Abstract
This chapter summarizes the application of electroretinogram, electrooculogram, and visual evoked potential in retinal toxicities caused by vigabatrin, hydroxychloroquine (Plaquenil), chloroquine, antipsychotics, cis-platinum, deferoxamine, digoxin, ethambutol, indomethacin, isotretinoin, ocular siderosis, phosphodiesterase type 5 Inhibitor, and quinine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electroretinogram
- Electrooculogram
- Visual evoked potential
- Retinal toxicity
- Vigabatrin
- Hydroxychloroquine (Plaquenil)
- Chloroquine
- Antipsychotics
- Cis-platinum
- Deferoxamine
- Digoxin
- Ethambutol
- Indomethacin
- Isotretinoin
- Ocular siderosis
- Phosphodiesterase type 5 inhibitor
- Quinine
Vigabatrin
Vigabatrin (VGB) , sold under the brand name Sabril, is an anticonvulsant medication inhibiting gamma-aminobutyric acid (GABA) degradation for the treatment of epilepsy. It is an irreversible inhibitor of GABA transaminase. By irreversibly binding to GABA-transaminase, thus metabolism of GABA is hurdled. The accumulation of GABA then damages the neurons in the eye and brain. It can reduce visual acuity, contrast sensitivity, retinal thickness, the a-wave and b-wave of scotopic and photic electroretinography (ERG), oscillatory potential (OP) amplitudes, 30-Hz flicker ERG amplitude, multifocal electroretinography (mfERG) amplitudes, electrooculography (EOG) Arden ratio, and the VEP amplitude from central or peripheral visual field and increase the latencies of ERG a-wave and b-wave [1,2,3,4,5,6,7,8,9,10,11]. Reduction in b-wave amplitudes and 30 Hz flicker amplitudes are usually the first signs of toxicity. There is also evidence that light exposure in mice administered VGB produces the taurine deficiency, causing the retinal degeneration [2, 12]. Dysregulated expression of transcripts in the mTOR pathway, GABA/glutamate transporters GABAA/B receptors, and metabotropic glutamate receptors 1/6 in the eye are associated with the reduction of VEP amplitude after VGB administration [13].
Complex full-field electroretinography (ffERG) changes can be found in children placed on vigabatrin for intractable epilepsy (Fig. 9.1). However, importantly, some ffERG changes can precede exposure to vigabatrin, underscoring that these patients may have preexisting changes in their retinal function related to their underlying condition. Thus, to correctly assess vigabatrin-mediated toxicity, obtaining baseline ffERGs is essential. With this caveat in mind, toxic effects during exposure to vigabatrin onto retinal function can affect both receptoral and post-receptoral responses and can vary from patient to patient (Fig. 9.1).
Electroretinographic findings in vigabatrin-mediated retinal toxicity. Importantly, both patients exhibited baseline abnormalities, highlighting the importance of obtaining baseline measurements to ascertain potential retinal toxicities of this or any other medication. A normal set of ERGs, shown on the top row of the figure. Patient 1. This boy was tested first at baseline before the age of 2 years old, when he had first been placed on vigabatrin for intractable epilepsy linked to subdural hematomas and encephalopathy, and signs of toxicity emerged 14 months later. Dark- and light-adapted flash ERGs demonstrated at baseline mixed a-wave amplitude reduction, a mild electronegative shape of the 10.0 cd·s·m-2 response, and borderline low amplitudes for all cone-driven responses. After 14 months of follow-up, there was evidence of rod-driven b-wave attenuation, further reduction in mixed a-wave, lack of a-wave growth at 10 cd·s·m-2 (consistent with diminished photoreceptor sensitivity), and loss of cone-driven b-wave and flicker ERG amplitudes (note the different scale for flicker responses compared to normal). ON–OFF ERGs performed at follow-up (not shown) indicated also OFF > ON response compromise. Taken together, these findings suggested adverse vigabatrin-mediated effects at the photoreceptor and post-receptoral levels (on bipolar cells themselves or at the photoreceptor-to-bipolar-cell synapse). Patient 2. This girl was tested first at baseline before the age of 2 years old, when she had first been placed on vigabatrin for intractable epilepsy, and signs of toxicity emerged after 33 months. ERGs at baseline show delayed rod-driven ERGs, borderline low mixed dark-adapted a- and b-wave response amplitudes, delayed mixed b-waves, reduced photopic a-wave amplitudes, and attenuated and delayed flicker ERG responses. Compared to baseline, follow-up flash ERGs 33 months later demonstrate rod-driven and mixed b-wave amplitude loss (electronegative ERG), reduction in the photopic b-waves [arrows] with an artifactual a-wave increase (due to increased electronegativity in the light-adapted responses as well) attributable to ON>OFF post-receptoral response compromise (not shown). The flicker ERG was overall stable
Hydroxychloroquine (Plaquenil) and Chloroquine
Hydroxychloroquine (HCQ) and chloroquine (CQ) are antimalarial agents commonly used in the treatment of lupus erythematosus, Sjogren’s disease, rheumatoid arthritis, and dermatological inflammations. HCQ is also used in the treatment of uncomplicated malaria caused by chloroquine-sensitive strains of Plasmodium species or in the prophylaxis in regions with chloroquine-resistant strains. HCQ inhibits movements of neutrophils and eosinophils and also impairs complement-dependent antigen–antibody reactions. Its antirheumatic properties are proposed to result from their interference with “antigen processing” in macrophages and other antigen-presenting cells [14].
