Abstract
Both shape memory alloy and superelastic properties of nitinol material have attracted substantial attentions in a wide range of medical applications, specifically endovascular devices. The device could be collapsed in a low profile with cooling (i.e., martensite phase) and expanded the original shape in an elevated temperature in the body (i.e., austenite phase). This chapter focuses on (1) mechanical and biological properties of nitinol, (2) manufacturing various endovascular devices, and (3) currently commercially available nitinol endovascular devices including guidewires, stents, percutaneous heart valves, occluders, and filters.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nitinol
- Endovascular device
- Superelasticity
- Shape memory alloy
- Manufacturing
- Guidewire
- Stent
- Occluder
- Heart valve
- Filter
4.1 Background on Nitinol
4.1.1 History of Nitinol
Shape memory behavior was first discovered in gold–cadmium (AuCd) alloy by Dr. Olander in 1932. The deformed AuCd alloy material in low temperature regains its original shape with the applied heat [1]. Many other alloys, such as CrMn, FePt, BaTiO3, and CuMn, were synthesized to achieve similar phase transformation behavior [2]. By late 1950s at the US Naval Ordnance Laboratory, Buehler and his colleagues discovered the shape memory behavior in nickel–titanium (NiTi) alloy while they worked on intermetallic compounds for heat shielding of missiles [2,3,4]. Nitinol is referred as NiTi in Ordnance Laboratory and became popular in various fields including aerospace, medical, and other industries due to its inexpensive cost for manufacturing and reliable performance. Nitinol has another unique property, superelasticity , in addition to shape memory behavior. Kurdiumov discovered superelastic behavior of metallic alloys in 1948 by investigating the elastic response of alloys that exhibit phase transformation upon varied stress levels applied on materials [5].
Nitinol has become one of the attractive alloy materials in numerous applications due to two unique properties, shape memory behavior and superelasticity. Currently available commercial products include buckling-resistant antennas, pipe-coupling devices, and eyeglass frames, which utilize superelastic behavior of nitinol [6]. There are more advanced types of applications that use shape memory behavior, which include light structure and engine rotors for aircrafts, biomedical robots, and micro-actuators. More recently, nitinol has been widely used in various medical applications. Dr. Andreasen has developed the first nitinol biomedical application for an orthodontic device utilizing the superelastic behavior of nitinol. Other applications include implantable medical devices, such as endovascular and orthopedic devices [7,8,9]. Nitinol is specifically beneficial in transcatheter-based devices such as stents, percutaneous heart valves, and vascular occluders, and filters since these devices can be easily collapsed and inserted to a small diameter delivery catheter in low temperature, then deployed to its original shape and dimension in body temperature showing superelastic property after the device delivery.
4.1.2 Macroscopic Behavior of Nitinol
4.1.2.1 Shape Memory Effect
Shape memory effect (SME) is the capability of the material, upon heating, to recover the permanent strain that occurs from the deformation in the martensitic phase [2]. Nitinol has a transformation (Tf) temperature between two different phases, austenite (i.e., above Tf) and martensite (i.e., below Tf). Each phase has different crystal structure that provides the material either to have shape memory effect and superelasticity. The transformation of this alloy could be altered via manipulation of compositional variation of Ni and Ti followed by heat treatment.
When the material is below the transformation temperature (i.e., martensite finish temperature, Mf), it deforms easily with the capability of reaching high level of strains, martensite phase. When the temperature is above the transformation temperature (i.e., austenite finish temperature, Af), the material exhibits superelastic behavior, austenite phase. Since the temperature is the main parameter for the phase transformation, it is sometimes called thermomechanical transformation. The schematic of phase transformation for the materials is shown in Fig. 4.1. When the material is deformed in martensitic phase, it could recover its deformation through heating to reach the austenite phase as shown in Fig. 4.1a [5].
4.1.2.2 Superelasticity
Elasticity is the capability of recovering from the deformation when the load is removed without generating plastic deformation, similar to the spring effect. Superelastic material is regarded as a very efficient spring. Nitinol compositions can be manipulated in order to attain specific transformation temperatures between phases. Superelasticity happens only to nitinol, when the material temperature lies above the austenite transformation threshold level as shown in Fig. 4.1b.
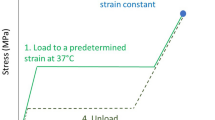
Nitinol gains relatively high merits over other traditional metals due to the stress-strain response, where nitinol could reach high levels of strains with fracture. Nitinol’s superelasticity could exceed the elastic limit of 10%; however, steel and copper fail to precede the elastic limit of 0.5% and 0.1%, respectively [8]. The stress-strain relation of nitinol, compared to steel, is shown in Fig. 4.2a. Another advantage of nitinol for the biomedical application is the exhibition of a similar response trend upon loading and unloading to biological tissues for their deformation as shown in Fig. 4.2b. This phenomenon is called pseudoelasticity, where the material is capable of having a plateau upon large deflections through loading, or recovered deformations through unloading, without noticeable change during loading.
For example, if nitinol is properly manufactured to have its austenite phase transformation temperature less than the body temperature, the material will be fully superelastic in the human body. In this case, nitinol biomedical devices can achieve high strain level, which is a crucial property for medical implants used in the body.
4.1.3 Microscopic View of Nitinol
4.1.3.1 Crystallography
Nitinol has two distinct phases and transforms from one to another by rearranging its crystal structure. The basic structure is composed of nickel and titanium atoms entangled in a single unit cell, which can be cubic, monoclinic, or triclinic structure [10]. The shape recovery of nitinol is considered as a solid–solid phase transformation, also called martensitic transformation. The unit cell in the martensitic transformation changes from cubic (B2: austenite phase) to monoclinic (B19’) structure by passing through intermediate phase of orthorhombic (B19).
