Abstract
The work offers for discussion the results of investigations of absorption spectra (gamma-ray nuclear resonance) of gallium antimonide doped with 57Fe isotope in the concentration range of the atomic mass of 1–3%, within the temperature range of 4.2–300 K, in the absence of an external magnetic field. Also, a model of the impurity center of iron in gallium antimonide is described.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Gallium antimonide—GaSb, is a group AIIIBV semiconductor with a narrow energy band, small effective mass of electrons, and high mobility. GaSb can be the main material whose crystal lattice is suitable for fabricating optoelectronic devices in the range of 0.8–4.3 μm. Studies of GaSb have been progressing of late due to the growing demand in new compatible optoelectronic materials, in optic fibers of 2–4 μm. Information on the research and development of devices based on GaSb is available, for example, in Milnes and Polyakov [1], Dutta et al. [2].
The study of the behaviour of the dopants from the transition group, such as Fe, Ni, Cr, Mn, in gallium antimonide is topical and challenging. Still, physical properties of GaSb doped with elements from the transition group have not been thoroughly investigated yet.
As is known, in order to modify electrical and optical properties of specific devices it is necessary to dope the material used. This is achieved, as a rule, by diffusing the element concerned in a semiconductor. The p-type GaSb is doped with 57Fe by diffusion, because it is this isotope that is responsible for high mobility and solubility of GaSb. To control the doping with 57Fe it is necessary to profoundly understand the mechanism of the diffusion of 57Fe. No doubt the study of the diffusion of 57Fe in GaSb is of interest in both theoretical and applied aspects.
Nowadays a variety of experimental technologies are used to study impurity atoms in semiconductors. Cutting-edge technologies of semiconductor materials make it possible to get samples that meet the requirements of practically every experimental method used in solid state physics. Most widely used are those to measure electrical conductivity, galvanomagnetic effects, photoconductivity, thermal and electrical conductivity, etc. All those methods, when used to study semiconductors, supply a lot of data on the impurity atoms in semiconductors. The methods mentioned may be called indirect methods, that is, the information obtained during the experiment concerns the charge carries but not the impurity atoms as such. The interpretation of these data, first of all, regarding the structure of the impurities centers and then the involvement, to a smaller or larger degree, of the arbitrary positions, should be very cautious and followed by the findings of direct methods.
Direct methods that can give direct information about the impurity atoms under investigation by the author of this article are: electronic paramagnetic resonance, nuclear magnetic resonance, nuclear quadrupole resonance, photoelectronic spectroscopy, perturbated angular correlation spectroscopy, etc. Among those is the Mössbauer spectroscopy, too. The role of the electronic paramagnetic resonance is well known, for example, when confirming the theory of the impurity centers in semiconductors. However those methods are not as universal as is the measurement of the electrical conductance or the Hall effect, that is, those methods mentioned above can be applicable to a limited number of semiconductor materials or impurities in them.
The features of the Mössbauer effect that allow it to compete with other direct methods are: (1) a possibility to obtain a number of parameters in a single experiment, which supplies direct information about impurity atoms and their surroundings; (2) an absolute selectivity of the isotope under study; (3) an insensibility to long-range rays.
In the past years, the literature in the field has accumulated a large volume of data on the impurities centers in semiconductors that became possible owing to the Mössbauer spectroscopy.
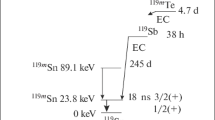
Figure 1 shows the schemes of the nuclear levels, revised and described by Mössbauer isotopes, and the schemes as the nuclear disintegration basis for these.
Schemes of decay of radioactive nuclei, resulting in the formation of the Mössbauer levels 57Fe, 73Ge, 83Kr,121Sn, 121Sb, 125Te, 129J, 133Cs, 151Eu şi 197Au [3]
These schemes demonstrate the difference between the results with the absorption spectroscopy and those with the emission one, and they will be further used to illustrate the findings for certain impurities.
In both of those versions of spectroscopy there is a radioactive source that emits Mössbauer quanta when certain nuclei pass from the excited into the ground state, as well as the absorption that has the same nuclei in the ground state and can absorb radiation at a corresponding resonance frequency. More widely used is the version with the absorption, that is, the object studied is an absorber—a standard radiation source with a single emission line. The sensibility for this type of spectroscopy is up to 1018 at/cm3.
