Abstract
It is established that schizophrenia represents an important public health challenge as it contributes to significant psychosocial disability worldwide (Whiteford et al., Lancet 382(9904):1575–86, 2013). Although its fundamental pathobiology remains elusive, neuroimaging studies provide compelling evidence that schizophrenia is associated with alterations in brain gray matter morphometry, which may be one mechanism that underpins the emergence and maintenance of the clinical symptoms of this disorder. This chapter reviews current evidence on the nature and extent of cross-sectional and longitudinal gray matter changes in patients with syndromal schizophrenia. We then identify the most consistent links between such alterations with psychopathology and highlight key potential pathogenetic and moderating mechanisms. The relevant neuroimaging literature is substantial and substantive. We focus on evidence from large-scale, multisite, magnetic resonance imaging (MRI) studies and meta-analyses as they have the advantage of greater power and precision in identifying and quantifying schizophrenia-related alterations in gray matter morphometry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Schizophrenia-Related Gray Matter Alterations
A number of analytical approaches have been used to assess case-control differences in structural MRI studies (see Chap. 1 for more details). Univariate analyses of group differences in neuroimaging typically employ either a region-of-interest (ROI) approach or voxel-based morphometry (VBM). In the former, case-control differences are estimated at the level of anatomical regions defined using brain atlases while the latter involves a voxel-wise comparison of brain tissue concentration or density between two groups. In meta-analyses of ROI-based studies, the pooled effect size of case-control differences is commonly expressed either as Cohen’s d (Cohen 1977) or Hedges’ g (Hedges 1981). Meta-analyses of VBM studies examine whether the brain-coordinates of case-control differences derived from the primary literature are statistically likely to cluster within specific brain regions. The Anatomical Likelihood Estimation (ALE) (Eickhoff et al. 2012) and Signed Differential Mapping (SDM) (Radua and Mataix-Cols 2009) are the most widely used algorithms for VBM-based meta-analyses. Univariate analyses, although informative, ignore potential covariation among brain regions or voxels. By contrast multivariate analyses are concerned with the identification of case-control differences in the spatial patterns of brain structural covariation. Several multivariate methods have been employed and these mainly include source based morphometry and machine learning algorithms. Source-based morphometry (SBM) (Xu et al. 2009) extracts spatially independent components (i.e., clusters) from MRI-derived voxelwise measures of gray matter concentration or volume. Machine learning approaches examine the predictive value of brain phenotypes in discriminating patients from healthy individuals and commonly include a recursive feature elimination framework (Guyon et al. 2002; Saeys et al. 2007). Multiple machine learning algorithms are available with support vector machines (SVM), discriminant and sparse canonical analyses, multiple kernel learning, deep learning and multiview learning being the most popular choices (Arbabshirani et al. 2017; Veronese et al. 2013).
1.1 Cross-Sectional Studies
1.1.1 Univariate Analyses in Patient Samples Unselected for Illness Duration
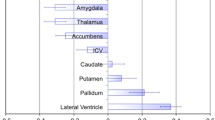
The consortium for Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) (http://enigma.ini.usc.edu/) is the largest international collaborative initiative in brain imaging. ENIGMA is organized in disorder-specific working groups which aim to quantify case-control differences in brain phenotypes extracted from MRI data using harmonized analysis and quality assurance protocols across all participating sites (Thompson et al. 2014, 2017). The ENIGMA schizophrenia working-group has leveraged the data within the consortium to create one of the largest datasets of gray matter ROIs in patients with schizophrenia and healthy individuals. Studies from the ENIGMA consortium (Chap. 21) have established that schizophrenia is associated with replicable but subtle case-control differences in the volume of subcortical structures (patients = 2028 and healthy individuals = 2540) (van Erp et al. 2016) (Fig. 2.1) and in regional cortical thickness and area (patients = 4474 and healthy individuals = 5089) (van Erp et al. 2018) (Fig. 2.2). Specifically, compared to healthy individuals, patients had smaller hippocampus (d = −0.46), amygdala (d = −0.31), thalamus (d = −0.31), accumbens (d = −0.25) and intracranial volumes (d = −0.12) and larger pallidum (d = 0.21) and lateral ventricles (d = 0.37) and global bilateral reductions in cortical thickness (left: d = −0.53; right: d = −0.51) and surface area (left: d = −0.25; right: d = −0.25) (Fig. 2.1). When controlling for these global effects, patients showed further regional changes in cortical thickness but not area. This accentuated regional cortical thinning was most pronounced in the fusiform gyrus, but was also present in multiple temporal regions (inferior, middle and superior temporal gyri and parahippocampal gyrus), in the ventrolateral prefrontal cortex (PFC; lateral orbitofrontal and inferior frontal gyrus), in the insula, and in the posterior anterior cingulate cortex (ACC) (Fig. 2.3). Notably, the motor (precentral gyrus), somatosensory (postcentral gyrus), and parietal (superior and inferior parietal cortex and paracentral lobule) cortices as well as the rostral ACC were significantly thicker in patients than in healthy individuals (Fig. 2.3). The findings of ENIGMA have been replicated in an independent Japanese sample of 884 patients with schizophrenia and 1680 healthy individuals from the consortium of the Cognitive Genetics Collaborative Research Organization (COCORO) (Okada et al. 2016) and are aligned with meta-analyses based on pooled effect size of case-control differences from 317 studies comprising over 18,000 patients (Haijma et al. 2013).
Subcortical volume differences between patients with schizophrenia and healthy individuals. Mean and standard error of the effect size (Cohen’s d) of regional case-control differences in subcortical volume (van Erp et al. 2016). The effect size for each subcortical volume was corrected for sex, age and intracranial volume (ICV). The effect size for ICV was corrected for sex and age. (From https://www.ncbi.nlm.nih.gov/pubmed/26283641)
Cortical map of differences in cortical thickness between patients with schizophrenia and healthy individuals. Cortical map of regional effect size (Cohen’s d) of case-control differences in regional cortical thickness controlling for age and gender (a) and age, sex, and global cortical thickness (b) (van Erp et al. 2018). (From https://www.ncbi.nlm.nih.gov/pubmed/29960671)
Regional differences in cortical thickness between patients with schizophrenia and healthy individuals. Mean and standard error of the effect size (Cohen’s d) of regional case-control differences in cortical thickness after controlling for age, sex, and global mean cortical thickness (van Erp et al. 2016). (From https://www.ncbi.nlm.nih.gov/pubmed/29960671)
Two meta-analyses, published a year apart used ALE to map gray matter changes in schizophrenia. Glahn et al. (2008) used data from 31 VBM studies, comprising 1195 patients with schizophrenia and 1262 healthy volunteers, while Fornito et al. (2009) used data from 37 VBM studies that collectively compared 1646 patients to 1690 healthy individuals. Not surprisingly the primary studies in these two meta-analyses showed significant overlap, which extended to the results reported. Schizophrenia-related reductions were more consistent for gray matter concentration than for volume although the findings largely converged (Fornito et al. 2009). The regions where gray matter density reductions in patients were most likely to be reported comprised the ACC, the parahippocampal, middle frontal and postcentral gyri, and the thalamus; conversely, patients had increased gray matter density in striatal regions (Fornito et al. 2009; Glahn et al. 2008). These findings were updated by Bora et al. (2011) who applied SDM to MRI data from 49 VBM studies comparing 1999 patients with schizophrenia and 2180 healthy individuals. Gray matter volume reductions were more likely to be located bilaterally in the ACC, the medial and inferior PFC, the superior temporal gyrus, the precentral gyrus and insula. Amongst the subcortical regions, schizophrenia-related reductions were also noted in the thalamus and the amygdala extending to the red nucleus in the midbrain.
1.1.2 Univariate Analyses in First-Episode Patients
Three separate meta-analyses have identified brain changes seen in patients with first-episode schizophrenia. Vita et al. (2006) and Steen et al. (2006) examined case-control differences in a very limited number of volumetric ROIs. The former study used data from 21 studies involving 551 first-episode patients with an approximate mean age of 25 years and 680 demographically matched healthy individuals. The latter extracted data contrasting 1424 patients with first-episode schizophrenia, with an approximate average age of 26 years, to 1315 matched healthy individuals. Although both studies found larger ventricles and smaller hippocampi, effect sizes were only provided by Vita et al. (ventricles: d = 0.39; hippocampi right d = −0.47 and left: d = −0.65). Radua et al. (2012) used SDM to analyse data from 25 VBM studies, contrasting 965 patients with first-episode psychosis, with an approximate mean age of 24 years, to 1040 matched healthy individuals. In patients, gray matter volume decrements clustered in the insula, operculum, the superior temporal gyrus and the medial frontal and anterior cingulate cortices while gray matter increased clustered in the precentral gyri.
1.1.3 Univariate Analyses in Early-Onset Schizophrenia
A relatively small number of studies primarily conducted by the National Institutes of Mental Health (NIMH) in the USA and centres in the Norway, Spain, and the UK have examined brain alterations in patients with childhood-onset (onset before the age of 13 years) or adolescent-onset (onset between the ages of 13–18 years) schizophrenia, henceforth jointly referred to as early onset psychosis (EOP). Although EOP patients represent less than 4% of schizophrenia cases arising from any birth cohort (Cannon et al. 1999), their study has the potential to provide information about the interaction between schizophrenia-related mechanisms and developmental processes that occur during adolescence (Giedd et al. 1999).
Studies in adolescent-onset schizophrenia have found reductions in total brain volume (Collinson et al. 2003; Matsumoto et al. 2001) and gray matter volume particularly within the frontal lobe (Moreno et al. 2005; Reig et al. 2011; Yoshihara et al. 2008) but no such differences in subcortical volumes (James et al. 1999; Juuhl-Langseth et al. 2012; Matsumoto et al. 2001).
Nearly all studies on childhood-onset schizophrenia derive from the NIMH cohort and collectively have found that patients, compared to age-matched healthy children, have lower total brain and thalamic volumes and larger caudate and globus pallidus (Frazier et al. 1996a, b; Jacobsen et al. 1996; Kumra et al. 2000).
1.1.4 Multivariate Pattern Analyses in Patients Unselected for Illness Duration
The study of Gupta et al. (2015) is of particular interest as they applied SBM to the largest MRI dataset to date comprising 936 healthy individuals and 784 patients with schizophrenia. Following age and sex regression, both decrements and increments in gray matter concentration were observed in schizophrenia. Decrements clustered within the superior, middle, medial and inferior frontal gyri, the superior and inferior temporal gyri, the fusiform gyrus, the insula, the cuneus and precuneus, and the cerebellar vermis and declive while increments were located in the brainstem (pons and ventral tegmental area).
Further, Kambeitz et al. (2015) conducted a meta-analysis of studies (n = 20) that applied machine learning to MRI data from patients with schizophrenia from healthy individuals. They found that, regardless of the specific algorithm, patients with schizophrenia could be distinguished from healthy individuals with a sensitivity of 76.4% and a specificity of 79.0%.
Similar findings were reported in two of the largest studies conducted to-date that applied machine learning algorithms to structural MRI data to discriminate healthy individuals from patients with first episode schizophrenia (n = 127) and from patients with schizophrenia (n = 387) at different stages of their illness (Rozycki et al. 2018; Squarcina et al. 2017). In both studies, regions within the medial prefrontal, temporolimbic and peri-Sylvian cortex, together with ventricular and pallidal enlargement, showed the higher discriminative power but the accuracy of diagnostic classification was modest and ranged from 72 to 77% (Rozycki et al. 2018).