The martensitic transformation process go through three changes to reach to the final phase as shown in Fig. 4.3 [10]. First, the material is in the cubic structure phase (B2) as the shaded cube shown in Fig. 4.3a, and then the material unit cell transforms into tetragonal shown in Fig. 4.3b. The orthorhombic (C) is formed as an intermediate phase, until homogenous shear force distorted the (B19) phase into the monoclinic (D: B19’). In the final stage, the nickel atom displaces its center position, making the plane lost the center of symmetry [10].
Martensitic distortions of the B2 crystal structure of NiTi. (a) The relation between the cubic B2 cell (shaded box) and the undistorted (tetragonal) B19 cell. (b) The orthorhombic B19 structure. (c) The distortion to the stress stabilized B19’ structure. (d)The BCO minimum-energy structure with further doubled conventional cell (shaded box). (Reprinted with permission from [10])
The transformation from B2 cubic to B19’ monoclinic achieves a large shape change that reaches 7%. This large deformation comes from the crystal asymmetry and produces hysteresis during the transformation phases [11]. While the hysteresis is an important feature to be considered for actuators or springs, it would be less important for the medical implants, specifically for the endovascular device, because superelasticity is a key property for the device, which is not associated with any phase transformation.
4.1.3.2 Martensitic Phase Transformation
The deformation in the martensitic phase transformation occurs without any atom diffusion; however, slip and twinning take place inside the crystal structure. Slip is the motion between slip planes, when the planes have sufficient force to overcome the interatomic forces between slip planes as shown in Fig. 4.4b. Defects in atomic arrangements cause dislocations, responsible for slip motion planes. Slip typically occurs randomly between atomic planes; however, the twinning needs cooperative displacement to happen, which is distinct from the slip, as shown in Fig. 4.4c. Twinning is a rare occurrence in the austenite phase, while it is more common in nitinol martensitic phase, which explains the shape memory behavior of nitinol.
4.1.3.3 Twin Boundary Motion
Twinning (or twin boundary motion) causes change in shape and volume during the martensitic transformation. The twins occur in alternating layers with different directions, which avoid large strains in the martensitic phase. The alternating layers in the twinning boundaries bond with the austenite phase with no twins, which generate an interface of minimum energy planes. The new interface does not produce significant fractures or dislocations as shown in Fig. 4.5 [12].
4.1.4 Other Properties of Nitinol for Endovascular Devices
4.1.4.1 Surface Properties
Biological response of the living organisms to the materials governs the material ability to be implanted in the body. Surface conditions such as roughness and chemical composition have significant influence on the biological response. Higher roughness tends to decrease the hydrophilicity of the surface, which increases protein adsorption (contaminations on the surface) that occurs on the hydrophobic surfaces [13, 14]. Manufacturing processes, such as electropolishing, reduces the roughness of the nitinol surface to minimize the protein adsorption. Also, the chemical composition of the surface can cause inflammatory response due to nickel allergy, upon being implanted inside the body. When nitinol’s surface is exposed to the atmosphere or various surface treatments, thin titanium oxide layer is generated, keeping nickel beneath the oxide layer [15]. Most metals including nitinol have high surface energy, which exhibit hydrophilicity. Hydrophilic surface could decrease the protein adsorption during the implantation.
4.1.4.2 Hemocompatibility
Endovascular device’s surface should be compatible with blood to minimize thrombosis formation on the device. Nitinol typically shows minimal thrombogenic response, similar to the excellent hemocompatible metallic biomaterials (e.g., stainless steel and titanium) [14, 16]. Nitinol devices that are placed in the blood vessel for a long time have demonstrated better performance of hemocompatibility by minimum protein adsorption, least possible of platelets adhesion, or blood clot formation on the implanted devices. Numerous surface modification strategies including surface coatings, heat treatment, chemical treatment, and electropolishing have been investigated to improve the hemocompatibility in nitinol surface [15, 17].
4.2 Manufacturing Processes of Nitinol Used for Endovascular Devices
Along with the growth of nitinol usage in biomedical devices, manufacturing processes have been developed to produce high-quality nitinol alloy because the composition of nickel and titanium is critical for determining the property of nitinol. The ratio between the two metals is typically 50:50%, and any change in this ratio alters the shape memory and superelastic properties. Any change in nickel or titanium percentage, even by 1%, could shift the transformation temperature with 100 °C [18, 19]. Higher amount of nickel modifies the properties of nitinol to be more superelastic at lower temperatures. Various manufacturing processes and the produced material properties are described in this section, since the manufacturing processes affect the material percentage that determines the nitinol’s property.
4.2.1 Drawing Process for Nitinol Wires and Tubes
Continuous production of nitinol wires using the strain annealing ensures the homogeneity of thermomechanical property of the whole product. Figure 4.6 shows the schematic of continuous production of nitinol wires. The main parts are (1) load control unit that regulates the tension during the drawing process, (2) furnace that controls the drawing temperature, and (3) speed control pulley that manipulates the drawing speed. Mechanical properties, final austenite temperature, and straightness are manipulated by temperature, tension, and speed in the drawing process [19]. The drawing is performed under a stress of 35–100 MPa with a temperature range of 450 °C–550 °C.
Nitinol wires undergo several steps through drawing process. Raw material should have 50.5%–51.0% of nickel to produce superelastic wires. Nonmetallic inclusions, micro- and macro-segregations should be avoided before the drawing process [19]. Pre-annealing is performed to form thin oxide layer to work as lubricant through the process; however, if the oxide layer becomes too thick, it will cause cracks in the wire surface [20]. An alternative to the oxide layer as lubricant is molybdenum disulfide, which shows a good lubrication performance in nitinol drawing process [20]. The drawing is performed using multi-passes in inert gas medium through monocrystalline dies. The final pass in drawing is the most important step to produce the desired dimensions and specific superelastic properties. Finally, any lubricant residue is eliminated from the drawn nitinol wire.
