological and the movement sensor approach, with their advantages and disadvantages, in more depth. Considerations on optimal aDBS parameter adjustments have to be addressed in the future for example frequency of adjustments (Habets et al. 2018).
Neurophysiological Approaches
Subcortical Neurophysiological Approaches
Neurophysiological recordings play an important role in DBS (see Chap. 6). Neurophysiological signals can be recorded from virtually the entire nervous system. However, until now, subcortical recordings only play a role in the intraoperative delineation of subcortical structures (Verhagen et al. 2015). The neural “signatures” derived from the neurophysiological recordings of different subcortical nuclei, however, not only help in the optimal placement of DBS leads but can also be used as a biomarker to indirectly quantify symptom severity. Since MRI is becoming more and more important in the targeting of DBS electrodes (Brodsky et al. 2017), it is likely that neurophysiological recordings will have the most important role in adapting DBS based on their correlation with the presence or severity of symptoms in the near future. In PD, the most well-known disruption of neural activity is increased neural population synchrony in the beta (13–30 Hz) frequency band measured by local field potentials (LFPs). Although enhanced beta oscillations are the subject of intense inquiry in the field DBS for almost two decades (e.g. Bronte-Stewart et al. 2009; Brown et al. 2001; Kuhn et al. 2008), their exact origin has not yet been revealed. Exaggerated synchronization in the beta band recorded in the STN is however correlated with the severity of contralateral bradykinesia and rigidity (Beudel et al. 2017). Contrary to bradykinesia and rigidity, the correlation between local field potential (LFP) characteristics is less outspoken, or at least more complex in tremor. From a power spectral density perspective, tremor is associated with more low-gamma (30–45 Hz) power (Beudel et al. 2015) and more high frequency (>200 Hz) oscillations (Hirschmann et al. 2016). In a recent study these power features were used in a classifier algorithm which led to an accuracy of up to 84% in predicting rest tremor in PD (Hirschmann et al. 2017). At present, these LFP analyses do not occur “real time” and lack sufficient accuracy. For these reasons, it is not yet possible to apply such classifier algorithms in embedded DBS systems and use them for aDBS.
The clinical appearance of rest tremor is suggested to be a compensational mechanism to encounter excessive beta synchronization associated with bradykinesia and rigidity (Helmich et al. 2012). A reported association between reduced subthalamic beta power and cortical-subthalamic coherence and tremor supports this hypothesis (Qasim et al. 2016). Furthermore, it is possible to identify two separate, potentially clinical relevant, beta bands: low-beta (11–15 Hz) and high-beta (19–27 Hz) (Blumenfeld et al. 2017; Priori et al. 2004). Different beta-bands are hypothesized to have frequency-specific patterns of functional connectivity between the basal ganglia and cortical motor areas (Hirschmann et al. 2011; Litvak et al. 2011; Oswal et al. 2016). In a recent study, bradykinesia improved similarly when 60-Hz and 140-Hz STN stimulations were applied, whereas high beta was attenuated by both paradigms and low beta was only attenuated by 140-Hz and amplified by 60-Hz stimulation (Blumenfeld et al. 2017). Another study showed that the suppression of local synchrony of high-beta oscillations within the STN was correlated to the amount of motor improvement during continuous DBS (cDBS) (Oswal et al. 2016). They suggest that high- and low-beta STN oscillations are coupled to different cortical motor areas via the hyper-direct and indirect pathways, respectively. Although these findings do not reach consensus yet, they all contribute to the understanding of beta-band modulation by clinical effective cDBS.
The effect of aDBS on the amplitude of STN LFPs is also questioned. Tinkhauser et al. (2017) found shorter beta “bursts” with lower amplitude aDBS compared to cDBS and no DBS. This showed that aDBS modulates the neural synchronization in the STN in a different way than cDBS, leading to shorter synchronization periods and lower burst amplitudes. Furthermore, Giannicola et al. demonstrated that STN DBS did not induce attenuation of increased beta oscillations in all patients as levodopa did. Moreover, the LFP attenuation induced by DBS was merely detectable if there was concomitant levodopa-induced beta attenuation (Giannicola et al. 2010).
An important limitation of most LFP experiments so far is the fact that they have all been conducted in the immediate postoperative phase, during which a reversible decreased impedance of the neural tissue around the implanted electrodes can distort results (Lempka et al. 2009). Some evidence, however, shows that DBS-induced modulation of subcortical beta LFPs in the STN is similar after 2 and 30 days after DBS-lead implantation (Rosa et al. 2010) and that this modulation is still observed 7 years after DBS lead implementation (Giannicola et al. 2012).
The first proof-of-concept for aDBS in a PD model in nonhuman primates was described by Rosin et al. (2011). In this landmark study, GPi aDBS was provided based on cortical spiking activity in the primary motor cortex (M1) using electrocorticography (ECoG) and resulted in a significant more robust decrease in bradykinesia relative to continuous DBS in African green monkeys. However, a delay was needed between recording of the biomarker and the effective stimulation. Johnson et al. (2016) used an STN aDBS paradigm in a PD model in a rhesus macaque and showed a clinical improvement in rigidity but not in bradykinesia. For this reason, they question the feasibility of solely using STN LFP beta power as biomarker. They suggest that aDBS systems might need multiple biomarkers specific for PD phenotypes and customized to patients’ symptom profile. One crucial detail in this experimental approach was that bradykinesia was assessed using cued movements that are less affected than spontaneous movements in parkinsonism (e.g. Nieuwboer et al. 2007).