It is worth noting here that the emission spectroscopy allows to partially avoid one of the main bottlenecks of the Mössbauer effect—a limited number of isotopes. From the schemes of Mössbauer levels of 119Sn it is clear that the transition for 119Sn can be used for five different impurities: cadmium, indium, stanium, antimonium, tellurium Fig. 1. Nowadays, the Mössbauer effect has been found with over 40 isotopes. However in semiconductor physics only 15 isotopes could be used. It is necessary to underline here that the existing isotopes are of high importance as doping elements for many semiconductor materials.
In spite of all its advantages, emission spectroscopy has two heavy drawbacks. First, the information based on the emission spectra turned to be complicated—it refers to the parent atom according to its position in the crystal lattice, the formation of different types of associations with the lattice defects, and to the daughter atom, depending on the electronic state. Second, radioactive transformations preceding the Mössbauer transition, could change the initial position of the state of the charge of the parent atom, which leads to difficulties in treating the emission spectra [4].
The main parameters of the Mössbauer spectra that are used in the present work are: isomer shift δ (it allows to identify electronic states and the charge of the impurity atoms), quadrupole splitting Δ (it allows to determine the local symmetry of the impurity center and to draw conclusions concerning the formation of the associations of such a center with other crystal defects), the width of the spectral line Γ (it contains the information on the weak hyperfine interactions).
2 Discussion of Experimental Results
Gallium antimonide doped with 57Fe, under investigation in this article, was obtained in the following way: first, the synthesis of GaSb in evacuated fused quartz ampoules, with pure Ga and Sb as well as Fe dopants in predetermined concentrations was carried out. Then the container with the respective components was placed in an electric furnace (with the T kept at 9000 °C), connected to the mechanical vibrator at the frequency of 50 Hz. Under the experimental conditions mentioned above, the synthesis took 24 h; then the electric furnace with the container was left for cooling. The following step in the technological process was the homogenisation and the growth of single crystals in an installation for zone melting.
Interesting physical phenomena have been revealed while investigating more profoundly (a) the properties of gallium antimonide doped with transition elements, and (b) gamma-ray nuclear resonance—the Mössbauer effect—in the iron atoms diffused in GaSb. For instance, gallium antimonide doped with the isotope 57Fe, enriched up to 99.99%, has been grown using the zone melting technique modified for the concentrations of 1–3% atomic mass.
Under investigation here were both p-type single crystals of GaSb with the charge carriers concentration of 4 × 1018 cm−3 and n-type single crystals of GaSb with the charge carriers concentration of 8 × 1016 cm−3. In order to get Mössbauer’s spectra, absorbants from different zones of the ingot were prepared. The spectra were registered in the range from the room temperature to the temperature of liquid nitrogen. The estimations and the results of the spectra processing, as well as of the experimental data were carried out using the program WMOSS (solid lines) and are plotted in Figs. 2, 3 and 4.
Another object of study was the valence of the Fe impurity atoms arising from the 57Co decay using Mössbauer’s spectroscopy. The picture of the 57Co radioactive decay is given in Fig. 2. Gamma-ray nuclear resonance spectra for GaSb doped with 57Fe depend on the conductivity type and the concentration of charge carriers.
In p-type samples Mössbauer’s spectra are in a form of a doublet, i.e. two specific peaks in a spectrum, with the quadrupole splitting \( \Delta E_{Q} \approx 1.2 \) mm s−1 and the isomer shift \( \delta \approx 0.5 \) mm s−1, whereas in n-type samples the quadrupole splitting is \( \Delta E_{Q} \approx 0.5 \) mm s−1 and the isomer shift is \( \delta \approx 0.4 \) mm s−1.
