Abstract
In 1972, there were some cases of poor laboratory practice in the United States. Therefore, the FDA decided to do some in-depth researches of 40 toxicology laboratories and increase the number of laws for chemical and pharmaceutical products [1]. They discovered a lot of fraudulent activities and poor laboratory practices such as uncalibrated equipment, wrong measurements, inaccurate data, and inadequate test systems. Accordingly, by setting GLP guidelines, final data can demonstrate a true reflection of results gained during the study [2]. One of the important goals of GLP is promoting safety, quality, consistency, and reliability of products, data, and services in the process of laboratory testing, to improve human health and environmental risk management. Moreover, it is important to know how scientists use quality setting to improve biological products and data. Finally, research authorities should prove that there are no changes in the quality of data [1, 3].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 What You Will Learn in This Chapter
-
The importance of understanding necessary information about good laboratory practice (GLP) as well as implementing relevant standard methods
-
The importance of maintaining biosafety in the laboratory and applying correct assessment of the potential risks in laboratory
-
The necessity of providing safety of individuals, environment, and chemicals in nonclinical laboratories
-
The necessity of conducting GLP guidelines under carefully controlled situations and also traceable, reproducible, and reliable data set
-
The importance of implementing standardized and validated procedures to support human clinical trials
-
The necessity of setting GLP guidelines by different regulatory agencies to consider organizational structure, documentation (record keeping), quality assurance, personnel training practices, responsibilities, good microbiological techniques (GMT), safety equipment, standard operating procedures (SOPs), records, and sample retention
3.2 Rationale and Importance
In 1972, there were some cases of poor laboratory practice in the United States. Therefore, the FDA decided to do some in-depth researches of 40 toxicology laboratories and increase the number of laws for chemical and pharmaceutical products [1]. They discovered a lot of fraudulent activities and poor laboratory practices such as uncalibrated equipment, wrong measurements, inaccurate data, and inadequate test systems. Accordingly, by setting GLP guidelines, final data can demonstrate a true reflection of results gained during the study [2]. One of the important goals of GLP is promoting safety, quality, consistency, and reliability of products, data, and services in the process of laboratory testing, to improve human health and environmental risk management. Moreover, it is important to know how scientists use quality setting to improve biological products and data. Finally, research authorities should prove that there are no changes in the quality of data [1, 3].
3.3 Good Laboratory Practice (GLP)
3.3.1 An Overview of General Rules
GLP is a set of techniques that provide safety of personnel, laboratory, and environment and also pave the way for better laboratory practices by eliminating poor practices. Safety assessment based on GLP guidelines is a key step before starting clinical trials [4]. Therefore, GLP guidelines have conducted to guarantee biosafety in laboratories [5, 6]. Accordingly, this chapter will describe biosafety aspects of GLP focusing on risk management approaches that should consider risk groups [7]. In accordance with risk groups, each country should design a national classification of microorganisms based on their possibility of increasing harm in humans, animals, and environments according to the following issues [6]:
-
Pathogenicity of the microorganism
-
Transmission modes and host range of the organism
-
Access to effective prevention plans which usually include prophylaxis by immunization, antisera administration, and sanitary measures
-
Access to effective treatment measures including antimicrobials, antivirals, and chemotherapeutic agents, passive immunization, and post-exposure vaccination [6, 8]
There are four risk groups based on the classification of World Health Organization (WHO). Infectious microorganisms are categorized in risk groups based on their relative risks. Risk group 1 includes organisms which do not cause disease in humans or animals, risk group 2 pathogens cause diseases such as infections in humans or animals, risk group 3 agents cause serious diseases, and finally risk group 4 organisms cause lethal diseases in humans [9]. The classification of risk groups is used for laboratory work only. In contrast to risk groups, biosafety levels prescribe practices, facility requirements, engineering controls, and personal protective equipment (PPE) for working with infectious microorganisms. Similar to risk groups, there are four biosafety levels which are level 1, 2, 3, and 4 (◘ Table 3.1) [4, 6].
