Abstract
Changes in immunologic pathways play a leading role in all stages of cancer. Proper immune function also associates with quantitative and qualitative aspects of nutrition [1, 2]. Therefore, overnutrition and imbalanced nutrition may affect development, progression, and therapeutic response of cancer [2]. Pro-inflammatory cytokines such as tumor necrosis factor (TNF), interferon-γ (IFN-γ), and interleukins 1 and 6 (IL-1 and IL-6) are important mediators of cancer complications such as cachexia [3]. A tumor can trigger the release of cytokines such as IL-6 [4], which is associated with an increase in lipolysis and proteolysis, which in turn affect the appetite and host neuroendocrine axis and induce anorexia and cachexia [4, 5]. Several neuropeptides such as neuropeptide Y (NPY) and adipokines such as leptin have been implicated in the pathogenesis of cancer cachexia syndrome [5, 6]. Thus, an imbalance of cytokine production, and neuropeptide and adipokine dysfunction as well as changes in microbiota (particularly in GI in the consequence of cancer and tumor suppressive agents) may be a major cause of the nutritional consequences of cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
24.1 Introduction
Changes in immunologic pathways play a leading role in all stages of cancer. Proper immune function also associates with quantitative and qualitative aspects of nutrition [1, 2]. Therefore, overnutrition and imbalanced nutrition may affect development, progression, and therapeutic response of cancer [2]. Pro-inflammatory cytokines such as tumor necrosis factor (TNF), interferon-γ (IFN-γ), and interleukins 1 and 6 (IL-1 and IL-6) are important mediators of cancer complications such as cachexia [3]. A tumor can trigger the release of cytokines such as IL-6 [4], which is associated with an increase in lipolysis and proteolysis, which in turn affect the appetite and host neuroendocrine axis and induce anorexia and cachexia [4, 5]. Several neuropeptides such as neuropeptide Y (NPY) and adipokines such as leptin have been implicated in the pathogenesis of cancer cachexia syndrome [5, 6]. Thus, an imbalance of cytokine production, and neuropeptide and adipokine dysfunction as well as changes in microbiota (particularly in GI in the consequence of cancer and tumor suppressive agents) may be a major cause of the nutritional consequences of cancer.
24.2 Role of Nutrition in Predisposition of Cancer from an Immunologic View
One of the known risk factors for cancer is obesity, especially with the modern lifestyle and low physical activity [3]. Dietary patterns have a significant effect on the cytokine profile; for instance, the high intake of saturated fats, especially in obese people, leads to infiltration of adipose tissue by macrophages producing IL-1β, IL-6, and macrophage inhibitory factor (MIF) [4,5,6]. Moreover, a decrease in the secretion of anti-inflammatory adipokines such as adiponectin may maintain pro-inflammatory signals and activate the production of C-reactive protein (CRP) by the liver [7, 8]. Based on previous studies, this chronic inflammatory process is related to an increased susceptibility to various types of cancer, including cancers of the gastrointestinal, respiratory, and genitourinary systems [9,10,11]. It has been evident that the inflammation is promoted by saturated fatty acids and their binding to the Toll-like receptors (TLR 2 and 4) activating pro-inflammatory factors such as nuclear factor-kappa B (NF-κB) [12]. Moreover, downregulation of autophagy and decreased cytoplasmic recycling of damaged organelles accelerate activation of inflammasome and complement components [13, 14]. Chronic inflammation dysregulates immune function from immunosurveillance to carcinogenic inflammasome by stimulating cellular turnover, increasing stem cell divisions, enhancing production of reactive oxygen species and metabolic rate locally [15]. Unresolved inflammation due to overnutrition provides a local immunosuppressive microenvironment by production of transforming growth factor beta (TGF-β) and myeloid-derived suppressor cells within the tumor lesion [16, 17]. Obesity also affects the microbiota leading to an intestinal dysbiosis and diminishes the bacterial and endotoxin barriers, which increases the risk of procarcinogenic metabolites presentation [18, 19]. Decreased autophagy also enhances aging process affecting immune profile by decreasing cytotoxic T-cells, thymic atrophy, and dendritic cells’ dysfunction [19,20,21].
Vice versa, intermittent fasting and adjusted low-carbohydrate/hypocaloric diet has beneficial effects on antagonizing the chronic inflammation process mediated by increased ketone-bodies, decreased risk factors of metabolic syndrome [1, 22,23,24,25]. Surprisingly this method can be used for boosting chemotherapy since it can increase the remodeling of the immune-cell infiltrate by an increased infiltrating cytotoxic T-cells and local depletion of regulatory T-cells [26, 27]. Treatment with one or several fasting cycles diminishes tumor growth, prevents cellular transformation, and upregulates autophagy [28,29,30].