1.2 Longitudinal Studies
Several studies have assessed gray matter morphometry in patients with a first episode psychosis or chronic schizophrenia at multiple time points after illness onset as well as examined the association of the longitudinal changes identified to clinical outcome. The challenge in this line of research is to disentangle brain alterations related to disease mechanism versus those that may be more closely associated with medication exposure and side-effects, substance use, and suboptimal lifestyle choices.
1.2.1 First-Episode Psychosis
Multiple research groups, mostly based in Australia, Europe, and North America have contributed to the literature on brain alterations following the first psychotic episode in adult (Andreasen et al. 2011; Cahn et al. 2002; DeLisi et al. 1997, 2004; Haukvik et al. 2016; Lieberman et al. 2001; Nakamura et al. 2007; Puri et al. 2001; Takahashi et al. 2009; Whitworth et al. 2005) and in EOP patients (Arango et al. 2012; Fraguas et al. 2012; Jacobsen et al. 1998; James et al. 2004; Keller et al. 2003; Reig et al. 2009; Sporn et al. 2003; Thompson et al. 2001). There is significant variability in the results reported, even between studies using cohorts with similar characteristics. Nevertheless, key meta-analyses of the literature have identified some consistent findings.
Fraguas et al. (2016) focused specifically on early onset psychosis. Their meta-analysis included longitudinal MRI studies (with a mean inter-scan of 2.46 years) that collectively involved 156 EOP patients (mean age at baseline 13.3–16.6 years) and 163 demographically matched healthy youth. They used a ROI approach to derive the effect size of case-control differences in longitudinal changes in total brain volume, total gray matter volume and in the gray matter volume of each cerebral lobe. Compared to healthy youth, EOP patients showed accelerated volume reduction in all the gray matter ROIs examined; the largest effect sizes were noted for the gray matter volume of the frontal (g = −0.43) and the occipital lobes (g = −0.79). These results resonate with the longitudinal changes in cortical thickness reported by Greenstein et al. (2006) in the NIMH cohort which only comprises youth with childhood-onset schizophrenia (n = 70) and healthy youth (n = 70) scanned repeatedly between the ages of 14 and 22 years. At younger ages, patients showed greater cortical thinning in anterior and posterior cortical regions. This pattern became more localized in the superior, middle, medial, and lateral orbital frontal gyri and in the superior and middle temporal gyri when the patients reached adulthood. Additionally during the follow-up period, the rate of cortical thinning in the superior parietal and postcentral gyri “normalized” while that in the superior and middle temporal gyri did not. Collectively, these findings have been interpreted as an exacerbation of the developmental changes taking place during adolescence (Thompson et al. 2001); in typically developing youth, there is a posterior-to-anterior wave of reduction in cortical volume and thickness which is assumed to reflect brain maturational processes in frontal and occipital regions that precede similar maturational changes in the lateral temporal cortex (Giedd et al. 1999; Gogtay et al. 2004).
Although EOP studies are informative, the typical onset of schizophrenia is in the third decade of life and therefore adult-onset datasets are more representative of the clinical population with schizophrenia. A meta-analysis by Vita et al. (2012) identified nine longitudinal studies of adults patients scanned within the first 24 months from onset that provided ROIs measures of gray matter of the whole-brain, the cerebral lobes and the auditory cortex on Heschl’s gyrus (HG). Compared to healthy individuals (n = 337), patients (n = 341) showed reduction of moderate effect size (range of d: −0.30 to −0.58) in all the gray matter measures examined with the exception of the HG where the effect size of case-control differences was large (right: g = −1.13; left: g = −1.33).
We also highlight the contribution of the Iowa Longitudinal Study that stands out based on sample size and duration of follow-up. The Iowa Longitudinal Study was conducted between 1987 and 2007 that recruited 542 patients at their initial psychotic presentation at a mean approximate age of 24 years. Repeated MRI scans (minimum 2 and maximum 5 scans) were available from 202 patients and 125 healthy individuals; the mean interval between first scan and last available scan was 7.2 years (Andreasen et al. 2011). Patients showed accentuated decrease in whole-brain and regional gray and white matter volume and increased enlargement of the lateral ventricles. With regards to gray matter volume, the slope of decline in patients was greater for the frontal lobe and the thalamus (Andreasen et al. 2011). However, progressive brain changes did not occur uniformly in patients or in healthy individuals. For example, 47% of patients were losing at least 0.5% and 34% were losing at least 1% of frontal gray matter volume (as compared with 36% and 23% in healthy individuals, respectively). The interaction between time and diagnostic group on all brain measures was statistically significant at the first inter-scan interval, which was at 2 years after intake, but not thereafter. Increased attrition with longer follow-up may have reduced the power to detect case-control differences. However, these findings raise the possibility that disease-mechanisms affecting brain integrity may indeed be most active during the early stages of schizophrenia. This interpretation may need to be considered in the context of a study by Schaufelberger et al. (2011) who conducted a 1-year follow-up of 39 patients with first-episode psychosis and 52 healthy individuals enrolled in a population-based case-control study. Although the sample size was small and the follow-up period was brief, the authors reported a significant group by time interaction in the superior temporal gyrus and the hippocampus due to gray matter increase in patients in these brain regions while no change was observable in the control group. This “gain” in patients was associated with prolonged remission suggesting that if disease mechanisms are indeed active in the early stages of the illness this effect may be limited to a subset of patients likely to have a more severe illness.
1.2.2 Patient Samples Unselected for Illness Duration
Several studies have examined progressive brain changes in patients of schizophrenia at different stages of their illness beyond the first episode (Mathalon et al. 2001; Mitelman et al. 2009; van Haren et al. 2008, 2011, 2016; Veijola et al. 2014; Wood et al. 2001) as have several reviews (Hulshoff Pol and Kahn 2008; Zipursky et al. 2013). Several meta-analyses have also provided a synthesis of the emerging patterns (Haijma et al. 2013; Kempton et al. 2010; Olabi et al. 2011).
More specifically, Kempton et al. (2010) focused their meta-analysis on the issue of lateral ventricular enlargement as an indirect measure of progressive brain loss; they included 13 studies that had measured the lateral ventricles or entire ventricular system at a minimum of two time points in a cumulative sample of 473 patients and 348 healthy individuals. Patients showed greater progressive ventricular enlargement than healthy individuals with a mean effect size g = 0.45; the effect size of case-control differences was similar for early stage (g = 0.49) and chronic (g = 0.41) patients.
Olabi et al. (2011) conducted a meta-analysis using data from 27 patient cohorts comprising 928 patients and 867 healthy individuals for whom baseline and follow-up a ROI volumetric data were available. The mean duration of illness at the baseline scan was 6.2 years (range: 0.3–18.7 years) and the mean inter-scan interval was 3.49 years. Greater progressive changes in patients were noted for the whole brain volume (d = −0.40) and the ventricular volume (d = 0.53). The slope of decline was steeper in patients for all gray matter ROIs but reached significance for the whole-brain (d = −0.52) and frontal lobe (d = −0.34). No significant time by diagnosis interaction was noted for subcortical structures although the reported confidence intervals were large, suggesting significant inter-study heterogeneity.
The results of these meta-analyses resonate with the findings from one of the largest longitudinal MRI datasets of patients with schizophrenia at the University Medical Center Utrecht (Hulshoff Pol et al. 2001). At baseline, the cohort comprised 159 patients with schizophrenia (with a mean duration of illness of 13.7 years) and 158 healthy participants. At intake, patients had smaller amygdala, hippocampus and thalamus and larger caudate and globus pallidus. They also showed evidence of cortical thinning in the inferior frontal, superior temporal and ventral occipital regions and in the precuneus, insula and posterior cingulate cortex (Hulshoff Pol et al. 2001) and greater volume in the superior parietal lobule and occipital pole (van Haren et al. 2011). After a mean interval of approximately 5 years, 96 patients and 113 healthy individuals were rescanned. At follow-up, both diagnostic groups showed progressive reductions in whole-brain and cerebral lobe gray matter volume (van Haren et al. 2008) and thickness, but the slope was steeper in patients particularly in frontal and temporal regions (van Haren et al. 2011). Subcortical progressive volumetric reductions were more pronounced in patients’ caudate, putamen, hippocampus, and amygdala (van Haren et al. 2016).
2 Associations Between Gray Matter Morphometry and the Clinical Features of Schizophrenia
Gray matter alterations in schizophrenia are thought to underpin disease expression but this association is complex. Several clinical features seem to correlate with symptom severity in cross-sectional investigations and the prognostic value of neuroimaging for clinical and functional outcomes. Outcomes were commonly defined using consensus criteria for clinical remission (e.g., Andreasen et al. 2005), type of course (e.g., non-remitting versus remitting) or overall function based on the General Assessment of Functioning (GAF) score. We focus on associations between gray matter morphometry and disease expression that were reported in the studies discussed in the preceding sections when such information was available. Additionally we highlight notable findings from the wider literature.
2.1 Cross-Sectional Studies
2.1.1 Associations Between Gray Matter Changes and Symptom Severity
In the ENIGMA studies, the association between gray matter ROIs and symptoms was assessed using both whole-brain and hypothesis-led ROI methods. In the whole-brain analyses, greater positive symptom severity correlated with cortical thickness in the left fusiform gyrus (r = 0.60), the left pars triangularis (r = −0.08), the left superior frontal gyrus (r = −0.07), the right inferior (r = −0.07), and the bilateral middle frontal gyrus (left r = −0.08; right r = −0.10) and middle temporal gyrus (left r = −0.07; right r = −0.06) (van Erp et al. 2018). Greater negative symptom severity correlated with widespread cortex thinning bilaterally (left and right r = −0.08) and larger lateral ventricular volume (van Erp et al. 2016, 2018). The hypothesis-led analyses focused on the superior temporal gyrus (STG) given its involvement in auditory hallucinations (Lee et al. 2018) and on PFC regions because of their association with negative symptoms (Millan et al. 2014). In these analyses, positive symptom severity was negatively related to STG thickness bilaterally (left: βstd = −0.05; right: βstd = −0.07) (Walton et al. 2017) while negative symptom severity negatively related with the thickness of the left medial orbitofrontal cortex (βstd = −0.07) and to a lesser extent with the thickness of the left lateral orbitofrontal gyrus and the pars opercularis (Walton et al. 2018).
However, the ENIGMA findings are only partially supported by other meta-analyses. The meta-analysis by Bora et al. (2011) reported a link between negative symptom severity and reduced gray matter density in the medial and lateral orbital PFC (as well as the insula) but only in chronic patients. Fusar-Poli et al. (2012), who focused on first-episode mediation-naïve patients, found a negative between reduced STG gray matter volume and overall psychopathology. Therefore the magnitude of the associations between gray matter changes and psychopathology is generally small and perhaps it is not surprising that such associations have not been always detectable (e.g., Gupta et al. 2015).
The complexity of the links between gray matter phenotypes and psychopathology is highlighted by the contradictory findings from machine learning studies. Kambeitz et al. (2015) found that diagnostic accuracy based on machine learning analysis of structural MRI data was better for patients with predominantly positive symptoms while Rozycki et al. (2018) reported better diagnostic classification accuracy for patients with greater negative symptom severity.