Despite conflicting biomarkers reported in nonhuman primate research on aDBS in a model of PD (Johnson et al. 2016; Rosin et al. 2011), current clinical evidence of aDBS in PD consists predominantly of studies based on STN beta band oscillations as input signal. The first proof-of-concept for aDBS in humans was described by Little et al. (2013) in which they showed that blind evaluation of unilateral STN aDBS resulted in superior improvement of tremor, bradykinesia, and rigidity compared to random stimulation and cDBS . It should be noted that these were patients with bilateral symptoms but the application of aDBS was only possible unilaterally. Bilateral aDBS showed to be feasible and improved motor UPDRS scores compared to no stimulation (Little et al. 2016a), without causing stimulation-induced dysarthria, which was observed during cDBS (Little et al. 2016b). Furthermore, aDBS acted in synergy with concurrent dopaminergic medication (Little et al. 2016a). Finally, both unilateral and bilateral aDBS approaches consumed less energy than cDBS. Rosa et al. (2017) described the superior effect of aDBS on levodopa-induced dyskinesias relative to cDBS over a longer period of observation, not exceeding a single-day assessment however (Arlotti et al. 2018).
As mentioned before, most aDBS experiments so far were performed in an acute postoperative period . However, Piña-Fuentes et al. (2017) recently demonstrated improvement in bradykinesia during aDBS in a chronically implanted patient. This effect occurred without the possible presence of a postoperative microlesional effect; however, the efficacy of aDBS applied on a longer term still has to be proven at group level. A first attempt for this has been made by Velisar et al. (2019). However, no formal comparison between cDBS and aDBS was made in this study.
Cortical Neurophysiological Recordings
Based on the hypothesis that dopamine denervation in the striatum leads to a diminished inhibiting function and to excessive oscillatory activity of the cortico-basal-ganglia pathways (de Hemptinne et al. 2013; Hammond et al. 2007; Oswal et al. 2016), researchers have explored features of cortical oscillations as a biomarker for aDBS in PD. Until now, experimental evidence for aDBS based on cortical oscillatory features is based on studies in nonhuman primates (see previous section).
Recent experiments in PD patients have shown excessive phase-amplitude-coupling (PAC) in M1 ECoG recordings. PAC is an emerging phenomenon in many fields within the neurosciences (Jensen and Colgin 2007) and describes how the amplitude of one frequency band correlates with the phase of another frequency band. An established example of PAC is the correlation between the amplitude of high-frequency oscillations (HFO) and the phase of beta oscillations in the STN and motor cortex in PD patients (van Wijk et al. 2016; de Hemptinne et al. 2013). Furthermore, this beta-HFO PAC decreases during STN DBS, parallel to a decrease of clinically assessed bradykinesia (de Hemptinne et al. 2015). Also, a narrow-band M1 gamma band (60–90 Hz) oscillation was identified to correlate with dyskinesias (Swann et al. 2016). Furthermore, M1 ECoG recordings showed a stable profile over a period of 12 months (Swann et al. 2018a).
Altogether, these findings show that ECoG signals might also be a potential neurophysiological biomarker for aDBS in PD. However, significant limitations have to be taken such as the risks of the implantation of additional cortical strips, the computational power that is needed to perform PAC analyses, and the validation of PAC with clinical symptomatology (van Wijk et al. 2016). Finally, the feasibility of chronic application and clinical benefit has to be proven for aDBS based on cortical recordings. Recently, a proof of principle of using narrow-band M1 gamma showed to be feasible and tolerated in two patients (Swann et al. 2018b).
External Sensor Approaches
Wearable sensors (i.e., “wearables”) objectively detect movement via accelerometers and/or gyroscopes (Grimaldi and Manto 2010). Accelerometers assess acceleration and velocity over one axis based on Newton’s second law (force = mass × acceleration). Gyroscopes assess angular velocity and provide information from more than one dimension, in addition to a single accelerometer. Differentiation between physiological movement and pathologic movement is based on kinematic features as mean amplitudes of tremor (or movement), average regularity between two amplitudes, mean frequencies , and peak frequencies (Jeon et al. 2017).
Because of the potential of wearables to detect motor symptoms in PD, more and more research is done to use these as an input signal for aDBS in PD (Sanchez-Ferro et al. 2016). This research field is however emerging into a very heterogeneous field due to the presence of a plethora of commercially available sensors and the fact many researchers and companies do not make their algorithms openly available.
In case of tremor, the severity or presence of symptoms can relatively easily be recorded using accelerometers and gyroscopes (Basu et al. 2013; Khobragade et al. 2015). Since tremor is highly dependent on the state of the patient (e.g., Parkinson tremor occurs especially in rest, whereas essential tremor typically occurs during actions), a high sampling frequency (at least in the order of a multitude of the tremor frequency) is necessary. In the first study describing aDBS based on tremor amplitude in PD (Malekmohammadi et al. 2016), a Bluetooth watch was wirelessly connected to an interface (the Nexus-D platform, by Metronic, Minneapolis, MN, USA) that was connected to a responsive neurostimulator (the Activa PC + S by Metronic). In this study, it was shown that aDBS was effective in decreasing tremor power with much lower voltages , and only half of the stimulation time was needed compared to cDBS in five tremor-dominant PD patients.
Although wearables are most often used for the detection of tremor until now, they also have a potential in assessing the presence and fluctuation of freezing of gait (Rodriguez-Martin et al. 2017), bradykinesia, and dyskinesia (Griffiths et al. 2012; Hasan et al. 2017) in PD. Bradykinesia and dyskinesia, assessed through an accelerometer on the wrist, correlated with the UPDRS motor score (for bradykinesia) and to the Abnormal Involuntary Movement Scale (for dyskinesia) (Griffiths et al. 2012). Note that these studies used only rest recordings , meaning that the participants did not have to do tasks while wearing the wearables. For this reason, it is of crucial importance that these paradigms are translated to more ecologically valid circumstances including different movements.