Influenced by this important effect of nutrition on the immune system, characteristics of the human diet can directly stimulate gastrointestinal malignancies [31]. A diet low in fiber and vegetables may affect the regulation of carbohydrate absorption and short chain fatty acid formation, which affects the metabolism of carcinogens [32]. This process is linked to colon cancer and its progression [33]; apparently, a decrease in fiber intake may allow more time for exposure of colon cells and the immune system to the potential carcinogens, affecting intestinal transit [34]. However, recently the anti-inflammatory effects of fiber and multiple distinct phytochemicals (e.g., enterolactone, flaxseed, lignin, and spermidine) on microbiome have been reported including increased proportion of Lactobacilli and Bifidobacteria [35, 36]. Moreover, based on the evidence used to draw conclusions about a gluten free diet in patients with celiac disease leading to cancer protection, it seems reasonable to consider gluten as a booster for cancer in celiac patients [37, 38]. Meat consumption is a risk factor for some cancers, especially colon, rectum, and prostate. Red meat consumption increases the risk of colon cancer by causing increased production of heterocyclic amines [39, 40]. On the other hand, a change in the normal diet and deficiency of vitamins or minerals may affect the adequacy of either innate immunity (phagocytic activity, chemotaxis of neutrophils, or release of cytokines from monocytes) or adaptive immunity (immunoglobulin production of B-cells or cell-mediated immunity) [41,42,43,44]. Many of the consequences of malnutrition in the regulation of signal transduction and immunoregulatory gene expression were first recognized in the early 1800s as nutrigenomics [44, 45]. The majority of these changes are reversible after administration of adequate nutrition supplements [46]. The following list of specific dietary factors has been studied in relation to the immune aspects of cancer.
24.2.1 Protein–Calorie Balance
The formation of lymphocytes, eosinophils, and vital immune system proteins such as thymic hormones, antibody (Ab) responses to T-cell dependent antigens (Ags), and Ab affinity are affected by protein–calorie imbalance [47]. It has long been recognized that caloric restriction with a well-balanced diet avoiding certain nutrient deficiencies can increase longevity and has cancer preventive effects in mammals [48].
24.2.2 Essential Fatty Acids
Essential fatty acids, mainly suggested by consumption of nuts, in our body can regulate the production of prostaglandins, prostacyclins, thromboxanes, and leukotrienes, causing a significant effect on the host immune system and regulation of inflammation and C-reactive proteins [49].
24.2.3 Antioxidants (Selenium, Vitamin E, and Vitamin C)
These nutrients have strong antioxidative effects and may reduce the risk of cancer by neutralizing reactive oxygen species or free radicals that can damage DNA [50, 51].
24.2.3.1 Vitamin A
Vitamin A plays an important role in protection against measles, white blood cell (WBC) function, resistance to carcinogens, and skin and mucous membrane defenses. Vitamin A precursor carotenoids, such as lycopene, have a potential effect on cancer prevention [52, 53].
24.2.4 Vitamin D
25-hydroxyvitamin D has been of interest based on ecologic studies on populations with greater exposure to ultraviolet light who had a lower risk of breast cancer, colon cancer, and prostate cancer. This vitamin regulates humoral Ab response, enhances organ specific cytotoxic T-cells, and supports a Th2-mediated anti-inflammatory profile of cytokines; therefore, its anticancer properties are strongly suggested [54,55,56].
24.2.5 Vitamin B6
Pyridoxine and its metabolite PLP (pyridoxal-5′ phosphate) induce immunosurveillance activation and Th1 cytokine-mediated immune responses. Epidemiologic studies and laboratory animal models have shown that vitamin B6 modulates the risk of cancer. It is not clear how vitamin B6 mediates this effect, but it has been reported that high dietary vitamin B6 attenuates and low dietary vitamin B6 increases the risk of cancer [55, 57,58,59].
24.2.6 Folate
Folate is important for DNA methylation, repair, and synthesis, which is also crucial for lymphocyte development [60, 61]. Epidemiologic studies have shown that low folic acid intake is associated with a higher risk of various cancers, most notably colon, breast, and probably cervical cancer. The fact that methylenetetrahydrofolate reductase, an enzyme predicted to reduce the risk of colon cancer, is associated with folate status supports the role of folate deficiency in cancers [62, 63].