2.1.2 Associations Between Gray Matter Changes and Outcome
In childhood-onset schizophrenia, whole-brain mean cortical thickness at baseline was positively associated with remission at 3 months post-scan (Greenstein et al. 2008). This finding was partially confirmed by Doucet et al. (2018) who used a machine learning approach to evaluate the predictive value of gray matter phenotypes for symptomatic improvement at 6-months in adult patients (n = 100) in the early stages of schizophrenia (duration of illness <5 years). Although symptomatic improvement showed moderate correlations with global cortical thickness and subcortical volumes, none of these phenotypes had significant prognostic value.
Two notable studies attempted to test whether machine learning applied to brain structural features can distinguish between patients with remitting versus non-remitting outcomes (also see Chap. 17). Zannetti and colleagues (2013) used MRI data from 62 patients with first-episode of schizophrenia and an equal number of matched healthy individuals; the diagnostic accuracy of the classifier was modest in distinguishing cases from controls (73.4%), but failed to discriminate between patients with remitting and non-remitting course, as the diagnostic accuracy achieved was marginally above chance (58.3%). Nieuwenhuis et al. (2017) expanded upon this effort by using data from a substantial sample of patients with first episode psychosis (n = 386) from centres in Australia, Brazil, the Netherlands, Spain, and the UK. Classification accuracy according to outcome was modest in some of the participating centres but did not generalize across centres; in fact when data from all the centres were analyzed into one model, the classification accuracy was at chance level.
2.2 Longitudinal Studies
None of the major meta-analyses of longitudinal studies have addressed the association with clinical outcome (Fraguas et al. 2016; Olabi et al. 2011; Vita et al. 2012) so we focus on the findings of the two largest longitudinal studies, the Iowa Longitudinal Study, which followed-up first-episode psychosis (Andreasen et al. 2011, 2013), and the study coordinated by the University Medical Center Utrecht that examined patients with schizophrenia at different stages of their illness (Hulshoff Pol et al. 2001; van Haren et al. 2008, 2011, 2016). The Iowa Longitudinal Study focused on the association between remission (defined as in Andreasen et al. 2005), relapse (defined as in Csernansky et al. 2002) and the rate of progressive brain changes in patients (n = 202). Over an average period of 7 years, the mean duration of relapse was 1.34 years. The early phases of the illness were characterized by multiple relapses of relatively short duration; as time progressed, the number of relapses decreased while their length increased. The length of the period spent in relapse (but not the number of relapses) was associated with greater loss in whole-brain and frontal gray matter volume (Andreasen et al. 2013). In the cohort from the University Medical Center Utrecht, patients (n = 96) were followed-up for an average period of 5 years at which point the median GAF score was used to divide patients into those with good (GAF = 66.7) and those with poor outcome (GAF = 38.2). The outcome groups did not differ in age, sex, scan interval, socioeconomic status, illness duration, or antipsychotic medication intake. However, compared to patients with good outcomes, those with poor outcomes showed more pronounced progressive cortical thinning in multiple temporal regions (superior and middle temporal and Heschl gyri), in the ACC and in the visual cortex (cuneus).
3 Potential Moderators
3.1 Sex
There are no consistent reports of a differential effect of sex on schizophrenia-related brain structural alterations either in cross-sectional or longitudinal studies. Whenever an effect of sex has been found it has been associated with greater brain structural deviance in male patients. For example, the ENIGMA studies found no interaction between diagnosis and sex on subcortical volume when correcting for multiple comparisons; at a nominal uncorrected statistical threshold, the proportion male patients was associated with more pronounced case-control differences in the accumbens and the amygdala (van Erp et al. 2016). Moreover, meta-analyses that examined the effect of sex found no interactions with diagnosis (Fraguas et al. 2016; Haijma et al. 2013; Kempton et al. 2010; Olabi et al. 2011) with the exception of Bora et al. (2011), who reported that male sex was associated with greater deviance in the gray matter of the PFC, the insula, the amygdala and the thalamus.
3.2 Age
Age and illness duration are commonly highly correlated and their independent contribution to schizophrenia-related gray matter morphometry can be difficult to disentangle. Age is a significant determinant of gray matter morphometry; a substantial body of literature suggests that gray matter regions cortical thickness and volume peak during late childhood and decrease thereafter (Ducharme et al. 2015; Good et al. 2001; Raz et al. 2005, 2010). The association between age and brain morphometry in schizophrenia is likely to involve complex interactions between age-related brain changes, primary effects of disease mechanisms, and secondary effects of prolonged medication exposure and patients’ suboptimal lifestyle. There is some evidence that the magnitude of case-control differences in schizophrenia may be influenced by developmental age (Chap. 15). As previously noted, the slope of cortical thinning was steeper in adolescence in the NIMH cohort of childhood-onset schizophrenia but became similar to that of healthy youth as patients approached adulthood (Greenstein et al. 2006). The evidence for older adults is somewhat conflicting. Kambeitz et al. (2015) found that machine learning algorithms attained better diagnostic sensitivity for chronic compared to first-episode patients indicating that case-control differences in schizophrenia may become more distinctive with age. By contrast, in the University Medical Center Utrecht cohort, excessive gray matter volume decrease in patients with schizophrenia over a 5-year follow-up was significant for those aged 18–46 years but not in older patients (van Haren et al. 2008). This observation has not been replicated in other studies including a further study from the same cohort that examined cortical thickness (van Haren et al. 2011).
3.3 Antipsychotic Medication
The effect of antipsychotic medication on brain morphology is subject to intense scrutiny and debate (See also Chap. 18). Antipsychotic drugs are the mainstay pharmacological treatment of schizophrenia and reduce dopaminergic neurotransmission albeit to a variable degree. The first-generation antipsychotics (FGA) are high-affinity antagonists of dopamine D2 receptors while the second-generation (SGA) antipsychotics have lower affinity for D2 receptors but different affinities for serotonergic, adrenergic, acetylcholine, and histamine receptors (Miyamoto et al. 2005). Antipsychotic exposure and illness severity are not independent because patients with a more severe illness are more likely to receive medication for longer periods and at higher doses. Disambiguating the effects of medication on gray matter integrity from those attributable to primary disease mechanism is the greatest challenge as most MRI studies include medicated patients.
3.3.1 Effect of Antipsychotic Medication on Striatal Volumes
Striatal enlargement has been associated with antipsychotic treatment in multiple studies (Gupta et al. 2015; Ho et al. 2011; van Haren et al. 2016) and meta-analyses (Haijma et al. 2013) and is absent in studies of medication-naïve patients (Fusar-Poli et al. 2012, 2013). The first indication that antipsychotics may causally affect striatal structure was provided by Chakos and colleagues in 1994 who reported an increase in the volume of the caudate nucleus in patients with first-episode schizophrenia following initiation of treatment with haloperidol, a prototypical FGA. Subsequent studies found that caudate enlargement can be reversed following withdrawal of antipsychotic medication or substitution of FGAs with SGAs (Chakos et al. 1995; Frazier et al. 1996b; Corson et al. 1999). Because the caudate is rich in dopamine D2 receptors, this FGA-induced enlargement has been attributed to remodelling caused by strong dopaminergic blockade which can induce ultrastructural changes in striatal neurons (Benes et al. 1985; Chakos et al. 1998; Meshul et al. 1992) and alterations in the dendritic morphology of cortical neurons (Lidow et al. 2001; Selemon et al. 1999) and in fronto-striatal connectivity (Tost et al. 2010).
Antipsychotics may also influence progressive striatal changes. In the University Medical Center Utrecht cohort, the cumulative annual intake of FGAs was inversely associated with progressive volume loss in the putamen (rho = 0.46) and caudate (rho = 0.38) over a 5-year period (van Haren et al. 2016); similarly, in the Iowa Longitudinal Study, higher antipsychotic doses were inversely associated with progressive volume loss in the caudate and putamen over a 7-year period (Ho et al. 2011).
3.3.2 Antipsychotic Treatment and Volume Changes in Other Subcortical Regions
The most convincing evidence for an association between antipsychotic exposure and subcortical volumes derives from the ENIGMA dataset (van Erp et al. 2016). Specifically, the proportion of medication-naive patients was negatively associated with the effect size of case-control differences in hippocampal volume. The effect size of case-control difference in ventricular enlargement was negatively associated with the proportion of patients treated with SGA and positively associated with the mean current antipsychotic dose in chlorpromazine equivalents (CPZE).
3.3.3 Antipsychotic Treatment and Changes in Cortical Thickness or Volume
Preclinical studies suggest several mechanisms that may lead to cortical thinning following exposure to both FGAs and SGAs. These include antipsychotic-induced microstructural changes in dendritic morphology (Dorph-Petersen et al. 2005; Vernon et al. 2011), which can be reversed upon discontinuation (Vernon et al. 2012). FGAs may compromise gray matter integrity by reducing cerebral flow and metabolism (Goff et al. 1995; Lahti et al. 2004; Miller et al. 1997; Molina et al. 2003; Wright et al. 1998) and by increasing oxidative stress and excitotoxicity (Goff et al. 1995; Wright et al. 1998). Conversely, SGAs (such as olanzapine and clozapine) may preserve cortical gray matter integrity by reducing excitotoxicity (Duncan et al. 2000) while enhancing dendritic resilience (Wang and Deutch 2008), neurogenesis (Halim et al. 2004; Wakade et al. 2002; Wang et al. 2004), and neurotrophic factor expression (Bai et al. 2003; Fumagalli et al. 2003). The association between antipsychotic dose and class has therefore been examined in multiple MRI studies comparing patients to healthy individuals and patients treated with FGAs or SGAs.
The ENIGMA consortium found evidence for an association between antipsychotic exposure and mostly global measures of cortical thickness (van Erp et al. 2018). In particular, mean hemispheric cortical thickness was inversely associated with exposure to FGAs or combinations of FGAs and SGAs. In addition, higher dose in CPZEs inversely correlated with cortical thickness in almost all cortical regions but more prominently in the superior frontal gyrus, the pars triangularis, the superior, middle and inferior temporal gyri, and the supramarginal gyrus. The magnitude of these associations however was small (|r| < 0.2). The meta-analysis by Radua et al. (2012) has provided further information about the localization of cortical changes associated with antipsychotic medication in first-episode patients. In a series of meta-regression analyses they found that the gray matter volume in medial PFC, the ACC, and the insula were significantly lower in medicated patients compared to healthy individuals. This reduction was greater than that observed in the same regions in a subgroup analysis that included only samples with antipsychotic-naïve patients. The meta-analysis by Haijma et al. (2013) focused on more global measures and reported that higher antipsychotic dose at the time of scanning was associated with lower whole-brain gray matter volume for both FGAs and SGAs. Importantly, a sub-analysis of antipsychotic-naive samples found that the effect-size of case-control differences was approximately 75% of that seen in medicated samples, indicating that the most significant reduction in whole-brain gray matter volume may predate treatment initiation.
