Abstract
Metastatic cancer cells are characterized by uncontrolled proliferation and invasive behavior. In solid tumors, mortality is caused by invasive and metastatic activity of tumor cells rather than their proliferative ability. Therefore, the treatment of solid tumors should be supplemented with drugs that suppress the ability of tumor cells to invade through the surrounding extracellular matrix and form secondary tumors. During invasion, metastatic cancer cells can utilize various invasion modes, which stresses the need to target mechanisms common to all invasion modes. Here, we summarize the concept of migrastatics, which refers to drugs targeting all forms of cancer cell invasion. We overview the most potential migrastatic candidates and discuss their advantages and disadvantages.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Migratory Phenotype of Cancer Cells
One of the characteristics of life is movement. Accordingly, cells have the intrinsic ability to migrate. However, complex multicellular organisms have developed mechanisms that ensure temporal and spatial regulation of cell movement in order to form complex multi-cellular structures, such as organs made of specialized tissues. In human, cell migration is preserved in specific types of cells, such as immune cells, and largely regulated to maintain homeostasis. Unsurprisingly, dysregulated migration of cells disrupts tissue integrity and is associated with various diseases, including the deadliest – metastatic cancer [1,2,3,4]. While characteristics such as uncontrolled growth, apoptosis evasion or increased angiogenesis are shared by both benign and metastatic cancer, only the latter is able to invade from the primary site to secondary sites and establish secondary tumors, i.e. metastases, which are the cause of death in over 90% of all cancer associated deaths [5, 6].
Overall, the ability to migrate is enabled by re-structuralizing the complex network of the cytoskeleton, composed of actin, microtubules and intermediate filaments. This process is organized by many signaling pathways converging on cytoskeleton regulatory proteins, which directly drive the structural changes [7] (Fig. 1). Interestingly, the same set of proteins that maintains epithelial polarity also drives the polarization in migrating cells by interacting with cytoskeletal proteins [8]. Underlying force generation necessary for cell body translocation is polymerization and contraction of actin, which are processes regulated by Rho, Rac and Cdc42 proteins from the family of Rho GTPases [7, 9, 10]. Rac and Cdc42 signaling regulates activation of many actin associated proteins, such as Wiskott-Aldrich Syndrome proteins (e.g. WASP, N-WASP and WAVE1/2), that drive polymerization of actin leading to the formation of actin-based protrusions. On the other hand, Rho activation is the key signaling event in regulation of actomyosin contractility, which is propelled by phosphorylation of myosin light chain (MLC) [11] (Fig. 2). Rho activates Rho-kinase (ROCK), which directly phosphorylates MLC [12]. Additionally, ROCK along with myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) deactivate myosin light chain phosphatase (MLCP), a negative regulator of MLC phosphorylation [13, 14].
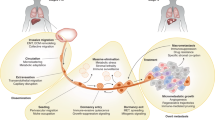
Overview of candidate migrastatics. Agents targeting actin organization (actin stabilizing drugs, actin destabilizing drugs), tropomyosin (TR100) and actomyosin contractility (kinase inhibitors, blebbistatin) are suitable migrastatic candidates by affecting actin polymerization and actomyosin contractility, which are processes underlying cancer cell motility. In result, migrastatic drugs inhibit dissemination of metastatic cells (blue) from the primary tumor (grey)
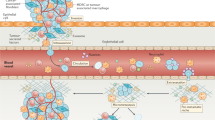
Actomyosin contractility as a suitable target for migrastatics. The activity of ROCK and MRCK kinases results in increased phosphorylation of myosin light chain, which activates actomyosin contractility. Drugs targeting ROCK or MRCK are candidates for efficient migrastatics as they act to inhibit actomyosin contractility, which is necessary for of all cell invasion modes
Various migration modes have been described, ranging from collective to single cell invasion, which differ in their requirements for actin protrusive forces and/or actomyosin contractility, resulting in diverse strategies of movement through the extracellular matrix (ECM). During collective migration cells move as a group of cells, which can be organized into clusters, sheets or strands that hold together by cell-cell adhesions. This type of migration is common during embryonic development or wound healing [15]. Cells at the leading front, referred to as leader cells, are responsible for proteolytical degradation of the extracellular matrix (ECM), which provides space for the group of cells to move forward [16, 17]. Unlike collective migration, single- cell migration does not require cell-cell adhesion. Cells invading as single cells adopt the protease- dependent mesenchymal invasion mode, protease-independent amoeboid mode, or various intermediate phenotypes. Mesenchymally migrating cells, such as fibroblasts, are characterized by elongated cell shape and numerous actin-based protrusions, and due to their proteolytical activity form tunnels through the ECM [18, 19]. Cells utilizing the amoeboid invasion mode enhance actomyosin contractility to dynamically deform their cell body, enabling them to push through pre-existing pores in the ECM. They are typically round with numerous membrane blebs that form due to high pressure inside the cells [20, 21]. A typical example of amoeboid migrating cells are leukocytes [22].
