Abstract
Tourette syndrome (TS) is a disorder manifested by motor and vocal tics, frequently associated with other behavioral disorders. While TS frequently improves as patients age, a subset of patients remain disabled by their condition and are candidates for surgical intervention. DBS has been shown to be effective in refractory TS patients, although the most appropriate target remains a subject of debate, with multiple intracranial DBS targets demonstrating similar clinical efficacy. We discuss the underlying physiology of TS, a history of neurosurgical intervention for this condition beginning with lesioning and proceeding to DBS, and review surgical technique, complications, and outcome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tourette syndrome
- DBS
- Medial thalamus
- Globus pallidus
- Tic disorder
- Internal capsule
- OCD
- ADHD
- Tourette Syndrome Association
- Movement disorder
Introduction
The history of the neurosurgical treatment of Gilles de la Tourette syndrome (TS) – whether via lesioning or stimulation – has been predicated on the notion that intervention at key nodes can improve disorder-specific circuit abnormalities. One major, unresolved question at the time of this writing is what or which are the key surgical nodes in TS, a question that is particularly challenging given the variable, multifaceted nature of TS, which often includes prominent obsessive-compulsive and attention-deficit behaviors alongside the hyperkinetic motor and vocal tic behaviors. Historically, as we shall review, surgical approaches have often targeted regions classically associated either with the presumed compulsive nature of tics akin to targets used for OCD (e.g., cingulum and anterior limb of the internal capsule) or regions more commonly associated with hyperkinetic movement disorders (e.g., the motor thalamus and posteroventral globus pallidus). Over time, subregions within the thalamus and pallidum, areas that represent a kind of crossroads between motor and limbic function, have emerged as stereotactic targets of choice, namely, the anteromedial globus pallidus and the dorsomedial thalamus. These two areas will receive the greater focus of our attention but not to the exclusion of other still-utilized targets.
The TS Network
A basic understanding of the underlying circuit-based abnormalities in TS provides a useful platform for better understanding the surgical history. The cause or causes of TS are currently unknown. Genetics clearly plays a role in many cases with particular interest focusing on the role of single nucleotide polymorphisms, but the exact genetic underpinning has yet to be identified [1, 2]. Subtle neuropathological abnormalities have been reported (e.g., changes in caudate and thalamic volumes) but not consistently [3, 4]. What has been repeatedly observed, however, is a difference in regional brain metabolism supporting a circuit-based pathophysiology or, more specifically, a cortico-striato-thalamo-cortical (CSTC) abnormality [5]. In this model, simplified for our present purposes, aberrant activation of striatal neurons with inhibitory connections to GPi/SN leads to disinhibition of thalamocortical projections. The result is an imbalance of the normal promotion of voluntary movements that leads to unwanted, specific motor patterns manifesting as tics. The prominent striatal role is supported by the symptomatic improvement following dopamine blocking and depleting therapies (the mainstay of medical management for decades) as well as evidence supporting metabolic “normalization” following successful surgical intervention [6].