Both HCQ and CQ belong to the quinolone family and have similar clinical indications and side effects, including retinal toxicity. Hobbs et al. first described the CQ-induced retinal toxicity in 1959 [15], and Shearer et al. first described the HCQ toxicity to retina in 1967 [16]. HCQ has much less retinal side effect so that CQ has almost been replaced by HCQ for the treatment of inflammatory diseases. The retinal damage induced by these medications is usually irreversible. In a portion of patients with long-term HCQ/CQ treatment, HCQ-/CQ-induced toxicity can be found in cornea and macula, possibly related to the factors of cumulated dosage, duration of use, age, concomitant use of certain medications (e.g., tamoxifen), and health status (e.g., renal disease). In the retina, it may cause bilateral Bull’s eye pattern of maculopathy (Fig. 9.2a), parafoveal damage of retina in early stage, and extramacular damage of retina in later stage. It affects ganglion cell layer, photoreceptors, and retinal pigment epithelium (RPE) by the inhibition of protein synthesis [17, 18] and peroxidation of lipid [19]. The risk of HCQ-/CQ-induced maculopathy is associated to the daily dose and the duration of HCQ/CQ administration [20, 21]. More damage may occur after the initial damage with continued use of HCQ/CQ [22]. Therefore, early screening of HCQ/CQ toxicity in these patients is necessary.
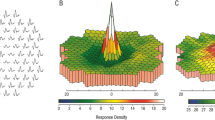
In mfERG test, HCQ-/CQ-induced maculopathy shows paracentral decrease of mfERG amplitude and prolongation of latency (Fig. 9.2b) [21, 23,24,25,26,27,28,29,30]. The ffERGs can be abnormal in some patients [31]. In the late stages of HCQ-induced retinopathy, ffERGs and other tests can be used together for the differential diagnosis [32]. For early detection of HCQ/CQ toxicity, mfERG, spectral domain optical coherence tomography (SD-OCT) (Fig. 9.2c), and fundus autofluorescence (FAF) (Fig. 9.2d) may have higher sensitivity than perimetry [24, 28, 33,34,35,36,37] and are part of the standard Plaquenil patient workup. In addition, mfERG can provide objective data of the retinal function across visual field [20].
Fundus imaging and mfERG in hydroxychloroquine retinopathy. (a) Fundus image of a hydroxychloroquine user showing Bull’s eye pattern of maculopathy. (b) mfERG result of a hydroxychloroquine user demonstrates marked reduction of mfERG response density and prolongation of mfERG implicit time in the parafoveal region (Adapted from Figure 1 [21]). (c) SD-OCT of a hydroxychloroquine user that shows significant decrease of the thickness of outer retina in the parafoveal region. (d) Fundus autofluorescence displays higher signal in the parafoveal region
Antipsychotics
Chlorpromazine (CPZ) and thioridazine are members of the first-generation antipsychotic class, also known as typical antipsychotics. Their mechanism of action depends on the blockage of postsynaptic D2 receptors in the mesolimbic and mesocortical pathways. Typical antipsychotics may also block muscarinic, alpha 1-adrenergic, histamine, and 5-HT2 receptors.
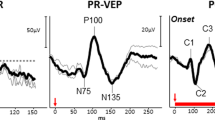
CPZ administration can cause latency prolongation of the ffERG mixed response and cone response, decrease of rod and cone b-wave amplitude, and latency prolongation of the oscillatory potentials (OPs). However, CPZ is not known to change parameters of the fVEP, pERG, and the retinocortical times calculated from the difference of pERG and pVEP latencies [38].
Thioridazine (also known as Mellaril) is a dopaminergic antagonist (similar to CPZ) first used as antipsychotic with indication for the management of schizophrenic patients who fail to respond adequately to the treatment with other first-line antipsychotic drugs.