Until now, there are several limitations of aDBS based on wearables. First, wearables will only be capable to detect motor symptoms and cannot provide information on possible non-motor symptoms or side effects of cDBS which also contribute to the quality of life of patients. Second, the development, validation, and reproducibility of sensing algorithms is slow because of the complexity of the differentiation between voluntary and pathologic movements and the fact that many researchers do not publish their used algorithms. Third, the hardware that has to record, transfer, and process the data does not meet all the necessary conditions yet, like wireless communication with the IPG. Although recent (experimental) platforms that have adopted Bluetooth interfacing expand future possibilities (e.g., Nexus-E by Medtronic). Which device should perform the computational processing, limitation of battery life, and guaranteed connectivity between all involved devices are issues that have to be solved in this field of research.
Essential Tremor
Thalamic cDBS is a safe and effective treatment for ET which results in long-term tremor reduction and functional improvement (Baizabal-Carvallo et al. 2014; Limousin et al. 1999). At present, several targets along the dentato-rubro-thalamic (DRT) tract are used for cDBS (Holslag et al. 2018), with the ventral intermediate nucleus (VIM) of the thalamus being used the most. However, there are limitations to this therapy. These are especially due to often-occurring side effects like speech and balance impairment and habituation over time (Baizabal-Carvallo et al. 2014; Barbe et al. 2011). In a more or less similar fashion as in PD, these limitations led to an interest in aDBS in ET.
Closed-Loop Approaches
In contrast to PD , a biomarker for aDBS in ET has to represent only tremor and no other motor symptoms. As stated earlier in the section on aDBS in PD, the understanding of the relation between tremor and (sub)cortical LFPs is still limited. For this reason, most experimental aDBS systems for ET used wearables, with or without additional surface electromyography (sEMG), as input.
Graupe et al. (2010) showed it was possible to suppress tremor without re-occurrence in one patient, by applying an alternating, non-continuous DBS paradigm. Based on visual inspection of unprocessed sEMG in the DBS-OFF period, they titrated DBS in such a way that it restarted before tremor reoccurred. The same group presented a proof-of-principle of an aDBS system that predicted tremor occurrence based on wrist accelerometer and sEMG data with a specificity of 86% and a sensitivity of 100% using a computational algorithm that was trained via a neural network (Basu et al. 2013). Based on this data, it was stated that stimulation could be OFF 30% of the time without tremor reoccurrence. However, in patients with severe tremor and short delay to tremor onset, this prediction would be harder.
Latest experimental designs for aDBS in ET have used wearables consisting of triaxial accelerometers. Cagnan et al. showed that not only the frequency of DBS was important in tremor suppression but also the timing of DBS pulse compared to the tremor-phase (Cagnan et al. 2014). They found a significant difference in tremor-amplitude modulation by phase-dependent stimulation in ET compared to PD tremor and hypothesized different underlying neural pathophysiological networks. Resonance of underlying tremor oscillators was kept responsible for this. Later they pioneered with phase-specific thalamic aDBS in ET and dystonic tremor and demonstrated that it modulates tremor amplitude, depending on the timing of DBS pulse with respect to the phase of the ongoing tremor. Phase-specific aDBS resulted in a comparable tremor suppression as high-frequency cDBS in selected ET and dystonic tremor patients as using less than half total electrical energy (Cagnan et al. 2017). The same group also showed that a similar phase-based approach might also be applied using noninvasive cortical stimulation (Brittain et al. 2013).
Another group tested two aDBS approaches in a single case study of an ET patient (Herron et al. 2017). One aDBS system was triggered by the presence of tremor measured via a smartwatch containing an accelerometer and a gyroscope, the other aDBS was triggered by recordings from four EMG electrodes on the patient’s arm. These aDBS approaches resulted in respectively 36% additional tremor and 85% battery savings, and 7% additional tremor and 53% battery savings compared to cDBS. The authors stated that there is a trade-off between the occurrence of tremor and the threshold to apply stimulation and consequently battery consumption to be determined in developing an aDBS system in ET. Furthermore, they add that it might be an individual choice how much tremor reduction a future aDBS system should achieve to be attractive for a patient. Altogether, promising progress is made in the development of aDBS systems for ET patients. At the moment, wearables containing accelerometers and gyroscope, and possibly additional EMG electrodes, are used as input signal to measure tremor presence or reoccurrence. Plausibly, individual patients and their clinician have to determine a trade-off in the future between the amount of desired tremor reduction and the amount of battery consumption and possibly side effects (see also section “Closed-Loop Approaches”).
aDBS in Dystonia
Dystonia is a movement disorder consisting of sustained or intermittent muscle contractions causing abnormal, often repetitive, movements or both. In medical refractory cases, dystonia can be treated with DBS (see Chap. 14). To date, responses to DBS are heterogeneous, and efficacy is difficult to predict, which makes it notoriously difficult to optimally titrate the therapy. For this reason, it would be of great benefit if a biomarker that is able to reliably detect symptom severity would become available. Especially in mobile dystonia, increased low-frequency (4–12 Hz) oscillations can be detected (Barow et al. 2014; Piña-Fuentes et al. 2019). Furthermore, the volatility of the power spectral density (PSD) of these low-frequency oscillations seems similar to that of beta oscillations in PD (Piña-Fuentes et al. 2018a, b), and their amplitude correlates with the severity of dystonia (Neumann et al. 2017). For this reason, pallidal low-frequency oscillations might be a suitable candidate for aDBS dystonia. However, low-frequency aDBS has so far been tested in only one patient as proof of principle (Piña-Fuentes et al. 2018a, b) with only surrogate endpoints. Future studies are needed to further explore the potential of pallidal low-frequency oscillations as biomarker for aDBS in dystonia. Furthermore, other biomarkers, derived from EMG and electrocorticography, will possibly be explored (Piña-Fuentes et al. 2018b).
aDBS in Epilepsy
Epilepsy is a chronic neurological disorder characterized by recurrent, unprovoked seizures. In most cases, epilepsy can be treated by antiepileptic medication. However, approximately in 30% of the patients, antiepileptic medication cannot achieve sufficient control of seizures (Kwan et al. 2010). In a selected group of these patients, surgical resection of the epileptic focus can be a treatment option. In patients where surgical resection did not have the desired effect or are not suitable for resective surgery, neuromodulation can be an option. At the moment, there are three neuromodulation therapies available for epilepsy; vagus nerve stimulation (VNS), DBS of the anterior nucleus of the thalamus, and responsive cortical/subcortical neurostimulation. Closed-loop forms of neuromodulation are already clinically applied in medical refractory epilepsy (see also section “Introduction”). This closed-loop approach is especially an elegant approach to treat epilepsy, since epilepsy is a paragon paroxysmal disorder in which seizures only occur in a very small fraction of time.