4.2.2 Laser-Cutting Process for Stent Fabrication
Laser cutting is a process of applying high-intensity light beam that swiftly heats the targeted area, which melts or/and vaporizes the material through its thickness. Palmaz-Schatz stent was the first approved stent for use in the United States in 1994, which was fabricated using a laser cutting process [21]. Laser cutting offers a couple of advantages over other manufacturing processes, including reliable dimensional accuracy, very small resolution, ease of automation, higher productivity, cut capability for most of materials, and suitable for complex structures. Nitinol laser cutting can be exploited using continuous wave and pulsed wave using different processes including Nd:YAG laser [22], fiber laser [23, 24], and ultrashort pulse laser [25]. The primary laser cutting parameters are cutting speed, laser power, pulse type–duration, and gas (oxygen, inert gas, or air). The main objective of choosing the laser machine and its parameters is to achieve good surface quality with high-dimensional accuracy, as well as minimum heat-affected zone that typically causes brittleness of nitinol surface, in addition to lesser consistent kerf width (distance between cut slot edges). The early laser-cut stents were fabricated using Nd:YAG laser; however, low efficiency and lifetime affect the kerf width consistency [26]. Recently, fiber laser and short-pulsed laser are commonly used for manufacturing the stent due to their reliability, efficiency, and long lifetime.
4.2.3 Joining and Welding Processes for Stents, Filters, Guidewires, and Occluders
Joining and welding processes of nitinol were recently investigated to overcome the drawbacks from laser cutting of the devices. The main problem from the laser cutting is the formation of wide heat-affected zone (relatively to joining process) that alters the microstructure and mechanical properties in nitinol. To minimize the heat-affected zone, wire-to-wire joining was proposed to fabricate the devices. In addition, the laser cutting process is limited by the dimensions of the tube to be cut in order to fabricate device, and laser or other types of welding could be one of the best options for the devices that have dimensions exceed the limits of the fabrication of nitinol tube. Laser-cut process used in stent fabrication typically removes more than 90% of materials, increasing the manufacturing cost, especially for larger devices. Nitinol-to-nitinol laser welding of can be performed using Nd:YAG laser [27, 28], CO2 laser [29], and tungsten inert gas welding [30]. Thermomechanical properties are usually maintained after the welding process. Nd:YAG laser welding preserves up to 75% of tensile strength of nitinol as well as 7% deformation for the superelastic welded parts [31]. Welding nitinol to dissimilar metal is quite challenging due to the formation of the brittle intermetallic compounds; however, nitinol was successfully welded to stainless steel [28, 32]. There are other types of joining techniques for nitinol such as crimping and swaging.
4.2.4 Subsequent Post-Processes
4.2.4.1 Thermal Annealing
The thermal annealing is performed in order to set the transformation temperature of the nitinol, which is essential to reconstruct the microstructure of the heat affect zone, resulted from the prior fabrication processes. On the one hand, the nitinol material is typically thermally annealed in the temperature around 500 °C to achieve superelastic property in a desired temperature [33]. On the other hand, the nitinol alloys are typically annealed in the temperature between 350 °C and 450 °C for better shape memory behavior. Both thermal annealing temperature and time significantly affect the formation of the oxide layer on the nitinol surface, which govern the thermomechanical and hemocompatible properties of the material [34]. Thermal annealing is also used for shape setting through the relaxation of the material at the desired equilibrium shape. Shape setting could be carried out at temperature around 500 °C using mandrel in order to have the desired geometry of the devices [35].
4.2.4.2 Electropolishing
Electropolishing is the final process for nitinol endovascular devices, which is a standard finishing process for stents, heaver valve fames, or any other implantable devices. This process is typically used for creating smooth surface with corrosion-resistive coating layer on the outer surface of the metal or alloys [36, 37]. The improved surface smoothness is beneficial for endovascular devices due to its enhanced biocompatibility property as implantable devices. Electropolishing reduces the free surface energy to remove any contamination from foreign materials outside the body; thus, thrombosis formation could be minimized [38]. In addition, the electropolishing finishing process has been proven to reduce the nickel concentration on the surface of nitinol alloys [39].
4.3 Nitinol Endovascular Devices
4.3.1 Guidewires
With the growth of endovascular procedures due to their minimally invasive nature, a guidewire is a very important device to deliver the drugs or devices to the location of the diseased or injured blood vessel. Endovascular procedure, sometimes called transcatheter-based procedure , requires a small incision typically in groin to access the vascular system. Due to the nature of less invasive surgery and less postsurgical trauma, endovascular procedures became more popular in the last two decades. A guidewire is typically used with a delivery catheter or sheath that is a kink-resistant composite tube [40, 41]. A guidewire is an ultrathin, elastic, kink-resistant, and sufficiently long metallic wire. A guidewire is used to direct the catheter to the desired location, applying stiffness in the tortuous or bifurcated vessels. The guidewire is first placed in the disease or injury locations in the blood vessel, and then, a catheter is delivered over the guidewire for easy access to the desired locations. Collapsed devices or drugs could be delivered at the location with the catheter (Fig. 4.7).
(a) Main components of guidewire and (b) penetrating tip design the lesion entry; (i) Straight tip or small bend to assist tip penetration, (ii) better navigation and more flexibility are achieved with the secondary bend for a tortuous segment, and (iii) J tips are used to allow returning to the lumen from the subintima
Figure 4.6a shows a typical structure of the guidewire that consisted of three main parts: central core, tip, and lubricous coating. The central core is the main part that extends through the guidewire, which controls the flexibility, tracking, steering, and support of the guidewire [42]. The central core material is either stainless steel or nitinol. While stainless steel has been extensively used for guidewires previously, however, nitinol is one of the most popular materials used in current guidewire manufacturing because it has superelasticity that is kink-resistant with high torqueability and steering capability [7, 18]. Kinking resistance is specifically very important in guidewire because permanent kinks on the guidewire in endovascular procedure cause difficulties in removing the wire without injuries inside the vascular system [43].