Surgical History
The first contemporary references to surgical intervention for TS involved anecdotal reports of patients who underwent frontal lobotomies and leukotomies in the 1950s (Stevens 1955; Baker 1960) often for treatment of comorbid psychiatric symptoms. Beginning in the 1960s, the medial thalamus – which was noted to have degenerated following these more indiscriminate procedures – was more specifically targeted for TS [7]. Cooper, an early pioneer of stereotactic lesioning for movement disorders, targeted the ventrolateral thalamus with some reported success, followed soon after by Dieckmann and Hassler who, drawing from their prior experience lesioning patients with OCD, theorized that tics were a form of “motor obsessional phenomena” [8]. They targeted the medial thalamus with a fairly extensive lesion that involved the rostral interlaminar, ventro-oralis, and centromedian-parafascicular nuclei. They reported improvement of 70–100% in 3 patients and, interestingly for the pre-DBS era, noted different symptomatic responses when testing stimulation frequencies prior to lesioning. Their approach, with its reported good results and safety outcomes, would provide a roadmap for the earliest DBS interventions some 35 years later and can thus be seen as an important landmark. At the time, however, they shared company with a variety of other approaches that included dentotomies, limbic leukotomies, and anterior cingulotomies [9, 10]. From the 1950s through the 1980s, approximately 65 cases were reported involving lesioning of these and other targets thought to be involved in the TS network. Reports often provided limited information on the precise location of the lesion and results generally lacked specifics in terms of pre/post tic evaluations. Furthermore, complications in the pre-DBS era were often considerable ranging from debilitating dysarthria (a not infrequent complication of bilateral thalamic lesioning), dystonia, ataxia, and hemiplegia [7, 11]. Interestingly, the data (limited though it is) suggested that targeting the cingulum, which was seen to be effective for OCD, was less effective for motor tics, suggesting that TS and OCD, while sharing many features, are not necessarily amenable to the same intervention [10]. The era of DBS, starting in the late 1980s, saw a rekindling of interest in possible surgical intervention for TS even if the ideal target remained a matter of conjecture. Targeting possibilities seemed to broaden rather than narrow with anecdotal reports of tics improving following STN DBS in a patient with Parkinson’s and TS, and following GPI DBS for patients with dystonia and TS [12, 13]. The first reported case of DBS specifically for TS was in 1999 by Vandewalle, who targeted Hassler’s aforementioned centromedian thalamic region and described the tics at 1 year as “abolished” [14]. Since that time, approximately 200 DBS cases, often as part of small case series, have been reported using as many as nine stereotactic targets, though largely focused on medial thalamic subregions, pallidal subregions, and the anterior limb of the internal capsule. Optimal candidates, optimal targets, and optimal programming approaches remain topics of debate with consensus being further hampered by the relatively small patient population requiring surgical intervention. Nevertheless, results continue to support the potential for improvement, sometimes dramatic and lasting, in properly selected patients as shall be discussed below.
Candidate Selection
Consensus guidelines for patient selection were proposed in 2006 and slightly revised in 2015 [15, 16]. Being a surgical procedure with its attendant risks (as discussed below), candidates should have sufficient burden from their TS and should have tried the commonly prescribed medications before being considered. The Yale Global Tic Severity Scale Score (YGTSS), which grades motor and vocal tics based on a number of variables with maximal score of 50 (or 100 if including the 50-point impairment score), is often used as a proxy of severity. The latest consensus guidelines suggest a score of 35/50 being indicative of sufficiently severe TS to warrant surgical. While this is not unreasonable, it should be borne in mind that the score cannot entirely stratify potential risk of harm from tics and, being report-based, can over- or underestimate severity at any given point in time. For example, a single, forceful neck-jerking tic that poses a risk for cervical myelopathy might result in a relatively low YGTSS in the absence of other more complex tics. Conversely, an individual could have a large variety of complex motor and vocal tics, all on the milder side and not impacting quality of life greatly despite a high YGTSS score. Therefore, understanding the risk of harm and impairment from tics is more important than a particular number, and this is now also acknowledged in the consensus algorithm. Although the evidence for various specific medications in treatment of TS is often weak, standard of care includes trials of alpha-adrenergic agents, dopamine blocking, and/or depleting medications. Not every medication within these classes needs to be tried, but clearly, treatment by an experienced TS specialist familiar with appropriate options is required before a patient is deemed medically refractory. In addition to medications, there is good evidence that cognitive behavioral therapy or habit reversal therapy is helpful to some TS patients and should be pursued prior to DBS. From a practical standpoint, it is not always easy to identify experienced cognitive behavioral specialists, insurance coverage can be challenging, and evidence for its efficacy in the most severe TS cases is lacking, but given the possibility for benefit, every effort to connect patients with a behavioral therapist prior to DBS should be undertaken.