Because of its serious retinal and cardiac side effects (thioridazine may increase the risk of potentially fatal ventricular arrhythmias), its use is presently recommended only for selected cases. Probably due to an idiosyncratic response, retinal toxicity has also been reported for short-term and low-dose administrations [39]. However, long-term thioridazine treatments are usually associated with the highest risk of retinopathy.
The earliest signs of thioridazine retinal toxicity is RPE mottling of the macula and perimacular regions. Full-blown retinopathy is characterized by nummular (coin shaped) patches of RPE loss in both posterior pole and periphery, with a RP-like appearance [40, 41] (Fig. 9.3). Decreased visual acuity, night blindness, paracentral scotomas, and loss of peripheral vision can all occur in thioridazine retinopathy. In more advanced stages of the disease, there is definite electrophysiological (ffERG, EOG, and mfERG) evidence of diffuse retinal compromise (Figs. 9.4 and 9.5).
The mechanism of retinal damage is still unclear, but it is thought to be similar to chlorpromazine, accumulating mainly in pigmented cells including the uveal level [42]. This presumed mechanism may explain the extensive choriocapillaris loss and RPE dysfunction and damage co-contributing to the retinal damage. Direct dopaminergic mechanisms have also been suspected, but not proven.
Thioridazine discontinuation is the only treatment available. Regression has been described, but only in early-diagnosed cases, and further progression of retinal damage over several years after discontinuation can (more commonly) occur [43].
Clinical and imaging findings in thioridazine retinopathy. (a) A large area of depigmentation across the entire posterior pole characterizes the fundus examination of this 70-year-old Caucasian woman with a previous longstanding history of treatment with thioridazine for a psychotic disorder. (b) Fundus autofluorescence imaging reveals much more clearly the nummular patches of hypo-autofluorescent lesions corresponding to the clinically visible atrophy of the RPE around the fovea and throughout the posterior pole, extending well beyond the arcades. There is central sparing but marked hyper-autofluorescence around the disc and in the foveal region. The nummular hypo-autofluorescent lesions are surrounded by a large halo of speckled hypo-autofluorescence indicative of further RPE suffering also in the periphery. (c) The OCT scan shows excellent central preservation of all layers, including the RPE, but sharp and discrete near-complete loss of RPE, EZ, ELM, and ONL just nasal to fovea and farther out in the temporal periphery. These clinical findings in the context of the thioridazine exposure are typical for thioridazine retinopathy
Electroretinographic findings in thioridazine retinopathy. The ffERG of the patient illustrated in Fig. 9.3 reveals both rod and cone response compromise, in an approximately similar ratio, and marked response delays. Findings were symmetric in both eyes both from the ffERG and clinical perspective
Multifocal electroretinography findings in thioridazine retinopathy, compared with normal values. (a) 3D plots of the response densities. (b) Average responses in 5 rings of different eccentricities. The grey bars indicate the normal ranges of the N1 and P1 components with 95% confidence interval, respectively. The mfERG response of this patient (same as in Figs. 9.3 and 9.4) reveals patchy loss of the response density across the macular region, with relative preservation of the foveal peak, accounting for the excellent visual acuity. An EOG was also measured (not shown), and the Arden ratio was ≤1.35 in each eye, consistent with widespread RPE compromise, again consistent with the history of thioridazine exposure. Had this patient not had this history, a workup for a possible autosomal recessive bestrophinopathy would have been in order
Cis-Platinum
Cis-platinum is a medicine used for the chemotherapy of bladder, testicular, ovarian, small and non-small cell lung, esophageal, breast, prostate, stomach, and cervical cancers. It is also used to treat mesothelioma, sarcomas, melanoma, multiple myeloma, neuroblastoma, and Hodgkin’s and non-Hodgkin’s lymphomas. Cis-platinum is an alkylating agent active during resting phase of the cell. Therefore, this drug is cell cycle nonspecific. The manifestation of electrophysiology of cis-platinum side effect includes reduction of ERG dark-adapted and light-adapted b-wave amplitudes, amplitude of OPs, amplitude of 30 Hz flicker ERG, and dark-adapted a-wave amplitude (Fig. 9.6) [44,45,46].
Deferoxamine
Deferoxamine (DFO) is an IV detoxification agent used in both acute iron intoxication and chronic iron overload. It is also used for the detoxification of aluminum intoxication. DFO chelates iron from ferritin and hemosiderin and enhances its elimination. Both ocular and auditory disturbances have been reported with this agent, suggesting pigmentary retinopathy and optic neuropathy can be caused by DFO. The ocular symptoms of its side effects include vision loss, dyschromatopsia, nyctalopia, and scotomas that can be partially recovered after the cessation of DFO administration. Electrophysiology can be used to monitor this side effect seen in the reduction of ERG responses of rod and cone pathways (Fig. 9.7), EOG Arden ratio, mfERG amplitude in central field, and the prolongation of VEP latency [47,48,49,50,51]. The visual function may be recovered after the cessation of the DFO administration.