The tip of guidewire is typically covered with very flexible coils, which guide the wire through the lesions without generating any potential complication such as blood vessel perforation or scratch. The tip design governs guidewire’s steering performance, flexibility, and pushing capability; therefore, the design is varied depending on the anatomy of the vasculature, e.g., tortuosity, bifurcations, diameter of vessels, and degree of bending needed during navigation. The tip design used for the guidewire depends on the vascular configuration. Figure 4.6b shows various designs used in guidewires; (i) the tip has a small angle bent to increase the penetration, (ii) secondary bend is added to allow better navigation, and (iii) the tip has “J” shape to allow returning to the lumen from the subintima. Finally, hydrophilic lubricous coating is applied on the entire guidewire to minimize any potential friction occurred during navigation, to increase physician control, and to provide smooth delivery without generating any thrombogenic issue in the blood stream.
The guidewire performance mainly affects the capability of the radiologists to operate the endovascular operation. Radiologists were requested to use three commercial guidewires and evaluate them in terms of specific parameters. The performance parameters were torque response, tortuous vessels navigation, radiopacity (ability to be seen in X-rays), balance, lubricity, and tip shape retention. The guidewires used in this survey are ZIPwire™ Hydrophilic Guide Wire, HiWire® Nitinol Core Wire Guide, and TERUMO GLIDEWIRE®. The evaluation is represented in Fig. 4.8 (data from [44]).
The rating of three representative commercial hydrophilic-coated guidewire characteristics, evaluated by 10 physicians in 40 different cases for every wire. (Data from [44])
4.3.2 Self-Expanding Stents
Stent is a mesh scaffold used to widen the narrowed blood vessel due to atherosclerosis or plaque formation, typically inside the coronary, intracranial, or peripheral arteries [45,46,47]. The stents are also used for blocking dilated artery (i.e., aneurysm) or for inserting a new conduit in the aneurysm locations in order to prevent potential rupture of aneurysms. Intravascular stenting technique was first investigated by Charles Dotter in 1969 [48]. This technique was developed to reconstruct vascular patency using transcatheter procedure, in a way other than percutaneous balloon angioplasty, which temporarily dilates the narrowed blood vessel with the inflated balloon to break the plaques or to push away the fatty substances to the wall [49]. Most of the devices in this era were coil spring-shaped stents. More than one decade later, Julio Palmaz developed a tube-shaped balloon expandable stainless steel stent for small artery applications [21].
Balloon inflation is needed to deploy the stainless steel stent to generate permanent plastic deformation in stent because stainless steel is ductile in the range of expansion from the delivery catheter size to the target artery size [50, 51]. Although balloon expandable stents have been widely used in earlier stent history, there are potential vessel wall damages that lead to severe restenosis (i.e., tissue ingrowth) due to the overexpanded balloon dilation to place the stent [52]. Therefore, self-expandable stents were widely investigated to replace the balloon expandable stents, which is simple to deploy, generating minimal intima damages [53]. One of the best metallic materials for self-expanding stents is nitinol as described in the earlier section (Sect. 1.2). There are primarily two types of nitinol stents: (1) laser-cut stent from thin wall nitinol tube for small diameter applications (e.g., coronary artery, neurovascular, or peripheral arterial stents) and (2) wire braided or bent stent for large diameter applications (e.g., thoracic or abdominal aortic aneurysm stent grafts). Figure 4.9 shows representative commercially available nitinol stent products for peripheral artery treatments. Figure 4.9a represents the Innova™ (Boston Scientific) stent, which can be used for proximal popliteal artery and superficial femoral artery. Figure 4.9b shows VIABAHN® (Gore Medical) stent graft, where the metallic backbone is covered by thin polymer, typically either expanded polytetrafluoroethylene (ePTFE) or Dacron polyester. This type of stent is used to graft the disease tissue and also to protect aortic–thoracic aneurysms from rupture [54, 55].
In addition, nitinol stent is non-ferromagnetic, which is MRI compatible. Patients who have stents in the body do not have problems for taking MRI in the future after the placement of the stents [56]. The common approach used for the stent delivery is keeping the stents in the cooler martensitic phase for crimping and then inserting into the catheter. Once the catheter reaches to the desired location inside the body, it is removed to deploy the stent with its nitinol’s own superelasticity.
Earlier stents were manufactured by transforming the nitinol wires into a coil as shown in Fig. 4.10a; however, due to low radial stiffness and collapsibility, these designs were abandoned. Current nitinol stents are wire-, sheet-, or tube-based designs. Wire-based stents are made of round or flat wires, which can be connected via welding or any other joining methods as shown in Fig. 4.10b. One advantage of wire-based stents is the ability to easily retrieve the stent in certain applications because the stent struts are typically closed-cell geometry (i.e., all wires are connected) [8]. Sheet-based stent is used to overcome the wire-based stent’s high collapsed volume due to the multiple crossed wires. The sheet is first laser cut, and then rolled up and welded to make the circular profile. While the sheet-based stent was preferred for a smaller stent, there were relatively bulky joining regions that should be avoided for better collapsing with a small catheter. Tube-based stents are seamless stents (Fig. 4.10c), manufactured by laser cutting from the nitinol tube, to avoid any potential fracture issue that may occur in the sheet-based stent due to the welding regions. The stents are annealed after the manufacturing processes to set the transformation temperature for the desired thermomechanical properties. Additionally, this thermal process eliminates any stress concentration zone that limits the localized brittleness. Radiopaque makers are added on both ends or any desired locations within the stent to help position the stent under fluoroscopic guidance.