Apart from physiological significance of the migration modes, they are utilized by cancer cells during metastasis. In this manner, cancer cells have evolved to be “masters” of invasion, as they can utilize all the above-mentioned modes, including many transient phenotypes. More importantly, they are able to switch among the modes in order to most efficiently adapt to surrounding conditions [23,24,25]. For example, when proteolytical degradation of the ECM is inhibited, cancer cells can undergo the mesenchymal-amoeboid transition (MAT) [26]. Further, upregulation of cell contractility by activation of the Rho/ROCK pathway results in MAT [27, 28]. Opposingly, amoeboid-mesenchymal transition (AMT) occurs when contractility is blocked, for example by ROCK inhibitors [28, 29]. Notably, the ECM itself affects the invasion mode – in dense, rigid ECM mesenchymal migration is preferred, whereas loose ECM promote the amoeboid invasion phenotype [30]. Further, non-cancer cells, such as tumor associated macrophages or cancer-associated fibroblasts, contribute to invasion plasticity by modulating the microenvironment by production of pro-invasive cytokines (e.g. IL6, IL8, VEGF, LIF etc.). Moreover, CAFs realign collagen fibers of the tumor stroma ECM, which facilitates invasion of cancer cells [31].
The ability of cancer cells to adopt various invasion modes is referred to as invasion plasticity and is the main reason why conquering metastatic cancer is still a mere desire rather than close reality. The focus of cancer therapy has been mostly on inhibition of proliferation; however, it is local invasion and metastasis, rather than proliferation, which are the dominant features of solid cancer and must be eliminated in order to achieve effective cancer treatment [32, 33]. Thus, cytostatic therapy should be complemented with a strategy against cancer cell invasion. For such strategy to be successful, all modes of cancer cell invasion must be targeted. Recently, the term “migrastatics” (from Latin “migrare” and Greek “statikos”) was proposed as a unifying name for drugs interfering with all modes of cancer cell invasion and consequently their ability to metastasize.
Due to the large variability of the invasion modes and even larger spectra of regulatory pathways, it is the common mechanism shared by all invasion modes which must be targeted. This requirement is fulfilled only by ultimate downstream effectors of cell migration, which are actin polymerization and actomyosin contractility. Unlike upstream regulators, which can be by-passed and compensated, there is no feasible way for a migrating cell to overcome inhibition of actin polymerization or contractility.
Migrastatic Drugs
Potential migrastatic candidates include drugs destabilizing actin cytoskeleton, such as cytochalasins, lantrunculins and Geodiamolide H. Further included are drugs stabilizing actin cytoskeleton, for example Jasplakinolide, chondramides and cucurbitacin E. Another class of candidates for migrastatic agents are those targeting contractility, such as TR-100, a tropomyosin inhibitor or blebbistatin, a non-muscle myosin II inhibitor. Many actomyosin targeting drugs are kinase inhibitors, for example ML-7 and ML-9 that inhibit MLCK or BDP5290, which inhibits MRCK. ROCK targeting inhibitors include fasudil, Y-27632, H-1152, Wf-536, RKI-1447 and RKI-18. DJ4 inhibits both ROCK and MRCK (Fig. 2). All the above mentioned have proven to decrease cancer cell invasion in vitro on model cancer cell lines. Additionally, in case of TR-100, ML-7 and fasudil in vivo data exist. For a complete set of references see [33].
Importantly, ROCK/PKA/PKB multi-kinase inhibitors have been demonstrated to abolish both amoeboid and mesenchymal invasion both in vitro and in vivo [34]. CCT129254 was shown to reduce invasiveness and metastatic ability of melanoma cells in vivo. Another tested compound, AT13148, showed similar abilities, however with increased toxicity [34]. Notably, AT13148 is currently being tested in clinical development for oncological applications [35]. As such, ROCK/PKA/PKB multi-kinase inhibitors represent the most promising migrastatic candidates up to date (Fig. 2).