24.2.7 Calcium
Many studies show that calcium may reduce the risk of colorectal cancer via direct and indirect effects. Calcium has a direct effect on proliferation, stimulating differentiation, and apoptosis in the colonic mucosa [64, 65]. Its indirect effect is binding to toxic secondary bile acids and ionized fatty acids to form insoluble soaps in the lumen of the colon [66].
24.2.8 Nutrition Overdose in Cancer
In addition to deficiency, an overdose of some micronutrients can also have an immunosuppressive effect, especially megadoses of vitamin E [67]. High doses of certain minerals such as chromium, copper, iron, manganese, and zinc also may induce cancer and immune dysfunction [68].
In summary, attenuated innate and adaptive immunity as a result of an inadequate diet leads to a higher risk of cancer and lower homeostasis for cancerous antigens, which could be resulted from reducing nutrient intake, increasing losses, and interfering with utilization due to altering metabolic pathways. Thus, nutrition may have a significant role in immune prevention and immune surveillance of cancer.
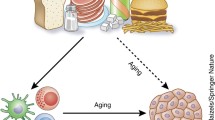
24.3 Aging as a Confounder of the Triangle of Nutrition, Immunity, and Cancer
Aging may be a confounder of the triangle of nutrition, immunity, and cancer (Fig. 24.1); however, neither the relationships nor the mechanisms of interaction are known. Unfortunately, only a few studies have considered that nutrition and immune function simultaneously decrease in elderly individuals [69]. It is known that increased age adversely affects the function of the immune system as well as nutrient intake habits [70]. Therefore, both immunosuppression (mainly due to decreased effectiveness of T and natural killer cells) and nutritional deficiencies (as defined by the 1989 recommended dietary allowances) in the elderly may have independent correlations with an increased risk of infection and neoplasia development [42].
One of the probable mechanisms that may affect both immunity and nutrition in old people is turnover fluctuations of cellular components in lysosomes or autophagy. Advanced age leads to a reduction in the autophagy of loading viral Ags and cross presentation of tumor Ags into MHC class I molecules, as well as pathogen killing [71,72,73]. Similarly, the capability of autophagy for energetic balance recycling of amino acids to maintain protein synthesis under starvation conditions and the capacity of intracellular lipid stores or glycogen mobilization are disturbed [74, 75]. However, only minimal information has been produced concerning human cancer initiation as a direct result of a specific dietary etiology in the elderly.
24.4 Microbiota as a Confounder of the Triangle of Nutrition, Immunity, and Cancer
Studies examining the composition of alimentary elements on the intestinal microbiome and the role of dysbiosis in different diseases states have uncovered associations with inflammation and tumorigenesis [76, 77]. Moreover, the impact of immunosuppressive and anticancer agents on the microbiota profile has been recorded [78,79,80,81]. High protein diet can increase the microbial diversity and proportion of Bifidobacteria, Lactobacilli, and Eubacterium Rectale but can decrease Bacteroides species. Similarly most of natural sugar can enhance incidence of Bifidobacteria rather than Bacteroides. Moreover high fat diet inhibits propagation of the lactic acid bacteria but provide an environment in favor of Clostridiales and Bacteroides. Probiotics also can change the microbiota by overpresentation of Bifidobacteria, Lactobacilli, aerobes/anaerobes, and lower presentation coliforms, Helicobacter pylori, Escherichia coli [82, 83].
Immunosurveillance profile (low short chain fatty acids, low lipopolysaccharide levels, low IL-6, and high IL-10) is associated with specific microbiome molecular patterns which usually can be linked with Mediterranean diet with dominancy of Bifidobacteria, Lactobacilli, Eubacteria, Bacteroides, and Prevotella. Studies that involve intake of a specific dietary component demonstrate how certain bacteria tend to respond to the nutrient-specific challenge. Protein, fats, digestible and non-digestible carbohydrates, and probiotics all induce shifts in the microbiome with secondary effects on host immunologic and metabolic markers suggesting maintaining healthy gut microbiome is critical to human health [84, 85].