Two key single-cohort studies have examined the association between antipsychotic exposure and progressive cortical changes. In the Iowa Longitudinal Study, patients were divided into those that received high (mean dose, 929.4 CPZE mg), intermediate (mean dose, 391.7 CPZE mg), and low daily antipsychotic dose (mean dose, 111.5 CPZE mg) (Ho et al., 211; Andreasen et al. 2013). Patients in the top tertile had smaller frontal gray matter volume compared to those in the lowest tertile; this association however was independent of follow-up duration. Conversely, in the University Medical Center Utrecht longitudinal cohort, cortical thickness reduction over the 5-year follow-up period was associated with antipsychotic class (van Haren et al. 2011), being more pronounced in those treatment with FGAs. The main SGA prescribed in this cohort was olanzapine; higher exposure to olanzapine over the follow-up period (in mg per year) was associated with a non-significant increase in whole-brain gray matter volume. A different pattern was observed in patients with emergent treatment resistance that were prescribed clozapine at any point during the follow-up period. These patients showed more pronounced cortical thinning in the left superior temporal cortex compared to all other patients, and those prescribed higher doses of clozapine (in mg per year) showed further thinning in the PFC and ACC.
Similar results were reported by Vita and colleagues who examined the association between antipsychotic dose and class (FGA or SGA) on ROIs involving whole-brain, frontal, temporal, and parietal gray matter volume derived from 18 longitudinal studies (range of follow-up: 1–7 years) yielding a cumulative sample of 1155 patients with schizophrenia and 911 healthy individuals (Vita et al. 2015). Patients treated with FGAs or a combination of FGAs and SGAs showed reductions in all gray matter measures over time; the greater the exposure to FGAs during the inter-scan period, both in terms of cumulative and mean daily dose, the greater the reduction in whole-brain gray matter volume. By contrast, the higher the mean daily dose of SGAs during the follow-up, the lower the reduction in whole brain gray matter volume. A larger ROI-based meta-analysis by Fusar-Poli et al. (2013) of 30 studies comprising MRI data from of 1046 schizophrenia patients and 780 controls over a median follow-up period of over 1-year, also confirmed that progressive whole-brain gray matter volume decreases and lateral ventricular enlargement were associated with greater cumulative exposure to higher antipsychotic doses. This meta-analysis however did not consider antipsychotic class.
The study by Lieberman et al. (2005) merits special attention because their sample of patients with first-episode schizophrenia (n = 263) was randomized to double-blind treatment with the SGA olanzapine, 5–20 mg/day, or the FGA haloperidol, 2–20 mg/day, for up to 104 weeks. This random assignment enabled investigators to disentangle the effect of antipsychotic class from patient- and physician characteristics that may drive medication regimes in clinical settings. Patients were assessed by MRI at weeks 0 (baseline), 12, 24, 52, and 104 and were compared to 58 healthy study participants. At study end, the sample with available data comprised 239 patients with a baseline MRI, and 161 patients with at least one follow-up assessment. By week 12, patients’ rate of whole-brain gray matter volume reduction was steeper in the haloperidol- versus the olanzapine-treatment group and the magnitude of this difference remained largely constant to the study end. These group differences were driven by volume loss in haloperidol-treated patients, which was absent in the olanzapine-treated patients. The same pattern was noted for frontal, temporal, and parietal gray matter volumes. The volume of the caudate showed greater enlargement in the haloperidol- compared with the olanzapine-treatment group beginning at week 24.
3.3.4 Gray Matter Alterations in Medication-Naïve Patients
Studies on medication-naïve patients are particularly useful in disentangling disease- versus medication-related brain structural changes. Fusar-Poli et al. (2012) identified 14 studies with structural MRI data, collectively contrasting 206 antipsychotic-naive patients with first-episode psychosis (mean age of 26.4 years) to 202 healthy individuals. They found consistent and significant gray matter reductions (g = 0.83) (but no increases) in patients compared to healthy individuals that clustered within the superior temporal gyrus, the insula, and the cerebellum. These findings were largely replicated in a further meta-analysis by Haijma et al. (2013) who also found that medication-naïve patients, compared to healthy individuals, had smaller intracranial (d = −0.14), whole-brain (d = −0.21), whole-brain gray matter (d = −0.36), thalamic (d = −0.68), and caudate (d = −0.38) volumes.
3.4 Substance Use
Substance use, and particularly cigarette smoking, cannabis and alcohol, is highly prevalent in schizophrenia, affecting approximately 40% of patients (de Leon and Diaz 2005; Hunt et al. 2018). In MRI studies of non-psychotic populations, cannabis (Martín-Santos et al. 2010), cigarette smoking (Gallinat et al. 2006), and alcohol abuse (Zahr and Pfefferbaum 2017) have all been associated with diminished brain tissue volumes. Therefore substance abuse has the potential to exacerbate volume reductions in schizophrenia. Supporting evidence has been provided mainly from the University Medical Center Utrecht cohort, by Rais et al. (2008) who reported that first-episode patients who continued to use cannabis over the 5-year follow-up period showed greater reduction in gray matter volumes and increased ventricular enlargement compared to those that did not abuse cannabis. The effect of cannabis was most pronounced in regions rich in cannabinoid receptors (CB1) within the dorsolateral PFC, the ACC, and the occipital cortex (Rais et al. 2010). However, in a review of the literature, Malchow et al. (2013) cautioned that the effect of cannabis on brain structure in patients with schizophrenia is inconsistent and is likely to be small. Finally, a meta-analysis of the relevant literature, showed that alcohol abuse in patients with schizophrenia was associated with thinner cortex and enlarged ventricles but the magnitude of this effect was commensurate to that seen in non-psychotic individuals (Lange et al. 2017).
3.5 Stress and Cardiometabolic Risk Factors
There is substantial support for elevated baseline activity of the hypothalamic-pituitary-adrenal (HPA) axis, the central stress response system, in patients with psychosis (Borges et al. 2013; Walker et al. 2008). It is generally known that HPA hyperactivity is associated with microstructural abnormalities and volume loss in the hippocampus, which is a key target region for glucocorticoids (Sapolsky 2003). At present, the link between hippocampal volume reduction and HPA activity is based on a sample of 24 patients with first-episode schizophrenia where an inverse association was found with cortisol levels both cross-sectionally and at 3-month follow-up (Mondelli et al. 2010).
Patients with schizophrenia are at risk of developing cardio-metabolic disease for several reasons including genetic predisposition to diabetes (Ryan et al. 2003), reduced physical activity (Rosenbaum et al. 2014), and the metabolic side-effects of antipsychotic medications (Mitchell et al. 2013). It has been estimated that at least a quarter of all medicated patients meet criteria for metabolic syndrome (Mitchell et al. 2013). In non-psychotic populations (Doucet et al. 2018; Medic et al. 2016; Yates et al. 2012), the presence of metabolic syndrome (comprising insulin resistance, obesity, dyslipidemia and hypertension), or merely increased body mass index (BMI), is associated with cortical thinning. There are only two studies that have addressed the effect of BMI on brain morphometry in patients with schizophrenia. Jørgensen et al. (2017) obtained MRI data from patients with first-episode schizophrenia (n = 78) and healthy individuals (n = 119) at intake and then 1 year later. Increases in BMI over the follow-up period showed a small negative association (β = −0.19) with whole-brain and whole-brain gray matter volume change, which was however similar in magnitude in both groups. Moser et al. (2018) used a multivariate machine approach to examine the effect of BMI on MRI measures from 100 patients with recent-onset schizophrenia (illness duration <5 years) while simultaneously modelling correlations with clinical and cognitive symptoms, substance use, psychological trauma, physical activity, and medication (Fig. 2.4). Even after accounting for these multiple variables, BMI was negatively and significantly associated the cortical thickness (r = −0.28).
Multivariate associations between behavioral, clinical and multimodal imaging phenotypes in psychosis. Right Panel: Association between neuroimaging and non-imaging variables across all imaging modalities. Left Panel: (a): Regional cortical thickness measures correlated most highly with non-imaging variate (top) and correlations between non-imaging variables and cortical thickness variate (bottom). (b): Subcortical volumetric measures correlated most highly with non-imaging variate (top) and correlations between non-imaging variables and subcortical volumes variate (bottom). (c): Regional task-related brain activation correlated most highly with non-imaging variate (top) and correlations between non-imaging variables and task-related brain activation variate (bottom). (d): Regional fractional anisotropy measures correlated most highly with non-imaging variate (top) and correlations between non-imaging variables and fractional anisotropy variate (bottom) Results from sparse canonical correlation analyses from Moser et al. (2018). (From https://www.ncbi.nlm.nih.gov/pubmed/29516092)
4 Overview of Findings
The studies presented in the preceding sections provide evidence for the following statements: (1) schizophrenia-related gray matter alterations consist mostly of subtle and widespread reductions in cortical and subcortical gray matter density and volume and in cortical thickness; these gray matter alterations occur in the context of a small but significant reduction in intracranial volume (ICV) in patients with schizophrenia (effect size −0.2) (Haijma et al. 2013); (2) tissue loss is most prominent in the temporal (inferior, middle and superior temporal and parahippocampal gyri), the occipital and the frontal (lateral orbitofrontal and inferior frontal gyrus) cortex, in the posterior ACC, and in the insula; notably regions within the motor (precentral gyrus), somatosensory (postcentral gyrus), and parietal (superior and inferior parietal cortex and paracentral lobule) cortex may show tissue gain; (3) gray matter tissue reductions are largely present at the time of the first psychotic episode and may be greatest early in the course of illness, mirroring the social and occupational deterioration which seems more pronounced in the early stages of psychosis (Häfner and Maurer 2006); (4) after illness onset, the slope of age-related decline in cortical regions appears to be steeper in patients with schizophrenia than in healthy individuals but of small magnitude; it is estimated that the cumulative loss of brain tissue after 20 years of illness is approximately 3% (Hulshoff Pol and Kahn 2008; Wright et al. 2000); (5) the association between gray matter alterations and clinical outcome is complex; the general pattern suggests that whole-brain gray matter reductions are more pronounced in patients that are more symptomatic and have poor clinical and functional outcomes; (6) sex differences in schizophrenia-related gray matter alterations are small, and when present they indicate greater deviance in male patients; (7) antipsychotic drugs, and particularly FGAs, have been associated with progressive striatal and ventricular enlargement and diffuse cortical thinning; (8) several factors including HPA dysregulation, substance abuse, stress, higher BMI, and cardiometabolic risk are likely to contribute to the gray matter alterations observed in schizophrenia (Fig. 2.5).
Maps of change in cortical thickness in millimeters and F values, showing reduction (blue) or excess (red) at study entry (patients with schizophrenia = 154, controls = 156) and during the 5-year interval (patients = 96, controls = 113) (van Haren et al., 2011; https://www.ncbi.nlm.nih.gov/pubmed/21893656)
5 Mechanistic Implications and Future Directions
Structural MRI as applied to the studies described above lacks the resolution to inform us about the nature of pathogenetic processes underlying gray matter changes in schizophrenia. Furthermore, the processes involved may not affect all patients in the same fashion. Studies that have applied multivariate clustering techniques to multimodal datasets from patients with schizophrenia (i.e., datasets that include clinical, genetic, cognitive and brain phenotypes) hint at the presence of multiple, partially overlapping syndromes that may be associated with a discrete set of risk factors (Arnedo et al. 2015; Zheutlin et al. 2018). No systematic examination, however, has been undertaken to date to examine the association between gray matter integrity and risk-conferring gene variants or other non-genetic risk factors. A meta-analyses of the limited neuroimaging studies available have implicated a four-marker haplotype in G72, a microsatellite and single nucleotide polymorphism in NRG1, DISC1, CNR1, BDNF, COMT and GAD1; these associations were of small or medium magnitude and were most frequently observed in frontal regions (Harari et al. 2017).
