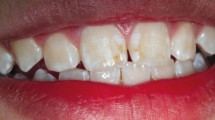
Abstract
Demarcated hypomineralized lesions of enamel, pathognomonic of MIH, are caused by the process of amelogenesis being altered or interrupted, most likely during the maturation phase. Increased protein concentrations are present in the lesion, primarily from serum, although some proteins involved in amelogenesis, such as amelogenin, have also been identified. The result of this disruption is enamel with decreased mineral density, decreased hardness and increased porosity and crystalline impurities, making it prone to demineralization and physical breakdown. The cause of enamel defects included in MIH and similar conditions including hypomineralized second primary molars is yet to be determined. Several hypotheses have been proposed, including childhood illness and genetic influences and a putative individual threshold of susceptibility; however, there is still conjecture on what the causative factors are.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The causative factors for MIH are still to be determined. Numerous researchers have identified associated factors; however, despite it being a prevalent condition in many communities worldwide, no consensus has been obtained regarding a single aetiological factor or a group of necessary factors [1,2,3]. Most putative factors identified to date involve childhood illness, medication taken during amelogenesis (such as antibiotics) and environmental toxins.
2 Amelogenesis
Amelogenesis is a complex process, and due to the stressful processes that the epithelially derived cells endure during enamel formation, the ameloblasts are sensitive to insults, both indirect and direct. There are two main phases of amelogenesis-secretion and maturation-with a transitional phase in between [4, 5].
2.1 Secretion
Amelogenesis commences with secretion of several enamel matrix proteins (EMPs) by elongated ameloblasts-namely, amelogenin (AMELX), ameloblastin (AMBN) and enamelin (ENAM). The matrix is gel-like in consistency, and initial mineralization starts during this period. The tight junctions (TJs) between the ameloblasts during the secretory phase may allow movement of substances, such as mineral ions, into the developing matrix whilst separating the developing tooth from the surrounding vascular tissue [5]. The secretion phase can be affected by genetic influences or direct trauma leading to less matrix being secreted, creating a quantitative defect-hypoplastic enamel. The metallopeptidase MMP20 is expressed during secretion, and MMP20-null mice have hypoplastic and hypomineralized enamel which cleaves from the dentino-enamel junction.
The secretory phase is followed by a short transitional phase during which the ameloblasts become squatter in shape and the production of matrix proteins is ceased. This is followed by the maturation phase [5].
2.2 Maturation
The majority of theorization over the past two decades identifies the maturation period of the ameloblast as the most likely period for the development of MIH enamel.
The maturation phase involves an increase in mineral density of the enamel-ameloblasts secrete proteolytic agents to cleave the matrix proteins and allow increasing mineralization. The major proteinase is the kallikrein-related peptidase (KLK4) and the metallopeptidases (MMP2, MMP3, MMP9); KLK4 dominates during maturation with KLK4 null mice manifesting hypomineralized enamel of normal thickness. Other activities, such as ion transport, EMP removal, pH balance and apoptosis, occur during this phase, giving multiple potential processes in which disturbances could lead to a qualitative defect-hypomineralization [5, 6].
3 Aberrations During Amelogenesis
Understanding the pathogenesis of hypomineralized enamel may lead to identification of potential aetiological factors. So, what are the possible ways the ameloblast can be affected to produce demarcated hypomineralized enamel lesions?
There is a potential for errors in the production of precursor proteins and mineral deposition leading to hypomineralized enamel, such as in the several hypocalcified and hypomature forms of amelogenesis imperfecta [7].
Mineral formation causes release of protons, which are neutralized by bicarbonate secretion by the ameloblast. If the bicarbonate pH moderation system was affected and less efficient, could this lead to hypomineralized enamel? Increased carbonate, beyond levels usually found during amelogenesis, has been reported in MIH enamel [8] (Fig. 4.1).
There are several mineral transporters of Ca2+ used by ameloblasts-null mutation mice for these transporters have severely hypomineralized enamel [6]. During the mid and late maturation phase, the Ca2+ transport is at its greatest-and if this is affected leading to lower ionic transport, it may decrease mineral density. Of the ions found in hypomineralized enamel, Mg2+ is an inhibitor of mineralization [6]. Thus, there are number of potential mineral processes that, if altered, could lead to demarcated hypomineralized lesions of enamel (Fig. 4.1).