24.5 Role of Cancer in Predisposition to Malnutrition from an Immunologic View
Despite the role of nutrition in either preventing or causing cancer in humans, malnutrition is a common problem (global percentage of 56.5%), and weight loss is often predictive of shortened survival in these patients [86]. In advanced stages of cancer, up to 35% of related deaths may be linked to improper diet [87, 88]. Moreover, a proportion of patients with malignancy develop cachexia, a progressive involuntary weight loss status that is attributed to clinic-pathologic factors of the tumor (origin, metastasis, and size), host immunity, and antitumor treatment (Fig. 24.2) [89]. During the development of cancer-associated cachexia, several Th2-dominant condition mediators such as IL-2 and TNF (prognostic markers) are implicated in appetite loss and metabolic disturbances, as well as leptin, IL-1, IL-6, IFN-γ, leukemia inhibitory factor, NPY, and proteoglycan 24 K [90, 91]. These immunologic and metabolic changes induce cancer cachexia syndrome, which is characterized by patient tissue wasting, anorexia, appetite loss, prolonged fatigue and lethargy, insulin resistance, microcytic anemia, hyperlipidemia, and hypoalbuminemia [92, 93]. Metabolic features of this syndrome include increases in the heterogeneity of energy requirement, substrate cycling and turnover, Cori cycle activity, and hepatic protein synthesis, as well as decreases in peripheral muscle protein synthesis, serum protein lipase activity, and plasma concentration of branched chain amino acids. In general, the severity of malnutrition and cachexia in digestive neoplasias is in highest percentages (from 79% in esophageal cancer to 40% in rectum cancers) due to the involvement of all predisposing factors described in Fig. 24.2 during the development of cancer and in chemotherapy or tumor resection. It should be noted that antitumor agents with their side effect on cells with high turnover may exacerbate malnutrition [94]. This could be explained by the competition between cancerous regions and normal cells of the gastrointestinal system to use nutrients to repair the adverse effects of antitumor drugs (hypermetabolic state) [95]. Briefly, impaired caloric intake, side effects of therapy, changes in taste and mood, pain and other adverse consequences of eating, obstruction, fistula, and malabsorption all promote malnutrition in cancer patients; therefore, well-nourished cancer patients with intact gastrointestinal integrity have lower morbidity and mortality than others [96]. It should be noted that cachexia after cancer differs from cachexia following starvation. Increased protein and glucose turnover, high whole body synthesis and catabolism, accelerated hepatic protein production (especially acute phase agents), increased serum free fatty acid levels, and depletion of fat stores were reported only in cancer patients. However, metabolic abnormalities and, paradoxically, impaired immune response are probable consequences of cancer cachexia, as explained in the previous section. Increased levels of immunosuppressive mediators (e.g., TGF-β), decreased C3 and delayed hypersensitivity response, and diminished numbers and activity of NK cells are the most common changes in the immune system of patients with cancer cachexia, leading to more infectious complications and poor prognosis [96]. Neutrophil chemotaxis, monocyte phagocytosis and cytotoxicity, number of T-cells, and proliferation of lymphocytes are also defective in patients with lung cancer. Phagocytic and bactericidal activities of neutrophils were low in hepatocellular carcinoma patients. In addition, surgical stress in cancer patients enhances Th2 and compromises the Th1/Th2 balance and expression of HLA-DR on monocytes, which is considered to be a central marker of immune paralysis after surgical trauma [97, 98]. Most of these immune parameters are also reduced during radiotherapy and chemotherapy because of their side effects on bone marrow. However, these factors are reversible after nutrition improvement [99].
24.6 Role of Nutritional Support in Immune Restoration of Cancer Patients
Adjuvant therapy of cancer patients by different nutritional support strategies (dietary counseling, oral nutritional supplements, enteral tube feeding, and parenteral tube feeding) is the mainstream recommendation to increase their quality of life and to obviate the risks associated with gastrointestinal complications and reverse malnutrition. However, there is no comprehensive approach based on the needs of cancer patients with cachexia or those with increased nutrient requirements. Several studies have shown the effectiveness of nutritional supply in groups of patients with malignancy that resulted in weight gain, increased appetite, increased energy and protein intake, reduced gastrointestinal toxicity, and enhanced immune function [100]. In the clinical setting with standard treatment protocols, it turns out that the implementation of nutrition support in patients with cancer is most effective when it is limited to special, well-described circumstances. Nonetheless, the potential advantages of some specific nutrients have been described and are outlined below [101].
24.6.1 Arginine
Arginine is a semi-essential amino acid with immunomodulatory potentials such as stimulated thymic growth and mononuclear cell response to mitogens, which enhances lymphokine-activated killer cell generation via a nitric oxide-mediated mechanism and stimulates the release of polyamines by the small intestine. In one randomized trial of malnourished patients with head and neck cancer, follow-up at 10 years indicated better survival in those who received supplemental arginine preoperatively [102].