The gray matter changes in patients with schizophrenia should be considered in the context of ICV reduction, a consistent finding in MRI studies of schizophrenia (Haijma et al. 2013). The expansion of the skull, which determines ICV, is driven by brain growth (O’Rahilly and Müller 2001) and reaches its maximum size at approximately 13 years of age (Blakemore 2012). The reduced ICV in patients suggests that the biological process leading to overt disease may be active early in development. As already discussed, there are progressive post-onset changes in brain and gray matter volume that are indicative of on-going disease related mechanisms that lead to further compromise in brain structural integrity.
Traditional models of schizophrenia emphasize neurotransmitter abnormalities mostly in the dopaminergic (Howes and Kapur 2009) and glutamatergic systems (Moghaddam and Javitt 2012). According to the dopamine hypothesis, schizophrenia is characterized by hyperactive transmission in the striatal areas and hypoactive transmission in the prefrontal cortex as proposed by Davis and colleagues in 1991 and updated more recently by Howes and Kapur in 2009. In vivo and in vitro studies suggest that excess dopamine can trigger inflammation, oxidative stress, apoptosis, and mitochondrial impairment (Junn and Mouradian 2001). For example, prolonged stimulation of cortical neurons by excessive dopamine can induce calcium-related excitotoxicity and mitochondrial dysfunction through mitochondrial complex I inhibition (Ben-Shachar 2017). With respect to glutamate, the primary excitatory neurotransmitter, gray matter pathology in schizophrenia may be linked to excitotoxic injury through excessive Ca2+ influx via the N-methyl-d-aspartate (NMDA-R) receptor (Moghaddam and Javitt 2012). Further, Ca2+ toxicity targets mitochondria leading to dysfunction, either directly or through oxidative inhibition of mitochondrial complexes I, II and V, ultimately activating death signals (Nicholls 2009; Davis et al. 2014).
Several other mechanisms have been proposed to explain the gray matter alterations observed in schizophrenia with most of the data derived from post-mortem studies. To-date, there is no convincing evidence that schizophrenia is associated with gliosis or neuronal cell loss or other histopathological changes commonly seen in degenerative disorders (Arnold et al. 1998; Baldessarini et al. 1997; Heckers et al. 1991; Pakkenberg 1993; Thune et al. 2001; Walker et al. 2002). By contrast, decreased cortical neuropil, relative to specimens from psychiatrically-healthy individuals, has been one of the most replicable post-mortem findings in schizophrenia (Selemon and Goldman-Rakic 1999). This reduction has been observed in multiple brain regions including primary sensory and associative cortices (Casanova et al. 2008). In a parallel line of research, replicable reductions have been noted in synaptic protein and mRNA levels in multiple cortical regions, although the precise distribution of these changes cannot be fully evaluated because of limited brain coverage of the available data. The most robust, but modest, reductions have been noted for synaptophysin (a neuron-specific presynaptic protein) particularly within the hippocampus, but also in dorsolateral PFC and ACC as these regions are preferentially sampled in schizophrenia research (Osimo et al. 2018). Additional but smaller reductions in other synaptic proteins (i.e., SNAP-25, PSD-95, synapsin and rab3A) have also been consistently noted in the hippocampus while data for other brain regions are very limited (Osimo et al. 2018). Whether these changes are developmental or whether they occur later in life remains unknown as post-mortem studies are restricted to chronic cases.
Some researchers have proposed that mitochondrial dysfunction (either primary or induced) may contribute to the neuronal, dendritic, and synaptic abnormalities seen in schizophrenia. While the brain comprises less than 2% of the human body mass, it consumes 20% of the energy expensed. ATP production is therefore crucial for cellular homeostasis, electrical conductivity, and synaptic function; neurons are critically dependent on the mitochondria to generate the requisite amounts of ATP. Evidence of abnormal energy generation in schizophrenia has been known since Looney and Childs (1934) who reported increased lactate and decreased glutathione in blood samples from schizophrenic patients. In post-mortem brain tissue from patients with schizophrenia, mitochondria are differentially affected depending on the brain region, cell type, and subcellular location. The overall pattern however indicates that mitochondria, particularly in or near the synapses and neuropil, are functionally compromised (Roberts 2017).
Mitochondrial DNA is susceptible to damage from reactive oxygen species (Corral-Debrinski et al. 1992) while release of mitochondrial DNA during cell stress can trigger inflammatory responses (López-Armada et al. 2013; Naik and Dixit 2011; Oka et al. 2012). There is substantial evidence implicating oxidative/nitrosative stress pathways in the pathophysiology of schizophrenia as recently shown in a meta-analysis of 61 independent cohorts comprising blood samples from 3002 patients with first-episode schizophrenia and 2806 healthy individuals; patients had lower total antioxidant status and docosahexaenoic acid (DHA) levels and higher levels of homocysteine, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) (Fraguas et al. 2018). Moreover, a recent meta-analysis of post-mortem studies (n = 41; 783 patients and 762 controls) by van Kesteren et al. (2017) confirmed that despite significant inter-study heterogeneity, both microglia density and the concentrations of pro-inflammatory proteins are increased in schizophrenia. These changes were particularly evident in temporal cortical regions. Direct in-vivo evidence that these mechanisms operate centrally is currently lacking. Neuroimaging studies attempting to quantify case-control differences in central pro-inflammatory markers have focused on the activation state of microglia. Activated microglia expresses higher levels of the 18-kDa translocator protein (TSPO) (Cosenza-Nashat et al. 2009) which can be measured in vivo using positron emission tomography (PET) radiotracers. Studies using this methodology have thus far failed to provide consistent support for an increase of TSPO in schizophrenia (Plaven-Sigray et al. 2018; Marques et al. 2018), and the heterogeneity between studies is at least partly attributable to on-going methodological challenges (Plavén-Sigray and Cervenka 2019).
Finally, there is also evidence for myelin-related dysfunction in schizophrenia, which could also account for the observed decreases in brain tissue. Maturational changes in intracortical myelination occur in late adolescence and early adulthood and coincide with the peak period of risk for schizophrenia (Bartzokis 2002; Paus et al. 2008; Rapoport et al. 2005; Weinberger 1987). Several oligodendrocyte/myelin related genes, such as neuregulin and its receptor ERBB4, are genetically associated with schizophrenia (Karoutzou et al. 2008; Norton et al. 2006). Post-mortem studies in schizophrenia have reported compromised integrity of the myelin sheath (Uranova et al. 2001), abnormal oligodendrocyte density and morphology (Uranova et al. 2004), and expression of genes associated with oligodendrocytes and myelin in multiple brain regions (Dratcheva et al. 2006; Hakak et al. 2001; Katsel et al. 2005). These findings do not seem attributable to antipsychotic exposure (Hakak et al. 2001; Konopaske et al. 2008). In vivo neuroimaging studies of intracortical myelination in schizophrenia have yielded variable results. Nevertheless, reduced intracortical myelin has been found in the prefrontal cortex (Bagary et al. 2003; Bohner et al. 2012; Price et al. 2010), temporal lobes (Foong et al. 2000) and primary/association visual cortex (Bachmann et al. 2011; Palaniyappan et al. 2013). A more recent and larger study reported myelin reduction in patients with schizophrenia compared to controls in the sensorimotor cortex, the transverse temporal gyri, the cuneus, and in the visual cortex (Jørgensen et al. 2016).
The small effect size of case-control differences in gray matter integrity and the multiplicity of potential mechanisms and moderators argue that future research efforts will require adequately powered large-scale projects comprising multiscale measures (e.g., genetic, molecular, neuroimaging, clinical, and cognitive) from patients and healthy individuals. It is widely accepted that adolescent mental health problems arise from complex interactions between these factors. Examination of these interacting influences requires advanced multivariate modelling and significant computing power in order to simultaneously handle high-dimensional datasets. This is an emerging field with multiple groups endeavoring to define optimal statistical algorithms and to test the validity of the resultant models (e.g., Nymberg et al. 2013).
Summary
-
Gray matter alterations in schizophrenia comprise subtle and widespread reductions in cortical and subcortical gray matter density and volume and in cortical thickness in the context of reduction in intracranial volume.
-
Gray matter tissue reductions seem to occur at the time of the first psychotic episode and may mirror the social and occupational deterioration observed in the early stages of psychosis.
-
After illness onset, the slope of age-related decline in cortical regions appears steeper in patients with schizophrenia than in healthy individuals; these age-related changes may be exacerbated by the use of first-generation antipsychotics.
-
Factors that may contribute to gray matter abnormalities in schizophrenia also include substance abuse, stress, and cardiometabolic dysregulation.
References
Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–9.
Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(7):672–9.
Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–15.
Arango C, Rapado-Castro M, Reig S, Castro-Fornieles J, González-Pinto A, Otero S, Baeza I, Moreno C, Graell M, Janssen J, Parellada M, Moreno D, Bargalló N, Desco M. Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry. 2012;69(1):16–26.
Arbabshirani MR, Plis S, Sui J, Calhoun VD. Single subject prediction of brain disorders in neuroimaging: promises and pitfalls. Neuroimage. 2017;145(Pt B):137–65.
Arnedo J, Svrakic DM, Del Val C, Romero-Zaliz R, Hernández-Cuervo H, Molecular Genetics of Schizophrenia Consortium, Fanous AH, Pato MT, Pato CN, de Erausquin GA, Cloninger CR, Zwir I. Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiatry. 2015;172(2):139–53.
Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry. 1998;55(3):225–32.
Bachmann S, Haffer S, Beschoner P, Viviani R. Imputation techniques for the detection of microstructural changes in schizophrenia, with an application to magnetization transfer imaging. Schizophr Res. 2011;132(1):91–6.
Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003;60(8):779–88.
Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71(1):127–31.
Baldessarini RJ, Hegarty JD, Bird ED, Benes FM. Meta-analysis of postmortem studies of Alzheimer’s disease-like neuropathology in schizophrenia. Am J Psychiatry. 1997;154:861–3.
Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27(4):672–83.
Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res. 1985;329(1–2):265–73.
Ben-Shachar D. Mitochondrial multifaceted dysfunction in schizophrenia; complex I as a possible pathological target. Schizophr Res. 2017;187:3–10.
Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406.
Bohner G, Milakara D, Witthaus H, Gallinat J, Scheel M, Juckel G, Klingebiel R. MTR abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Schizophr Res. 2012;137(1–3):85–90.
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yücel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127(1–3):46–57.
Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38(5):603–11.
Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002–10.
Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, Murray RM. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 1999;56(5):457–63.
Casanova MF, Kreczmanski P, Trippe J 2nd, Switala A, Heinsen H, Steinbusch HW, Schmitz C. Neuronal distribution in the neocortex of schizophrenic patients. Psychiatry Res. 2008;158(3):267–77.
Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151(10):1430–6.
Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345(8947):456–7.
Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry. 1998;44(8):675–84.
Cohen J. Statistical power analysis for the behavioral sciences: Routledge; 1977.
Collinson SL, Mackay CE, James AC, Quested DJ, Phillips T, Roberts N, Crow TJ. Brain volume, asymmetry and intellectual impairment in relation to sex in early-onset schizophrenia. Br J Psychiatry. 2003;183:114–20.
Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992;2(4):324–9.
Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156(8):1200–4.
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–28.
Csernansky JG, Mahmoud R, Brenner R, Risperidone-USA-79 Study Group. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16–22.
Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–86.
Davis J, Moylan S, Harvey BH, Maes M, Berk M. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512–29.
de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2–3):135–57.
DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74(3):129–40.
DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130(1):57–70.
Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–61.
Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb Cortex. 2018;28(3):988–97.
Dracheva S, Davis KL, Chin B, Woo DA, Schmeidler J, Haroutunian V. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis. 2006;21(3):531–40. https://doi.org/10.1016/j.nbd.2005.08.012.
Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Pérez JM, Labbe A, Evans AC, Karama S, Brain Development Cooperative Group. Trajectories of cortical surface area and cortical volume maturation in normal brain development. Data Brief. 2015;5:929–38.
Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of the effects of clozapine, risperidone, and olanzapine on ketamine-induced alterations in regional brain metabolism. J Pharmacol Exp Ther. 2000;293(1):8–14.
Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–61.
Foong J, Maier M, Barker GJ, Brocklehurst S, Miller DH, Ron MA. In vivo investigation of white matter pathology in schizophrenia with magnetisation transfer imaging. J Neurol Neurosurg Psychiatry. 2000;68(1):70–4.
Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108(1–3):104–13.
Fraguas D, Gonzalez-Pinto A, Micó JA, Reig S, Parellada M, Martínez-Cengotitabengoa M, Castro-Fornieles J, Rapado-Castro M, Baeza I, Janssen J, Desco M, Leza JC, Arango C. Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr Res. 2012;137(1–3):58–65.
Fraguas D, Díaz-Caneja CM, Pina-Camacho L, Janssen J, Arango C. Progressive brain changes in children and adolescents with early-onset psychosis: a meta-analysis of longitudinal MRI studies. Schizophr Res. 2016;173(3):132–9.
Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, Leza JC, Arango C. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2018;45:742–51. https://doi.org/10.1093/schbul/sby125.
Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kassen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996a;53(7):617–24.
Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry. 1996b;153(4):564–6.
Fumagalli F, Molteni R, Roceri M, Bedogni F, Santero R, Fossati C, Gennarelli M, Racagni G, Riva MA. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. J Neurosci Res. 2003;72(5):622–8.
Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38(6):1297–307.
Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37(8):1680–91.
Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24(6):1744–50.
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3.
Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774–81.
Goff DC, Tsai G, Beal MF, Coyle JT. Tardive dyskinesia and substrates of energy metabolism in CSF. Am J Psychiatry. 1995;152(12):1730–6.
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–9.
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36.
Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, Rapoport J, Gogtay N. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47(10):1003–12.
Greenstein DK, Wolfe S, Gochman P, Rapoport JL, Gogtay N. Remission status and cortical thickness in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47(10):1133–40.
Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J, Segall J, Franke B, Zwiers MP, Arias-Vasquez A, Buitelaar J, Fisher SE, Fernandez G, van Erp TG, Potkin S, Ford J, Mathalon D, McEwen S, Lee HJ, Mueller BA, Greve DN, Andreassen O, Agartz I, Gollub RL, Sponheim SR, Ehrlich S, Wang L, Pearlson G, Glahn DC, Sprooten E, Mayer AR, Stephen J, Jung RE, Canive J, Bustillo J, Turner JA. Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull. 2015;41(5):1133–42.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46(1–3):389–422.
Häfner H, Maurer K. Early detection of schizophrenia: current evidence and future perspectives. World Psychiatry. 2006;5(3):130–8.
Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–38.
Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4746–51.
Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29(6):1063–9.
Harari JH, Díaz-Caneja CM, Janssen J, Martínez K, Arias B, Arango C. The association between gene variants and longitudinal structural brain changes in psychosis: a systematic review of longitudinal neuroimaging genetics studies. NPJ Schizophr. 2017;3:40. https://doi.org/10.1038/s41537-017-0036-2.
Haukvik UK, Hartberg CB, Nerland S, Jørgensen KN, Lange EH, Simonsen C, Nesvåg R, Dale AM, Andreassen OA, Melle I, Agartz I. No progressive brain changes during a 1-year follow-up of patients with first-episode psychosis. Psychol Med. 2016;46(3):589–98.
Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia: a stereological study. Arch Gen Psychiatry. 1991;48(11):1002–8.
Hedges L. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–28.
Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–37.
Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–62.
Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34(2):354–66.
Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58(12):1118–25.
Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990-2017: systematic review and meta-analysis. Drug Alcohol Depend. 2018;191:234–58.
Jacobsen LK, Giedd JN, Vaituzis AC, Hamburger SD, Rajapakse JC, Frazier JA, Kaysen D, Lenane MC, McKenna K, Gordon CT, Rapoport JL. Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry. 1996;153(3):355–61.
Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, Lenane MC, Rapoport JL. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry. 1998;155(5):678–85.
James AC, Crow TJ, Renowden S, Wardell AM, Smith DM, Anslow P. Is the course of brain development in schizophrenia delayed? Evidence from onsets in adolescence. Schizophr Res. 1999;40(1):1–10.
James AC, James S, Smith DM, Javaloyes A. Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am J Psychiatry. 2004;161(6):1023–9.
Jørgensen KN, Nerland S, Norbom LB, Doan NT, Nesvåg R, Mørch-Johnsen L, Haukvik UK, Melle I, Andreassen OA, Westlye LT, Agartz I. Increased MRI-based cortical grey/white-matter contrast in sensory and motor regions in schizophrenia and bipolar disorder. Psychol Med. 2016;46(9):1971–85.
Jørgensen KN, Nesvåg R, Nerland S, Mørch-Johnsen L, Westlye LT, Lange EH, Haukvik UK, Hartberg CB, Melle I, Andreassen OA, Agartz I. Brain volume change in first-episode psychosis: an effect of antipsychotic medication independent of BMI change. Acta Psychiatr Scand. 2017;135(2):117–26.
Junn E, Mouradian MM. Apoptotic signaling in dopamine-induced cell death: the role of oxidative stress, p38 mitogen activated protein kinase, cytochrome c and caspases. J Neurochem. 2001;78(2):374–83.
Juuhl-Langseth M, Rimol LM, Rasmussen IA Jr, Thormodsen R, Holmén A, Emblem KE, Due-Tønnessen P, Rund BR, Agartz I. Comprehensive segmentation of subcortical brain volumes in early onset schizophrenia reveals limited structural abnormalities. Psychiatry Res. 2012;203(1):14–23.
Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, Falkai P, Koutsouleris N. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 2015;40(7):1742–51.
Karoutzou G, Emrich HM, Dietrich DE. The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol Psychiatry. 2008;13(3):245–60.
Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res. 2005;79(2-3):157–73. https://doi.org/10.1016/j.schres.2005.06.007.
Keller A, Castellanos FX, Vaituzis AC, Jeffries NO, Giedd JN, Rapoport JL. Progressive loss of cerebellar volume in childhood-onset schizophrenia. Am J Psychiatry. 2003;160(1):128–33.
Kempton MJ, Stahl D, Williams SC, DeLisi LE. Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophr Res. 2010;120(1–3):54–62.
Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63(8):759–65.
Kumra S, Giedd JN, Vaituzis AC, Jacobsen LK, McKenna K, Bedwell J, Hamburger S, Nelson JE, Lenane M, Rapoport JL. Childhood-onset psychotic disorders: magnetic resonance imaging of volumetric differences in brain structure. Am J Psychiatry. 2000;157(9):1467–74.
Lahti HH, Weiler MA, Medoff DR, Frey KN, Hardin M, Tamminga CA. Clozapine but not haloperidol reestablishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29(1):171–8.
Lange EH, Nerland S, Jørgensen KN, Mørch-Johnsen L, Nesvåg R, Hartberg CB, Haukvik UK, Osnes K, Melle I, Andreassen OA, Agartz I. Alcohol use is associated with thinner cerebral cortex and larger ventricles in schizophrenia, bipolar disorder and healthy controls. Psychol Med. 2017;47(4):655–68.
Lee WH, Kennedy NI, Bikson M, Frangou S. A computational assessment of target engagement in the treatment of auditory hallucinations with transcranial direct current stimulation. Front Psych. 2018;9:48. https://doi.org/10.3389/fpsyt.2018.00048.
Lidow MS, Song ZM, Castner SA, Allen PB, Greengard P, Goldman-Rakic PS. Antipsychotic treatment induces alterations in dendrite- and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biol Psychiatry. 2001;49(1):1–12.
Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49(6):487–99.
Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M, HGDH Study Group. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–70.
Looney J, Childs H. The lactic acid and glutathione content of the blood of schizophrenic patients. J Clin Invest. 1934;13(6):963–8.
López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response? Mitochondrion. 2013;13(2):106–18.
Malchow B, Hasan A, Fusar-Poli P, Schmitt A, Falkai P, Wobrock T. Cannabis abuse and brain morphology in schizophrenia: a review of the available evidence. Eur Arch Psychiatry Clin Neurosci. 2013;263(1):3–13.
Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, Sommer IEC, Howes OD. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med. 2018;49:1–11. https://doi.org/10.1017/S0033291718003057.
Martín-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P. Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2010;40(3):383–98.
Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(2):148–57.
Matsumoto H, Simmons A, Williams S, Pipe R, Murray R, Frangou S. Structural magnetic imaging of the hippocampus in early onset schizophrenia. Biol Psychiatry. 2001;49(10):824–31.
Medic N, Ziauddeen H, Ersche KD, Farooqi IS, Bullmore ET, Nathan PJ, Ronan L, Fletcher PC. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes (Lond). 2016;40(7):1177–82.
Meshul CK, Janowsky A, Casey DE, Stallbaumer RK, Taylor B. Effect of haloperidol and clozapine on the density of “perforated” synapses in caudate, nucleus accumbens, and medial prefrontal cortex. Psychopharmacology (Berl). 1992;106(1):45–52.
Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24(5):645–92.
Miller DD, Andreasen NC, O’Leary DS, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology. 1997;17(4):230–40.
Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–18.
Mitelman SA, Canfield EL, Newmark RE, Brickman AM, Torosjan Y, Chu KW, Hazlett EA, Haznedar M, Shihabuddin L, Buchsbaum MS. Longitudinal assessment of gray and white matter in chronic schizophrenia: a combined diffusion-tensor and structural magnetic resonance imaging study. Open Neuroimaging J. 2009;3:31–47. https://doi.org/10.2174/1874440000903010031.
Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104.
Moghaddam B, Javitt D. From the revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15.
Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, Palomo T, Desco M. Cerebral metabolism and risperidone treatment in schizophrenia. Schizophr Res. 2003;60(1):1–7.
Mondelli V, Pariante CM, Navari S, Aas M, D’Albenzio A, Di Forti M, Handley R, Hepgul N, Marques TR, Taylor H, Papadopoulos AS, Aitchison JK, Murray RM, Dazzan P. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res. 2010;119(1–3):75–8.
Moreno D, Burdalo M, Reig S, Parellada M, Zabala A, Desco M, Baca-Baldomero E, Arango C. Structural neuroimaging in adolescents with a first psychotic episode. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1151–7.
Moser DA, Doucet GE, Lee WH, Rasgon A, Krinsky H, Leibu E, Ing A, Schumann G, Rasgon N, Frangou S. Multivariate associations among behavioral, clinical, and multimodal imaging phenotypes in patients with psychosis. JAMA Psychiatry. 2018;75(4):386–95.
Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–20.
Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in first episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62(7):773–83.
Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta. 2009;1787(11):1416–142.