It is postulated that fluoride stresses the ameloblast (especially endoplasmic reticulum function) and, due to the effects of this stress, leads to decreased matrix cleavage and removal due to downregulation of KLK4 during the maturation phase; however, amelogenin and MMP20 are not downregulated during the secretion phase. Therefore, increased enamel porosity and decreased hardness may be due to retained proteins and peptides (mainly related to amelogenin) inhibiting crystal growth [9, 10]. It is unknown if childhood illnesses and other putative aetiological factors for demarcated defects could have a similar effect on the ameloblast as fluoride.
There is the possibility that during the secretion stage, matrix proteins are changed in structure, later leading to poor cleavage and/or reduced clearance by ameloblasts. Disruption to this endocytosis of cleaved EMPs could lead to increased residual protein content and decreased mineral density. However, this would seem unlikely as it would be expected in this case that more remnants of matrix proteins in lesion enamel would be present. While traces of amelogenin have been found in MIH-affected enamel, this was not the major protein identified; rather, serum albumin predominates with levels up to 15 times that of healthy enamel reported [11, 12].
Ameloblasts provide a well-sealed barrier between the enamel matrix and the vascular system around the developing tooth, allowing maintenance of different ionic concentrations on either side of the cells [6, 13, 14]. The TJs between the ameloblasts maintain this seal, and the intercellular permeability depends on the constituents of the TJs, which are tight in maturation but leaky in the secretory stage [6].
Albumin is not synthesized by ameloblasts; therefore, it must be of a source extrinsic to the matrix [15]: albumin leaking between ameloblasts has been discussed by Robinson and colleagues including that it is a normal minor component of the enamel matrix during the secretory phase [16]. It has been suggested that albumin can bind to developing enamel crystals and once bound inhibits further growth; however, it is believed that the crystals developing during the secretory phase are protected from this effect by the intact amelogenins surrounding them, and so the albumin is present in a free state [17, 18]. As amelogenins are degraded and removed during transition and maturation phases, albumin may gain access to the now unprotected crystals and become bound: it is postulated that excessive levels of albumin exposure during this window of mineral binding opportunity could result in Hypomineralization defects [16].
Robinson and colleagues suggested hyperaemia associated with trauma as a situation leading to excessive albumin in the maturing enamel tissue; however, TJs can also be affected by cell stressors such as fluoride [10, 14, 16]. Animal studies have found that inflammatory mediators and serum can adversely affect the function of TJs in retinal, renal and salivary gland cells [19,20,21]. This suggests that there is a multitude of local and systemic circumstances that could allow increased amounts of passive paracellular ion movement and blood proteins into the developing tooth germ during the maturation phase when the TJ is meant to be tight.
Additionally, cellular stress may lead not to impairment of function but rather to apoptosis of ameloblasts and therefore the possibility of unregulated ingress of ions and blood proteins into the developing enamel matrix. Although there is no evidence of this possibility at present, ameloblasts are, for a part of their lifespan, highly specialized secretory cells; in order to perform this high-load function, such cells must often employ specific coping strategies. In the case of the ameloblast, the unfolded protein response (UPR) is utilized; under more extreme circumstances, this system can switch from promoting cell survival to “cutting its losses” and promoting cell death [9].
4 Genetic Influences
It is becoming increasingly apparent that no single environmental cause exists and that MIH and the related hypomineralized second primary molars (HSPM) are likely to have complex aetiologies with a contribution from genetic factors [22]. In particular, as certain variants of amelogenesis imperfecta have some similarities in appearance to MIH, genetic influences have been postulated for many years. Vieira has stated that approximately 20% of MIH variation is explained by genetics [23]. However, environmental factors have been the focus of most studies with genetic studies being scarce. Family and twin studies can be used to determine the heritability, that is, the genetic contribution to the variation in a disease, as a percentage from 0 to 100 [24].