The appropriate minimum age at which surgery be considered has been a matter of debate. TS often naturally wanes in early adulthood and so performing brain surgery on a minor who might improve with time alone has been viewed with some apprehension. In the initial proposed algorithm from the Tourette Syndrome Association [15], the suggested minimum age was 25, at which point the likelihood of natural attenuation was assumed diminishing. Arguing against this conservative approach was the contention that severe TS in younger patients could be associated with significant physical and psychosocial disability, and thus performing an intervention earlier could have a meaningful long-term impact. The revised TSA algorithm [16] took a more nuanced stand, recommending that surgery only be considered in patients under 18 in cases where a multidisciplinary team and local ethic committee reviews the circumstances of a given case and weighs the relative risks and benefits. Our current approach, particularly given the lack of FDA approval for the indication at present, is to have all cases reviewed by a multidisciplinary committee not involved in the case with the addition of a pediatric specialist for patients under 18 and, though not absolute, will generally not perform surgery in patients under 16.
TS often keeps company with other neuropsychiatric and behavioral symptoms including depression, anxiety, OCD, and ADHD. The response to these does not always follow suit even following successful reduction in tic severity (OCD, for example, is common and sometimes improves alongside tics but can remain unchanged or even worsen despite improvement in tics in some cases). It is thus helpful to understand how much of a potential candidate’s quality of life is impaired by tics and ideally select those whose benefit would not be undercut by significant, persistent depression or OCD. Optimizing and understanding comorbid factors prior to surgery and often working as part of a multispecialty team are vital to optimal outcomes. A particular emphasis on identifying suicidal or addictive behaviors that could potentially worsen following DBS (especially if results are not as positive as hoped for) is of paramount importance. Along these lines, framing patient expectations is a very important part of candidate selection. As will be detailed below, outcomes are variable and nonresponders difficult to predict prospectively, so patients should understand the potential for no or minimal improvement. Thoughtfully consider whether a patient’s expectations are realistic.
In summary, a reasonable candidate is an otherwise healthy individual with a clear diagnosis of TS who has failed an adequate trial of medications and behavioral therapy as assessed by an experienced specialist and has significant impairment resulting chiefly if not exclusively from the motor and/or vocal tics with reasonable expectations and understanding of the possible outcomes and risks.
The Surgical Targets
The recent publication by international Tourette Syndrome Deep Brain Stimulation Public Database and Registry [17], pooling case data from multiple centers and reporting on 185 patients, reports the most common surgical as the centromedian thalamic region (57.1%), followed by the anterior globus pallidus internus (25.2%), posterior GPi (15.3%), and the anterior limb of the internal capsule (2.5%). There is currently insufficient evidence to suggest which target affords the most clinical benefit, as no significant difference in outcome was noted between targets.
Surgical Technique
As in DBS for the more common movement disorders (PD, ET, dystonia), the surgical technique varies from center to center. Challenges unique to the TS population include a relatively young patient age, as well as the potential for an increased rate of surgical complications due to the presence of self-mutilatory and/or OCD behavior in a substantial fraction of these patients [18]. While the authors’ surgical target (medial thalamus, described as CM/Pf/Voi depending on the publication) has remained the same over our 10-year experience, we have modified our technique due to both our own clinical experience as well as the introduction of adjunctive technology.
Staging
Our preference is to perform the surgery in a staged fashion, with simultaneous bilateral cranial lead placement as an inpatient procedure, followed by generator placement 1–2 weeks later as an outpatient.
Choice of generator
We have moved from placing bilateral single-channel devices to dual channel rechargeable devices, given the aforementioned young patient age as well as the real-world experience of insurance company denials of generator replacement surgery, despite having approved the initial implantation.
Anesthetic technique
For lead placement, we have utilized both the traditional method of awake/conscious sedation surgery with agents such as propofol and dexmedetomidine, which provides the opportunity for both microelectrode recording and macrostimulation to assess for both clinical efficacy and side effects. Given the severe and violent tics experienced by many of our patients resulting in difficulty with maintaining an appropriate level of conscious sedation, combined with the introduction of intraoperative CT, we have begun to perform all our of lead placement surgeries under general anesthesia, with anesthetic techniques that still allow for microelectrode recording. We utilize frame-based stereotaxis with MRI-CT fusion, with the MRI scan performed in the weeks prior to surgery under general anesthesia. CT scanning is performed following frame placement for the purposes of stereotactic target calculation and after each lead is placed to confirm lead placement.