Closed-Loop VNS
In the past years, attempts have been made to develop closed-loop VNS systems. Although VNS is not a form of DBS, the developments of closed-loop VNS are also of relevance for the aDBS field in epilepsy since similar biomarkers might be used. The concept of closed-loop VNS arose from magnet-activated VNS. Magnet-activated VNS allows the caregiver or patient himself to apply additional stimulation at the time of seizure onset. Two studies tested whether magnet-activated VNS was feasible and effective in epilepsy treatment. Results showed that magnet-activated VNS could have a positive effect, but they also revealed that especially caregivers were involved in activating the additional stimulation, because most patients were not able to (Boon et al. 2001; Morris 3rd 2003). This disadvantage formed the inspiration for the search of an automatic closed-loop VNS system and for suitable biomarkers.
One potential biomarker for epilepsy is changes in heart rate, since heart rate has shown to increase in over 70% of partial seizures (Eggleston et al. 2014). Cardiac-based closed-loop systems were proposed and examined by several groups. Those proof-of-concept studies showed that cardiac-based VNS, with an increase in heart rate as biomarker, is feasible and has potential in treating epilepsy patients (Jeppesen et al. 2010; Osorio 2014; Shoeb et al. 2009; van Elmpt et al. 2006). These promising results led to the development of a cardiac-based closed-loop VNS system; the AspireSR (Cyberonics, Houston, USA).
In 2015, the AspireSR was approved by the FDA. The AspireSR automatically delivers additional stimulation when the heart rate, measured by an ECG sensor in the pulse generator, exceeds a prespecified threshold at the onset or during a seizure. A prospective multicenter study evaluated the performance of AspireSR in 31 epilepsy patients. This study showed that the AspireSR detected more than 80% of seizures accompanied by cardiac changes. Detection of the seizures occurred close to or even before seizure onset. This study therefore concluded that the AspireSR and its implemented cardiac-based seizure detection algorithm could detect seizures with cardiac changes (Boon et al. 2015). These results were stretched even further by a recent cardiac-based seizure detection algorithm, which showed that heart rate variability can be used for the early detection and potentially for the prediction of seizures with a sensitivity of 94.1% (Pavei et al. 2017). The SenTiva (LivaNova, London, UK) was approved by the FDA in 2017. This closed-loop VNS system also responds to cardiac changes and collects and logs events as body position, because these might be associated with seizures. Because of its recent approval, the performance of this system still has to be evaluated. Although there are promising results of cardiac-based closed-loop VNS, it is unclear yet if this stimulation approach is more effective than open-loop VNS.
Closed-Loop Responsive Cortical/Subcortical Stimulation
Previous studies showed that brief bursts of electrical stimulation could abort after discharges, which are similar to spontaneous epileptiform activity, by inducing electrical stimulation in an appropriate manner at seizure onset (Kros et al. 2015; Lesser et al. 1999; Motamedi et al. 2002). These studies paved the way for the development of closed-loop responsive stimulation using epileptiform activity, measured by subdural recordings, as biomarker.
In 2004, closed-loop responsive cortical stimulation was first applied in epilepsy (Kossoff et al. 2004). In this experiment in four patients, externalized electrodes from cortical grids were used to record neural activity that was automatically analyzed. When electrographic seizures occurred, cortical stimulation was provided. The main findings were that electrographic seizures were suppressed and the number of clinical seizures reduced during the responsive neurostimulation trials, with no major side effects. One year later, an integrated bedside system performing closed-loop responsive stimulation (Peters et al. 2001) was evaluated in eight patients undergoing intracranial monitoring (Osorio et al. 2005). This was done by connecting electroencephalogram equipment with a constant current stimulator in a closed-loop way. By doing this, electrical stimulation could be provided when clinical or electrographic seizures occurred. Four of the eight patients showed a ≥50% reduction in seizures with this responsive stimulation algorithm (Osorio et al. 2005). These proof-of-concept studies indicated that closed-loop responsive neurostimulation was feasible and effective.
In 2008, the same procedure was conducted in a patient with a fully implanted device (Anderson et al. 2008), called the responsive neurostimulation system (RNS, NeuroPace, Mountain View, USA). This system includes an implanted neurostimulator in the cranium, and one or two recording and stimulating depth and/or cortical strip leads placed at the seizure foci. Safety and efficacy of the RNS have been established in a feasibility study (n = 65), a randomized multicenter double-blinded controlled trial (n = 191) and a 7-year long-term extension study (n = 230) (Bergey et al. 2015; Heck et al. 2014; Morrell and RNS System in Epilepsy Study Group 2011). First, it was shown that seizures were reduced in the closed-loop responsive cortical stimulation group compared to a sham stimulation group (Morrell and RNS System in Epilepsy Study Group 2011). Five months after implantation, all subjects received closed-loop responsive stimulation to complete 2 years of follow-up. Here it was shown that after 1 year the median percent reduction in seizures was 44% and after 2 years even 53% (Heck et al. 2014). The 7-year follow-up study showed that reductions in seizures increased up to 60% and 66% respectively 3 and 6 years after implantation (Bergey et al. 2015). These studies therefore concluded that closed-loop responsive neurostimulation to the seizure focus was well tolerated and acceptably safe, and moreover reduced the frequency of seizures. Ever since, the RNS has been successfully implanted in many patients with intractable epilepsy, not only using cortical grids but also using DBS leads (Geller et al. 2017; Jobst et al. 2017). Future studies will be necessary to optimize stimulation algorithms and to compare closed-loop responsive neurostimulation with nonresponsive neurostimulation in epilepsy.
