Abstract
Hydrogels are crosslinked polymeric networks with a large number of hydrophilic domains. They can expand in numerous solvents and aqueous environments without dissolving owing to the chemical or physical bonds formed between polymer chains. During the past decades, hydrogels have been designed using synthetic or natural polymers like proteins or polysaccharides for biomedical applications such as tissue engineering. Due to its biocompatibility and its structure, most commonly used in tissue engineering is collagen, the most abundant structural protein of the extracellular matrix, which is predominantly found in fibrous connective tissues. In the present study to obtain hydrogels alongside collagen was used also dextran, a polysaccharide derived from glucose condensation. The crosslinking was made under the influence of riboflavin, which is a water-soluble vitamin that plays an important role in the production of energy in the body. In addition, the hydrogels have been exposed to physical treatments like UV radiation and lyophilization. The hydrogels were characterized using FT-IR spectroscopy and to highlight the hydrogels porous was used microscopy in phase contrast and fluorescence microscopy. The citocompatibility tests (MTT) indicated normal values for the cells viability in the presence of hydrogels. For detection of living cells the hydrogels a treatment with calcein AM solution was used and for detection of living cell nuclei was used the DAPI solutions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Tissue engineering is a modern field of the regenerative medicine which integrates all the technologies using live cells or biomaterials (synthetic or natural) with the purpose of promote the quality of human life by obtaining tissues and bioartificial organs, outside the body, in vitro. Natural polymers, specifically collagens and polysaccharides, are frequently used for hydrogel preparation because of their biocompatibility and chemical structure. Hydrogels consist of a water swollen network of crosslinked polymer chains that can have a wide range of physical and biological properties depending on the composition of the polymer and the nature of the crosslinks [1, 2].
Until now, biocompatible and biodegradable hydrogels have been designed using polysaccharides and functionalized polysaccharides for biomedical applications such as tissue engineering. Due to the advantage of biocompatibility and biodegradability, the natural polymers such as collagen and dextran have been studied as the potential matrix for tissue engineering applications [3,4,5,6,7].
Collagen is the most abundant structural protein of the extra cellular matrix (ECM), showing good attachment/adhesion to different kinds of cells and has been studied for several biomedical applications. Is one of the most used polymers in biomaterials field, due to its excellent properties in biocompatibility, biodegradability, with well-established structure, biologic profile, and in vivo response [1, 8].
Dextran is a nontoxic, hydrophilic bacterial polysaccharide that is broadly applicable in the biomedical field owing to its biocompatibility and biodegradability. It is mainly composed of linear α-1,6-linked d-glucopyranose residues with allow percentage of α-1,2-, α-1,3- and α-1,4-linked side chains [9].
Dextran is present in the extracellular matrix (ECM) and shows superior properties of interaction with human body tissues. It is known that dextran promotes adhesion between proteins and cells. It is commonly used to decrease vascular thrombosis, reduce inflammatory response and prevent ischemia–reperfusion injury in organ transplantation in which dextran acts as a mild reactive oxygen species scavenger and reduces platelet activation in excess. Dextran has volume expansive properties and therefore its inclusion can improve blood flow [10, 11].
A new trend in the crosslinking of the hydrogels takes in consideration biological active molecules, like riboflavin, an essential water-soluble vitamin with unique biological and physicochemical properties [12, 13].
The objective of this study was to develop a new method for obtaining hydrogels based on collagen and dextran, made by crosslinking with riboflavin. The future objectives of the study are related to increasing the biocompatibility of hydrogels and the population of these materials with cells through the encapsulation process.
2 Materials and Methods
2.1 Materials
The hydrogels were obtained by using 1% collagen solution (with initial pH = 2, which was adjusted to pH = 6 by the addition of NaOH 1M) and 1% dextran solution. Initial collagen solution was kindly donated by Lohmann & Rauscher GmbH & Co. KG, Germany, and Dextran sulfate sodium salt from Leuconostoc spp. from Sigma-Aldrich (average Mw >500,000). The riboflavin as crosslinking agent was purchased from Sigma-Aldrich and used at a concentration of 1% in bidistilled water.
2.2 Methods
For the crosslinking reaction the following compositions of hydrogels were chosen: 100% collagen; 75% collagen-25% dextran; 50% collagen-50% dextran. The final volume of each hydrogel composition was 1500 μl and was made in the wells of 6 well-culture plates for cell cultures. Thus, after mixing collagen and dextran in the desired ratio, a 120 μl of riboflavin solution was added to each composition, followed by 15 min exposure to UV radiation in the aim to achieve the crosslinking. The wavelength of UV radiation was 365 nm and distance between hydrogel solution and UV source was 100 mm.