Nieuwenhuis M, Schnack HG, van Haren NE, Lappin J, Morgan C, Reinders AA, Gutierrez-Tordesillas D, Roiz-Santiañez R, Schaufelberger MS, Rosa PG, Zanetti MV, Busatto GF, Crespo-Facorro B, McGorry PD, Velakoulis D, Pantelis C, Wood SJ, Kahn RS, Mourao-Miranda J, Dazzan P. Multi-center MRI prediction models: predicting sex and illness course in first episode psychosis patients. Neuroimage. 2017;145(Pt B):246–53.
Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O’Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(1):96–101.
Nymberg C, Jia T, Ruggeri B, Schumann G. Analytical strategies for large imaging genetic datasets: experiences from the IMAGEN study. Ann N Y Acad Sci. 2013;1282:92–106.
O’Rahilly R, Müller F. Human embryology and teratology. 3rd ed. New York: Wiley-Liss; 2001.
Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–5.
Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, Yasuda Y, Fujimoto M, Watanabe Y, Yahata N, Nemoto K, Hibar DP, van Erp TGM, Fujino H, Isobe M, Isomura S, Natsubori T, Narita H, Hashimoto N, Miyata J, Koike S, Takahashi T, Yamasue H, Matsuo K, Onitsuka T, Iidaka T, Kawasaki Y, Yoshimura R, Watanabe Y, Suzuki M, Turner JA, Takeda M, Thompson PM, Ozaki N, Kasai K, Hashimoto R, COCORO. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21(10):1460–6.
Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88–96.
Osimo EF, Beck K, Marques TR, Howes OD. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol Psychiatry. 2018;24:549–61. https://doi.org/10.1038/s41380-018-0041-5.
Pakkenberg B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry. 1993;34(11):768–72.
Palaniyappan L, Al-Radaideh A, Mougin O, Gowland P, Liddle PF. Combined white matter imaging suggests myelination defects in visual processing regions in schizophrenia. Neuropsychopharmacology. 2013;38(9):1808–15.
Plavén-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, Mizrahi R, Pomper MG, Rusjan P, Veronese M, Wang Y, Cervenka S.Positron Emission Tomography Studies of the Glial Cell Marker Translocator Protein in Patients With Psychosis: A Meta-analysis Using Individual Participant Data. Biol Psychiatry. 2018;84(6):433–42. https://doi.org/10.1016/j.biopsych.2018.02.1171.
Plavén-Sigray P, Cervenka S. Meta-analytic studies of the glial cell marker TSPO in psychosis - a question of apples and pears? Psychol Med. 2019;49(10):1624–28. https://doi.org/10.1017/S003329171800421X.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–57.
Price G, Cercignani M, Chu EM, Barnes TR, Barker GJ, Joyce EM, Ron MA. Brain pathology in first-episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. Neuroimage. 2010;49(1):185–92.
Puri BK, Hutton SB, Saeed N, Oatridge A, Hajnal JV, Duncan L, Chapman MJ, Barnes TR, Bydder GM, Joyce EM. A serial longitudinal quantitative MRI study of cerebral changes in first-episode schizophrenia using image segmentation and subvoxel registration. Psychiatry Res. 2001;106:141–50.
Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195(5):393–402.
Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, Fusar-Poli P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36(10):2325–33.
Rais M, Cahn W, Van Haren N, Schnack H, Caspers E, Hulshoff Pol H, Kahn R. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165(4):490–6.
Rais M, van Haren NE, Cahn W, Schnack HG, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2010;20(12):855–65.
Rapoport JL, Addington AM, Frangou S. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10(5):434–49.
Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89.
Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51(2):501–11.
Reig S, Moreno C, Moreno D, Burdalo M, Janssen J, Parellada M, Zabala A, Desco M, Arango C. Progression of brain volume changes in adolescent-onset psychosis. Schizophr Bull. 2009;35(1):233–43.
Reig S, Parellada M, Castro-Fornieles J, Janssen J, Moreno D, Baeza I, Bargalló N, González-Pinto A, Graell M, Ortuño F, Otero S, Arango C, Desco M. Multicenter study of brain volume abnormalities in children and adolescent-onset psychosis. Schizophr Bull. 2011;37(6):1270–80.
Roberts RC. Postmortem studies on mitochondria in schizophrenia. Schizophr Res. 2017;187:17–25.
Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75(9):964–74.
Rozycki M, Satterthwaite TD, Koutsouleris N, Erus G, Doshi J, Wolf DH, Fan Y, Gur RE, Gur RC, Meisenzahl EM, Zhuo C, Yin H, Yan H, Yue W, Zhang D, Davatzikos C. Multisite machine learning analysis provides a robust structural imaging signature of schizophrenia detectable across diverse patient populations and within individuals. Schizophr Bull. 2018;44(5):1035–44.
Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160(2):284–9.
Saeys Y, Inza I, Larrañaga P. A review of feature selection techniques in bioinformatics. Bioinformatics. 2007;23(19):2507–17.
Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28(11):1735–42.
Schaufelberger MS, Lappin JM, Duran FL, Rosa PG, Uchida RR, Santos LC, Murray RM, McGuire PK, Scazufca M, Menezes PR, Busatto GF. Lack of progression of brain abnormalities in first-episode psychosis: a longitudinal magnetic resonance imaging study. Psychol Med. 2011;41(8):1677–89.
Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45(1):17–25.
Selemon LD, Lidow MS, Goldman-Rakic PS. Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry. 1999;46(2):161–72.
Sporn AL, Greenstein DK, Gogtay N, Jeffries NO, Lenane M, Gochman P, Clasen LS, Blumenthal J, Giedd JN, Rapoport JL. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am J Psychiatry. 2003;160(12):2181–9.
Squarcina L, Castellani U, Bellani M, Perlini C, Lasalvia A, Dusi N, Bonetto C, Cristofalo D, Tosato S, Rambaldelli G, Alessandrini F, Zoccatelli G, Pozzi-Mucelli R, Lamonaca D, Ceccato E, Pileggi F, Mazzi F, Santonastaso P, Ruggeri M, Brambilla P, GET UP Group. Classification of first-episode psychosis in a large cohort of patients using support vector machine and multiple kernel learning techniques. Neuroimage. 2017;145(Pt B):238–45.
Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8.
Takahashi T, Wood SJ, Soulsby B, McGorry PD, Tanino R, Suzuki M, Velakoulis D, Pantelis C. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr Res. 2009;108(1–3):49–56.
Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650–5.
Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro R, Jahanshad N, Schumann G, Franke B, Wright MJ, Martin NG, Agartz I, Alda M, Alhusaini S, Almasy L, Almeida J, Alpert K, Andreasen NC, Andreassen OA, Apostolova LG, Appel K, Armstrong NJ, Aribisala B, Bastin ME, Bauer M, Bearden CE, Bergmann O, Binder EB, Blangero J, Bockholt HJ, Bøen E, Bois C, Boomsma DI, Booth T, Bowman IJ, Bralten J, Brouwer RM, Brunner HG, Brohawn DG, Buckner RL, Buitelaar J, Bulayeva K, Bustillo JR, Calhoun VD, Cannon DM, Cantor RM, Carless MA, Caseras X, Cavalleri GL, Chakravarty MM, Chang KD, Ching CR, Christoforou A, Cichon S, Clark VP, Conrod P, Coppola G, Crespo-Facorro B, Curran JE, Czisch M, Deary IJ, de Geus EJ, den Braber A, Delvecchio G, Depondt C, de Haan L, de Zubicaray GI, Dima D, Dimitrova R, Djurovic S, Dong H, Donohoe G, Duggirala R, Dyer TD, Ehrlich S, Ekman CJ, Elvsåshagen T, Emsell L, Erk S, Espeseth T, Fagerness J, Fears S, Fedko I, Fernández G, Fisher SE, Foroud T, Fox PT, Francks C, Frangou S, Frey EM, Frodl T, Frouin V, Garavan H, Giddaluru S, Glahn DC, Godlewska B, Goldstein RZ, Gollub RL, Grabe HJ, Grimm O, Gruber O, Guadalupe T, Gur RE, Gur RC, Göring HH, Hagenaars S, Hajek T, Hall GB, Hall J, Hardy J, Hartman CA, Hass J, Hatton SN, Haukvik UK, Hegenscheid K, Heinz A, Hickie IB, Ho BC, Hoehn D, Hoekstra PJ, Hollinshead M, Holmes AJ, Homuth G, Hoogman M, Hong LE, Hosten N, Hottenga JJ, Hulshoff Pol HE, Hwang KS, Jack CR Jr, Jenkinson M, Johnston C, Jönsson EG, Kahn RS, Kasperaviciute D, Kelly S, Kim S, Kochunov P, Koenders L, Krämer B, Kwok JB, Lagopoulos J, Laje G, Landen M, Landman BA, Lauriello J, Lawrie SM, Lee PH, Le Hellard S, Lemaître H, Leonardo CD, Li CS, Liberg B, Liewald DC, Liu X, Lopez LM, Loth E, Lourdusamy A, Luciano M, Macciardi F, Machielsen MW, Macqueen GM, Malt UF, Mandl R, Manoach DS, Martinot JL, Matarin M, Mather KA, Mattheisen M, Mattingsdal M, Meyer-Lindenberg A, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meisenzahl E, Melle I, Milaneschi Y, Mohnke S, Montgomery GW, Morris DW, Moses EK, Mueller BA, Muñoz Maniega S, Mühleisen TW, Müller-Myhsok B, Mwangi B, Nauck M, Nho K, Nichols TE, Nilsson LG, Nugent AC, Nyberg L, Olvera RL, Oosterlaan J, Ophoff RA, Pandolfo M, Papalampropoulou-Tsiridou M, Papmeyer M, Paus T, Pausova Z, Pearlson GD, Penninx BW, Peterson CP, Pfennig A, Phillips M, Pike GB, Poline JB, Potkin SG, Pütz B, Ramasamy A, Rasmussen J, Rietschel M, Rijpkema M, Risacher SL, Roffman JL, Roiz-Santiañez R, Romanczuk-Seiferth N, Rose EJ, Royle NA, Rujescu D, Ryten M, Sachdev PS, Salami A, Satterthwaite TD, Savitz J, Saykin AJ, Scanlon C, Schmaal L, Schnack HG, Schork AJ, Schulz SC, Schür R, Seidman L, Shen L, Shoemaker JM, Simmons A, Sisodiya SM, Smith C, Smoller JW, Soares JC, Sponheim SR, Sprooten E, Starr JM, Steen VM, Strakowski S, Strike L, Sussmann J, Sämann PG, Teumer A, Toga AW, Tordesillas-Gutierrez D, Trabzuni D, Trost S, Turner J, Van den Heuvel M, van der Wee NJ, van Eijk K, van Erp TG, van Haren NE, van’t Ent D, van Tol MJ, Valdés Hernández MC, Veltman DJ, Versace A, Völzke H, Walker R, Walter H, Wang L, Wardlaw JM, Weale ME, Weiner MW, Wen W, Westlye LT, Whalley HC, Whelan CD, White T, Winkler AM, Wittfeld K, Woldehawariat G, Wolf C, Zilles D, Zwiers MP, Thalamuthu A, Schofield PR, Freimer NB, Lawrence NS, Drevets W, Alzheimer’s Disease Neuroimaging Initiative, EPIGEN Consortium, IMAGEN Consortium, Saguenay Youth Study (SYS) Group. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8(2):153–82.