Digoxin
Digoxin is a drug in the cardiac glycoside family of medications for the treatment of some cardiac disorders, including heart failure, atrial fibrillation and atrial flutter. Its side effects include abnormal vision, irregular heartbeat, nausea, loss of appetite, confusion, and breast enlargement. The symptoms of the visual disorder include blurry vision, central scotomas, photopsia, abnormal color vision, and xanthopsia [52,53,54,55]. Studies show ERG or mfERG in the central field in some patients treated with digoxin is abnormal [56,57,58,59]. Some studies with animals also confirmed the side effect of digoxin on retinal cone and rod photoreceptors displayed as abnormal ERG results and other tests of visual function and morphology [60,61,62,63].
Ethambutol
Ethambutol (EMB) is mainly used to treat tuberculosis. Its side effects include ethambutol-induced optic neuropathy and retinopathy. For early detection of ethambutol-induced retinopathy, mfERG can show the decrease of P1 amplitudes and/or prolonged P1 latencies in the central, bitemporal, peripheral, nasal, or the whole tested field even before the visual symptoms occur [64,65,66,67,68,69]. These papers suggest mfERG is probably suitable for early diagnosis of ethambutol-induced retinopathy.
Indomethacin
Indomethacin is a nonsteroidal, anti-inflammatory drug that can be used to treat fever, pain, and inflammation. Indomethacin is a reversible inhibitor of cyclooxygenase 1 and 2, leading to decreased production of prostaglandins. Indomethacin can cause retinopathy reflected in significant reduction of rod and cone responses of ERG and the Arden ratio of EOG [70].
Isotretinoin
Isotretinoin is a medication primarily used to treat severe acne unresponsive to conventional therapy and occasionally used in the treatment and prevention of cancer. Isotrentinoin’s exact mechanism of action is unknown. However, isotretinoin may induce apoptosis. Due to its high teratogenicity, isotretinoin is distributed under a risk evaluation and mitigation strategy (REMS) program called iPledge, intended to prevent fetal exposure to the drug. The ocular side effect of isotretinoin can be manifested by the reduction of scotopic ERG amplitudes and Arden ratio of EOG [71,72,73].
Ocular Siderosis
Ocular siderosis (OS) is caused by retained iron-containing intraocular foreign body after a penetrating ocular injury (Fig. 9.8a) that can cause significant vision loss. Pigmentary retinopathy and cataract are common in OS. The degree of retinal damage and recovery is affected by many factors, such as location and size of the foreign body, which can be monitored by the decrease and improvement of ffERG amplitudes (Fig. 9.8b) [74,75,76,77,78,79], mfERG latency [80], the relationship of a-wave and b-wave amplitudes [81], or the Arden ratio of EOG [82].
Phosphodiesterase (PDE) Type 5 Inhibitors (Erectile Dysfunction Medication)
Phosphodiesterase (PDE) type 5 inhibitors , such as sildenafil, tadalafil, vardenafil, udenafil, and avanafil, are commonly used in erectile dysfunction and pulmonary hypertension. PDE5 inhibition leads to decreased degradation of cGMP leading to vasodilation. Sexual stimulation leads to a physiological release of nitric oxide activating guanylate cyclase and production of cGMP. Increased levels of cGMP from the medication leads to smooth muscle relaxation and increased blood flow to the penis. However, these drugs may cause transient retinal disorders or optic neuropathy [83, 84].
The side effect in patients includes abnormal color vision, blurred vision, photophobia, ocular pain, and conjunctival hyperemia [85]. Decrease of amplitude and prolongation of latency in ffERG and mfERG were observed in some subjects or animals without preexisting ocular disorders after sildenafil administration [86,87,88,89,90,91]. Decrease of response in retinal ganglion cells in rat after sildenafil administration was also observed [92]. However, sildenafil increased the a-wave and b-wave amplitude in a rat model of neonatal hypoxia-ischemia [93] and the b-wave amplitude in a mouse carrier of retinitis pigmentosa [88]. In addition, both sildenafil and tadalafil may cause central serous chorioretinopathy [94, 95] and damage photoreceptors [96]. Sildenafil, tadalafil, and udenafil may cause optic neuropathy [91, 97,98,99,100,101,102,103,104,105,106,107,108]. Therefore, it is complicated to explain the electrophysiologic results of the users of PDE5 inhibitor for the diagnosis of other ocular disorders.