24.6.2 Glutamine
Glutamine is the most abundant amino acid in the human body and the preferential fuel of rapidly dividing cells such as lymphocytes and macrophages [103]. However, supplementing glutamine in the diets of patients with cancer may be counterproductive because glutamine (which is essential for fast growing cells in culture) may promote accelerated tumor growth. A meta-analysis of studies that used parenteral glutamine postoperatively showed it was associated with a shorter hospital stay and a lower incidence of infectious complications [104].
24.6.3 Branched Chain Amino Acids
L-valine, L-leucine, and L-isoleucine can improve the immune response and maintain serum albumin level in the course of hepatocellular carcinoma recurrence [105].
24.6.4 Nucleotides, Long-Chain
Omega-3 polyunsaturated fatty acids and eicosapentaenoic acid. These lipid agents have anti-inflammatory, anticachectic, immunomodulating, and antitumor effects [106,107,108].
24.6.5 Fructooligosaccharides
This group of functional fibers associated with increased lactic acid bacteria acts as an immunomodulator by stimulating IgA synthesis, promoting mucin production, modulating inflammatory cytokines, and decreasing Ag absorption [90].
24.6.6 Bioactive Compounds
Agaricaceae fungus consisting of ergosterol, oleic acid, and triterpenes may inhibit neovascularization induced by tumors and therefore attenuate cancer progression [109].
24.6.7 Antioxidants (Vitamin E and Vitamin C)
Since chemotherapy may induce mucositis and bleomycin in particular induces chromosomal damage in lymphocytes, the administration of vitamins C and E may reduce the side effects of therapy [110].
24.6.8 Vitamin A
This fat-soluble vitamin can increase the numbers of NK cells or regulatory lymphocytes in cancer patients [89]. A recent study showed that all-trans retinoic acid can potentiate the chemotherapeutic effect of cisplatin by inducing differentiation of tumor initiating cells in liver cancer [111].
24.7 Concluding Remarks
In summary, due to the safety and cost-effectiveness of oral dietary therapies, nutrition counseling and the implementation of nutritional supplements should be the initial approaches to nutritional support [112]. Even though parenteral nutrition may also lead to weight gain and improvement in nitrogen balance in patients with cancer, it does not clearly improve serum albumin levels or alter whole body protein turnover even with prolonged administration. Therefore, when nutrition support is chosen as a therapy, the use of enteral nutrition is preferred if the gastrointestinal tract is functional [113, 114]. The use of parenteral nutrition should be limited to malnourished cancer patients who are receiving active anticancer treatment, whose gastrointestinal tract is not functional or who cannot tolerate enteral nutrition, and who are anticipated to be unable to meet their nutrient requirements for 14 days or more [113]. Moreover, it is proposed that preoperative and postoperative immune-nutrition intervention by total parenteral nutrition using a lipid-based regimen is the method of choice in cancer patients who have undergone major surgery to reduce immune dysfunction without enhancing tumor growth (increased augmentation of lymphocyte blastogenesis and production of helper T-lymphocyte lymphokine IL-2, increased ICAM-1 level, and decreased IL-4 and IL-10 values) [114, 115]. This observed preference of parenteral nutrition is marginal, and enteral methods are always the preferable route for cancer patients with an intact digestive system. It is also reported that complement components and lymphocyte response may be better with enteral rather than parenteral nutrition [115, 116].
References
Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–50.
Font-Burgada J, Sun B, Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 2016;23(1):48–62.
Dossus L, Kaaks R. Nutrition, metabolic factors and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22(4):551–71.
Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–8.
Schaffler A, Muller-Ladner U, Scholmerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev. 2006;27(5):449–67.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83.
Matsubara M, Namioka K, Katayose S. Decreased plasma adiponectin concentrations in women with low-grade C-reactive protein elevation. Eur J Endocrinol. 2003;148(6):657–62.
Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomark Prev. 2005;14(10):2413–8.
Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33(4):335–51.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7.
Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–85.
Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11(6):467–78.
Doerner SK, Reis ES, Leung ES, Ko JS, Heaney JD, Berger NA, et al. High-fat diet-induced complement activation mediates intestinal inflammation and neoplasia, independent of obesity. Mol Cancer Res. 2016;14(10):953–65.
Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–61.
Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human tumor-infiltrating myeloid cells: phenotypic and functional diversity. Front Immunol. 2017;8:86.