(a) Intracoil stent (IntraTherapeutics). (b) Wire-based stents connected using welding, symphony stent (Boston Scientific). (c) Laser-cut nitinol tubular stents, Memotherm (Bard Angiomed). (All images are reprinted with the permission from [8])
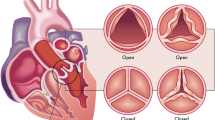
4.3.3 Percutaneous Heart Valve Frame
Symptomatic aortic stenosis (AS) is considered one of the main valvular heart disease, which is an important source of cardiovascular morbidity and death worldwide [57]. Aortic stenosis is the narrowing of the valve that delivers high pressure oxygenated blood from the heart to the aortic artery, which carries the blood to the main abdominal organs and the lower part of the body. The standard treatment of aortic valve stenosis is open chest aortic valve replacement; however, most of the patients are elderly who may not survive due to the open-surgery complications. Thus, one-third of the patients are rejected for surgery [58]. Less invasive techniques were adapted to overcome the open-chest complications. Percutaneous aortic valve replacement has developed as a new promising technique in the recent years as minimum invasive operation for symptomatic (AS) treatment [59, 60]. The first percutaneous valve was proposed in the early 1990s using balloon expanded valve stent [61]. With the aid of superelasticity in nitinol alloy (i.e., self-expanding device) nitinol-based aortic valve was used for the minimally invasive treatment of aortic stenosis. The metallic backbone is manufactured using laser cutting in a similar way of stent. Figure 4.11a shows a representative commercially available nitinol heart valve which is CoreValve Revalving™ System that is integrated with porcine pericardial trileaflet valve sewn to the nitinol backbone using ePTFE sutures [62]. The placement of the valve is performed under fluoroscopic guidance as shown in Fig. 4.11b. Figure 4.11c shows the implanted heart valve after a few months, which shows that the nitinol valve replaced the aortic valve well.
(a) CoreValve Revalving System profile (reprint with the permission from [57]). (b) In situ Coronal MRI image CoreValve prosthesis, after six months of implantation. (Reprint with the permission from [58]). (c)Fluoroscopic guidance for implanting the CoreValve prosthesis. (Reprint with the permission from [58])
4.3.4 Atrial Septal Defect (ASD) Occluder
Atrial septal defect (ASD) is a hole that occurs in the septum, the wall that separates between the upper chambers of the heart. This defect allows the oxygenated blood, comes from the lungs in the left atria, to induce a leakage to the poor-oxygenated blood in the right atria. Figure 4.12 is the schematic of the atrial septal defect. The patients with this defect suffer from fatigue, arrhythmias, and congestive blood failure [63]. ASD is a common congenital heart defect with an occurrence rate of 3.78/10000 live births, considered the fourth frequent form of congenital heart disease [64]. Even though open-heart surgical operation is widely accepted to repair the defect, exploiting minimally invasive alternative has been recently developed to overcome any complication found in the open-heart surgery [65].
King and Mills did the first endovascular occluder trial to repair ASD during cardiac catheterization in 1974. They used the opposed pair of stainless steel umbrellas covered with Dracon [63]. This device had many problems including structural failure and inability to recapture [66]. With the significant development of shape memory alloys, nitinol was used to repair the atrial septal defect with the self-expanding, self-centered, and repositionable properties.
The ASD occluder was named the Amplatzer, which consists of two round disks of nitinol mesh. The mesh was made from 0.004- to 0.005-inch nitinol wire, which is tightly woven into two flat buttons, and the disks were linked using connecting waist [66], as shown in Fig. 4.13a. The whole device can be delivered via either 6Fr or 7Fr sheath (Fig. 4.13b), depending on the diameter wire and expanded size of the occluders [67]. The two flat retention meshes extend to the radial direction beyond the central waist (Fig. 4.13c) to work as secure anchors. The nitinol mesh is covered with Dacron. The Amplatzer is deployed with ultrasound and fluoroscopic guide to ensure optimal positioning [65]. Figure 4.13d shows cineradiographic frames of the ASD occluder implanted inside human body. The occluder demonstrates good positioning and whole closure.
(a) Amplatzer septal occluder made of two round disks from 0.005-in. nitinol wire, that was tightly woven into with a 4-mm connecting waist (arrowheads). (Reprint with the permission from [66]). (b) Adaptor tube (arrowheads) used for the occluder delivery. (Reprint with the permission from [66]). (c) Mechanisms of leakage-proof closure with the two retention disks, which are angled inward. The left atrial retention disk is slightly bigger than the right, to ensure overfitted clamping against around the defect. (Reprint with the permission from [67]). (d) Good positioning and complete closure of the Amplatzer are shown using Levo-phase of pulmonary arteriogram. (Reprint with the permission from [66])
4.3.5 Vena Cava Filter
The vena cava is a large vein that carries the deoxygenated blood to the heart. The human body has two venae cavae: the superior vena cava that carries the blood from head and upper body and the inferior vena cava that carries blood from the lower body. Clot formed in these veins could travel to various locations and may cause fatal complications in the brain or lungs with the clots or fragmentation from clots (i.e., clot embolization) [68]. Due to the device’s large deployed diameter, it is always challenging to deploy endovascular devices [69]. However, these filter devices can be easily collapsed in low temperature region (i.e., martensite phase) by cooling, then, the filters recovers its original shape in the higher temperature (i.e., austenite phase) such as human body temperature, which is called shape memory effect of nitinol material. The first nitinol filter was presented by M. Simon in 1977. Shape memory effect of nitinol was used to make the filter recover its predefined configuration, when the filter is heated by body temperature upon implanting. Figure 4.14a shows the filter inside the body, with full recovery and good positioning [70]. The test results for vena cava demonstrate the capability of IVC filter to collect the blood clots [68, 71, 72]. More recently, new filter devices have been introduced. Figure 4.14b shows the commercially available Optease vena cava filter (Cordis), which is used as a temporary filter. The filter consists of six diamond-shaped laser-cut nitinol struts. The filter contains a self-centering portion and the upper hook for easy retrieval of the filter. Another commercial nitinol filter is Denali vena cava filter (Bard, Fig. 4.14c). The filter consists of 12 laser-cut nitinol appendages, which can be used for temporary or permanent placement of the device.