aDBS in Tourette’s Syndrome
Tourette’s syndrome (TS) is a neuropsychiatric disorder, characterized by both motoric and phonic tics and often accompanied by psychiatric comorbidities. For most patients, pharmacological and/or behavioral therapies are effective, but a subset of TS patients is refractory to these treatments. DBS has emerged as an established therapy for severe, drug-refractory TS. There is a growing body of evidence showing a robust degree of tic improvement due to DBS in the globus pallidus internus (GPi) or in the centromedian-parafascicular region of the thalamus in TS (Dowd et al. 2017; Kefalopoulou et al. 2015; Servello et al. 2016). Despite these positive short-term results of DBS in TS, after 12–78 months, the balance between effects and side effects of thalamic DBS, like reduced levels of energy, visual disturbances, or memory problems, becomes less favorable (Smeets et al. 2018). Since TS is a paroxysmal disorder, in which tics only occur in a fraction of time, aDBS might also be applied in TS with a potential of better tic suppression and less stimulation-induced side effects.
Recent research has shown that neurophysiological signals, like LFPs, measured in the GPi, GPe, and thalamus, have specific patterns during tics (Bour et al. 2015; Israelashvili et al. 2017; Jimenez-Shahed et al. 2016; Maling et al. 2012). Tics were found to be associated with increased high frequency and gamma band activity in the GPi (Jimenez-Shahed et al. 2016), and tics were preceded by repetitive coherent thalamo-cortical discharges (Bour et al. 2015). Furthermore, increased thalamic gamma band activity following DBS treatment was associated with reduced tic severity (Maling et al. 2012), and tic-related transient rate changes were found in individual GP neurons (Israelashvili et al. 2017). In addition to these tic-specific neurophysiological patterns, LFPs can be recorded and analyzed from DBS electrodes. Consequently, it was hypothesized that LFPs might be useful in detecting tics, and LFP-based aDBS approaches might be feasible in TS.
Shute et al. (2016) however used chronically implanted thalamic and cortical subdural electrodes to identify neurophysiological signatures of tics and to develop a tic detector. This study also examined the consistency and reproducibility of neurophysiological tic signatures. Tics of two TS patients were detected by a thalamo-cortical increase of low frequency (1–10 Hz) coherence. Over the course of 6 months, long complex tics were detected with an average sensitivity of 88.6% and specificity of 96.3%. As mentioned, this study used cortical subdural electrodes in addition to the DBS leads to optimize tic detection. This can be seen as a limitation, because the proposed tic detector requires an additional device to be implanted. Marceglia et al. proposed an aDBS system for TS based on their own and previous results on thalamic LFP patterns during tics. They hypothesized that DBS may be delivered continuously with the optimized parameter set for each patient. DBS parameters should be adapted if thalamic alpha (8–12 Hz) power decreases at least 20% and if both alpha and low frequency (2–7 Hz) power subsequently increases by 150% at 250 ms after initial alpha decrease. At the time when low frequency power has decreased by more than 50% of its peak value, the aDBS system should change the parameters again into the continuous baseline settings (Marceglia et al. 2017).
Although aDBS systems for TS have been proposed, there has only been one proof-of-concept study for aDBS in TS so far (Molina et al. 2018). This recent single-case study examined chronic aDBS in a TS patient , which had bilateral DBS implantation in the centromedian-parafascicular region of the thalamus. The implanted DBS device was the RNS, which was originally designed for the treatment of epilepsy (see section “Neurophysiological Approaches”). Tics were detected by a personalized tic detector. In the case of the presented patient, an increase in power spectral density between 5 and 15 Hz occurred during the patient’s tics. The main finding in this proof of principle study was that aDBS was safe and well tolerated and as effective as conventional scheduled duty cycle stimulation with regard to improvements in tic severity scores compared to baseline, but with a 36% increase in expected battery longevity (Molina et al. 2018).
In conclusion, aDBS in TS is still in its pioneering phase. This is also because cDBS for TS is not established yet. Larger, randomized, and blinded follow-up studies should be performed which should also include other promising DBS targets like the GPi and which should examine the impact of aDBS on side effects and psychiatric comorbidities.
Obsessive Compulsive Disorder
Obsessive compulsive disorder (OCD) is a neuropsychiatric disease characterized by unwanted and recurrent obsessions (disturbing thoughts) and/or compulsions (repetitive behaviors). DBS of the ventral striatum/ventral capsule is indicated for severe, chronic, and otherwise intractable OCD (Provenza et al. 2019). OCD might be a candidate for aDBS because there is a delicate balance between understimulation , which could lead to insufficient improvement of OCD symptoms, and overstimulation, which could lead to hypomania (Widge et al. 2016). The normalizing effect of DBS on nucleus accumbens activity could be a candidate for aDBS in OCD (Figee et al. 2013). The heterogenic character of OCD and the dynamic disease states over days, weeks, or months is challenging in finding a suitable input signal for aDBS. Psychosocial monitoring or electrophysiology seems to be the first subjects to investigate.