Surgical targeting
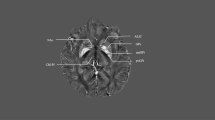
The choice of target, by definition, determines the method used for anatomic target identification. Whereas the pallidal and capsular targets can be visualized on MRI, individual thalamic nuclei including the centromedian region remain difficult if not impossible to target directly, and thus indirect anterior-posterior commissure-based targeting remains the primary targeting method. Our thalamic coordinates are 5 mm lateral to midline, 4 mm posterior to the midcommissural point, and on the AC-PC plane (Z = 0). These coordinates reflect the location of the electrode tip, which corresponds to Hassler’s substantia periventricularis (Spv). The deepest (most ventral) contact is rarely used, and the actual locus of stimulation in our experience maps to the junction of the Voi/CmPf [19] (Fig. 31.1). For this rather medial target, lateral approach angles (usually over 30 degrees in the coronal plane), are necessary. Sagittal angles are similar to those traditionally used for the Vim thalamic and STN targets, from 50 to 70 degrees posterior in the sagittal plane (Fig. 31.2).
Image from the Schaltenbrand and Wahren stereotactic atlas, axial slice at 2.0 mm above the AC-PC plane. The yellow marker represents the average area of stimulation as calculated using the postoperative imaging studies. This point corresponds to the calculated average area of stimulation in our series. (From Dowd et al. [19]. Reprinted with permission from Journal of Neurosurgery)
Microelectrode Recording and Macrostimulation
Our findings during microelectrode recording (MER) of the medial thalamus are similar to those reported in the literature [20]. We have found that the cessation of thalamic bursting activity can be useful to identify the laterality of the trajectory. Thalamic bursting cells are usually encountered until the microelectrode tip exits the thalamus and enters the SPv, approximately 2 mm above the target. Loss of thalamic activity earlier in the trajectory suggests a medial deviation, and continued thalamic activity closer to target suggests the opposite, namely, a lateral offset.
As the medial thalamus is distant from the corticospinal tracts as well as the medial lemniscus, motor and sensory effects are not seen with macrostimulation. In awake patients, ventral stimulation can result in a subjective complaint of dizziness. Whereas direct tic suppression is difficult to confirm intraoperatively, stimulation slightly dorsal to the tip will, in some patients, elicit a sensation of “calmness.”
Outcomes
A pithy distillation of TS outcomes across numerous studies would be to state that approximately 50% of patients improve about 50%. A more rigorous examination of the data, however, leads to a far more nuanced and cautious assessment of the results along with greater awareness of the imperfections and limitations of the relatively small and variable data. The vast majority of the reported outcomes derive from small, retrospective, non-blinded, non-placebo-controlled case series. Different age ranges, different rating scales, different brain targets, different stimulation paradigms, and different follow-up periods make it hard to glean a clear picture let alone make convincing comparisons across different studies. Despite these important caveats, reported outcomes from approximately 200 patients (at time of this writing), including a handful of small, randomized trials and published meta-analyses, allow for some general remarks. Rather than delving into the details of each small case series, we will highlight an illustrative few that will hopefully provide context for better understanding of some pooled analyses.