A Brazilian study of 167 pairs of twins aged 8-15 years reported identical monozygotic twins were more concordant for MIH than nonidentical dizygotic twins, suggestive of a strong genetic influence on aetiology [25]. However, a twin study of six-year-old Australian twins failed to find a similar difference, even after adjusting for known risk factors [26]. DNA-based studies have also found some, albeit weak, evidence of possible genetic aetiological factors (Table 4.1). A genome-wide association study (GWAS) failed to find any genome-wide significant results but may have been underpowered to detect anything other than very large effect sizes. The authors did nevertheless suggest a gene locus near SCUBE1 (signal peptide, CUB domain and EGF-like domain containing 1) on chromosome 22 that may be important in MIH [27].
A more targeted study of genes known to be involved in tooth development revealed an association between specific markers and MIH [28]. While some genetic variants increased the susceptibility to MIH, others appeared to be protective. Importantly, AMELX, the gene that encodes amelogenin, was not associated with MIH, suggesting that the aetiology and pathophysiology of MIH is not linked to genetic alteration of amelogenin secretion. More recently, family-based candidate gene studies seem to provide stronger evidence of a genetic influence on MIH. A number of genes (including AMELX, ENAM, AMBN and MMP20) involved in the various stages and processes of amelogenesis, including secretion and degradation of enamel matrix proteins, have been associated with MIH in this way [29].
Further, interactions between amelogenesis and immune-related genes have been suggested to have an additive effect on MIH susceptibility [30]. Inflammatory-related cytokines can induce angiogenesis in the developing tooth germ-possibly increasing the risk of entry of blood proteins into the developing tooth [31]. The authors speculated that the association could be due to the regulation of KLK4 and MMP20 by TGF-β [30]. Increased prevalence of MIH has also been linked to children affected by “allergies”, although the pathological mechanism is unclear [32].
The importance of epigenetics in complex diseases is increasingly recognized and may apply to MIH/HSPM. Epigenetics describes the mitotically stable modifications of genes, associated with differences in DNA expression within different tissues [33]. Aside from cell lineage specification, epigenetics appears to mediate environmental influences on the gene expression. The most commonly studied epigenetic modification is DNA methylation, the addition of a methyl (–CH3) group at the cytosine of cytosine-guanine (CpG) dinucleotides. Despite recent interest, epigenetic dental studies are rare [34]. However, a recent very small (n = 10) epigenome-wide association study identified differential methylation of nine genes involved in cartilage, bone, tooth and neural development that may be associated with hypodontia [35]. Further studies investigating the role of epigenetic factors in HSPM/MIH would improve understanding of the aetiology of the condition and the mechanisms by which the disease is mediated by environmental factors.
Both a genetic predisposition and an environmental systemic cause would be expected to affect teeth in a stable, temporal pattern. However, in the case of MIH and HSPM, the cause must be able to explain why teeth forming at the same time can be affected to varying degrees. The potential reasons why there are variable numbers of teeth affected by MIH and variable lesion presentation and characteristics between teeth and within single teeth remain uncertain [36]. It has been proposed that the mechanism may be related to variations in mechanical forces in the tooth germ and variable gene expression influencing specific areas of the tooth [36]. Therefore, localized factors may influence the occurrence of MIH. The distribution of MIH lesions is restricted to the occlusal two-thirds of the crown, and it has been theorized that this may be due to more dramatic changes in molecular signalling occurring during the maturation of the occlusal two-thirds involving the initiation and development of the dentine-pulp complex. Epigenetic factors can be site-specific and could potentially impart different influences on teeth forming at the same time, albeit in different locations. However, there are no epigenetic studies of MIH/HSPM, and these areas of research need further investigation.
5 Potential Aetiological Factors
Numerous potential aetiological factors have been proposed for MIH. Three reviews have determined that no specific factor(s) with a high-quality evidence base has been identified [1,2,3]. Limitations of the existing studies include lack of consistency in design and reporting and that the majority are cross-sectional and retrospective in nature, introducing recall bias and limiting any conclusions on causation. This is especially problematic given the possibility of combined factor effects and the likelihood a child might experience multiple putative factors over the relevant time frames. However, a consistent finding is that childhood illness, especially those that induce pyrexia, is associated with MIH.