Future Directions
At present aDBS has only been clinically implemented in epilepsy. Although accumulating evidence shows that aDBS might also be implemented in movement disorders (Meidahl et al. 2017) and neuropsychiatric disorders (Marceglia et al. 2017), this needs to be proven in clinical and ecologically valid studies in which non-motor aspects are also investigated. This crucially depends on the availability of DBS hardware with closed-loop properties. The next step from the currently available hardware that is able to connect hardware with closed-loop properties outside the body to the IPG (e.g., applied in Malekmohammadi et al. 2016) is to develop and test IPGs with closed-loop properties “on board.” These might be systems with LFP/EcOG computational analysis on board, devices that can (wirelessly) receive accelerometer or (surface) EMG signals, or devices that have accelerometers or gyroscopes embedded in the IPG. Critical limitations in the development of this hardware is the required energy consumption, which is influenced by how many samples are required per time unit. Currently, the research focus in aDBS has been on high sampling rates (>100 samples/s). Based on the volatility and burst behavior of LFPs in PD (Tinkhauser et al. 2017), these high (and continuous) sampling frequencies are necessary for “burst-based” aDBS in PD. However, it needs to be explored whether other strategies, e.g., briefly sensing beta power every half hour, would be equally beneficial. Furthermore, the integration of closed-loop properties in other emerging stimulation paradigms like “coordinated reset” (Adamchic et al. 2014) and the development of more refined and adaptive modulations of increased synchronization (Cagnan et al. 2017; Popovych et al. 2017) will shape the future of aDBS.
References
Adamchic I, Hauptmann C, Barnikol UB, et al. Coordinated reset neuromodulation for Parkinson’s disease: proof-of-concept study. Mov Disord. 2014;29(13):1679–84.
Anderson WS, Kossoff EH, Bergey GK, Jallo GI. Implantation of a responsive neurostimulator device in patients with refractory epilepsy. Neurosurg Focus. 2008;25(3):E12.
Arlotti M, Marceglia S, Foffani G, Volkmann J, Lozano AM, Moro E, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology. 2018;90:e971–6.
Baizabal-Carvallo JF, Kagnoff MN, Jimenez-Shahed J, et al. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry. 2014;85(5):567–72.
Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol. 2011;258(3):434–9.
Barow E, Neumann W-J, Brücke C, Huebl J, Horn A, Brown P, et al. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain. 2014;137:3012–24.
Basu I, Graupe D, Tuninetti D, et al. Pathological tremor prediction using surface electromyogram and acceleration: potential use in ‘ON-OFF’ demand driven deep brain stimulator design. J Neural Eng. 2013;10(3):036019.
Bergey GK, Morrell MJ, Mizrahi EM, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810–7.
Beudel M, Little S, Pogosyan A, et al. Tremor reduction by deep brain stimulation is associated with gamma power suppression in Parkinson’s disease. Neuromodulation. 2015;18(5):349–54.
Beudel M, Oswal A, Jha A, et al. Oscillatory beta power correlates with Akinesia-rigidity in the Parkinsonian subthalamic nucleus. Mov Disord. 2017;32(1):174–5.
Blumenfeld Z, Koop MM, Prieto TE, et al. Sixty-hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations. Mov Disord. 2017;32(1):80–8.
Bogdan DI, van Laar T, Oterdoom DLM, Drost G, van Dijk JMC, Beudel M. Optimal parameters of deep brain stimulation in essential tremor: a meta-analysis and novel programming strategy. Abstract # 1395, Movement disorders conference. 2019.
Boon P, Vonck K, Van Walleghem P, et al. Programmed and magnet-induced vagus nerve stimulation for refractory epilepsy. J Clin Neurophysiol. 2001;18(5):402–7.
Boon P, Vonck K, van Rijckevorsel K, et al. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure. 2015;32:52–61.
Bour LJ, Ackermans L, Foncke EM, et al. Tic related local field potentials in the thalamus and the effect of deep brain stimulation in Tourette syndrome: report of three cases. Clin Neurophysiol. 2015;126(8):1578–88.
Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23(5):436–40.
Brodsky MA, Anderson S, Murchison C, et al. Clinical outcomes of asleep vs awake deep brain stimulation for Parkinson disease. Neurology. 2017;89(19):1944–50.
Bronte-Stewart H, Barberini C, Koop MM, et al. The STN beta-band profile in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215(1):20–8.
Brown P, Oliviero A, Mazzone P, et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21(3):1033–8.
Cagnan H, Little S, Foltynie T, et al. The nature of tremor circuits in parkinsonian and essential tremor. Brain. 2014;137(Pt 12):3223–34.
Cagnan H, Pedrosa D, Little S, et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain. 2017;140(1):132–45.
Chen CC, Brucke C, Kempf F, et al. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr Biol. 2006;16(22):R952–3.
de Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(12):4780–5.
de Hemptinne C, Swann NC, Ostrem JL, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18(5):779–86.
Delgado JM, Delgado-García JM, Grau C. Mobility controlled by feedback cerebral stimulation in monkeys. Physiol Behav. 1976;16:43–9.
Dowd RS, Pourfar M, Mogilner AY. Deep brain stimulation for Tourette syndrome: a single-center series. J Neurosurg. 2017:1–9.
Eggleston KS, Olin BD, Fisher RS. Ictal tachycardia: the head-heart connection. Seizure. 2014;23(7):496–505.
Fasano A, Lozano AM. The FM/AM world is shaping the future of deep brain stimulation. Mov Disord. 2014;29(2):161–3.
Figee M, Luigjes J, Smolders R, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–7.
Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994–1004.
Giannicola G, Marceglia S, Rossi L, et al. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson’s disease. Exp Neurol. 2010;226(1):120–7.
Giannicola G, Rosa M, Servello D, et al. Subthalamic local field potentials after seven-year deep brain stimulation in Parkinson’s disease. Exp Neurol. 2012;237(2):312–7.
Graupe D, Basu I, Tuninetti D, et al. Adaptively controlling deep brain stimulation in essential tremor patient via surface electromyography. Neurol Res. 2010;32(9):899–904.
Graupe D, Tuninetti D, Slavin KV, Basu I. Closed-loop electrochemical feedback system for DBS. J Neurosurg. 2014;121(3):762–3.
Griffiths RI, Kotschet K, Arfon S, et al. Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J Parkinsons Dis. 2012;2(1):47–55.
Grimaldi G, Manto M. Neurological tremor: sensors, signal processing and emerging applications. Sensors (Basel). 2010;10(2):1399–422.