Apart from direct inhibition of cancer cell motility, migrastatic drugs could further decrease cancer cell invasion by affecting signaling within the tumor stroma and organization of the surrounding ECM. Various non-cancer cells that support tumor growth and invasion reside in the tumor stroma, such as tumor-associated macrophages , which produce cytokines known to promote invasive behavior of cancer cells, and also non-cancer cells, such as cancer-associated fibroblasts [36]. These signaling circuits are largely dependent on actomyosin contractility, which makes them possible targets of migrastatic drugs [37]. For example, direct macrophage contact with cancer cells activates RhoA signaling [38]. Vice versa, amoeboid cells with high myosin II activity recruit monocytes and stimulate their differentiation into TAMs [39]. Importantly, actomyosin contractility is essential for the activity of cancer-associated fibroblasts, which remodel the ECM by aligning collagen fibers. This increases ECM stiffness, which is known to facilitate cancer cell invasion [40]. Taken together, inhibition of Rho/ROCK signaling by migrastatics could disrupt the complex pro-malignant interactions within the tumor stroma and in reduce cancer cell dissemination.
As with all drugs, the main concern with migrastatics lies in possible toxicity [41]. After all, it was already mentioned here that the migration modes are employed also by non-cancer cells; and interfering with actin polymerization and contractility can be expected to affect both normal and cancer cells. Nevertheless, it can be anticipated, based on published examples, that concentrations targeting cancer cells, but not healthy cells, can be determined using suitable in vivo models. Moreover, the recent advancements in drug delivery to neoplastic tissues may disclose further potential of migrastatics when targeted directedly [42].
Migrastatic Therapy, Challenges and Advantages
It is important to stress that migrastatic therapy is not intended to replace the conventional anti- proliferative strategy. Rather, administering migrastatic agents should suitably complement cytotoxic therapy and increase its benefit. Due to aberrant cytokinesis caused by migrastatics, the tumor cells may be more responsive to DNA-modifying drugs, such alkylators or nucleoside analogs [43]. Also, it has already been shown that combination of actin-binding drugs with mitosis targeting microtubule drugs may result in reinforced inhibition of proliferation, as already documented in case of cytochalasin B and vincristine [44]. In fact, migrastatics themselves may have anti-proliferative effects. For example, inhibition of ROCK kinases decreases proliferation and loss of both ROCK isoforms blocks tumor formation in mice [45].
Moreover, during conventional cytotoxic treatment, tumor cells are prone to undergo Darwinian selection of drug-resistant clones, and these will inevitably overgrow the susceptible population [46] (Fig. 3). Additionally, this can result in treatment-induced metastasis [47]. However, it is anticipated that resistance to migrastatics will not provide cells with proliferative advantage, as in the case of acquired resistance to cytotoxic drugs. Overall, suitable combination or sequential administration of cytotoxic drugs and migrastatics may be especially effective in inhibiting cancer cell dissemination.
Comparison of cytotoxic therapy and migrastatic therapy. Cytotoxic therapy reduces cell proliferation and thus tumor size. However, this is accompanied by accumulation of drug- resistant clones (pink and red) due to their proliferative advantage. Also, cell invasion is not primarily targeted, and metastases can be formed. On the other hand, migrastatic therapy is not expected to decrease tumor size, but impair cancer cell invasion and in result reduce secondary tumors. Moreover, drug-resistant clones do not gain proliferative advantages, decreasing the risk of gaining drug-resistant tumors. Altogether, combining anti-proliferative cytotoxic therapy and anti-invasive migrastatic therapy may offer novel therapeutic possibilities
Administration of migrastatic drugs should be long term, which is associated with higher risk of adverse effects. The effective dose of migrastatics for metastasis prevention could be, however, lower than what is necessary to stop cell to migrate. It was shown that the effectivity of cancer cell dissemination is very low – despite large number of cells escaping the primary tumor only very small number of cells successfully give rise secondary tumors [48]. The invasive phenotype of cancer cells is required any many steps of metastatic cascade including tissue invasion on primary site, extravasation, intravasation and tissue invasion on secondary site. Therefore, the effect of slowing down cancer cell migration on metastases formation is potentially multiplied by several folds.
In summary, migrastatic therapy provides a novel approach to anti-metastatic therapy. By targeting the basic processes of cell migration, migrastatics target and inhibit all modes of cell invasion. In combination with conventional treatment they allow synergistic impairment of tumor cells. Hopefully, migrastatics will provide a fundamental shift in the treatment of metastatic tumors.