Chen W, Ten Dijke P. Immunoregulation by members of the TGFbeta superfamily. Nat Rev Immunol. 2016;16(12):723–40.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72.
Lamas O, Marti A, Martinez JA. Obesity and immunocompetence. Eur J Clin Nutr. 2002;56(Suppl 3):S42–5.
Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, et al. Obesity accelerates thymic aging. Blood. 2009;114(18):3803–12.
Macia L, Delacre M, Abboud G, Ouk TS, Delanoye A, Verwaerde C, et al. Impairment of dendritic cell functionality and steady-state number in obese mice. J Immunol. 2006;177(9):5997–6006.
Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest. 2015;125(12):4592–600.
Pietrocola F, Demont Y, Castoldi F, Enot D, Durand S, Semeraro M, et al. Metabolic effects of fasting on human and mouse blood in vivo. Autophagy. 2017;13(3):567–78.
Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166(6):1512–25 e12.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9.
Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra27.
Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, et al. Fasting-mimicking diet reduces HO-1 to promote T cell T-cell-mediated tumor cytotoxicity. Cancer Cell. 2016;30(1):136–46.
Madeo F, Pietrocola F, Eisenberg T, Kroemer G. Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov. 2014;13(10):727–40.
Woolf EC, Syed N, Scheck AC. Tumor metabolism, the ketogenic diet and beta-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122.
Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95.
Campos FG, Logullo Waitzberg AG, Kiss DR, Waitzberg DL, Habr-Gama A, Gama-Rodrigues J. Diet and colorectal cancer: current evidence for etiology and prevention. Nutr Hosp. 2005;20(1):18–25.
Brockman DA, Chen X, Gallaher DD. Consumption of a high beta-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr. 2013;52(7):1743–53.
Green CJ. Fibre in enteral nutrition: a new era? Nutr Hosp. 2002;17(Suppl 2):1–6.
Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6(2):41–51.
Han M, Wang C, Liu P, Li D, Li Y, Ma X. Dietary fiber gap and host gut microbiota. Protein Pept Lett. 2017;24(5):388–96.
Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol Spectr. 2015;3(3).
Daien CI, Pinget GV, Tan JK, Macia L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: an overview. Front Immunol. 2017;8:548.
Maukonen J, Saarela M. Human gut microbiota: does diet matter? Proc Nutr Soc. 2015;74(1):23–36.
Ollberding NJ, Wilkens LR, Henderson BE, Kolonel LN, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: the Multiethnic Cohort Study. Int J Cancer. 2012;131(7):E1125–33.
Marques-Vidal P, Ravasco P, Ermelinda Camilo M. Foodstuffs and colorectal cancer risk: a review. Clin Nutr. 2006;25(1):14–36.
Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition. 2001;17(1 Suppl):S1–20.
Wardwell L, Chapman-Novakofski K, Herrel S, Woods J. Nutrient intake and immune function of elderly subjects. J Am Diet Assoc. 2008;108(12):2005–12.
Mahima IAM, Verma AK, Tiwari R, Karthik K, Chakraborty S, et al. Immunomodulators in day to day life: a review. Pak J Biol Sci. 2013;16(17):826–43.
Meydani SN, Erickson KL. Nutrients as regulators of immune function: introduction. FASEB J. 2001;15(14):2555.
Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57(Suppl 1):S66–9.
Keusch GT. The history of nutrition: malnutrition, infection and immunity. J Nutr. 2003;133(1):336S–40S.
Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98.
Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. 2014;13:38–45.
Vineyard KR, Warren LK, Kivipelto J. Effect of dietary omega-3 fatty acid source on plasma and red blood cell membrane composition and immune function in yearling horses. J Anim Sci. 2010;88(1):248–57.
Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40(11):1213–32.
Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94.
Niu ZY, Wei FX, Liu FZ, Qin XG, Min YN, Gao YP. Dietary vitamin A can improve immune function in heat-stressed broilers. Animal. 2009;3(10):1442–8.
Yano H, Ohtsuka H, Miyazawa M, Abiko S, Ando T, Watanabe D, et al. Relationship between immune function and serum vitamin A in Japanese black beef cattle. J Vet Med Sci. 2009;71(2):199–202.
Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301–23.
Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27(23):3757–63.
Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100(3):450–4.
Choi SW, Friso S. Vitamins B6 and cancer. Subcell Biochem. 2012;56:247–64.
Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109(3):1–9.
Galluzzi L, Vitale I, Senovilla L, Olaussen KA, Pinna G, Eisenberg T, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012;2(2):257–69.