3.3.2 Good Laboratory Practice Training
Trained and well-organized personnel play a key role in the successful performance of procedures in a laboratory. On the other hand, it is necessary to have systems that assess persistent competency and trained personnel to convince suitable responsibility and communication during study conduct. Therefore, all personnel should receive direct and accurate training information to complete and perform their tasks [10]. After initial staff training, qualification assessments should be performed and recorded for all components of the training and practical responsibilities. As a result, the clinical education program should meet all the personnel needs and be documented and available to all laboratory staff [10, 11].
3.4 Biosafety Concepts in the Laboratory
Laboratory biosafety refers to protective measures, containment principles, and relevant technologies to prevent accidental exposure to pathogens and toxins. The association between four biosafety levels and infectious microorganisms based on their potential risks are demonstrated in ◘ Fig. 3.1. Effective implementation of biosafety in a laboratory is the base of laboratory biosecurity [7]. Accordingly, laboratory biosecurity refers to personal and organizational security measures that prevent misuse, loss, diversion, or deliberate release of pathogens and toxins [12]. On the other hand, risk assessment is an essential part of a biosafety program, which collects information about the type of available organisms, their physical location, and also identification of staff who are responsible for the maintenance of organisms [13]. Moreover, professional and ethical competency of all employees faced with dangerous pathogens that are permanently accessed to sensitive materials has a central role in laboratory biosafety programs. As a result, assessing competency of employees, training specific security issues, and complying accurate protection approach against pathogens are rational tools for promoting biosecurity in the laboratories [12]. Therefore, it is necessary to determine the biosafety level, type of microorganisms, available facilities, arrangements, skills, and procedures for performing a safe performance in laboratories [4, 14].
Biosafety levels (BSL). Microorganisms classified by four risk groups and accordingly biosafety levels are divided into four classes. Biosafety level 1 refers to low-risk microorganisms such as Nonpathogenic strains of Escherichia coli and Lactobacillus, while biosafety level 4 refers to high-risk microorganisms such as Ebola and Marburg viruses. Additionally, biosafety levels 2 and 3 are located between 1 and 4 [7, 9]
3.5 Good Microbiological Techniques
The aim of good microbiological techniques (GMT) is improving public health and also lifestyle. Each laboratory must take safety measures to eliminate or reduce potential hazards. GMT plays a pivotal role in laboratory safety and is based on the prevention of contamination [15].
Because of improper collecting, transferring, and handling of samples in laboratory, humans will be prone to risk of infections [16]. To prevent contamination in laboratories, some procedures should be considered as follows:
-
Transferring of Infectious Substances: Transportation of infectious substances and materials should be done in accordance with national and international standards and rules. These rules describe how to properly use packaging materials and other transportation requirements to reduce potential damages and possible infections. For example, using a triple packaging system is essential for transporting potentially infectious substances [17].
-
Specimen Receipt: A particular room for receiving a large number of specimens should be considered [18].
-
Opening Packages: For opening packages, a disinfectant must be available and opened in biological safety cabinets (BSCs) [18].
-
Avoiding Ingestion of Infectious Agents and Contact with Skin and Eyes: Personnel should wear disposable gloves during microbiological manipulations and avoid touching mouth and eyes; moreover, they should not eat and drink in the laboratory [16].
-
Disinfection and Sterilization: Basic knowledge of disinfection and sterilization is essential for laboratory safety. As highly contaminated materials cannot be disinfected or sterilized quickly, it is important to know the principles of initial cleansing as disinfecting. In this regard, general rules apply to all known pathogens. Accordingly, specific conditions should be used to eliminate contamination depending on the type of testing, nature, and source of contaminations. As a result, preclearing materials are essential to provide suitable disinfection and sterilization. Some types of chemical germicides are used as disinfectant (◘ Table 3.2) [7, 17].
-
Waste Disposal: Contaminated materials should be removed from laboratory daily. Moreover, most instruments, laboratory clothes, and glassware should be reused or recycled. It is important that all infectious materials are decontaminated, incinerated, and autoclaved [19].