Habets JG, Heijmans M, Kuijf ML, et al. An update on adaptive deep brain stimulation in Parkinson’s disease. Mov Disord. 2018;33(12):1834–43.
Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30(7):357–64.
Hasan H, Athauda DS, Foltynie T, Noyce AJ. Technologies assessing limb bradykinesia in Parkinson’s disease. J Parkinsons Dis. 2017;7(1):65–77.
Hauser RA, McDermott MP, Messing S. Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol. 2006;63(12):1756–60.
Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS system pivotal trial. Epilepsia. 2014;55(3):432–41.
Helmich RC, Hallett M, Deuschl G, et al. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135(Pt 11):3206–26.
Herron JA, Thompson MC, Brown T, et al. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg. 2017;127(3):580–7.
Hirschmann J, Ozkurt TE, Butz M, et al. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. Neuroimage. 2011;55(3):1159–68.
Hirschmann J, Butz M, Hartmann CJ, et al. Parkinsonian rest tremor is associated with modulations of subthalamic high-frequency oscillations. Mov Disord. 2016;31(10):1551–9.
Hirschmann J, Schoffelen JM, Schnitzler A, van Gerven MAJ. Parkinsonian rest tremor can be detected accurately based on neuronal oscillations recorded from the subthalamic nucleus. Clin Neurophysiol. 2017;128(10):2029–36.
Holslag JAH, Neef N, Beudel M, et al. Deep brain stimulation for essential tremor: a comparison of targets. World Neurosurg. 2018;110:e580–4.
Horgan J. The Forgotten Era of Brain Chips. Scientific American. 2005;293(4):66–73.
Horn A, Reich M, Vorwerk J, et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82(1):67–78.
Israelashvili M, Smeets AY, Bronfeld M, et al. Tonic and phasic changes in anteromedial globus pallidus activity in Tourette syndrome. Mov Disord. 2017;32(7):1091–6.
Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci. 2007;11(7):267–9.
Jeon H, Lee W, Park H, et al. Automatic classification of tremor severity in Parkinson’s disease using a wearable device. Sensors (Basel). 2017;17(9)
Jeppesen J, Beniczky S, Fuglsang-Frederiksen A, et al. Detection of epileptic-seizures by means of power spectrum analysis of heart rate variability: a pilot study. Technol Health Care. 2010;18(6):417–26.
Jimenez-Shahed J, Telkes I, Viswanathan A, Ince NF. GPi oscillatory activity differentiates tics from the resting state, voluntary movements, and the unmedicated parkinsonian state. Front Neurosci. 2016;10:436.
Jobst BC, Kapur R, Barkley GL, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58(6):1005–14.
Johnson LA, Nebeck SD, Muralidharan A, et al. Closed-loop deep brain stimulation effects on parkinsonian motor symptoms in a non-human primate—is beta enough? Brain Stimul. 2016;9(6):892–6.
Kefalopoulou Z, Zrinzo L, Jahanshahi M, et al. Bilateral globus pallidus stimulation for severe Tourette’s syndrome: a double-blind, randomised crossover trial. Lancet Neurol. 2015;14(6):595–605.
Khobragade N, Graupe D, Tuninetti D. Towards fully automated closed-loop deep brain stimulation in Parkinson's disease patients: a LAMSTAR-based tremor predictor. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2616–9.
Kossoff EH, Ritzl EK, Politsky JM, et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45(12):1560–7.
Kros L, Rooda OH, De Zeeuw CI, Hoebeek FE. Controlling cerebellar output to treat refractory epilepsy. Trends Neurosci. 2015;38(12):787–99.
Kuhn AA, Kempf F, Brucke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28(24):6165–73.
Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–77.
Lee KH, Lujan JL, Trevathan JK, et al. WINCS harmoni: closed-loop dynamic neurochemical control of therapeutic interventions. Sci Rep. 2017;7:46675.
Lempka SF, Miocinovic S, Johnson MD, et al. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng. 2009;6(4):046001.
Lesser RP, Kim SH, Beyderman L, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999;53(9):2073–81.
Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66(3):289–96.
Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74(3):449–57.
Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016a;87(7):717–21.
Little S, Tripoliti E, Beudel M, et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry. 2016b;87(12):1388–9.
Litvak V, Jha A, Eusebio A, et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain. 2011;134(Pt 2):359–74.
Malekmohammadi M, Herron J, Velisar A, et al. Kinematic adaptive deep brain stimulation for resting tremor in Parkinson’s disease. Mov Disord. 2016;31(3):426–8.
Maling N, Hashemiyoon R, Foote KD, et al. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndrome. PLoS One. 2012;7(9):e44215.
Marceglia S, Rosa M, Servello D, et al. Adaptive deep brain stimulation (aDBS) for Tourette syndrome. Brain Sci. 2017;8(1)
Martinez-Fernandez R, Pelissier P, Quesada JL, et al. Postoperative apathy can neutralise benefits in quality of life after subthalamic stimulation for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87(3):311–8.
Maxwell JC. I. On governors. Proc R Soc Lond. 1868;(16):270–283.
Meidahl AC, Tinkhauser G, Herz DM, et al. Adaptive deep brain stimulation for movement disorders: the long road to clinical therapy. Mov Disord. 2017;32(6):810–9.
Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303(6):322–4.
Molina R, Okun MS, Shute JB, et al. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J Neurosurg. 2018;129(2):308–14.
Moro E, Poon YY, Lozano AM, et al. Subthalamic nucleus stimulation: improvements in outcome with reprogramming. Arch Neurol. 2006;63(9):1266–72.
Morrell MJ, RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304.
Morris GL 3rd. A retrospective analysis of the effects of magnet-activated stimulation in conjunction with vagus nerve stimulation therapy. Epilepsy Behav. 2003;4(6):740–5.
Motamedi GK, Lesser RP, Miglioretti DL, et al. Optimizing parameters for terminating cortical afterdischarges with pulse stimulation. Epilepsia. 2002;43(8):836–46.