Thalamic DBS for TS
As mentioned above, the medial thalamus was an early target for TS in the pre-DBS era and was the first dedicated target for DBS in 1999 with Vandewalle reporting a 72–90% reduction in tic severity in 3 patients at up to 72 months follow-up [14, 21, 22]. The thalamus has subsequently remained the most common target but where precisely within the medial thalamus is targeted has varied across different centers. For example, the Milan-Bergamot group, with the largest thalamic case series published to date, targeted approximately 2 mm anterior to Vandewalle’s target and reported a 24–72% improvement in the YGTSS in 18 patients at up to 18 months follow-up [23]. Interestingly, while the tic reduction was highly statistically significant, there was a surprising lack of concordance between patient and physician perceptions of outcome in nearly half the cases. This disconnect speaks perhaps to the complexity of issues TS patients deal with that are not captured by a tic-related rating scale. Our group reported results in 13 patients who had undergone medial thalamic DBS and found a 50% reduction at last follow-up (ranging from 6 months to 5 years [19]. Closer inspection of the improvement on a case-by-case basis revealed a separation between marked responders and mild to minimal responders with no clear prospectively differentiating features. Notably, all subjects including those with less robust YGTSS reductions reported that they would repeat the procedure knowing what they know now. Two blinded, crossover studies highlight, in part, the challenges of performing such studies in this patient population. Maciunas et al. performed a double-blind, randomized stimulation versus sham stimulation study in 5 patients over 4 weeks followed by open, unblinded stimulation for 3 months [24]. A video assessment, which was selected as the primary outcome, did demonstrate significant overall improvement comparing stimulation versus non-stimulation, but the YGTSS, selected as a secondary outcome, did not reach significance perhaps in part due to the small number and in part perhaps related to varying overall outcomes with 3 of 5 patients demonstrating a more robust improvement. No clear factors appeared to distinguish the responders from the 2 nonresponders. Ackermans et al. also undertook a blinded study but faced recruitment challenges with only 8 subjects over 4 years and only 6 who completed 1 year follow-up with a randomized OFF versus ON assessment at 3 months [25]. At 3 months there was a 37% improvement ON versus OFF stimulation (though not statistically significant) with a 49% improvement at 1 year, again with varying individual degrees of improvement. Despite improvement, all patients reported a sense of diminished energy and visual complaints without associated, objective findings on examination.
Pallidal DBS for TS
Pallidal DBS results are likewise chiefly derived from case series. A small double-blind trial of 3 patients implanted with both pallidal and thalamic electrodes suggested a greater response to pallidal stimulation at 20–60 months follow-up compared with thalamic or thalamic and pallidal together [26]. Though generalizing from a small study is difficult, it did generate increased interest in pallidal stimulation. As with the medial thalamus, targeting has varied with some centers using the traditional posteroventral “motor” target and others using a more anteromedial target with some studies including a mix of the two. One of the largest case series involving both pallidal targets (though predominantly the anteromedial target) involved 15 patients, 13 of whom successfully completed a double-blind, crossover trial of on versus off stimulation evaluations for two three-month periods followed by open-label on stimulation follow-up of up to 36 months [27]. Improvement during the blinded phase was modest and not statistically significant, but open-labeled follow-up demonstrated a 40% improvement in the YGTSS with associated improvements in quality of life scales. Martinez-Torres et al. also reported a mixed cohort of anteromedial and posteroventral GPI DBS in 5 patients and found variable degrees of improvement, slightly more robust with the anteromedial target compared with the posteroventral [28]. In an open-label series of 17 patients targeting the anteromedial GPI, Sachdev et al. reported a mean reduction of 54% in the YGTSS in patients followed up to 46 months with 12 of the 17 improving over 50% and all but one reporting some perceived benefit [29]. Eleven of the 17 also reported some improvement in their OCD symptoms.
Other Targets for TS (ALIC, STN)
The anterior limb of the internal capsule is the approved target for OCD, and as TS shares obsessive/compulsive features, it is perhaps not surprising that it has been used to treat TS as well. The majority of ALIC reports for TS are limited to single case reports [30,31,32,33]. The small numbers make generalizable conclusions difficult particularly as results have varied from worsening of tics [30] to dramatic reduction of tics and OCD [33]. In one open-label case report, a patient received a 25% improvement in YGTSS global severity following ALIC but experienced apathy or hypomania with adjustments. Following a lead fracture, the ALIC electrodes were replaced with medial thalamic electrodes resulting in a 50% overall improvement without stimulation-associated mood issues despite considerably higher stimulation parameters [34]. The STN is another potential if seldom utilized contender in the busy field of potential TS targets. Stimulation of the STN, often preferred for Parkinson’s, has been reported to improve OCD [35] and, in a single case report, improved tics in a patient suffering from both PD and TS [13].