3.6 Laboratory Equipment
3.6.1 General Equipment
General laboratory equipment mainly include pipettes, centrifuges, freezers, incubators, hot plates, coolers, stirrers, water baths, bunsen burners, scales, fume hoods, and also microscopes [18, 20].
3.6.2 Safety Equipment
As aerosols are critical sources of contamination, their dispersion should be reduced. Harmful aerosols can be released by the most of laboratory activities such as mixing, blending, sonicating, grinding, shaking, and centrifuging [21]. Therefore, to achieve contamination control and personnel and product safety, it is seriously recommended that all procedures be performed in BSCs [22]. As a result, not only implementing safety equipment in laboratory is important but also laboratory operator should be trained to ensure safety of equipment regularly. Moreover, it is necessary to implement a system for monitoring equipment calibration [21].
3.6.2.1 Biological Safety Cabinets (BSCs)
BSCs are designed to protect personnel, laboratory environments, and materials against contamination by pathogens. BSCs are classified into three groups based on biosafety levels (◘ Table 3.3) [7, 22].
3.6.2.2 Pipetting Aids, Homogenizers, Sonicators, and Specimen Containers
-
Pipettes and pipetting aids [7]:
-
Pipetting by mouth is prohibited. Therefore, personnel must always use a pipetting aid.
-
All pipettes should have cotton caps to reduce contamination.
-
Never blow air into a fluid containing infectious agents, and do not mix infectious agents by blowing and suction.
-
Liquids should not be removed by force from the pipette [18, 20].
-
-
Homogenizers and sonicators can reduce small pathogens from liquids and should be used in BSCs or be covered with shields during working [7].
-
Specimen containers should be made by high resistance glass, metal, or plastic and should not leak when the cap is in place. No material should remain on the outer surface of the container. It is better to label the dishes properly before filling for easy identification and avoiding hazards [7, 18]. Moreover, specimen containers should be placed on secondary containers such as boxes and racks to prevent their spillage and leakage. Secondary metal or plastic containers should be autoclavable [18].
3.6.2.3 Personal Protective Equipment and Clothing
PPE are important to reduce high risks of contact with aerosols, accidental inoculations, and splashes. Some of the PPEs include the following [23, 24]:
-
Laboratory coats and gowns: Laboratory coats protect personnel from workplace hazards such as chemical splashes, chemicals spills, or biological materials such as blood and tissue specimens. Therefore, long-sleeved and fully buttoned coats are better choices. On the other hand, personnel should not wear laboratory coats and gowns when they are outside the laboratory [23, 24].
-
Gloves: Latex, vinyl, or nitrile gloves are used to protect against infectious agents, blood, and body fluids [23].
-
Face protection devices: Eyes and face protection devices including safety spectacles, safety goggles, face shield, face respirators, and masks protect personnel from hazards that may cause serious injury on the face and eye. Safety goggles and face shield are the best choices to protect staff from chemical splashes, while safety spectacles are not suitable. Moreover, masks with filters are used for protecting against gases, toxic vapors, aerosols, and microorganisms [24].
3.7 Safety Against Fire, Electrocution, and Chemicals
Personnel in microbiological laboratories are exposed to hazardous chemicals as well as pathogenic microorganisms [25]. Therefore, they should be aware of the toxic effects of chemicals, exposure pathways, and potential hazards. Personnel may be exposed to dangerous chemicals through skin contact, needle sticks, ingestion, and inhalation [25]. On the other hand, personnel may face a variety of hazards such as fire, electricity, radiation, and noise. A summary of these hazards and relevant preventive actions is presented in ◘ Table 3.4 [26].
3.8 Biosafety Instruction
Risk assessment process is the basis of biosafety [7]. Risk assessment steps should be carried out by individuals who are well trained and those with the good knowledge of organisms, tools, methods, animal models, and equipment [13]. Accordingly, laboratory director or researcher is responsible for providing risk assessment equipment and facilities in collaboration with safety and staff who are responsible for laboratory biosafety. Risk assessment measures should be reviewed regularly and revised as necessary. Revisions will be provided using scientific literatures and other relevant information sources [16].