Neumann W-J, Horn A, Ewert S, Huebl J, Brücke C, Slentz C, et al. A localized pallidal physiomarker in cervical dystonia. Ann Neurol. 2017;82:912–24.
Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–40.
Osorio I. Automated seizure detection using EKG. Int J Neural Syst. 2014;24(2):1450001.
Osorio I, Frei MG, Sunderam S, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57(2):258–68.
Oswal A, Beudel M, Zrinzo L, et al. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain. 2016;139(Pt 5):1482–96.
Pavei J, Heinzen RG, Novakova B, et al. Early seizure detection based on cardiac autonomic regulation dynamics. Front Physiol. 2017;8:765.
Peters TE, Bhavaraju NC, Frei MG, Osorio I. Network system for automated seizure detection and contingent delivery of therapy. J Clin Neurophysiol. 2001;18(6):545–9.
Piña-Fuentes D, Little S, Oterdoom M, et al. Adaptive DBS in a Parkinson’s patient with chronically implanted DBS: a proof of principle. Mov Disord. 2017;32(8):1253–4.
Piña-Fuentes D, van Zijl JC, van Dijk JMC, Little S, Tinkhauser G, Oterdoom DLM, et al. The characteristics of pallidal low-frequency and beta bursts could help implementing adaptive brain stimulation in the parkinsonian and dystonic internal globus pallidus. Neurobiol Dis. 2018a;121:47–57.
Piña-Fuentes D, Beudel M, Little S, van Zijl J, Elting JW, Oterdoom DLM, et al. Toward adaptive deep brain stimulation for dystonia. Neurosurg Focus. 2018b;45:E3–8.
Piña-Fuentes D, van Dijk JMC, Drost G, van Zijl JC, van Laar T, Tijssen MAJ, et al. Direct comparison of oscillatory activity in the motor system of Parkinson’s disease and dystonia: a review of the literature and meta-analysis. Clin Neurophysiol. 2019;130:917–24.
Popovych OV, Lysyansky B, Tass PA. Closed-loop deep brain stimulation by pulsatile delayed feedback with increased gap between pulse phases. Sci Rep. 2017;7(1):1033.
Priori A, Foffani G, Pesenti A, et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol. 2004;189(2):369–79.
Provenza NR, Matteson ER, Allawala AB, et al. The case for adaptive neuromodulation to treat severe intractable mental disorders. Front Neurosci. 2019;13:152.
Qasim SE, de Hemptinne C, Swann NC, et al. Electrocorticography reveals beta desynchronization in the basal ganglia-cortical loop during rest tremor in Parkinson’s disease. Neurobiol Dis. 2016;86:177–86.
Rodriguez-Martin D, Sama A, Perez-Lopez C, et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS One. 2017;12(2):e0171764.
Rosa M, Marceglia S, Servello D, et al. Time dependent subthalamic local field potential changes after DBS surgery in Parkinson’s disease. Exp Neurol. 2010;222(2):184–90.
Rosa M, Arlotti M, Marceglia S, et al. Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov Disord. 2017;32(4):628–9.
Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72(2):370–84.
Sanchez-Ferro A, Elshehabi M, Godinho C, et al. New methods for the assessment of Parkinson’s disease (2005 to 2015): a systematic review. Mov Disord. 2016;31(9):1283–92.
Servello D, Zekaj E, Saleh C, et al. Sixteen years of deep brain stimulation in Tourette’s syndrome: a critical review. J Neurosurg Sci. 2016;60(2):218–29.
Shoeb A, Pang T, Guttag J, Schachter S. Non-invasive computerized system for automatically initiating vagus nerve stimulation following patient-specific detection of seizures or epileptiform discharges. Int J Neural Syst. 2009;19(3):157–72.
Shute JB, Okun MS, Opri E, et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. Neuroimage Clin. 2016;12:165–72.
Smeets AY, Duits AA, Leentjens AF, et al. Thalamic deep brain stimulation for refractory Tourette syndrome: clinical evidence for increasing disbalance of therapeutic effects and side effects at long-term follow-up. Neuromodulation. 2018;21(2):197–202.
Swann NC, de Hemptinne C, Miocinovic S, et al. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson’s disease. J Neurosci. 2016;36(24):6445–58.
Swann NC, de Hemptinne C, Miocinovic S, et al. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease. J Neurosurg. 2018a;128(2):605–16.
Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng. 2018b;15(4):046006.
Tinkhauser G, Pogosyan A, Little S, et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain. 2017;140(4):1053–67.
van Elmpt WJ, Nijsen TM, Griep PA, Arends JB. A model of heart rate changes to detect seizures in severe epilepsy. Seizure. 2006;15(6):366–75.
van Wijk BC, Beudel M, Jha A, et al. Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson’s disease. Clin Neurophysiol. 2016;127(4):2010–9.
Velisar A, Syrkin-Nikolau J, Blumenfeld Z, Trager MH, Afzal MF, Prabhakar V, et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019;12:868.
Verhagen R, Zwartjes DG, Heida T, et al. Advanced target identification in STN-DBS with beta power of combined local field potentials and spiking activity. J Neurosci Methods. 2015;253:116–25.
Widge AS, Licon E, Zorowitz S, et al. Predictors of hypomania during ventral capsule/ventral striatum deep brain stimulation. J Neuropsychiatry Clin Neurosci. 2016;28(1):38–44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Beudel, M., Heijmans, M., Habets, J.G.V., Kubben, P.L. (2020). Future Perspectives: Adaptive Deep Brain Stimulation. In: Temel, Y., Leentjens, A., de Bie, R., Chabardes, S., Fasano, A. (eds) Fundamentals and Clinics of Deep Brain Stimulation. Springer, Cham. https://doi.org/10.1007/978-3-030-36346-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-36346-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36345-1
Online ISBN: 978-3-030-36346-8
eBook Packages: MedicineMedicine (R0)