Quinine
Quinine is used in the treatment of uncomplicated malaria where resistance to chloroquine has been documented. Quinine is also used in the prevention of nocturnal leg cramps. The exact mechanism of antimalarial activity is not known. However, it is thought quinine can inhibit nucleic acid synthesis, protein synthesis, and glycolysis. The amplitudes of ffERG, mfERG, VEP, and EOG can be reduced after the administration of quinine [109,110,111,112,113,114].
References
Hebert-Lalonde N, et al. Electrophysiological evidences of visual field alterations in children exposed to vigabatrin early in life. Pediatr Neurol. 2016;59:47–53.
Tao Y, et al. The vigabatrin induced retinal toxicity is associated with photopic exposure and taurine deficiency: an in vivo study. Cell Physiol Biochem. 2016;40(5):831–46.
Kjellstrom U, Andreasson S, Ponjavic V. Attenuation of the retinal nerve fibre layer and reduced retinal function assessed by optical coherence tomography and full-field electroretinography in patients exposed to vigabatrin medication. Acta Ophthalmol. 2014;92(2):149–57.
Ruether K, et al. Electrophysiologic evaluation of a patient with peripheral visual field contraction associated with vigabatrin. Arch Ophthalmol. 1998;116(6):817–9.
Harding GF, et al. Electro-oculography, electroretinography, visual evoked potentials, and multifocal electroretinography in patients with vigabatrin-attributed visual field constriction. Epilepsia. 2000;41(11):1420–31.
Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ. 1997;314(7075):180–1.
Gross-Tsur V, et al. Visual impairment in children with epilepsy treated with vigabatrin. Ann Neurol. 2000;48(1):60–4.
Daneshvar H, et al. Symptomatic and asymptomatic visual loss in patients taking vigabatrin. Ophthalmology. 1999;106(9):1792–8.
Schroeder CE, et al. Effects of high-dose gamma-vinyl GABA (vigabatrin) administration on visual and somatosensory evoked potentials in dogs. Epilepsia. 1992;33 Suppl 5:S13–25.
Cosi V, et al. Effects of vigabatrin on evoked potentials in epileptic patients. Br J Clin Pharmacol. 1989;27(Suppl 1):61S–8S.
Ponjavic V, Andreasson S. Multifocal ERG and full-field ERG in patients on long-term vigabatrin medication. Doc Ophthalmol. 2001;102(1):63–72.
Yang J, et al. Vigabatrin-induced retinal toxicity is partially mediated by signaling in rod and cone photoreceptors. PLoS One. 2012;7(8):e43889.
Vogel KR, et al. mTOR inhibition mitigates molecular and biochemical alterations of vigabatrin-induced visual field toxicity in mice. Pediatr Neurol. 2017;66:44–52 e1.
Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(2 Suppl 1):82–91.
Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2(7101):478–80.
Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (plaquenil) therapy. Am J Ophthalmol. 1967;64(2):245–52.
Ivanina TA, et al. A study of the mechanisms of chloroquine retinopathy. II. Chloroquine effect on protein synthesis of retina. Ophthalmic Res. 1989;21(3):272–7.
Gonasun LM, Potts AM. In vitro inhibition of protein synthesis in the retinal pigment epithelium by chloroquine. Investig Ophthalmol. 1974;13(2):107–15.
Ivanina TA, et al. A study of the mechanisms of chloroquine retinopathy. I. Chloroquine effect on lipid peroxidation of retina. Ophthalmic Res. 1989;21(3):216–20.
Marmor MF, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123(6):1386–94.
Maturi RK, Yu M, Weleber RG. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol. 2004;122(7):973–81.
Pasadhika S, Fishman GA. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye (Lond). 2010;24(2):340–6.
So SC, et al. Evaluation of hydroxychloroquine retinopathy with multifocal electroretinography. Ophthalmic Surg Lasers Imaging. 2003;34(3):251–8.
Michaelides M, et al. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol. 2011;129(1):30–9.
Kellner U, Kraus H, Foerster MH. Multifocal ERG in chloroquine retinopathy: regional variance of retinal dysfunction. Graefes Arch Clin Exp Ophthalmol. 2000;238(1):94–7.
Lai TY, et al. Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am J Ophthalmol. 2005;140(5):794–807.
Chang WH, et al. A novel method for screening the multifocal electroretonogram in patients using hydroxychloroquine. Retina. 2008;28(10):1478–86.
Lyons JS, Severns ML. Using multifocal ERG ring ratios to detect and follow Plaquenil retinal toxicity: a review: review of mfERG ring ratios in Plaquenil toxicity. Doc Ophthalmol. 2009;118(1):29–36.