4.4 Summary
Many endovascular devices, which are commercially available and under research, take advantage of the mechanical and biological properties of nitinol for the treatment of various physiological problems. Both shape memory alloy and superelastic properties are utilized to achieve the capability of delivery of the endovascular devices with minimum invasive techniques, and self-expanding performance. Manufacturing processes influence thermomechanical behaviors of nitinol, hence should be carefully selected for various devices. Nitinol alloy have been applied for various endovascular devices, including guidewires, stents, percutaneous heart valves, filters, and occluders, demonstrating excellent performance in terms of hemocompatibility, surface properties, and mechanical reliability. Many nitinol endovascular devices are currently under research; therefore, these could be used in the future for better clinical outcomes, helping patients who have vascular diseases or injuries.
References
A. Ölander, An electrochemical investigation of solid cadmium-gold alloys. J. Am. Chem. Soc. 54(10), 3819–3833 (1932)
C.M. Wayman, J.D. Harrison, W.J. Bryan, Shape memory effect. JOM 41, 26–28 (1989)
W.J. Buehler, J.V. Gilfrich, R.C. Wiley, Effect of low-temperature phase changes on the mechanical properties of alloys near composition TiNi. J. Appl. Phys. 34(5), 1475–1477 (1963)
F.E. Wang, W.J. Buehler, S.J. Pickart, Crystal structure and a unique “martensitic” transition of TiNi. J. Appl. Phys. 36(10), 3232–3239 (1965)
D.J. Rabkin, E.V. Lang, D.P. Brophy, Nitinol properties affecting uses in interventional radiology. J. Vasc. Interv. Radiol. 11(3), 343–350 (2000)
S. Yamamoto, H.E. Wilczek, T. Iwata, M. Larsson, H. Gjertsen, G. Söderdahl, G. Solders, B.G. Ericzon, Long-term consequences of domino liver transplantation using familial Amyloidotic polyneuropathy grafts. Transpl. Int. 20(11), 926–933 (2007)
C.D.J. Barras, K.A. Myers, Nitinol – its use in vascular surgery and other applications. EJVES Extra 19(6), 564–569 (2000)
D. Stoeckel, A. Pelton, T. Duerig, Self-expanding nitinol stents: Material and design considerations. Eur. Radiol. 14(2), 292–301 (2004)
A.R. Pelton, D. Stöckel, T.W. Duerig, Medical uses of nitinol. Mater. Sci. Forum 328(May 1999), 327–328 (2000)
X. Huang, G.J. Ackland, K.M. Rabe, Crystal structures and shape-memory behaviour of NiTi. Nat. Mater. 2(5), 307–311 (2003)
M. Meyers and K. Chawla, “Solid Solution, Preciptation, and Dispersion Hardening,” Mechanical Behavior of Materials, 558–591 (2009)
K. Otsuka, C. Wayman, Shape Memory Materials (Cambridge university press, Cambridge, 1998)
N.J. Hallab, K.J. Bundy, K. O’Connor, R.L. Moses, J.J. Jacobs, Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 7(1), 55–71 (2001)
L. Ponsonnet, V. Comte, A. Othmane, C. Lagneau, M. Charbonnier, M. Lissac, N. Jaffrezic, Effect of surface topography and chemistry on adhesion, orientation and growth of fibroblasts on nickel-titanium substrates. Mater. Sci. Eng. C 21(1–2), 157–165 (2002)
S.A. Shabalovskaya, Surface, corrosion and biocompatibility aspects of nitinol as an implant material. Biomed. Mater. Eng. 12(1), 69–109 (2002)
D.A. Armitage, T.L. Parker, D.M. Grant, Biocompatibility and hemocompatibility of surface-modified NiTi alloys. J. Biomed. Mater. Res. Part A 66(1), 129–137 (2003)
S. Sapatnekar, K.M. Kieswetter, K. Merritt, J.M. Anderson, L. Cahalan, M. Verhoeven, M. Hendriks, B. Fouache, P. Cahalan, Blood–biomaterial interactions in a flow system in the presence of bacteria: Effect of protein adsorption. J. Biomed. Mater. Res. 29(2), 247–256 (1995)
T. W. Duerig, K. Melton, and D. Stöckel, Engineering aspects of shape memory alloys: Butterworth-Heinemann (2013)
L. M. Schetky and M. Wu, “Issues in the further development of Nitinol properties and processing for medical device applications,” in Medical device materials: proceedings from the materials & processes for medical devices conference. 271–276 (2003, 2004)
S. Shabalovskaya, J. Anderegg, J. Van Humbeeck, Critical overview of Nitinol surfaces and their modifications for medical applications. Acta. Biomater. 4, 447–467 (2008)
J. Palmaz, Progress in radiology balloon-expandable. Am. J. Roentgenol. 150, 1263–1269 (1988)
R. Pfeifer, D. Herzog, M. Hustedt, S. Barcikowski, Pulsed Nd : YAG laser cutting of NiTi shape memory alloys — Influence of process parameters. J. Mater. Process. Tech. 210(14), 1918–1925 (2010)
C.A. Biffi, A. Tuissi, Nitinol laser cutting: Microstructure and functional properties of femtosecond and continuous wave laser processing. Smart Mater. Struct. 26, 035006 (2017)
L. Liu, D. Bo, L. Yi, F. Tong, Y. Fu, Fiber laser micromachining of thin NiTi tubes for shape memory vascular stents. Appl. Phys. A Mater. Sci. Process. 122(7), 1–9 (2016)
