Abstract
Autism spectrum disorder (ASD) is a rapidly growing global pandemic that affects an estimated 1 in 59–68 children. It is a complex disease with both genetic and environmental etiologies. Due to the rapid increase in the incidence of ASD, environmental causes for ASD are gaining attention. Efforts to probe several environmental exposures that could contribute to causing ASD are underway. In this regard, this chapter is directed towards understanding prenatal exposure to key environmental factors i.e., drugs and dietary nutrients that may act via the same molecular pathway - epigenetics as a potential etiological factor for ASD. Epigenetic regulation is a molecular mechanism known to be a significant contributor to neurodevelopmental disorders. It also offers a means to explain how environmental exposures can impact genetics. We discuss the impact of maternal exposures to certain drugs, and dietary intake, on the developing fetus during pregnancy. Maternal Exposure to some drugs during gestation are associated with a higher risk of ASD, while exposure to other dietary compounds may offer promise to rescue epigenetic regulatory insults related to ASD. However, more work in this important area is still required, nevertheless preliminary research already has important implications in the understanding, prevention and treatment of ASD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction to NDs in General and ASD in Particular
Autism spectrum disorder (ASD) is an umbrella term used to describe an expanse of neurodevelopmental disorders (NDs), mainly characterized by deficits in social interaction and communication and repetitive patterns of behavior (see Box 1 for a summarized definition of ASD from the most recent edition of the American Psychiatric Association handbook, The Diagnostic and Statistical Manual of Mental Disorders (DSM-5), a globally accepted standard [1]). In this context it is important to note that ASD is one of several types of NDs. Symptoms found in ASD can also be found in other types of ND, notably in intellectual disability (ID) [2], as each is an umbrella term encompassing a wide spectrum of disease presentations.
Both ASD and ID are not considered single disorders, rather they are on a spectrum defined by a set of common criteria that are broad in nature. ASD often appears as a co-morbidity in children primarily diagnosed with ID and vice versa, with reports of up to 70% of ASD patients’ having ID [3, 4]. In addition, several other co-morbidities, such as; major congenital anomalies, blindness, deafness, motor dysfunction, cerebral palsy, and epilepsy for example, are found among patients with ID/ASD or both [4]. This is important as it implies that evidence for causation of other ND conditions and co-morbidities, when discussing prenatal exposure to possible ASD environmental risk factors, may also play a role in the development of ASD. Therefore in this chapter, while we will focus on ASD, we will not restrict ourselves to it alone, and consider what is known about the role of epigenetics in prenatal exposures to the etiology of ND in general, when necessary.
Currently, NDs are among the most commonly diagnosed conditions, globally. According to a parental survey, around 15% of children aged between 3 and 17 years were affected by NDs, in the USA alone. These include ASD, attention deficit hyperactivity disorder (ADHD), learning disabilities, ID, cerebral palsy, seizures, stuttering or stammering, moderate to profound hearing loss, blindness, and other developmental delays [5, 6]. A study in 2016 found that an estimated 1 in every 68 children in the USA had ASD [7], and it is currently recognized as one of the most common disorders worldwide [8]. However, an update of the estimated prevalence of ASD among children in the USA released by the Centers for Disease Control and Prevention (CDC) reported a 15% increase from 2012 to 2014 (1 in 68 children in 2012 to 1 in 59 children in 2014) [9]. Estimated ASD prevalence was at 2.47% among US children and adolescents in 2014–2016 (95% confidence intervals, 2.20–2.73%) [9, 10]. Alarmingly these data do not stand alone, as ASD is currently recognized as being a burgeoning global pandemic [11, 12].
Therefore taken together, NDs are among the most prevalent disorders globally, and as they appear in childhood, they present an extreme burden of cost of care over lifespan, accounting for costs greater than that combined for heart disease, cancer and stroke [13]. This underscores the urgent need to find effective prophylactic and therapeutic strategies. The first step toward combating any disease condition is to understand what causes it. We will now provide an overview of what is known about causes and other risk factors for ASD below.
Box 1 Autism Spectrum Disorder Definition as given by DMS V [1]
ASD is diagnosed when all five of the following major symptoms are present in a child.
-
A.
Persistent deficits in social communication and social interaction across multiple contexts.
-
B.
Restricted, repetitive patterns of behavior, interests, or activities, as manifested by at least two of the following, currently or by history (examples are illustrative).