Meta-Analyses
Two recent reports, one a meta-analysis based on review of the published literature and another a meta-analysis of data from the International Deep Brain Stimulation Registry and Database for Tourette Syndrome [17, 36], convey a broader sense of the outcomes to date. Baldermann et al. reviewed 57 articles consisting of 156 cases, 78 being thalamic, 64 being pallidal, and 9 ALIC [36]. Median age at time of surgery was 30 (15–60) with a mean improvement 53% (mainly derived from changes in YGTSS from median 83 to 35). Reduction in motor tics was about equivalent to reduction in vocal tics at 39% and 40%, respectively, with >50% of patients improving by >50%. In comparing outcomes across targets, they found the median YGTSS improvement following thalamic DBS to be 48% compared with Gpi-pl at 58%, Gpi-am at 55%, and ALIC at 44%. OCD scores, measured using the YBOCS, had a mean improvement of 31% (median 16 to 11) and were similar across targets. There was a trend toward more improvement in younger patients, but no particular target was unequivocally superior nor were any determining factors identifiable in terms of separating responders from nonresponders. The DBS Registry and Database encompasses the pooled data from 31 actively involved DBS centers across 9 countries and recently reported 12-month data on 163 patients (many of whom are also included in the aforementioned meta-analysis). In terms of demographics, the population was 72% male with a mean age at surgery 29.5 (youngest being 13). Fifty-seven percent received thalamic stimulation, while 25% received anterior pallidal, 15% posterior pallidal, and 3% ALIC. The pooled improvement in the YGTSS was 44.1% with vocal responding slightly more than motor tics. Most improvement was obtained by 6 months and maintained at 1 year, and no significant differences were clearly perceived between targets though the most robust improvement compared with baseline was noted in the anterior pallidal cohort though not to a point where any clear recommendations could be made in regard to preferred target selection.
Complications
Although the risk of serious adverse events following DBS at experienced centers is low, it has been repeatedly noted that the risk of complications appear to be higher in the TS population compared with indications such as Parkinson’s disease [19, 23, 29]. Reasons for this may include the presence of obsessive behaviors such as picking at incision sites or compulsive twiddling of the pulse generator resulting in infection or hardware malfunction (both of which occurred in our patient cohort and required hardware removal without lasting sequelae) [37]. Serious intraoperative complications at experienced centers appear to be relatively uncommon and were reported as 1.3% in the International TS DBS Registry. A thalamic hemorrhage at the lead tip in one patient resulted in a gaze palsy [25]. Postoperative infections and hardware malfunctions likewise appear to be more common in TS, reported as occurring in 2.5% of patients in the Registry. Servello, in reviewing all DBS cases, reported a higher incidence of infectious complication in patients who received DBS for TS [18]. It does not appear that a particular target is inherently more or less risk-prone. As many TS patients receive DBS at a relatively young age, the compounded risk of long-term indwelling hardware and IPG replacements also needs consideration. Stimulation-related complications – though generally reversible – are not infrequent in TS. The TS Registry reported as many as 30% of patients experiencing a stimulation-related side effect, perhaps not surprising given the high stimulation parameters used in some patients. Thalamic stimulation has been associated with a subjective feeling of decreased energy and visual disturbances [25] despite a lack of objective neuroophthalmological findings. Dysarthria and paresthesias are also frequent though, again, typically amenable to reprogramming. Higher anxiety levels have occasionally been associated with anterior GPI stimulation [38] as has worsening of mood, impulsivity, and imbalance [29]. Although OCD has often improved or remained unchanged following DBS for TS, there have been thalamic and pallidal cases were OCD has worsened despite an improvement in tics [19, 23].