5.1 Environmental Contaminants
The connection between breastfeeding, the contamination of breast milk with dioxin and developmental defects of enamel (DDEs) including MIH was proposed around 20 years ago by Alaluusua and colleagues in a cohort of breast-fed children [37]. This difference was corroborated in a study of adults who were children in Seveso, Italy, at the time of an accidental dioxin exposure in 1976 [38]. In a more recent study of Finnish children, a marked difference was found in prevalence of MIH between urban and rural children, and it was speculated that differences in comparatively greater urban industrialisation may play a role, with the urban children (21.3%) having close to double the prevalence of MIH compared with their rural counterparts (11.5%), putatively due to environment toxicants [39].
In a recently published paper, an increased prevalence (approximately 2×) of DDE, including demarcated hypomineralized lesions, in an area of Vietnam affected by dioxin-contaminated herbicide exposure compared with a non-affected area was reported. The limitations of the study were that it was conducted in adults and the DDE index was used; thus, specific MIH prevalence data are difficult to derive [40].
Several studies using rat models have implicated bisphenol A (BPA) as a causative factor in MIH [41,42,43]. Exposure of the rat to BPA increased the albumin content of the enamel and increased the expression of enamelin and decreased the expression of KLK4. A greater effect on males compared with females has also been reported-mechanism unknown [42, 44].
There was an enhanced (greater) effect of BPA with concomitant exposure to fluoride [42]. This could be because AMELX and ENAM genes are modulated by BPA, whilst KLK4 and MMP are modulated by fluoride.
One needs to interpret animal models with some caution, especially those with a continuously growing tooth used as the expression of the phenotype; however, they do shed light onto the condition in ways human experimentation could never do.
Chronic fluoride ingestion is associated with increased prevalence of diffuse hypomineralized defects of enamel, and teeth forming at the time of the fluoride toxicity to the ameloblasts will be affected-distinct from the appearance of MIH. The risk of fluorosis depends on the age and extent of the fluoride ingestion, and also genetic factors are involved [45]. There has been no positive association determined between fluoride ingestion and MIH [1, 2].
5.2 Birth “Complications”
Most pre-, peri- and postnatal studies are cross-sectional, and therefore claims can be made about association only. There is little evidence of association between prenatal events and MIH, although some evidence exists regarding HSPM, in vitro fertilisation and maternal smoking later in pregnancy [1,2,3, 26]. Maternal smoking during pregnancy has not been found to be associated with MIH [3].
There is conflicting evidence related to peripartum events such as premature birth, caesarean birth and birth complications. In their systematic review, Silva and colleagues found heterogeneity in the methodology and reporting of peripartum events, restricting the drawing of any conclusions regarding risk factors [26]. Wu and colleagues reported that premature birth and low birth weight both increased the risk of MIH; although, like Silva, they commented that the heterogeneity of data was high and there may be a publication bias for papers with positive associations [3, 46]. More recently, a positive association between increased risk for MIH and infant hypoxia during delivery as well as caesarean section was reported in a French cohort; no association was determined for prematurity [47]. In a Brazilian study, a low (<7) Apgar score was associated with diffuse and demarcated opacities in primary teeth [48]; however, in a Norwegian study, no association between MIH and an Apgar score ≤5 was determined [49].
5.3 Early Childhood
In early childhood, up to the age of 3 or 4, the influence of a variety of factors, especially related to health and including pyrexia/fever, chicken pox, asthma, otitis media, etc., has been investigated broadly. There has been variability in the definition of “illness”, limiting the conclusions drawn [3]. Many studies have determined association of MIH with early childhood fever and “respiratory disease”, which includes pneumonia and asthma.
The influence of vitamin D on dental development has been known for many decades [50]. With respect to MIH, results are conflicting. In a German study, a positive relationship between lower vitamin D (25(OH)D) levels and increased prevalence of MIH was determined [51]; however, van der Tas and colleagues reported no link between vitamin D levels at 6 years of age with MIH or HSPM in Dutch children [52]. In a recent study, a link with higher levels of vitamin D and HSPM was reported; however, the authors stated that caution should be taken when interpreting the results as they could be influenced by unknown confounding factors [26]. A recent Danish paper reported that high dose Vitamin D supplementation of pregnant women may reduce the prevalence of MIH significantly [53].