References
Wedlich, D. 2006. Cell migration in development and disease. New Jersey: Wiley.
Binamé, F., G. Pawlak, P. Roux, and U. Hibner. 2010. What makes cells move: Requirements and obstacles for spontaneous cell motility. Molecular BioSystems 6: 648–661.
Ridley, A.J., M.A. Schwartz, K. Burridge, R.A. Firtel, M.H. Ginsberg, G. Borisy, et al. 2003. Cell migration: Integrating signals from front to back. Science 302: 1704–1709.
Micuda, S., D. Rosel, A. Ryska, and J. Brabek. 2010. ROCK inhibitors as emerging therapeutic candidates for sarcomas. Current Cancer Drug Targets (Bentham Science Publishers) 10: 127–134.
Lazebnik, Y. 2010. What are the hallmarks of cancer? Nature Reviews Cancer (Nature Publishing Group) 10: 232–233.
Hanahan, D., and R.A. Weinberg. 2011. Hallmarks of cancer: The next generation. Cell (Elsevier Inc.) 144: 646–674.
Fife, C.M., J.A. McCarroll, and M. Kavallaris. 2014. Movers and shakers: Cell cytoskeleton in cancer metastasis. British Journal of Pharmacology (England) 171: 5507–5523.
Gandalovičová, A., T. Vomastek, D. Rosel, and J. Brábek. 2016. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget 7 (18): 25022–25049.
Lauffenburger, D., and F. Horwitz. 1996. Cell migration: A physically integrated molecular process. Cell 84: 359–369.
Mitchison, T.J., and L.P. Cramer. 1996. Actin-based cell motility and cell locomotion. Cell 84: 371–379.
Spiering, D., and L. Hodgson. 2011. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhesion & Migration 5: 170–180.
Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, et al. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). The Journal of Biological Chemistry 271: 20246–20249.
Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, et al. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248.
Wilkinson, S., H.F. Paterson, and C.J. Marshall. 2005. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nature Cell Biology 7: 255–261.
Friedl, P., and D. Gilmour. 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews. Molecular Cell Biology (Nature Publishing Group) 10: 445–457.
Friedl, P., J. Locker, E. Sahai, and J.E. Segall. 2012. Classifying collective cancer cell invasion. Nature Cell Biology 14: 777–783.
Haeger, A., K. Wolf, M.M. Zegers, and P. Friedl. 2015. Collective cell migration: Guidance principles and hierarchies. Trends in Cell Biology 25: 556–566.
Friedl, P., and K. Wolf. 2008. Tube travel: The role of proteases in individual and collective cancer cell invasion. Cancer Research 68: 7247–7249.
Tolde, O., D. Rosel, R. Janostiak, P. Vesely, and J. Brabek. 2012. Dynamics and morphology of focal adhesions in complex 3D environment. Folia Biologica (Praha) 58: 177–184.
Sabeh, F., R. Shimizu-Hirota, and S.J. Weiss. 2009. Protease-dependent versus-independent cancer cell invasion programs: Three-dimensional amoeboid movement revisited. The Journal of Cell Biology 185: 11–19.
Charras, G., and E. Paluch. 2008. Blebs lead the way: How to migrate without lamellipodia. Nature Reviews. Molecular Cell Biology 9: 730–736.
Friedl, P., S. Borgmann, and E.B. Brocker. 2001. Amoeboid leukocyte crawling through extracellular matrix: Lessons from the Dictyostelium paradigm of cell movement. Journal of Leukocyte Biology 70: 491–509.
Yilmaz, M., and G. Christofori. 2010. Mechanisms of motility in metastasizing cells. Molecular Cancer Research 8: 629–642.
Friedl, P., and K. Wolf. 2010. Plasticity of cell migration: A multiscale tuning model. The Journal of Cell Biology 188: 11–19.
Panková, K., D. Rösel, M. Novotný, and J. Brábek. 2010. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cellular and Molecular Life Sciences 67: 63–71.
Wolf, K., I. Mazo, H. Leung, K. Engelke, U.H. von Andrian, E.I. Deryugina, et al. 2003. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. The Journal of Cell Biology 160: 267–277.
Rösel, D., J. Brábek, O. Tolde, C.T. Mierke, D.P. Zitterbart, C. Raupach, et al. 2008. Up-regulation of Rho/ROCK signaling in sarcoma cells drives invasion and increased generation of protrusive forces. Molecular Cancer Research 6: 1410–1420.
Sahai, E., and C.J. Marshall. 2003. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nature Cell Biology 5: 711–719.