Caveats and Conclusions
Despite the heterogeneous nature of the data, mainly derived from small case series with varying targets, methodologies, and outcome measures, there is a collective sense that DBS is an often (if variably) effective treatment option for refractory TS. Few of the studies allow for a conclusive determination as to what constitutes the best target, optimal stimulation parameters, or the most likely responders. These remain major limitations in our present understanding. Further hampering a clinical consensus is the relatively small number of patients requiring DBS, making a large multicenter, blinded study difficult to accomplish. The best means forward appears to be aggregated data as is being undertaken by the International Registry, which continues to publish outcomes data in hopes of providing a clearer picture. At this point, the authors feel safe stating that there is ample case-based evidence to warrant consideration of DBS for severe medication refractory TS targeting either the globus pallidus or median thalamus. However, the variable degree of response – including possibility of non-response – along with the higher incidence of complications needs to be explicitly explained to prospective candidates, particularly to potentially more vulnerable younger patients. Ideally, continued thoughtful and systematic collection of pre- and postsurgical data will allow for a more straightforward assessment of DBS’s place in the armamentarium.
References
Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 2013;18(6):721–8.
Mufford M, Cheung J, Jahanshad N, van der Merwe C, Ding L, Groenewold N, et al. Concordance of genetic variation that increases risk for tourette syndrome and that influences its underlying neurocircuitry. Transl Psychiatry. 2019;9(1):120.
Greene DJ, Williams Iii AC, Koller JM, Schlaggar BL, Black KJ, The Tourette Association of America Neuroimaging C. Brain structure in pediatric Tourette syndrome. Mol Psychiatry. 2017;22(7):972–80.
Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60(4):415–24.
Pourfar M, Feigin A, Tang CC, Carbon-Correll M, Bussa M, Budman C, et al. Abnormal metabolic brain networks in Tourette syndrome. Neurology. 2011;76(11):944–52.
Jo HJ, McCairn KW, Gibson WS, Testini P, Zhao CZ, Gorny KR, et al. Global network modulation during thalamic stimulation for Tourette syndrome. Neuroimage Clin. 2018;18:502–9.
Babel TB, Warnke PC, Ostertag CB. Immediate and long term outcome after infrathalamic and thalamic lesioning for intractable Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 2001;70(5):666–71.
Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris). 1970;123(2):89–100.
Beckers W. Gilles de la Tourette’s disease based on five own observations. Arch Psychiatr Nervenkr (1970). 1973;217(2):169–86.
Kurlan R, Kersun J, Ballantine HT Jr, Caine ED. Neurosurgical treatment of severe obsessive-compulsive disorder associated with Tourette’s syndrome. Mov Disord. 1990;5(2):152–5.
Asam U, Karrass W. Gilles de la Tourette syndrome and psychosurgery. Acta Paedopsychiatr. 1981;47(1):39–48.
Hwynn N, Tagliati M, Alterman RL, Limotai N, Zeilman P, Malaty IA, et al. Improvement of both dystonia and tics with 60 Hz pallidal deep brain stimulation. Int J Neurosci. 2012;122(9):519–22.
Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie T, Limousin P. Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology. 2009;72(20):1787–9.
Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724.
Mink JW, Walkup J, Frey KA, Como P, Cath D, Delong MR, et al. Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord. 2006;21(11):1831–8.
Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord. 2015;30(4):448–71.
Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, Porta M, Servello D, Meng FG, et al. Efficacy and safety of deep brain stimulation in Tourette syndrome: the international Tourette syndrome deep brain stimulation public database and registry. JAMA Neurol. 2018;75:353.
Servello D, Sassi M, Gaeta M, Ricci C, Porta M. Tourette syndrome (TS) bears a higher rate of inflammatory complications at the implanted hardware in deep brain stimulation (DBS). Acta Neurochir. 2011;153(3):629–32.