5.4 Medication
The association between childhood medications and MIH, like many other factors, is not specific, despite several research projects over the years [2, 54]. Antibiotics such as amoxicillin and erythromycin, as well as anti-asthma and chemotherapeutic drugs have been investigated, with a recent systematic review concluding that no specific drugs can be identified as causing MIH at this time [54,55,56,58]. The main issue is that in most cases the effect of the disease cannot be separated from the effect of the resultant medication, as the studies are either retrospective or cross-sectional; it would also be unethical to undertake a longitudinal trial and not provide appropriate medication to a control group.
6 Overview of MIH Aetiology
MIH aetiological factors are still uncertain. It is likely that childhood illnesses and genetic factors are involved, and possibly there is an individual threshold for susceptibility-as many children without relevant medical histories are severely affected by MIH and vice versa.
The evidence base is limited by issues with heterogeneity of existing research, especially regarding the indices used (EAPD, mDDE) and definitions of illnesses. More recently, the number of publications using the EAPD index has increased, allowing more valid comparison.
The importance of early diagnosis and appropriate treatment planning cannot be underestimated. Therefore, if a child has had HSPM, then due to increased risk, the presence of MIH should be determined as soon after the eruption of the first permanent molars as possible.
Hopefully, the aetiological factors involved in MIH (and HSPM) will be discovered; however, in the meantime, more quality longitudinal research projects are needed-both multisite and standardized.
References
Alaluusua S. Aetiology of molar-incisor hypomineralisation: a systematic review. Eur Arch Paediatr Dent. 2010;11(2):53–8. https://doi.org/10.1007/bf03262713.
Crombie F, Manton D, Kilpatrick N. Aetiology of molar–incisor hypomineralization: a critical review. Int J Paediatr Dent. 2009;19(2):73–83. https://doi.org/10.1111/j.1365-263X.2008.00966.x.
Silva MJ, Scurrah KJ, Craig JM, Manton DJ, Kilpatrick N. Etiology of molar incisor hypomineralization - a systematic review. Community Dent Oral Epidemiol. 2016;44(4):342–53. https://doi.org/10.1111/cdoe.12229.
Wright JT, Carrion IA, Morris C. The molecular basis of hereditary enamel defects in humans. J Dent Res. 2015;94(1):52–61. https://doi.org/10.1177/0022034514556708.
Lacruz R, Habelitz S, Wright J, Paine M. Dental enamel formation and implications for oral health and disease. Physiol Rev. 2017;97(3):939–93. https://doi.org/10.1152/physrev.00030.2016.
Bronckers ALJJ. Ion transport by ameloblasts during amelogenesis. J Dent Res. 2017;96(3):243–53. https://doi.org/10.1177/0022034516681768.
Crawford PJM, Aldred M, Bloch-Zupan A. Amelogenesis imperfecta. Orphanet J Rare Dis. 2007;2:17–27. https://doi.org/10.1186/1750-1172-2-17.
Crombie FA, Manton DJ, Palamara JEA, Zalizniak I, Cochrane NJ, Reynolds EC. Characterisation of developmentally hypomineralised human enamel. J Dent. 2013;41(7):611–8. https://doi.org/10.1016/j.jdent.2013.05.002.
Brookes SJ, Barron MJ, Dixon MJ, Kirkham J. The unfolded protein response in amelogenesis and enamel pathologies. Front Physiol. 2017;8:653. https://doi.org/10.3389/fphys.2017.00653.
Suzuki M, Shin M, Simmer JP, Bartlett JD. Fluoride affects enamel protein content via TGF-β1-mediated KLK4 inhibition. J Dent Res. 2014;93(10):1022–7. https://doi.org/10.1177/0022034514545629.
Mangum JE, Crombie FA, Kilpatrick N, Manton DJ, Hubbard MJ. Surface integrity governs the proteome of hypomineralized enamel. J Dent Res. 2010;89(10):1160–5. https://doi.org/10.1177/0022034510375824.
Farah RA, Monk BC, Swain MV, Drummond BK. Protein content of molar-incisor hypomineralisation enamel. J Dent. 2010;38(7):591–6. https://doi.org/10.1016/j.jdent.2010.04.012.
Pham C-D, Smith CE, Hu Y, JC-C H, Simmer JP, Y-HP C. Endocytosis and enamel formation. Front Physiol. 2017;8:529. https://doi.org/10.3389/fphys.2017.00529.