The culture plate with the three crosslinked hydrogel compositions was frozen at −19 °C overnight then dried using freeze-drying method to obtain a porous structure (Labconco LtD freeze-dryer). Successive washes of the lyophilized hydrogels were carried out with 70% ethyl alcohol to remove traces of riboflavin. The bacterial decontamination of the hydrogels was proceed in 70% aqueous sterile ethyl alcohol solution, followed by washing with bidistilled water and swelling for 48 h in saline phosphate buffer (HBSS).
Dried porous supports were characterized using FT-IR spectroscopy and phase contrast microscopy.
The biocompatibility of the collagen/dextran hydrogels crosslinked with riboflavin was analyzed through MTT cytotoxicity evaluation and capacity to be populated by living cells.
The MTT assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was performed by the direct contact technique. The MTT reagent being pale yellow in solution can be reduced through oxidative reaction of living cells to dark blue formazan. Thus, the amount of the formazan in the cell culture is directly proportional to the cell viability rate [14].
The MTT assay was performed in 12-well culture plates seeded with 3 × 104 cells/well primary fibroblasts from Albino rabbit dermis. One small piece of the hydrogels, about 6 mm diameter, was put on the bottom of each culture well, over which the cell suspension was added. The cells and hydrogels were incubated for 72 h then MTT reaction was performed using 1 ml of 0.25 mg/ml MTT solution. The absorbance of the formazan solution was measured using Tecan plate-reader spectrophotometer at the 570 nm wavelength. The cell viability was calculated as percent of formazan absorbance in the cell cultures incubated with the hydrogels, compared to controls (cultures without hydrogels). The experiment of each hydrogel was performed in triplicate.
The capacity of the hydrogels to be populated with living cell was performed after MTT test. The cells were seeded on the hydrogel surface as was described for MTT test. A vital staining with green fluorescence was performed to highlight the cells on the materials. Therefore, a Calcein AM solution (Sygma-Aldrich) in a concentration of 2 µl/ml of HBBS (without phenol red) was used. The staining was carried out in the dark condition, for 30 min.
The living cells were observed and analyzed after 72 h, using Leica DMIL optical microscope in fluorescence mode, at excitation/extinction wavelength of 455/530 nm. The presence of the cell in the hydrogel structure was analyzed as well by DAPI staining of the cell nuclei, using broadly used method. The blue fluorescent cell nuclei were analyzed using Leica DMIL optical microscope in fluorescence mode, at excitation/extinction wavelength of 358/461 nm.
3 Results and Discussions
Hydrogels based on collagen and dextran were obtained by crosslinking with riboflavin (Figs. 1 and 2).
The FT = IR result as for crosslinked hydrogels are presented in Fig. 3.
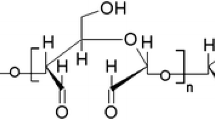
Collagen-specific absorption bands occur at the following wavelengths: valency vibrations of –OH—3317 cm−1, valence vibrations of –C–H from –CH2—2982 cm−1; Amide I—1643 cm−1; Amide II 1551 cm−1; Amide III—1234 cm−1; Amide IV—795 cm−1. The collagen stretch bands overlap with OH bands of dextran. As the dextran concentration increases, the amide band intensity decreases.
The microscopic analysis of the porous structure of hydrogels is showed in images A, B and C from Fig. 4.
The images from the Fig. 4 show the evenly distributed porous structure with large and interconnected pores. Each pore exceeds the size of 100 μm. This structure reveals an architecture with a potentially capacity to be populated with cells.
The MTT assay, which was performed as direct contact with cells, proved an acceptable cytocompatibility of the hydrogels. Cells viability after 72 h of contact with materials was 81–82% for collagen 100%; 73% for 75% collagen-25% dextran; and 73–74% for 50% collagen-50% dextran. It can be concluded that the collagen component increase the cytocompatibility of the hydrogels (Fig. 5).
The differences were observed between control and analyzed hydrogels that could be explained by the technical errors which are often occurs in the direct contact MTT tests. This supposition is based on the capacity of the hydrogels to be populated with cells, which was proved through Calcein AM and DAPI staining and shown in the Fig. 6.