3.8.1 Assessment of Microbiological Hazards
To identify microbiological hazards, microbiological risk assessment must be performed according to hazard identification, exposure assessment, hazard characterization, and risk characterization [27].
-
Hazard identification: Hazard identification identifies chemical, biological, and physical agents which can cause an adverse effect on health which is related to the presence of a pathogen in food [27].
-
Exposure assessment: Exposure assessment provides a qualitative or quantitative estimation of the intake of a microbiological hazard in a specific food or a range of foods [27].
-
Hazard characterization: The main point in hazard characterization is the association between the amount of exposure to a chemical, biological, or physical agent and the adverse impact on health [13, 27].
-
Risk characterization: Risk characterization is a combination of hazard identification, exposure assessment, and hazard characterization [27].
3.8.2 The Importance of Proper Documentation
Documentation plays a critical role in the quality system of laboratory which relies on the recording of vital information. Documents should be stored in laboratory and must be available to all laboratory staff. Policies and programs should be followed by biosafety officers. Therefore, every aspect of a new device or a new drug should be recorded, examined, revised, updated, submitted, and archived. In addition, the competency of all staff should be assessed, monitored, and recorded based on qualification and training programs and in relation with the responsibilities [28].
3.8.3 Standard Operating Procedures (SOPs)
SOP is a document which develops based on GLP program. Therefore, SOP content should comply with GLP rules [16]. According to the information obtained from risk assessment processes, biosafety levels, and testing facilities, SOPs are developed to provide the highest level of quality and safety during work [29].
3.8.4 Guidelines for Basic Laboratories: Biosafety Levels 1 and 2
WHO has recognized that biosafety in laboratory is one of the most important international issues. In 1983, WHO published the first edition of the laboratory biosafety manual. Then, codes of practice for safe exposure to microorganisms were developed, and countries were encouraged to enforce these regulations. Since 1983, many countries have applied specific recommendations to develop their codes of practice. Guidelines for basic laboratories (biosafety levels 1 and 2) can be generalized to implement in laboratories with any biological level (► Boxes 3.1 and 3.2) [7]. Also, guidelines for containment laboratories (biosafety level 3) and the maximum containment laboratories (biosafety level 4) are developed on the basis of guidelines for basic laboratories [30].
3.8.5 Guidelines for Containment Laboratories: Biosafety Level 3
Containment laboratories – biosafety level 3 – are designed to use for procedures involved in group 3 microorganisms or group 2 with a high concentration [7]. This type of laboratories requires more restricted guidelines and rules than basic ones (► Box 3.3 [9] and Box 3.4 [31]).
3.8.6 Guidelines for Maximum Containment Laboratories: Biosafety Level 4
Maximum containment laboratories are designed as a workplace which is involved with group 4 microorganisms. Controlling of these laboratories should be supervised by national health authorities [32] (► Boxes 3.5 and 3.6 [7, 32]).
3.8.7 Guidelines on Laboratory Animal Facilities
Personnel who use animals for laboratory practices and diagnostic purposes should be morally committed to take care of them and avoid any unnecessary harm. Accordingly, adequate food and water, as well as a hygienic and comfortable place, should be provided for animals. For security reasons, animal house should be a unit completely independent of the laboratory. If connected to the laboratory, it should be completely separated from the public parts of the laboratory with perfect disinfectant procedures [33]. Animal laboratory equipment can be designated based on a risk assessment protocol, risk group microorganisms, and biosafety levels 1, 2, 3, and 4. A summary of methods and safety equipment used according to animal facility biosafety level (ABSL) can be seen in ◘ Table 3.5 [7].