Rácz R, Földes A, Bori E, Zsembery Á, Harada H, Steward MC, et al. No change in bicarbonate transport but tight-junction formation is delayed by fluoride in a novel ameloblast model. Front Physiol. 2017;8:940. https://doi.org/10.3389/fphys.2017.00940.
Couwenhoven RI, Davis C, Snead ML. Mouse ameloblasts do not transcribe the albumin gene. Calcif Tissue Int. 1989;45(6):367–71. https://doi.org/10.1007/bf02556008.
Robinson C, Brookes SJ, Kirkham J, Bonass WA, Shore RC. Crystal growth in dental enamel: the role of amelogenins and albumin. Adv Dent Res. 1996;10(2):173–80. https://doi.org/10.1177/08959374960100020901.
Robinson C, Kirkham J, Brookes SJ, Shore RC. The role of albumin in developing rodent dental enamel: a possible explanation for white spot hypoplasia. J Dent Res. 1992;71(6):1270–4. https://doi.org/10.1177/00220345920710060101.
Robinson C, Shore RC, Kirkham J, Stonehouse NJ. Extracellular processing of enamel matrix proteins and the control of crystal growth. J Biol Buccale. 1990;18(4):355–61.
Zhang LW, Cong X, Zhang Y, Wei T, Su YC, Serrão ACA, et al. Interleukin-17 impairs salivary tight junction integrity in Sjögren’s syndrome. J Dent Res. 2016;95(7):784–92. https://doi.org/10.1177/0022034516634647.
Zhuang Y, Hu C, Ding G, Zhang Y, Huang S, Jia Z, et al. Albumin impairs renal tubular tight junctions via targeting the NLRP3 inflammasome. Am J Physiol Renal Physiol. 2015;308(9):F1012–F9. https://doi.org/10.1152/ajprenal.00509.2014.
Chang C-W, Wang X, Caldwell RB. Serum opens tight junctions and reduces ZO-1 protein in retinal epithelial cells. J Neurochem. 1997;69(2):859–67. https://doi.org/10.1046/j.1471-4159.1997.69020859.x.
Vieira AR, Kup E. On the etiology of molar-incisor hypomineralization. Caries Res. 2016;50(2):166–9. https://doi.org/10.1159/000445128.
Vieira AR. On the genetics contribution to molar incisor hypomineralization. Int J Paediatr Dent. 2019;29(1):2–3. https://doi.org/10.1111/ipd.12439.
Wray NVP. Estimating trait heritability. Nat Educ. 2008;1:29.
Teixeira R, Andrade NS, Queiroz LCC, Mendes FM, Moura MS, Moura L, et al. Exploring the association between genetic and environmental factors and molar incisor hypomineralization: evidence from a twin study. Int J Paediatr Dent. 2018;28(2):198–206. https://doi.org/10.1111/ipd.12327.
Silva MJ, Kilpatrick NM, Craig JM, Manton DJ, Leong P, Burgner D, et al. Etiology of hypomineralized second primary molars: a prospective twin study. J Dent Res. 2019;98(1):77–83. https://doi.org/10.1177/0022034518792870.
Kuhnisch J, Thiering E, Heitmuller D, Tiesler CMT, Grallert H, Heinrich-Weltzien R, et al. Genome-wide association study (GWAS) for molar-incisor hypomineralization (MIH). Clin Oral Investig. 2014;18(2):677–82. https://doi.org/10.1007/s00784-013-1054-8.
Jeremias F, Koruyucu M, Kuchler EC, Bayram M, Tuna EB, Deeley K, et al. Genes expressed in dental enamel development are associated with molar-incisor hypomineralization. Arch Oral Biol. 2013;58(10):1434–42. https://doi.org/10.1016/j.archoralbio.2013.05.005.
Jeremias F, Pierri RAG, Souza JF, Fragelli CMB, Restrepo M, Finoti LS, et al. Family-based genetic association for molar-incisor hypomineralization. Caries Res. 2016;50(3):310–8. https://doi.org/10.1159/000445726.