The authors in [5,6,7] have found a broad line in the gamma resonance (Mössbauer) spectra for GaSb. It is clearly seen there that for the Mössbauer impurity atoms, the parameters of those spectra have to be dependent, first of all, on the properties of the crystal lattice. From here, one could assume that in the same semiconductor, but in its different types and in different concentrations of the charge carriers, the Mössbauer spectra for the impurities of the electrically active atoms could be different relative to the parameters. Using Mössbauer’s parameters, one can see that iron in the crystal lattice in AIIIBV substitutes the atoms of elements for group III and has the configuration 3d5. As is known, elements from the Fe group in AIIIBV compounds should form acceptor levels [8]. In AIIIBV compounds with a broad energy band, GaSb being one of them, for the samples of the p-type, at T < 295 K, all impurity centers are not ionized, and those centers could be trivalent ions with the incomplete tetrahedral bonding arrangement, which leads to the appearance of the electric field gradient on 57Fe nuclei as well as to a greater quadrupole splitting. With the rise of temperature, the impurity centers become ionised and hence can be formally described as the movement of holes from the impurity atoms. Thereby Fe atoms are complementing their bondings, thus reducing the quadrupole splitting. A process similar to ionisation of the impurities centers can be observed when inserting Fe atoms in the n-type samples. No doubt when the concentration of the charge carriers in the n-type samples is less that the concentration of the impurity atoms then the gamma-ray resonance (Mössbauer) spectrum should be composed of the overlapping of two types of spectra. Measuring the dependence of the quadrupole splitting temperature of the gamma-ray (Mössbauer) resonance of 57Fe in GaSb allows to find out the nature of the impurity atoms as well as their energy levels.
The Mössbauer spectra for the samples of 1 and 3% concentrations were collected on a Model MS4 WRC spectrometer (SEE Co, Edina, MN), at the Centre “Physical Chemistry and Nanomaterials” of the Institute of Chemistry of the Academy of Sciences of Moldova. The conditions and facilities of the experiment were: the temperature range of 4.5–300 K, with the closed circuit; a system with refrigerated helium; and the temperature sensor of the model W106. The source was a 57Co (0.74 GBq) in Rh matrix; isomer shift refers to the metal α-Fe at 298 K. Experimental data were analysed using the program WMOSS (WEB Cercetare, Edina, MN).
3 Conclusions
As a result of the investigations it was found out that in GaSb doped with 57Fe gamma-ray resonance spectra depend on the conductivity type and the concentration of charge carriers. Estimations of the temperature dependence of the quadrupole splitting of the gamma-ray resonance (Mössbauer) spectra allowed to determine the nature of the electrical activity of the Mössbauer impurity atoms and their energy levels. The data obtained alow to conclude that iron doped in gallium antimonide makes the latter to have the configuration of 3d5, to be a trivalent impurity, with a tetrahedral structure, thus broadening its bondings.
References
Milnes, A.G., Polyakov, A.Y.: Solid-State Electron. 36, 803 (1993)
Dutta, P.S., Bhat, H.L., Kumar, V.: The physics and technology of gallium antimonide: an emerging optoelectronic material. J. Appl. Phys. 81(9), 5821–5870 (1997)
Nistriuk, I.V., Seregin, P.P.: Application of the Mössbauer emission spectroscopy in physics of semiconductors. Kishinev, 123 p (1982)
Seregin, P.P., Nasredinov, F.S., Vasilev, L.N.: Phys. St. Sol. A45(1), 11–45 (1978)
Beloserskii, G.N., Nemilov, Yu.A., Tomolov, S.B., Shvedchikov, A.V.: Fizika tverdogo tela 7, 3607 (1965)
Beloserskii, G.N., Gusev I.A., Nemilov, Yu.A., Shvedchikov, A.V.: Fizika tverdogo tela 8, 2112 (1966)
Basetsky, V.Ya., Veits, B.N., Grigalis, V.Ya., Lisin, Yu.D., Taksar, I.M.: Fizika tverdogo tela 10, 2852 (1968)
Veisberg, I.: Novel Semiconductor Materials, p. 153. Metallurgizdat, Moscow (1964)
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Mihalache, A. (2020). Mössbauer Effect in 57Fe-Doped Gallium Antimonide. In: Tiginyanu, I., Sontea, V., Railean, S. (eds) 4th International Conference on Nanotechnologies and Biomedical Engineering. ICNBME 2019. IFMBE Proceedings, vol 77. Springer, Cham. https://doi.org/10.1007/978-3-030-31866-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-31866-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31865-9
Online ISBN: 978-3-030-31866-6
eBook Packages: EngineeringEngineering (R0)