3.8.8 Guidelines for Laboratory Commissioning
The aim of the laboratory commissioning is defining a regular process of monitoring and also collection and verification of documents to ensure that all of the structural components of a GLP-based laboratory are installed, inspected, tested, and approved correctly in accordance with the international or national standards. On the other hand, laboratories are dynamic and complex environments that can be adapted to healthcare needs [28]. All biomedical laboratories should be certified to ensure that they are on the correct way focusing on the following considerations [7]:
-
Engineering controls are carried out properly and consistently as they are designed.
-
Administrative controls are in accordance with the established protocols and are performed in a suitable site.
-
PPE is provided based on established criteria to be suitable for the tasks.
-
Materials and wastes are completely decontaminated. Proper waste management practices are carried out quickly.
-
For the general safety of the laboratory, including chemical, electrical, and physical safety, there are well-defined procedures which are conducted properly.
3.9 Safety Checklist
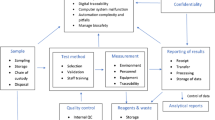
A proper safety checklist helps director to assess the safety and security of the laboratory. Some important points which should be considered in safety checklist of GLP are indicated in ◘ Fig. 3.2.
Safety checklist. Important points which should be considered in the safety checklist of GLP [7]. To ensure proper establishment, there should be answer to questions about laboratory premises; staff facilities and services; safety against fire, chemicals, and electrical hazards and also suitable heating, lighting, and ventilation system; and more importantly laboratory biosafety and biosecurity
3.10 Challenges and Future Perspectives
In recent years, following the advancement in technology and industrialization, biomedical sciences has being dramatically grown. Therefore, to avoid several challenges, appropriate codes of practice and protocols are needed. GLP standards are developed to direct laboratories in a right manner. Accordingly, GLP has been popularized throughout the world. Few people can estimate contemporary challenges about GLP regulatory which is faced today [34]. The aim of GLP is not only decreasing the adverse events of biological products but also improving human health and safety of environment [3]. GLP is also a protocol for nonclinical laboratory researches which can help scientists to perform biomedical researches perfectly. There are several challenges such as issues surrounding record keeping. For example, their reliability and trustworthy especially due to limited access to electronic systems and internet are serious limitations. To overcome these challenges, environmental protection agency (EPA) implements standard requirements that are equipped to receive information electronically for data submission [34]. Other challenges include very old information which is not in accordance with GLP guidelines, heavy costs and regulatory burdens, insufficient staff, and inadequate technical training. Accordingly, there are some procedures that can enhance the quality of the research such as monitoring GLP compliance through regulatory inspections, optimization, and electronic records including signatures, electronic system, internet, etc. [35]. The most important procedure is optimization [36]. It is predictable that other novel methods based on using burgeoning technologies including nanotechnology, biocides, computer modeling, electronic data, and record keeping using the fastest supercomputer with the ability to perform 1000 trillion calculations per second will be developed in the near future [34, 36].
Take-Home Messages
-
In 1983, the WHO has developed codes of practice for safe exposure to microorganisms and encouraged countries to enforce these regulations.
-
Biosafety is a set of rules that are used to handle the hazards of living organisms and also isolate them in an enclosed laboratory. The base of biosafety is the risk assessment process.
-
Risk assessment should be carried out by individuals who are well trained and those with the good knowledge of organisms, tools, methods, animal models, and equipment.
-
GPL is a set of techniques that provide safety and quality in the laboratory and environment and also safety of personnel.
-
GLP can be used as a practical standard to direct nonclinical laboratory researches.
-
Microorganisms are classified into four risk groups based on their possibility of harm in humans, animals, and environments.
-
Laboratory facilities are designed and classified based on biosafety levels 1, 2, 3, and 4.
-
There are some procedures that can enhance the biosafety and quality control of the laboratories such as using electronic records, internet, optimization, etc.
References
Baldeshwiler AM. History of FDA good laboratory practices. Qual Assur J. 2003;7:157–61.
Sasaki M, Hinotsu S, Kawakami K. Good laboratory practice (GLP) status of Asian countries and its implementation in non-clinical safety studies in pharmaceutical drug development. J Toxicol Sci. 2009;34(5):493–500.