Dowd RS, Pourfar M, Mogilner AY. Deep brain stimulation for Tourette syndrome: a single-center series. J Neurosurg. 2018;128:596–604.
Bour LJ, Ackermans L, Foncke EM, Cath D, van der Linden C, Visser Vandewalle V, et al. Tic related local field potentials in the thalamus and the effect of deep brain stimulation in Tourette syndrome: report of three cases. Clin Neurophysiol. 2015;126(8):1578–88.
Visser-Vandewalle V, Temel Y, Boon P, Vreeling F, Colle H, Hoogland G, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99(6):1094–100.
Visser-Vandewalle V, Kuhn J. Deep brain stimulation for Tourette syndrome. Handb Clin Neurol. 2013;116:251–8.
Porta M, Servello D, Zanaboni C, Anasetti F, Menghetti C, Sassi M, et al. Deep brain stimulation for treatment of refractory Tourette syndrome: long-term follow-up. Acta Neurochir. 2012;154(11):2029–41.
Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(5):1004–14.
Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011;134(Pt 3):832–44.
Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65(7):952–7.
Kefalopoulou Z, Zrinzo L, Jahanshahi M, Candelario J, Milabo C, Beigi M, et al. Bilateral globus pallidus stimulation for severe Tourette’s syndrome: a double-blind, randomised crossover trial. Lancet Neurol. 2015;14(6):595–605.
Martinez-Fernandez R, Zrinzo L, Aviles-Olmos I, Hariz M, Martinez-Torres I, Joyce E, et al. Deep brain stimulation for Gilles de la Tourette syndrome: a case series targeting subregions of the globus pallidus internus. Mov Disord. 2011;26(10):1922–30.
Sachdev PS, Mohan A, Cannon E, Crawford JD, Silberstein P, Cook R, et al. Deep brain stimulation of the antero-medial globus pallidus interna for Tourette syndrome. PLoS One. 2014;9(8):e104926.
Burdick A, Foote KD, Goodman W, Ward HE, Ricciuti N, Murphy T, et al. Lack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive-compulsive disorder. Neurocase. 2010;16(4):321–30.
Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, et al. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery. 2005;57(4 Suppl):E403; discussion E
Kuhn J, Lenartz D, Huff W, Mai JK, Koulousakis A, Maarouf M, et al. Transient manic-like episode following bilateral deep brain stimulation of the nucleus accumbens and the internal capsule in a patient with Tourette syndrome. Neuromodulation. 2008;11(2):128–31.
Neuner I, Podoll K, Lenartz D, Sturm V, Schneider F. Deep brain stimulation in the nucleus accumbens for intractable Tourette’s syndrome: follow-up report of 36 months. Biol Psychiatry. 2009;65(4):e5–6.
Shields DC, Cheng ML, Flaherty AW, Gale JT, Eskandar EN. Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg. 2008;86(2):87–91.
Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121–34.
Baldermann JC, Kohl S, Visser-Vandewalle V, Klehr M, Huys D, Kuhn J. Deep brain stimulation of the ventral capsule/ventral striatum reproducibly improves symptoms of body dysmorphic disorder. Brain Stimul. 2016;9(6):957–9.
Pourfar M, Budman C, Mogilner A. A case of deep brain stimulation in Tourette’s complicated by Twiddler’s syndrome. Mov Disord Clin Pract. 2015;2(2):192.
Cannon E, Silburn P, Coyne T, O’Maley K, Crawford JD, Sachdev PS. Deep brain stimulation of anteromedial globus pallidus interna for severe Tourette’s syndrome. Am J Psychiatry. 2012;169(8):860–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pourfar, M.H., Mogilner, A.Y. (2020). Gilles de la Tourette Syndrome: Deep Brain Stimulation. In: Pouratian, N., Sheth, S. (eds) Stereotactic and Functional Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-030-34906-6_31
Download citation
DOI: https://doi.org/10.1007/978-3-030-34906-6_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34905-9
Online ISBN: 978-3-030-34906-6
eBook Packages: MedicineMedicine (R0)