Tsang AC, et al. Hydroxychloroquine and chloroquine retinopathy: a systematic review evaluating the multifocal electroretinogram as a screening test. Ophthalmology. 2015;122(6):1239–1251 e4.
Moschos MN, et al. Assessing hydroxychloroquine toxicity by the multifocal ERG. Doc Ophthalmol. 2004;108(1):47–53.
Tzekov RT, Serrato A, Marmor MF. ERG findings in patients using hydroxychloroquine. Doc Ophthalmol. 2004;108(1):87–97.
Nair AA, Marmor MF. ERG and other discriminators between advanced hydroxychloroquine retinopathy and retinitis pigmentosa. Doc Ophthalmol. 2017;134(3):175–83.
Marmor MF, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–22.
Young B, Eggenberger E, Kaufman D. Current electrophysiology in ophthalmology: a review. Curr Opin Ophthalmol. 2012;23(6):497–505.
Farrell DF. Retinal toxicity to antimalarial drugs: chloroquine and hydroxychloroquine: a neurophysiologic study. Clin Ophthalmol. 2012;6:377–83.
Nebbioso M, et al. Retina in rheumatic diseases: standard full field and multifocal electroretinography in hydroxychloroquine retinal dysfunction. Clin Exp Optom. 2011;94(3):276–83.
Nebbioso M, Grenga R, Karavitis P. Early detection of macular changes with multifocal ERG in patients on antimalarial drug therapy. J Ocul Pharmacol Ther. 2009;25(3):249–58.
Bartel P, et al. Effects of chlorpromazine on pattern and flash ERGs and VEPs compared to oxazepam and to placebo in normal subjects. Electroencephalogr Clin Neurophysiol. 1990;77(5):330–9.
Neves MS, Jordon K, Dragt H. Extensive chorioretinopathy associated with very low dose thioridazine. Eye (Lond). 1990;4(Pt 5):767–70.
Hagopian V, Stratton DB, Busiek RD. Five cases of pigmentary retinopathy associated with thioridazine administration. Am J Psychiatry. 1966;123(1):97–100.
Kozy D, Doft BH, Lipkowitz J. Nummular thioridazine retinopathy. Retina. 1984;4(4):253–6.
Persad S, et al. Phototoxicity of chlorpromazine on retinal pigment epithelial cells. Curr Eye Res. 1988;7(1):1–9.
Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501–26.
Katz BJ, et al. Persistent severe visual and electroretinographic abnormalities after intravenous Cisplatin therapy. J Neuroophthalmol. 2003;23(2):132–5.
Marmor MF. Negative-type electroretinogram from cisplatin toxicity. Doc Ophthalmol. 1993;84(3):237–46.
Wilding G, et al. Retinal toxicity after high-dose cisplatin therapy. J Clin Oncol. 1985;3(12):1683–9.
Simon S, et al. Desferrioxamine-related ocular toxicity: a case report. Indian J Ophthalmol. 2012;60(4):315–7.
Kaplinsky C, et al. Deferoxamine (Desferal)-induced ocular toxicity. Pediatr Hematol Oncol. 1988;5(4):293–7.
Haimovici R, et al. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology. 2002;109(1):164–71.
Schmidt D, Finke J. Bull’s-eye maculopathy with deferoxamine treatment. Klin Monatsbl Augenheilkd. 2004;221(3):204–9.
Kertes PJ, Lee TK, Coupland SG. The utility of multifocal electroretinography in monitoring drug toxicity: deferoxamine retinopathy. Can J Ophthalmol. 2004;39(6):656–61.
Lely AH, van Enter CH. Large-scale digitoxin intoxication. Br Med J. 1970;3(5725):737–40.
Butler VP Jr, et al. Digitalis-induced visual disturbances with therapeutic serum digitalis concentrations. Ann Intern Med. 1995;123(9):676–80.
Robertson DM, Hollenhorst RW, Callahan JA. Ocular manifestations of digitalis toxicity. Discussion and report of three cases of central scotomas. Arch Ophthalmol. 1966;76(5):640–5.
Lawrenson JG, et al. Acquired colour vision deficiency in patients receiving digoxin maintenance therapy. Br J Ophthalmol. 2002;86(11):1259–61.
Renard D, et al. Spectrum of digoxin-induced ocular toxicity: a case report and literature review. BMC Res Notes. 2015;8:368.
Piltz JR, et al. Digoxin toxicity. Recognizing the varied visual presentations. J Clin Neuroophthalmol. 1993;13(4):275–80.
Weleber RG, Shults WT. Digoxin retinal toxicity. Clinical and electrophysiological evaluation of a cone dysfunction syndrome. Arch Ophthalmol. 1981;99(9):1568–72.