N.M.D.W.A. Boor, W.O.Z.L.L. Li, Picosecond laser micromachining of nitinol and platinum – Iridium alloy for coronary stent applications. Appl. Phys. A Mater. Sci. Process., 607–617 (2012)
L. Chen, L. Chen, Laser cutting for medical device (stent) – Yesterday, today and tomorrow, ICALEO® 2008 Congr. Proc., 607 (2008)
L. Quintino, R.M. Miranda, U.N. De Lisboa, Welding shape memory alloys with NdYAG lasers. Soldag. e Inspecção 17, 210–217 (2012)
G.R. Mirshekari, A. Saatchi, A. Kermanpur, S.K. Sadrnezhaad, Laser welding of NiTi shape memory alloy: Comparison of the similar and dissimilar joints to AISI 304 stainless steel. Opt. Laser Technol. 54, 151–158 (2013)
Y.T. Hsu, Y.R. Wang, S.K. Wu, C. Chen, Effect of CO2 laser welding on the shape-memory and corrosion characteristics of TiNi alloys. Metall. Mater. Trans. A 32(March), 569–576 (2001)
J.P. Oliveira, D. Barbosa, F.M.B. Fernandes, R.M. Miranda, Tungsten inert gas (TIG) welding of Ni-rich NiTi plates: Functional behavior. Smart Mater. Struct. 25(3), 03LT01 (2016)
M.H. Wu, Fabrication of nitinol materials and components. Mater. Sci. Forum 395, 285–292 (2002)
H.M. Li, D.Q. Sun, X.L. Cai, P. Dong, W.Q. Wang, Laser welding of TiNi shape memory alloy and stainless steel using Ni interlayer. Mater. Des. 39, 285–293 (2012)
M. Drexel, G. Selvaduray, A. Pelton, The effects of cold work and heat treatment on the properties of nitinol wire. Med. Device Mater. IV Proc. Mater. Process. Med. Devices Conf. 2007, 114–119 (2008)
L. Xu, R. Wang, Y. Liu, The optimization of annealing and cold-drawing in the manufacture of the Ni-Ti shape memory alloy ultra-thin wire. Int. J. Adv. Manuf. Technol. 55(9–12), 905–910 (2011)
Vojtěch, D., Influence of heat treatment of shape memory NiTi alloy on its mechanical properties, Proceeding 19th conference Metall. Mater. “Metal 2010,” 2010, pp. 867–871
W. Simka, M. Kaczmarek, A. Baron-Wiecheć, G. Nawrat, J. Marciniak, J. Zak, Electropolishing and passivation of NiTi shape memory alloy. Electrochim. Acta 55(7), 2437–2441 (2010)
R. Venugopalan, C. Trépanier, Assessing the corrosion behaviour of nitinol for minimally-invasive device design. Minim. Invasive Ther. Allied Technol. 9(2), 67–73 (2000)
B. Thierry, M. Tabrizian, O. Savadogo, L. Yahia, Effects of sterilization processes on NiTi alloy: Surface characterization. J. Biomed. Mater. Res. 49(1), 88–98 (2000)
A. Michiardi, C. Aparicio, J.A. Planell, F.J. Gil, New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 77(2), 249–256 (2006)
Dankelman, J., Grimbergen, C. A., and Stassen, H. G., 2004, Engineering for Patient Safety
L. Aklog, D.H. Adams, G.S. Couper, R. Gobezie, S. Sears, L.H. Cohn, A.F. Carpentier, D.B. Skinner, Techniques and results of direct-access minimally invasive mitral valve surgery: A paradigm for the future. J. Thorac. Cardiovasc. Surg. 116(5), 705–715 (1998)
C. Walker, “Guidewire selection for peripheral vascular interventions,” Endovasc. Today 5, 80–83 (2013)
T. Anson, Shape memory alloys - medical applications. Mater. World 212, 745–747 (2010)
A. Shah, C. Lau, S.W. Stavropoulos, A. Nemeth, M.C. Soulen, J.A. Solomon, J.I. Mondschein, A.A. Patel, R.D. Shlansky-goldberg, M. Itkin, J.L. Chittams, S.O. Trerotola, Comparison of physician-rated performance characteristics of hydrophilic-coated guide wires. J. Vasc. Interv. Radiol. 19, 400–405 (2008)
Y. Chun, D.S. Levi, K.P. Mohanchandra, F. Vinuela, F. Vinuela, G.P. Carman, Thin film nitinol microstent for aneurysm occlusion. J. Biomech. Eng. 131(5), 051014 (2009)
R. Uflacker, J. Robison, Endovascular treatment of abdominal aortic aneurysms : A review. Eur. Radiol. 11, 739–753 (2001)
H.A. Gary, A.W. Crane, J.A. Kaufman, S.C. Geller, D.C. Brewster, C. Fan, R.P. Cambria, G.M. Lamuraglia, J.P. Gertler, W.M. Abbott, A.C. Waltman, A.W. Crane, Endovascular repair of abdominal aortic aneurysms: Current status and future directions. Am. J. Roentgenol. 175, 289–302 (2000)
C.T. Dotter, Transluminally-placed coilspring endarterial tube grafts. Long-term patency in canine popliteal artery. Investig. Radiol. 4(5), 329–332 (1969)
J.C. Palmaz, Intravascular stenting: From basic research to clinical application. Cardiovasc. Intervent. Radiol. 15(5), 279–284 (1992)
R.A. Schatz, J.C. Palmaz, F.O. Tio, F. Garcia, O. Garcia, S.R. Reuter, Balloon-expandable intracoronary stents in the adult dog. Circulation 76(2), 450–457 (1987)
A. Buchwald, C. Unterberg, G. Werner, H. Kreuzer, V. Wiegand, E. Voth, Initial clinical results with the Wiktor stent: A new balloon-expandable coronary stent. Clin. Cardiol. 14(5), 374–380 (1991)
W. Kurre, F. Brassel, R. Brüning, J. Buhk, B. Eckert, S. Horner, M. Knauth, T. Liebig, J. Maskova, D. Mucha, V. Sychra, M. Sitzer, M. Sonnberger, M. Tietke, J. Trenkler, B. Turowski, J. Berkefeld, Complication rates using balloon-expandable and self-expanding stents for the treatment of intracranial atherosclerotic stenoses. Neuroradiology 54(1), 43–50 (2012)