Thompson PM, Andreassen OA, Arias-Vasquez A, Bearden CE, Boedhoe PS, Brouwer RM, Buckner RL, Buitelaar JK, Bulayeva KB, Cannon DM, Cohen RA, Conrod PJ, Dale AM, Deary IJ, Dennis EL, de Reus MA, Desrivieres S, Dima D, Donohoe G, Fisher SE, Fouche JP, Francks C, Frangou S, Franke B, Ganjgahi H, Garavan H, Glahn DC, Grabe HJ, Guadalupe T, Gutman BA, Hashimoto R, Hibar DP, Holland D, Hoogman M, Pol HEH, Hosten N, Jahanshad N, Kelly S, Kochunov P, Kremen WS, Lee PH, Mackey S, Martin NG, Mazoyer B, McDonald C, Medland SE, Morey RA, Nichols TE, Paus T, Pausova Z, Schmaal L, Schumann G, Shen L, Sisodiya SM, Smit DJA, Smoller JW, Stein DJ, Stein JL, Toro R, Turner JA, van den Heuvel MP, van den Heuvel OL, van Erp TGM, van Rooij D, Veltman DJ, Walter H, Wang Y, Wardlaw JM, Whelan CD, Wright MJ, Ye J. ENIGMA Consortium. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 2017;145(Pt B):389–408.
Thune JJ, Uylings HB, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35(1):15–21.
Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat Neurosci. 2010;13(8):920–2.
Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55(5):597–610.
Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67(2–3):269–75.
van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MW, Koenders L, de Haan L, Veltman DJ, Satterthwaite TD, Wolf DH, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller BA, Preda A, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NE, Pol HE, Ophoff RA, Kahn RS, Roiz-Santiañez R, Crespo-Facorro B, Wang L, Alpert KI, Jönsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh AM, Lawrie SM, Hashimoto R, Thompson PM, Turner JA. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–52.
van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, Pearlson GD, Yao N, Fukunaga M, Hashimoto R, Okada N, Yamamori H, Bustillo JR, Clark VP, Agartz I, Mueller BA, Cahn W, de Zwarte SMC, Hulshoff Pol HE, Kahn RS, Ophoff RA, van Haren NEM, Andreassen OA, Dale AM, Doan NT, Gurholt TP, Hartberg CB, Haukvik UK, Jørgensen KN, Lagerberg TV, Melle I, Westlye LT, Gruber O, Kraemer B, Richter A, Zilles D, Calhoun VD, Crespo-Facorro B, Roiz-Santiañez R, Tordesillas-Gutiérrez D, Loughland C, Carr VJ, Catts S, Cropley VL, Fullerton JM, Green MJ, Henskens FA, Jablensky A, Lenroot RK, Mowry BJ, Michie PT, Pantelis C, Quidé Y, Schall U, Scott RJ, Cairns MJ, Seal M, Tooney PA, Rasser PE, Cooper G, Shannon Weickert C, Weickert TW, Morris DW, Hong E, Kochunov P, Beard LM, Gur RE, Gur RC, Satterthwaite TD, Wolf DH, Belger A, Brown GG, Ford JM, Macciardi F, Mathalon DH, O’Leary DS, Potkin SG, Preda A, Voyvodic J, Lim KO, McEwen S, Yang F, Tan Y, Tan S, Wang Z, Fan F, Chen J, Xiang H, Tang S, Guo H, Wan P, Wei D, Bockholt HJ, Ehrlich S, Wolthusen RPF, King MD, Shoemaker JM, Sponheim SR, De Haan L, Koenders L, Machielsen MW, van Amelsvoort T, Veltman DJ, Assogna F, Banaj N, de Rossi P, Iorio M, Piras F, Spalletta G, McKenna PJ, Pomarol-Clotet E, Salvador R, Corvin A, Donohoe G, Kelly S, Whelan CD, Dickie EW, Rotenberg D, Voineskos AN, Ciufolini S, Radua J, Dazzan P, Murray R, Reis Marques T, Simmons A, Borgwardt S, Egloff L, Harrisberger F, Riecher-Rössler A, Smieskova R, Alpert KI, Wang L, Jönsson EG, Koops S, Sommer IEC, Bertolino A, Bonvino A, Di Giorgio A, Neilson E, Mayer AR, Stephen JM, Kwon JS, Yun JY, Cannon DM, McDonald C, Lebedeva I, Tomyshev AS, Akhadov T, Kaleda V, Fatouros-Bergman H, Flyckt L, Karolinska Schizophrenia Project, Busatto GF, Rosa PGP, Serpa MH, Zanetti MV, Hoschl C, Skoch A, Spaniel F, Tomecek D, Hagenaars SP, McIntosh AM, Whalley HC, Lawrie SM, Knöchel C, Oertel-Knöchel V, Stäblein M, Howells FM, Stein DJ, Temmingh HS, Uhlmann A, Lopez-Jaramillo C, Dima D, McMahon A, Faskowitz JI, Gutman BA, Jahanshad N, Thompson PM, Turner JA. Biol Psychiatry. 2018;84(9):644–54.
van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106–13.
van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68(9):871–80.
van Haren NE, Schnack HG, Koevoets MGJC, Cahn W, Hulshoff Pol HE, Kahn RS. Trajectories of subcortical volume change in schizophrenia: a 5-year follow-up. Schizophr Res. 2016;173(3):140–5.
van Kesteren CF, Gremmels H, de Witte LD, Hol EH, Van Gool AH, Falkai PH, Kahn RS, Sommer IE. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on post-mortem brain studies. Transl Psychiatry. 2017;7:e1075. https://doi.org/10.1038/tp.2017.4.
Veijola J, Guo JY, Moilanen JS, Jääskeläinen E, Miettunen J, Kyllönen M, Haapea M, Huhtaniska S, Alaräisänen A, Mäki P, Kiviniemi V, Nikkinen J, Starck T, Remes JJ, Tanskanen P, Tervonen O, Wink AM, Kehagia A, Suckling J, Kobayashi H, Barnett JH, Barnes A, Koponen HJ, Jones PB, Isohanni M, Murray GK. Longitudinal changes in total brain volume in schizophrenia: relation to symptom severity, cognition and antipsychotic medication. PLoS One. 2014;9(7):e101689. https://doi.org/10.1371/journal.pone.0101689.
Vernon AC, Natesan S, Modo M, Kapur S. Effect of chronic antipsychotic treatment on brain structure: a serial magnetic resonance imaging study with ex vivo and postmortem confirmation. Biol Psychiatry. 2011;69(10):936–44.
Vernon AC, Natesan S, Crum WR, Cooper JD, Modo M, Williams SC, Kapur S. Contrasting effects of haloperidol and lithium on rodent brain structure: a magnetic resonance imaging study with postmortem confirmation. Biol Psychiatry. 2012;71(10):855–63.
Veronese E, Castellani U, Peruzzo D, Bellani M, Brambilla P. Machine learning approaches: from theory to application in schizophrenia. Comput Math Methods Med. 2013;2013:867924. https://doi.org/10.1155/2013/867924.
Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82(1):75–88.
Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. https://doi.org/10.1038/tp.2012.116.
Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 2015;78(6):403–12.
Wakade CG, Mahadik SP, Waller JL, Chiu FC. Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res. 2002;69(1):727–9.
Walker MA, Highley JR, Esiri MM, McDonald B, Roberts HC, Evans SP, et al. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. Am J Psychiatry. 2002;159(5):821–8.
Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216.
Walton E, Hibar DP, van Erp TG, Potkin SG, Roiz-Santiañez R, Crespo-Facorro B, Suarez-Pinilla P, Van Haren NE, de Zwarte SM, Kahn RS, Cahn W, Doan NT, Jørgensen KN, Gurholt TP, Agartz I, Andreassen OA, Westlye LT, Melle I, Berg AO, Mørch-Johnsen L, Faerden A, Flyckt L, Fatouros-Bergman H, Karolinska Schizophrenia Project Consortium (KaSP), Jönsson EG, Hashimoto R, Yamamori H, Fukunaga M, Preda A, De Rossi P, Piras F, Banaj N, Ciullo V, Spalletta G, Gur RE, Gur RC, Wolf DH, Satterthwaite TD, Beard LM, Sommer IE, Koops S, Gruber O, Richter A, Krämer B, Kelly S, Donohoe G, McDonald C, Cannon DM, Corvin A, Gill M, Di Giorgio A, Bertolino A, Lawrie S, Nickson T, Whalley HC, Neilson E, Calhoun VD, Thompson PM, Turner JA, Ehrlich S. Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 2017;135(5):439–47.
Walton E, Hibar DP, van Erp TGM, Potkin SG, Roiz-Santiañez R, Crespo-Facorro B, Suarez-Pinilla P, van Haren NEM, de Zwarte SMC, Kahn RS, Cahn W, Doan NT, Jørgensen KN, Gurholt TP, Agartz I, Andreassen OA, Westlye LT, Melle I, Berg AO, Morch-Johnsen L, Færden A, Flyckt L, Fatouros-Bergman H, Karolinska Schizophrenia Project Consortium (KaSP), Jönsson EG, Hashimoto R, Yamamori H, Fukunaga M, Jahanshad N, De Rossi P, Piras F, Banaj N, Spalletta G, Gur RE, Gur RC, Wolf DH, Satterthwaite TD, Beard LM, Sommer IE, Koops S, Gruber O, Richter A, Krämer B, Kelly S, Donohoe G, McDonald C, Cannon DM, Corvin A, Gill M, Di Giorgio A, Bertolino A, Lawrie S, Nickson T, Whalley HC, Neilson E, Calhoun VD, Thompson PM, Turner JA, Ehrlich S. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med. 2018;48(1):82–94.
Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33(6):1276–86.
Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29(7):1230–8.
Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–86.
Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, Walch T, Wanko C, Weiss EM, Stuppaeck CH, Fleischhacker WW. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res. 2005;140(3):225–37.
Wood SJ, Velakoulis D, Smith DJ, Bond D, Stuart GW, McGorry PD, Brewer WJ, Bridle N, Eritaia J, Desmond P, Singh B, Copolov D, Pantelis C. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res. 2001;52(1–2):37–46.
Wright AM, Bempong J, Kirby ML, Barlow RL, Bloomquist JR. Effects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesia. Brain Res. 1998;788(1–2):215–22.
Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25.
Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp. 2009;30(3):711–24.
Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32(9):2060–7.
Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Ann General Psychiatry. 2008;7:25. https://doi.org/10.1186/1744-859X-7-25.
Zahr NM, Pfefferbaum A. Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res. 2017;38(2):183–206.
Zanetti MV, Schaufelberger MS, Doshi J, Ou Y, Ferreira LK, Menezes PR, Scazufca M, Davatzikos C, Busatto GF. Neuroanatomical pattern classification in a population-based sample of first-episode schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;43:116–25.
Zheutlin AB, Chekroud AM, Polimanti R, Gelernter J, Sabb FW, Bilder RM, Freimer N, London ED, Hultman CM, Cannon TD. Multivariate pattern analysis of genotype-phenotype relationships in schizophrenia. Schizophr Bull. 2018;44(5):1045–52.
Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39(6):1363–72.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Frangou, S., Kahn, R.S. (2020). Gray Matter Involvement in Schizophrenia: Evidence from Magnetic Resonance Imaging Studies. In: Kubicki, M., Shenton, M. (eds) Neuroimaging in Schizophrenia . Springer, Cham. https://doi.org/10.1007/978-3-030-35206-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-35206-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35205-9
Online ISBN: 978-3-030-35206-6
eBook Packages: MedicineMedicine (R0)