Thus, A1, B1, and C1 represent the microscopic analysis of the hydrogels treated with calcein AM solution for the detection of living cells, and the images A2, B2, C2 represent the microscopic analysis of the hydrogels stained with the DAPI solution for the detection of cell nuclei. From the images it can be seen that cells are present in the hydrogel pores. The cells are evenly distributed in the each type of hydrogel. The nuclei with a normal shape and no fragmentation were found by DAPI staining.
4 Conclusions
The preliminary study showed that hydrogels based on collagen and dextran crosslinked with riboflavin have potential for tissue engineering applications. These hydrogels are translucent in biological media, are non-cytotoxic and allow cell adhesion inside of their porous structure. The future objectives of the study are related to revels the potential benefits of the presence of the dextran in the hydrogel structure for a longer cell growth inside the material and possibility to use the reticulation technique for cells encapsulation.
The initiative of this project was to improve the materials used in surgery through the availability of financially accessible and highly efficient products.
Tissue engineering and, implicitly, regenerative medicine provide through the bioartificial tissues a new chance for patients who have suffered various traumas. The possibilities offered by these hydrogels are many, besides being used to replace a certain affected portion of an organ, these materials can also be used as dressings to heal burns or wounds difficult to heal, diagnostic devices, contact lenses and patches applicable to the skin for controlled drug delivery.
References
Geckil, H., Xu, F., Zhang, X., Moon, S., Demirci, U.: Engineering hydrogels as extracellular matrix mimics. Nanomedicine 5, 469–484 (2010)
Ferreira, L.S., Gerecht, S., et al.: Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomater 28(17), 2706–2717 (2007)
Punnida, N., Akimasa, M., et al.: Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties. Carbohydr. Polym. 2014, 131–141 (2018)
Chen, Y.M., Sun, L., et al.: Self-healing and photoluminescent carboxymethyl cellulose-based hydrogels. Eur. Polym. J. 94, 501–510 (2017)
Geng, X., Mo, X., Fan, L., Yin, A., Fang, J.: Hierarchically designed injectable hydrogel from oxidized dextran, amino gelatin and 4-arm poly(ethylene glycol)-acrylate for tissue engineering application. J. Mater. Chem. 22, 25130–25139 (2012)
Lisman, A., Butruk, B., Wasiak, I., Ciach, T.: Dextran/Albumin hydrogel sealant for Dacron(R) vascular prosthesis. J. Biomater. Appl. 28, 1386–1396 (2014)
Zhang, X., Yang, Y., Yao, J., Shao, Z., Chen, X.: Strong collagen hydrogels by oxidized dextran modification. ACS Sustain. Chem. Eng. 2(5), 1318–1324 (2014)
Caliari, S.R., Burdick, J.A.: A practical guide to hydrogels for cell culture. Nat. Meth. 13, 405–414 (2016)
Bachelder, E.M., Beaudette, T.T., Broaders, K.E.: Acetal-derivatized dextran: an acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc. 13, 10494–10495 (2008)
Robless, P., Okonko, D., et al.: Dextran reduces in vitro platelet aggregation in peripheral arterial disease. Platelets 15(4), 215–222 (2004)
Dubniks M, Persson J, Grande PO (2019) Comparison of the plasma volume expanding effects of 6% dextran 70, 5%albumin, and 6% HES 130/0.4 after hemorrhage in the guinea pig. J. Trauma 67(6), 1200–1204
Tirella, A., Ahluwalia, A.: Riboflavin and collagen: new crosslinking methods to tailor the stiffness of hydrogels. Seria Ştiinţele Vieţii 26(2), 243–249 (2016)
Beztsinna, N., Sole, M., Taib, N., Bestel, I.: Bioengineered riboflavin in nanotechnology. Biomaterials 80, 121–133 (2016)
Malich, G., Markovic, B., Winder, C.: The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 124, 179–192 (1997)
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Butnaru, M., Lucaci, A.M., Cosman, B.P., Verestiuc, L. (2020). Hydrogels Based on Collagen and Dextran for Bioartificial Tissues. In: Tiginyanu, I., Sontea, V., Railean, S. (eds) 4th International Conference on Nanotechnologies and Biomedical Engineering. ICNBME 2019. IFMBE Proceedings, vol 77. Springer, Cham. https://doi.org/10.1007/978-3-030-31866-6_71
Download citation
DOI: https://doi.org/10.1007/978-3-030-31866-6_71
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31865-9
Online ISBN: 978-3-030-31866-6
eBook Packages: EngineeringEngineering (R0)