Madreperla SA, Johnson MA, Nakatani K. Electrophysiologic and electroretinographic evidence for photoreceptor dysfunction as a toxic effect of digoxin. Arch Ophthalmol. 1994;112(6):807–12.
Landfried B, et al. Digoxin-induced retinal degeneration depends on rhodopsin. Cell Death Dis. 2017;8(3):e2670.
Kinoshita J, et al. Digoxin-induced reversible dysfunction of the cone photoreceptors in monkeys. Invest Ophthalmol Vis Sci. 2014;55(2):881–92.
Maehara S, et al. Detection of cone dysfunction induced by digoxin in dogs by multicolor electroretinography. Vet Ophthalmol. 2005;8(6):407–13.
Deeti S, O’Farrell S, Kennedy BN. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J Pharmacol Toxicol Methods. 2014;69(1):1–8.
Kandel H, et al. Visual function in patients on ethambutol therapy for tuberculosis. J Ocul Pharmacol Ther. 2012;28(2):174–8.
Lai TY, et al. Multifocal electroretinogram demonstrated macular toxicity associated with ethambutol related optic neuropathy. Br J Ophthalmol. 2005;89(6):774–5.
Behbehani RS, et al. Multifocal ERG in ethambutol associated visual loss. Br J Ophthalmol. 2005;89(8):976–82.
Kardon RH, Morrisey MC, Lee AG. Abnormal multifocal electroretinogram (mfERG) in ethambutol toxicity. Semin Ophthalmol. 2006;21(4):215–22.
Lai TY, et al. Multifocal electroretinography changes in patients on ethambutol therapy. Eye (Lond). 2009;23(8):1707–13.
Liu Y, et al. Multifocal electroretinographic abnormalities in ethambutol-induced visual loss. J Neuroophthalmol. 2008;28(4):278–82.
Graham CM, Blach RK. Indomethacin retinopathy: case report and review. Br J Ophthalmol. 1988;72(6):434–8.
Madke B, Prasad K, Kar S. Isotretinoin-induced night blindness. Indian J Dermatol. 2015;60(4):424.
Weleber RG, et al. Abnormal retinal function associated with isotretinoin therapy for acne. Arch Ophthalmol. 1986;104(6):831–7.
Mollan SP, et al. Does use of isotretinoin rule out a career in flying? Br J Ophthalmol. 2006;90(8):957–9.
Kannan NB, et al. Management of ocular siderosis: visual outcome and electroretinographic changes. J Ophthalmol. 2016;2016:7272465.
Mumcuoglu T, et al. An animal model (guinea pig) of ocular siderosis: histopathology, pharmacology, and electrophysiology. Curr Eye Res. 2015;40(3):314–20.
Faure C, et al. Functional and high resolution retinal imaging assessment in a case of ocular siderosis. Doc Ophthalmol. 2014;128(1):69–75.
Kuhn F, et al. Improvement of siderotic ERG. Eur J Ophthalmol. 1992;2(1):44–5.
Declercq SS. Desferrioxamine in ocular siderosis: a long-term electrophysiological evaluation. Br J Ophthalmol. 1980;64(8):626–9.
Declercq SS, Meredith PC, Rosenthal AR. Experimental siderosis in the rabbit: correlation between electroretinography and histopathology. Arch Ophthalmol. 1977;95(6):1051–8.
Gupta S, et al. Sensitivity of multifocal electroretinography (mfERG) in detecting siderosis. Can J Ophthalmol. 2015;50(6):485–90.
Schechner R, et al. A long term follow up of ocular siderosis: quantitative assessment of the electroretinogram. Doc Ophthalmol. 1990;76(3):231–40.
Schocket SS, Lakhanpal V, Varma SD. Siderosis from a retained intraocular stone. Retina. 1981;1(3):201–7.
Eltony SA, Abdelhameed SY. Effect of chronic administration of sildenafil citrate (Viagra) on the histology of the retina and optic nerve of adult male rat. Tissue Cell. 2017;49(2. Pt B):323–35.
Coca MN, et al. Bilateral posterior ischemic optic neuropathy associated with the use of Sildenafil for pulmonary hypertension. Can J Ophthalmol. 2016;51(3):e96–9.
Moschos MM, Nitoda E. Pathophysiology of visual disorders induced by phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drug Des Devel Ther. 2016;8:3407–13.
Luu JK, et al. Acute effects of sildenafil on the electroretinogram and multifocal electroretinogram. Am J Ophthalmol. 2001;132(3):388–94.
Kinoshita J, et al. Sildenafil-induced reversible impairment of rod and cone phototransduction in monkeys. Invest Ophthalmol Vis Sci. 2015;56(1):664–73.