Bussaneli DG, Restrepo M, Fragelli CMB, Santos-Pinto L, Jeremias F, Cordeiro RCL, et al. Genes regulating immune response and amelogenesis interact in increasing the susceptibility to molar-incisor hypomineralization. Caries Res. 2019;53(2):217–27. https://doi.org/10.1159/000491644.
Kobayashi-Kinoshita S, Yamakoshi Y, Onuma K, Yamamoto R, Asada Y. TGF-β1 autocrine signalling and enamel matrix components. Sci Rep. 2016;6:33644. https://doi.org/10.1038/srep33644. https://www.nature.com/articles/srep33644#supplementary-information.
Frascino S, Frascino A, Rezende KM, Imparato JC, Pignatari S. Molar-incisor enamel hypomineralization cross-sectional prevalence evaluation in oral-breathing allergic children. Clin Lab Res Dent. 2017:1–6. https://doi.org/10.11606/issn.2357-8041.clrd.2017.134317.
Barros SP, Offenbacher S. Epigenetics: connecting environment and genotype to phenotype and disease. J Dent Res. 2009;88(5):400–8. https://doi.org/10.1177/0022034509335868.
Townsend G, Richards L, Hughes T, Pinkerton S, Schwerdt W. Epigenetic influences may explain dental differences in monozygotic twin pairs. Aust Dent J. 2005;50(2):95–100. https://doi.org/10.1111/j.1834-7819.2005.tb00347.x.
Wang J, Sun K, Shen Y, Xu Y, Xie J, Huang R, et al. DNA methylation is critical for tooth agenesis: implications for sporadic non-syndromic anodontia and hypodontia. Sci Rep. 2016;6:19162. https://doi.org/10.1038/srep19162.
Vieira AR, Manton DJ. On the variable clinical presentation of molar-incisor hypomineralization. Caries Res. 2019;53(4):482–8, accepted for publication.
Alaluusua S, Lukinmaa P-L, Koskimies M, Pirinen S, Hölttä P, Kallio M, et al. Developmental dental defects associated with long breast feeding. Eur J Oral Sci. 1996;104(5–6):493–7. https://doi.org/10.1111/j.1600-0722.1996.tb00131.x.
Alaluusua S, Calderara P, Gerthoux Pier M, Lukinmaa P-L, Kovero O, Needham L, et al. Developmental dental aberrations after the dioxin accident in seveso. Environ Health Perspect. 2004;112(13):1313–8. https://doi.org/10.1289/ehp.6920.
Wuollet E, Laisi S, Salmela E, Ess A, Alaluusua S. Background factors of molar-incisor hypomineralization in a group of Finnish children. Acta Odontol Scand. 2014;72(8):963–9. https://doi.org/10.3109/00016357.2014.931459.
Ngoc VTN, Huong LT, Van Nhon B, Tan NTM, Van Thuc P, Hien VTT, et al. The higher prevalence of developmental defects of enamel in the dioxin-affected region than non-dioxin-affected region: result from a cross-sectional study in Vietnam. Odontology. 2019;107(1):17–22. https://doi.org/10.1007/s10266-018-0358-1.
Jedeon K, De la Dure-Molla M, Brookes SJ, Loiodice S, Marciano C, Kirkham J, et al. Enamel defects reflect perinatal exposure to bisphenol a. Am J Pathol. 2013;183(1):108–18. https://doi.org/10.1016/j.ajpath.2013.04.004.
Jedeon K, Houari S, Loiodice S, Thuy TT, Le Normand M, Berdal A, et al. Chronic exposure to bisphenol a exacerbates dental fluorosis in growing rats. J Bone Miner Res. 2016;31(11):1955–66. https://doi.org/10.1002/jbmr.2879.
Jedeon K, Marciano C, Loiodice S, Boudalia S, Lavier MCC, Berdal A, et al. Enamel hypomineralization due to endocrine disruptors. Connect Tissue Res. 2014;55:43–7. https://doi.org/10.3109/03008207.2014.923857.
Jedeon K, Berdal A, Babajko S. Impact of three endocrine disruptors, Bisphenol a, Genistein and Vinclozolin on female rat enamel. Bull Group Int Rech Sci Stomatol Odontol. 2016;53(1):28–32.