Jena G, Chavan S. Implementation of good laboratory practices (GLP) in basic scientific research: translating the concept beyond regulatory compliance. Regul Toxicol Pharmacol. 2017;89:20–5.
Bolon B, Baze W, Shilling CJ, Keatley KL, Patrick DJ, Schafer KA. Good laboratory practice in the academic setting: fundamental principles for nonclinical safety assessment and GLP-compliant pathology support when developing innovative biomedical products. ILAR J. 2018;59:18–28.
Wang Q, Zhou WM, Zhang Y, Wang HY, Du HJ, Nie K, et al. Good laboratory practices guarantee biosafety in the Sierra Leone-China friendship biosafety laboratory. Infect Dis Poverty. 2016;5(1):62.
Armstrong E. Principles of health and safety and good laboratory practice. In: Remesh V, editor. Biomolecular and bioanalytical techniques: theory, methodology and applications. Hoboken: Wiley; 2019. p. 1–15.
World Health Organization. Laboratory biosafety manual. Geneva: World Health Organization; 2004.
De Vos FJ, De Decker M, Dierckx RA. The good laboratory practice and good clinical practice requirements for the production of radiopharmaceuticals in clinical research. Nucl Med Commun. 2005;26(7):575–9.
Zaki AN. Biosafety and biosecurity measures: management of biosafety level 3 facilities. Int J Antimicrob Agents. 2010;36:S70–S4.
Ezzelle J, Rodriguez-Chavez IR, Darden JM, Stirewalt M, Kunwar N, Hitchcock R, et al. Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J Pharm Biomed Anal. 2008;46(1):18–29.
Brunetti MM. Critical aspects in the application of the principles of good laboratory practice (GLP). Ann Ist Super Sanita. 2002;38(1):41–5.
Salerno RM, Gaudioso J, Brodsky BH. Laboratory biosecurity handbook. Boca Raton: CRC Press; 2007.
Becker RA, Janus ER, White RD, Kruszewski FH, Brackett RE. Good laboratory practices and safety assessments. Environ Health Perspect. 2009;117(11):A482–A3.
Haeckel R. The meaning of good laboratory practice (GLP) for the medical laboratory. Clin Chem Lab Med. 1999;37(2):169.
Gouveia BG, Rijo P, Goncalo TS, Reis CP. Good manufacturing practices for medicinal products for human use. J Pharm Bioallied Sci. 2015;7(2):87–96.
Worl Health Organization. Handbook: good laboratory practice (GLP): quality practices for regulated non-clinical research and development. Geneva: World Health Organization; 2010.
Stevens W. Good clinical laboratory practice (GCLP): the need for a hybrid of good laboratory practice and good clinical practice guidelines/standards for medical testing laboratories conducting clinical trials in developing countries. Qual Assur. 2003;10(2):83–9.
Washington JA. Laboratory procedures in clinical microbiology: Springer Science & Business Media. 2nd edition, New York. 1985.
Padmanabhan K, Barik D. Health hazards of medical waste and its disposal. In: Energy from toxic organic waste for heat and power generation. Elsevier. Kidlington, United Kingdom 2019. p. 99–118.
McGinnis MR. Laboratory handbook of medical mycology. Saint Louis: Elsevier; 2012.
Todd CA, Sanchez AM, Garcia A, Denny TN, Sarzotti-Kelsoe M. Implementation of good clinical laboratory practice (GCLP) guidelines within the external quality assurance program oversight laboratory (EQAPOL). J Immunol Methods. 2014;409:91–8.
Whistler T, Kaewpan A, Blacksell SD. A biological safety cabinet certification program: experiences in Southeast Asia. Appl Biosaf. 2016;21(3):121–7.
Tomas ME, Kundrapu S, Thota P, Sunkesula VC, Cadnum JL, Mana TSC, et al. Contamination of health care personnel during removal of personal protective equipment. JAMA Intern Med. 2015;175(12):1904–10.
Verbeek JH, Ijaz S, Mischke C, Ruotsalainen JH, Mäkelä E, Neuvonen K, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2016;4:CD011621.