Nivison-Smith L, et al. Sildenafil alters retinal function in mouse carriers of retinitis pigmentosa. Exp Eye Res. 2014;128:43–56.
Jagle H, et al. Dose-dependency and time-course of electrophysiologic short-term effects of VIAGRA: a case study. Doc Ophthalmol. 2005;110(2–3):247–54.
Luke M, et al. Effects of phosphodiesterase type 5 inhibitor sildenafil on retinal function in isolated superfused retina. J Ocul Pharmacol Ther. 2005;21(4):305–14.
McKoy JM, et al. Sildenafil- and tadalafil-associated optic neuropathy: implications for men after prostate cancer treatment. Commun Oncol. 2009;6(2):78–80.
Martins J, et al. Sildenafil acutely decreases visual responses in ON and OFF retinal ganglion cells. Invest Ophthalmol Vis Sci. 2015;56(4):2639–48.
Jung S, et al. Sildenafil improves functional and structural outcome of retinal injury following term neonatal hypoxia-ischemia. Invest Ophthalmol Vis Sci. 2016;57(10):4306–14.
Roy R, et al. Central serous chorioretinopathy following oral tadalafil intake. Clin Exp Optom. 2014;97(5):473–4.
Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina. 2008;28(4):606–9.
Coscas F, et al. Optical coherence tomography in tadalafil-associated retinal toxicity. Eur J Ophthalmol. 2012;22(5):853–6.
Peter NM, Singh MV, Fox PD. Tadalafil-associated anterior ischaemic optic neuropathy. Eye (Lond). 2005;19(6):715–7.
Egan R, Pomeranz H. Sildenafil (Viagra) associated anterior ischemic optic neuropathy. Arch Ophthalmol. 2000;118(2):291–2.
Cunningham AV, Smith KH. Anterior ischemic optic neuropathy associated with viagra. J Neuroophthalmol. 2001;21(1):22–5.
Boshier A, Pambakian N, Shakir SA. A case of nonarteritic ischemic optic neuropathy (NAION) in a male patient taking sildenafil. Int J Clin Pharmacol Ther. 2002;40(9):422–3.
Dheer S, Rekhi GS, Merlyn S. Sildenafil associated anterior ischaemic optic neuropathy. J Assoc Physicians India. 2002;50:265.
Pomeranz HD, et al. Sildenafil-associated nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2002;109(3):584–7.
Gruhn N, Fledelius HC. Unilateral optic neuropathy associated with sildenafil intake. Acta Ophthalmol Scand. 2005;83(1):131–2.
Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (viagra): a report of seven new cases. J Neuroophthalmol. 2005;25(1):9–13.
Escaravage GK Jr, Wright JD Jr, Givre SJ. Tadalafil associated with anterior ischemic optic neuropathy. Arch Ophthalmol. 2005;123(3):399–400.
Bollinger K, Lee MS. Recurrent visual field defect and ischemic optic neuropathy associated with tadalafil rechallenge. Arch Ophthalmol. 2005;123(3):400–1.
Egan RA, Fraunfelder FW. Viagra and anterior ischemic optic neuropathy. Arch Ophthalmol. 2005;123(5):709–10.
Kim IG, Kim DY. Anterior ischemic optic neuropathy associated with udenafil. Korean J Ophthalmol. 2012;26(3):235–8.
Moloney JB, Hillery M, Fenton M. Two year electrophysiology follow-up in quinine amblyopia. A case report. Acta Ophthalmol. 1987;65(6):731–4.
Su D, et al. Quinine toxicity: multimodal retinal imaging and electroretinography findings. Retin Cases Brief Rep. 2017;11 Suppl 1:S102–6.
Gangitano JL, Keltner JL. Abnormalities of the pupil and visual-evoked potential in quinine amblyopia. Am J Ophthalmol. 1980;89(3):425–30.
Sato S. Toxic effects on the visual system of diaminodiphenoxybutane, quinine, and ethambutol in conscious dogs. Fundam Appl Toxicol. 1985;5(4):777–84.
Saeed MU, et al. Relatively spared central multifocal electroretinogram responses in acute quinine toxicity. BMJ Case Rep. 2011;2011.
Verdon W. Clinical electrophysiology in quinine induced retinal toxicity. Optom Vis Sci. 2008;85(1):17–26.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yu, M., Senatore, A., Iannaccone, A., Kheir, W.J., Creel, D. (2019). Characteristics of Visual Electrophysiology in Retinal Toxicities. In: Yu, M., Creel, D., Iannaccone, A. (eds) Handbook of Clinical Electrophysiology of Vision. Springer, Cham. https://doi.org/10.1007/978-3-030-30417-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-30417-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30416-4
Online ISBN: 978-3-030-30417-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)