Duthie SJ, Narayanan S, Blum S, Pirie L, Brand GM. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000;37(2):245–51.
McGlynn AP, Wasson GR, O’Reilly SL, McNulty H, Downes CS, Chang CK, et al. Low colonocyte folate is associated with uracil misincorporation and global DNA hypomethylation in human colorectum. J Nutr. 2013;143(1):27–33.
Eichholzer M, Luthy J, Moser U, Fowler B. Folate and the risk of colorectal, breast and cervix cancer: the epidemiological evidence. Swiss Med Wkly. 2001;131(37–38):539–49.
Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case-control study. Lancet. 2001;358(9297):1935–40.
Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, et al. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res. 2009;2(3):213–23.
Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313(22):1381–4.
Fedirko V, Bostick RM, Flanders WD, Long Q, Sidelnikov E, Shaukat A, et al. Effects of vitamin d and calcium on proliferation and differentiation in normal colon mucosa: a randomized clinical trial. Cancer Epidemiol Biomark Prev. 2009;18(11):2933–41.
Morante M, Sandoval J, Gomez-Cabrera MC, Rodriguez JL, Pallardo FV, Vina JR, et al. Vitamin E deficiency induces liver nuclear factor-kappaB DNA-binding activity and changes in related genes. Free Radic Res. 2005;39(10):1127–38.
Boyle P, Autier P, Bartelink H, Baselga J, Boffetta P, Burn J, et al. European code against cancer and scientific justification: third version (2003). Ann Oncol. 2003;14(7):973–1005.
Drewnowski A, Shultz JM. Impact of aging on eating behaviors, food choices, nutrition, and health status. J Nutr Health Aging. 2001;5(2):75–9.
Sebastian RS, Cleveland LE, Goldman JD, Moshfegh AJ. Older adults who use vitamin/mineral supplements differ from nonusers in nutrient intake adequacy and dietary attitudes. J Am Diet Assoc. 2007;107(8):1322–32.
Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27(5):675–84.
English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10(5):480–7.
Li F, Wang L, Burgess RJ, Weinshilboum RM. Thiopurine S-methyltransferase pharmacogenetics: autophagy as a mechanism for variant allozyme degradation. Pharmacogenet Genomics. 2008;18(12):1083–94.
Cuervo AM, Macian F. Autophagy, nutrition and immunology. Mol Asp Med. 2012;33(1):2–13.
Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13(5):495–504.
Carruba G, Cocciadiferro L, Di Cristina A, Granata OM, Dolcemascolo C, Campisi I, et al. Nutrition, aging and cancer: lessons from dietary intervention studies. Immun Ageing. 2016;13:13.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73.
Karin M, Jobin C, Balkwill F. Chemotherapy, immunity and microbiota--a new triumvirate? Nat Med. 2014;20(2):126–7.
Poutahidis T, Kleinewietfeld M, Erdman SE. Gut microbiota and the paradox of cancer immunotherapy. Front Immunol. 2014;5:157.
Nelson MH, Diven MA, Huff LW, Paulos CM. Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res. 2015;2015:368736.
Bashiardes S, Tuganbaev T, Federici S, Elinav E. The microbiome in anti-cancer therapy. Semin Immunol. 2017;32:74–81.
Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr. 2013;4(1):16–28.
Russo E, Taddei A, Ringressi MN, Ricci F, Amedei A. The interplay between the microbiome and the adaptive immune response in cancer development. Ther Adv Gastroenterol. 2016;9(4):594–605.
Zitvogel L, Daillere R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. 2017;15(8):465–78.
Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165(2):276–87.
Zoico E, Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev. 2002;60(2):39–51.
Elia M, van der Schueren MA V B-d, Garvey J, Goedhart A, Lundholm K, Nitenberg G, et al. Enteral (oral or tube administration) nutritional support and eicosapentaenoic acid in patients with cancer: a systematic review. Int J Oncol. 2006;28(1):5–23.
Elia M, Russell CA, Stratton RJ. Malnutrition in the UK: policies to address the problem. Proc Nutr Soc. 2010;69(4):470–6.
Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 2000;34(3):137–68.
Akbulut G. New perspective for nutritional support of cancer patients: enteral/parenteral nutrition. Exp Ther Med. 2011;2(4):675–84.
Liu MY, Tang HC, Hu SH, Yang HL, Chang SJ. Influence of preoperative peripheral parenteral nutrition with micronutrients after colorectal cancer patients. Biomed Res Int. 2015;2015:535431.
Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol. 2010;26(2):146–51.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95.