National Research Council. Prudent practices in the laboratory: handling and management of chemical hazards, updated version. Washington, DC: National Academies Press; 2011.
Asiry S, Ang L-C. Laboratory safety: chemical and physical hazards. Methods Mol Biol. 2019;1897:243–52.
Hoornstra E, Notermans S. Quantitative microbiological risk assessment. Int J Food Microbiol. 2001;66(1–2):21–9.
Gumba H, Waichungo J, Lowe B, Mwanzu A, et al. Implementing a quality management system using good clinical laboratory practice guidelines at KEMRI-CMR to support medical research. Wellcome Open Res. 2019;3:137.
Tolba RH, Riederer BM, Weiskirchen R. Standard operating procedures in experimental liver research: time to achieve uniformity. Lab Anim. 2015;49(1 Suppl):1–3.
Burnett LC, Lunn G, Coico R. Biosafety: guidelines for working with pathogenic and infectious microorganisms. Curr Protoc Microbiol. 2009;13(1):1A.1.1–1A.1.14.
Day D, Xiang J, Mo J, Clyde M, Weschler C, Li F, et al. Combined use of an electrostatic precipitator and a high-efficiency particulate air filter in building ventilation systems: effects on cardiorespiratory health indicators in healthy adults. Indoor Air. 2018;28(3):360–72.
Xia H, Huang Y, Ma H, Liu B, Xie W, Song D, et al. Biosafety level 4 laboratory user training program, China. Emerg Infect Dis. 2019;25(5):e1–4.
Feary J, Fitzgerald B, Schofield S, Potts J, Canizales J, Jones M, et al. Evidence based code of best practice for animal research facilities: results of the SPIRAL study. In: 28th international congress of the European-Respiratory-Society (ERS); 2018.
Liem FE, Lehr MJ. Future issues including broadening the scope of the GLP principles. Ann Ist Super Sanita. 2008;44(4):335–40.
Adamo JE, Bauer G, Berro MM, Burnett BK, Hartman MKA, Masiello LM, et al. A roadmap for academic health centers to establish good laboratory practice-compliant infrastructure. Acad Med. 2012;87(3):279.
Kendall G, Bai R, Błazewicz J, De Causmaecker P, Gendreau M, John R, et al. Good laboratory practice for optimization research. J Oper Res Soc. 2016;67(4):676–89.
Further Reading
Books
Armstrong E. Principles of health and safety and good laboratory practice. In: Remesh V, editor. Biomolecular and bioanalytical techniques: theory, methodology and applications. Wiley: Hoboken; 2019. p. 1–15.
World Health Organization. Laboratory biosafety manual. Geneva: World Health Organization; 2004.
World Health Organization. Handbook: good laboratory practice (GLP): quality practices for regulated non-clinical research and development. Geneva: World Health Organization; 2010.
Online Resources
Bolon B, Baze W, Shilling CJ, Keatley KL, Patrick DJ, Schafer KA. Good laboratory practice in the academic setting: fundamental principles for nonclinical safety assessment and GLP-compliant pathology support when developing innovative biomedical products. ILAR J. 2018;59(1):18–28. https://www.ncbi.nlm.nih.gov/pubmed/30566589.
Jena GB, Chavan S. Implementation of good laboratory practices (GLP) in basic scientific research: translating the concept beyond regulatory compliance. Regul Toxicol Pharmacol. 2017;89:20–5. https://www.ncbi.nlm.nih.gov/pubmed/28713068.
Liem FE, Lehr MJ. Future issues including broadening the scope of the GLP principles. Ann Ist Super Sanita. 2008;44(4):335–40. https://www.ncbi.nlm.nih.gov/pubmed/19351991.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sheikh-Hosseini, M. et al. (2020). Principles of Good Laboratory Practice (GLP). In: Arjmand, B., Payab, M., Goodarzi, P. (eds) Biomedical Product Development: Bench to Bedside. Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-35626-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-35626-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35625-5
Online ISBN: 978-3-030-35626-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)