Sanz-Moreno, V., G. Gadea, J. Ahn, H. Paterson, P. Marra, S. Pinner, et al. 2008. Rac activation and inactivation control plasticity of tumor cell movement. Cell 135: 510–523.
Van Goethem, E., R. Poincloux, F. Gauffre, I. Maridonneau-Parini, and V. Le Cabec. 2010. Matrix architecture dictates three-dimensional migration modes of human macrophages: Differential involvement of proteases and podosome-like structures. Journal of Immunology 184: 1049–1061.
Malik, R., P.I. Lelkes, and E. Cukierman. 2015. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends in Biotechnology 33: 230–236.
Steeg, P.S. 2016. Targeting metastasis. Nature Reviews. Cancer 16: 201–218.
andalovičová, A., D. Rosel, M. Fernandes, P. Veselý, P. Heneberg, V. Čermák, et al. 2017. Migrastatics—Anti-metastatic and anti-invasion drugs: Promises and challenges. Trends in Cancer 3: 391–406.
Sadok, A., A. McCarthy, J. Caldwell, I. Collins, M.D. Garrett, M. Yeo, et al. 2015. Rho kinase inhibitors block melanoma cell migration and inhibit metastasis. Cancer Research 75: 2272–2284.
Feng, Y., P.V. LoGrasso, O. Defert, and R. Li. 2016. Rho kinase (ROCK) inhibitors and their therapeutic potential. Journal of Medicinal Chemistry 59: 2269–2300.
Noy, R., and J.W. Pollard. 2016. Tumor-associated macrophages: From mechanisms to therapy. Immunity 41: 49–61.
Rodriguez-Hernandez, I., G. Cantelli, F. Bruce, and V. Sanz-Moreno. 2016. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research 5: 783. F1000 Faculty Rev.
Roh-Johnson, M., J.J. Bravo-Cordero, A. Patsialou, V.P. Sharma, P. Guo, H. Liu, et al. 2014. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33: 4203–4212.
Georgouli, M., C. Herraiz, E. Crosas-Molist, B. Fanshawe, O. Maiques, A. Perdrix, et al. 2019. Regional activation of myosin II in cancer cells drives tumor progression via a secretory cross-talk with the immune microenvironment. Cell 176: 757–774.e23.
Samuel, M.S., J.I. Lopez, E.J. McGhee, D.R. Croft, D. Strachan, P. Timpson, et al. 2011. Actomyosin- mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell (United States) 19: 776–791.
Gewirtz, D.A., M.L. Bristol, and J.C. Yalowich. 2010. Toxicity issues in cancer drug development. Current Opinion in Investigational Drugs (England): 612–614.
Rosenblum, D., N. Joshi, W. Tao, J.M. Karp, and D. Peer. 2018. Progress and challenges towards targeted delivery of cancer therapeutics. Nature Communications 9: 1410.
Trendowski, M. 2014. Exploiting the cytoskeletal filaments of neoplastic cells to potentiate a novel therapeutic approach. Biochimica et Biophysica Acta 1846: 599–616.
Kolber, M.A., and P. Hill. 1992. Vincristine potentiates cytochalasin B-induced DNA fragmentation in vitro. Cancer Chemotherapy and Pharmacology (Germany) 30: 286–290.
Kumper, S., F.K. Mardakheh, A. McCarthy, M. Yeo, G.W. Stamp, A. Paul, et al. 2016. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. eLife 5: e12994.
Rosel, Daniel, Michael Fernandes, Victoria Sanz-Moreno, and Jan Brábek. 2019. Migrastatics: Redirecting R&D in solid Cancer towards metastasis? Trends in Cancer 5 (12): 755–756.
Ebos, J.M.L. 2015. Prodding the beast: Assessing the impact of treatment-induced metastasis. Cancer Research 75: 3427–3435.
Luzzi, K.J., I.C. MacDonald, E.E. Schmidt, N. Kerkvliet, V.L. Morris, A.F. Chambers, et al. 1998. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. The American Journal of Pathology 153: 865–873.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gandalovičová, A., Rosel, D., Brábek, J. (2020). Migrastatics – Anti-metastatic Drugs Targeting Cancer Cell Invasion. In: Bizzarri, M. (eds) Approaching Complex Diseases. Human Perspectives in Health Sciences and Technology, vol 2. Springer, Cham. https://doi.org/10.1007/978-3-030-32857-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-32857-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32856-6
Online ISBN: 978-3-030-32857-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)