Flint TR, Fearon DT, Janowitz T. Connecting the metabolic and immune responses to cancer. Trends Mol Med. 2017;23(5):451–64.
Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. Biomed Res Int. 2014;2014:168407.
Yamagishi A, Morita T, Miyashita M, Kimura F. Symptom prevalence and longitudinal follow-up in cancer outpatients receiving chemotherapy. J Pain Symptom Manag. 2009;37(5):823–30.
Seelaender MC, Batista ML. Adipose tissue inflammation and cancer cachexia: the role of steroid hormones. Horm Mol Biol Clin Invest. 2014;17(1):5–12.
Tsoli M, Robertson G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol Metab. 2013;24(4):174–83.
Tong H, Isenring E, Yates P. The prevalence of nutrition impact symptoms and their relationship to quality of life and clinical outcomes in medical oncology patients. Support Care Cancer. 2009;17(1):83–90.
Murphy G, McCormack V, Abedi-Ardekani B, Arnold M, Camargo MC, Dar NA, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28(9):2086–93.
Caccialanza R, Pedrazzoli P, Cereda E, Gavazzi C, Pinto C, Paccagnella A, et al. Nutritional support in cancer patients: A Position Paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J Cancer. 2016;7(2):131–5.
Buijs N, van Bokhorst-de van der Schueren MA, Langius JA, Leemans CR, Kuik DJ, Vermeulen MA, et al. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am J Clin Nutr. 2010;92(5):1151–6.
Savarese DM, Savy G, Vahdat L, Wischmeyer PE, Corey B. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev. 2003;29(6):501–13.
Wang Y, Jiang ZM, Nolan MT, Jiang H, Han HR, Yu K, et al. The impact of glutamine dipeptide-supplemented parenteral nutrition on outcomes of surgical patients: a meta-analysis of randomized clinical trials. J Parenter Enter Nutr. 2010;34(5):521–9.
Kakazu E, Kondo Y, Kogure T, Ninomiya M, Kimura O, Iwata T, et al. Supplementation of branched-chain amino acids maintains the serum albumin level in the course of hepatocellular carcinoma recurrence. Tohoku J Exp Med. 2013;230(4):191–6.
Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131(7):1918–27.
Takagi K, Yamamori H, Furukawa K, Miyazaki M, Tashiro T. Perioperative supplementation of EPA reduces immunosuppression induced by postoperative chemoradiation therapy in patients with esophageal cancer. Nutrition. 2001;17(6):478–9.
Waitzberg DL, Torrinhas RS. Fish oil lipid emulsions and immune response: what clinicians need to know. Nutr Clin Pract. 2009;24(4):487–99.
Fortes RC, Novaes MR, Recova VL, Melo AL. Immunological, hematological, and glycemia effects of dietary supplementation with Agaricus sylvaticus on patients’ colorectal cancer. Exp Biol Med. 2009;234(1):53–62.
Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37(1):1–18.
Zhang Y, Guan DX, Shi J, Gao H, Li JJ, Zhao JS, et al. All-trans retinoic acid potentiates the chemotherapeutic effect of cisplatin by inducing differentiation of tumor initiating cells in liver cancer. J Hepatol. 2013;59(6):1255–63.
Piquet MA, Ozsahin M, Larpin I, Zouhair A, Coti P, Monney M, et al. Early nutritional intervention in oropharyngeal cancer patients undergoing radiotherapy. Support Care Cancer. 2002;10(6):502–4.
Bozzetti F. Rationale and indications for preoperative feeding of malnourished surgical cancer patients. Nutrition. 2002;18(11–12):953–9.
Bozzetti F. Nutritional support in oncologic patients: where we are and where we are going. Clin Nutr. 2011;30(6):714–7.
Bozzetti F, Gavazzi C, Miceli R, Rossi N, Mariani L, Cozzaglio L, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7–14.
Ponton F, Wilson K, Cotter SC, Raubenheimer D, Simpson SJ. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7(12):e1002223.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Abolhassani, H., Mohammadzadeh Honarvar, N., Mosby, T.T., Mahmoudi, M. (2020). Nutrition, Immunity, and Cancers. In: Rezaei, N. (eds) Cancer Immunology. Springer, Cham. https://doi.org/10.1007/978-3-030-30845-2_24
Download citation
DOI: https://doi.org/10.1007/978-3-030-30845-2_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30844-5
Online ISBN: 978-3-030-30845-2
eBook Packages: MedicineMedicine (R0)