Küchler EC, Dea Bruzamolin C, Ayumi Omori M, Costa MC, Antunes LS, Pecharki GD, et al. Polymorphisms in nonamelogenin enamel matrix genes are associated with dental fluorosis. Caries Res. 2018;52(1–2):1–6. https://doi.org/10.1159/000479826.
Wu X, Wang J, Li Y-h, Z-y Y, Zhou Z. Association of molar incisor hypomineralization with premature birth or low birth weight: systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2018:1–9. https://doi.org/10.1080/14767058.2018.1527310.
Garot E, Manton D, Rouas P. Peripartum events and molar-incisor hypomineralisation (MIH) amongst young patients in Southwest France. Eur Arch Paediatr Dent. 2016;17(4):245–50. https://doi.org/10.1007/s40368-016-0235-y.
Pinto GS, Costa FS, Machado TV, Hartwig A, Pinheiro RT, Goettems ML, et al. Early-life events and developmental defects of enamel in the primary dentition. Community Dent Oral Epidemiol. 2018;46(5):511–7. https://doi.org/10.1111/cdoe.12408.
Sidaly R, Schmalfuss A, Skaare AB, Sehic A, Stiris T, Espelid I. Five-minute Apgar score <= 5 and molar incisor Hypomineralisation (MIH) - a case control study. BMC Oral Health. 2016;17:7. https://doi.org/10.1186/s12903-016-0253-5.
Sheldon M, Bibby BG, Bales MS. The relationship between microscopic enamel defects and infantile debilities. J Dent Res. 1945;24(2):109–16. https://doi.org/10.1177/00220345450240020201.
Kuhnisch J, Thiering E, Kratzsch J, Heinrich-Weltzien R, Hickel R, Heinrich J, et al. Elevated serum 25(OH)-vitamin D levels are negatively correlated with molar-incisor hypomineralization. J Dent Res. 2015;94(2):381–7. https://doi.org/10.1177/0022034514561657.
van der Tas JT, Elfrink MEC, Heijboer AC, Rivadeneira F, Jaddoe VWV, Tiemeier H, et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dentist Oral Epidemiol. 2018;46(4):343–51. https://doi.org/10.1111/cdoe.12372.
Nørrisgaard PE, Haubek D, Kühnisch J, et al. Association of high-dose vitamin D supplementation during pregnancy with the risk of enamel defects in offspring: a 6-year follow-up of a randomized clinical trial. JAMA Pediatr. 2019;73(10):924–30. https://doi.org/10.1001/jamapediatrics.2019.2545.
Jälevik B. Prevalence and diagnosis of molar-incisor-hypomineralisation (MIH): a systematic review. Eur Arch Paediatr Dent. 2010;11(2):59–64. https://doi.org/10.1007/bf03262714.
Serna C, Vicente A, Finke C, Ortiz AJ. Drugs related to the etiology of molar incisor hypomineralization a systematic review. J Am Dent Assoc. 2016;147(2):120–30. https://doi.org/10.1016/j.adaj.2015.08.011.
Kuscu OO, Sandalli N, Dikmen S, Ersoy O, Tatar I, Turkmen I, et al. Association of amoxicillin use and molar incisor hypomineralization in piglets: visual and mineral density evaluation. Arch Oral Biol. 2013;58(10):1422–33. https://doi.org/10.1016/j.archoralbio.2013.04.012.
Laisi S, Ess A, Sahlberg C, Arvio P, Lukinmaa PL, Alaluusua S. Amoxicillin may cause molar incisor hypomineralization. J Dent Res. 2009;88(2):132–6. https://doi.org/10.1177/0022034508328334.
Wuollet E, Laisi S, Salmela E, Ess A, Alaluusua S. Molar–incisor hypomineralization and the association with childhood illnesses and antibiotics in a group of Finnish children. Acta Odontol Scand. 2016;74(5):416–22. https://doi.org/10.3109/00016357.2016.1172342.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Manton, D.J., Crombie, F.A., Silva, M.J. (2020). The Pathogenesis and Aetiology of MIH: More Questions Than Answers. In: Bekes, K. (eds) Molar Incisor Hypomineralization. Springer, Cham. https://doi.org/10.1007/978-3-030-31601-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-31601-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31600-6
Online ISBN: 978-3-030-31601-3
eBook Packages: MedicineMedicine (R0)