R. Beyar, R. Shofti, E. Grenedier, M. Henry, O. Globerman, M. Beyar, Self-expandable nitinol stent for cardiovascular applications: Canine and human experience. Catheter. Cardiovasc. Diagn. 32(2), 162–170 (1994)
R.K. Greenberg, K. West, K. Pfaff, J. Foster, D. Skender, S. Haulon, J. Sereika, L. Geiger, S.P. Lyden, D. Clair, L. Svensson, B. Lytle, Beyond the aortic bifurcation: Branched endovascular grafts for thoracoabdominal and aortoiliac aneurysms. J. Vasc. Surg. 45(5), 879–886 (2006)
M. Grabenwöger, D. Hutschala, M.P. Ehrlich, F. Cartes-Zumelzu, S. Thurnher, J. Lammer, E. Wolner, M. Havel, Thoracic aortic aneurysms: Treatment with endovascular self-expandable stent grafts. Ann. Thorac. Surg. 69(2), 441–445 (2000)
T. Duerig, A. Pelton, D. Sto, An overview of nitinol medical applications. Mater. Sci. Eng. A 275, 149–160 (1999)
A. Zajarias, A.G. Cribier, Outcomes and safety of percutaneous aortic valve replacement. J. Am. Coll. Cardiol. 53(20), 1829–1836 (2009)
J. Baan, Z.Y. Yong, K.T. Koch, J.P. Henriques, B.J. Bouma, S.G. de Hert, J. van der Meulen, J.G. Tijssen, J.J. Piek, B.A. de Mol, Percutaneous implantation of the CoreValve aortic valve prosthesis in patients at high risk or rejected for surgical valve replacement: Clinical evaluation and feasibility of the procedure in the first 30 patients in the AMC-UvA. Neth. Heart J. 18(1), 18–24 (2010)
E. Grube, J.C. Laborde, B. Zickmann, U. Gerckens, T. Felderhoff, B. Sauren, A. Bootsveld, L. Buellesfeld, S. Iversen, First report on a human percutaneous transluminal implantation of a self-expanding valve prothesis for interventional treatment of aortic valve stenosis. Catheter. Cardiovasc. Interv. 66(4), 465–469 (2005)
Y. Boudjemline, P. Bonhoeffer, Steps toward percutaneous aortic valve replacement. Circulation 105(6), 775–778 (2002)
H.R. Andersen, L.L. Knudsen, J.M. Hasenkam, Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur. Heart J. 13(5), 704–708 (1992)
M.B. Leon, S. Kodali, M. Williams, M. Oz, C. Smith, A. Stewart, A. Schwartz, M. Collins, J.W. Moses, Transcatheter aortic valve replacement in patients with critical aortic stenosis: Rationale, device descriptions, early clinical experiences, and perspectives. Semin. Thorac. Cardiovasc. Surg. 18(2), 165–174 (2006)
T.D. King, S.L. Thompson, C. Steiner, N.L. Mills, Secundum atrial septal defect: Nonoperative closure during cardiac catheterization. JAMA J. Am. Med. Assoc. 235(23), 2506–2509 (1976)
G.C. Emmanoulides, H.D. Allen, R. T, Heart Disease in Infants, Children and Adolescents, Including the Fetus and Young Adults (Williams and Wilkins, Baltimore, 1995)
J.G. Murphy, B.J. Gersh, M.D. McGoon, D.D. Mair, C.J. Porter, D.M. Ilstrup, D.C. McGoon, F.J. Puga, J.W. Kirklin, G.K. Danielson, Long-term outcome after surgical repair of isolated atrial septal defect. N. Engl. J. Med. 323(24), 1645–1650 (1990)
B.D. Thanopoulos, C.V. Laskari, G.S. Tsaousis, A. Zarayelyan, A. Vekiou, G.S. Papadopoulos, Closure of atrial septal defects with the Amplatzer occlusion device: Preliminary results. J. Am. Coll. Cardiol. 31(5), 1110–1116 (1998)
M.J.A. Sharafuddin, X. Gu, J.L. Titus, M. Urness, J.J. Cervera-Ceballos, K. Amplatz, Transvenous closure of Secundum atrial septal defects: Preliminary results with a new self-expanding nitinol prosthesis in a swine model. Circulation 95(8), 2162–2168 (1997)
M. Simon, R. Kaplow, E. Salzman, D. Freiman, A vena cava filter using thermal shape memory alloy experimental aspects. Radiology 125(1), 89–94 (1977)
E. Bruckheimer, A.G. Judelman, S.D. Bruckheimer, I. Tavori, G. Naor, B.T. Katzen, In vitro evaluation of a retrievable low-profile nitinol vena cava filter. J. Vasc. Interv. Radiol. 14(4), 469–474 (2003)
P.A. Poletti, C.D. Becker, L. Prina, P. Ruijs, H. Bounameaux, D. Didier, P.A. Schneider, F. Terrier, Long-term results of the Simon nitinol inferior vena cava filter. Eur. Radiol. 8(2), 289–294 (1998)
E. Engmann, M.R. Asch, Clinical experience with the antecubital Simon nitinol IVC filter. J. Vasc. Interv. Radiol. 9(5), 774–778 (1998)
B. FThomas, M. Kinney, C. Steven, M. Rose, E. Karl, M. Weingarten, M. Karim Valji, B. Steven, M. Oglevie, C. Anne, M. Roberts, IVC Filter Tilt and Asymmetry: Comparison of the over-the-Wire Stainless-Steel and Titanium. J. Vasc. Interv. Radiol. 8, 1029–1037 (1997)
Z. Jing, H. Mao, W. Dai, Endovascular Surgery and Devices (Springer Singapore, Singapore, 2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Elsisy, M., Chun, Y. (2021). Materials Properties and Manufacturing Processes of Nitinol Endovascular Devices. In: Bártolo, P.J., Bidanda, B. (eds) Bio-Materials and Prototyping Applications in Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-35876-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-35876-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35875-4
Online ISBN: 978-3-030-35876-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)