-
1.
Stereotyped or repetitive motor movements, use of objects, or speech (e.g., simple motor stereotypies, lining up toys or flipping objects, echolalia, idiosyncratic phrases).
-
2.
Insistence on sameness, inflexible adherence to routines, or ritualized patterns of verbal or nonverbal behavior (e.g., extreme distress at small changes, difficulties with transitions, rigid thinking patterns, greeting rituals, need to take same route or eat same food every day).
-
3.
Highly restricted, fixated interests that are abnormal in intensity or focus (e.g., strong attachment to or preoccupation with unusual objects, excessively circumscribed or perseverative interest).
-
4.
Hyper- or hypo-reactivity to sensory input or unusual interests in sensory aspects of the environment (e.g., apparent indifference to pain/temperature, adverse response to specific sounds or textures, excessive smelling or touching of objects, visual fascination with lights or movement).
-
1.
-
C.
Symptoms must be present in the early developmental period (but may not fully manifest until social demands exceed limited capacities or may be masked by learned strategies in later stages of life).
-
D.
Symptoms cause clinically significant impairment in social, occupational, or other important areas of current functioning.
-
E.
These disturbances are not better explained by ID (intellectual developmental disorder) or global developmental delay. ID and ASD frequently co-occur; to make comorbid diagnoses of ASD and ID, social communication should be below that expected for general developmental level.
Note: Individuals with a well-established DSM-IV diagnosis of autistic disorder, Asperger’s disorder, or pervasive developmental disorder not otherwise specified should be given the diagnosis of ASD. Individuals who have marked deficits in social communication, but whose symptoms do not otherwise meet criteria for autism spectrum disorder, should be evaluated for social (pragmatic) communication disorder.
2 ASD Etiology
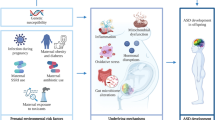
ASD is considered a complex disorder; one caused by the interaction of genetics with the environment. Although ASD is known to be highly heritable [14, 15], only 10–20% of patients are diagnosed with a definitive genetic cause [15]. Very low diagnostic yield despite comprehensive genetic screens has led to a significant “missing heritability” problem in ASD research.
The missing heritability conundrum has shifted attention to the environmental component as a likely explanation for the increased rates of ASD diagnoses. Several environmental risk factors have been suggested to contribute to the development of ASD in existing literature, including air pollution, pesticide exposure via food and otherwise, plastics, psychosocial and socio-economic factors that influence lifestyle and family, maternal obesity and metabolic conditions such as diabetes during pregnancy, maternal mental illness, prenatal and delivery complications and the use of certain supplements and medications during pregnancy [16, 17]. Importantly, the breadth of possible environmental risk factors, taken together with the extremely low percentage of ASD caused by 100% penetrant genetic factors (i.e., only a small fraction of ASD patients are found to have disease due to purely genetic causes, despite it being known to be a highly heritable disorder), emphasizes that ASD is truly a complex disorder. Multifactorial “causal pies” [16] comprising of more than one environmental and/or genetic factor that act in concert, may often be what causes this disorder to manifest.
It is possible that some environmental factors exert their adverse developmental effects via mechanisms that influence the genome, in which context a genome regulatory mechanism termed epigenetics is of particular interest (see Box 2 for a brief primer on Epigenetics). Epigenetics refers to control of the genome by external factors by a process termed epigenetic regulation. These factors are of two main types: (a) enzymes that catalyze epigenetic regulatory reactions, which themselves are encoded by genes, such as DNA methyltransferases, which add methyl groups onto the DNA strand, and (b) chemical moieties which are substrates (e.g., methyl groups, ethyl groups, etc.), that are supplied by the cellular environment. Physiological disturbances of either via maternal environmental exposures during prenatal developmental, may have the potential to contribute to ND/ASD development.
3 Epigenetics and ASD
The initial interest in epigenetic deregulation as a mechanism important in the development of ASD was fueled by the observation that several single gene disorders, that include ASD in their presentation, are caused by perturbation of the genes that encode enzymes involved in epigenetic regulation [18,19,20]. The subsequent recognition that genes having a role in epigenetic programming are among the most frequently mutated genes related to ASD [21, 22], further shone the spotlight on epigenetic deregulation as a possible prime etiological mechanism for ASD/ND. Recently, epigenetic deregulation was recognized to be as significant a cause for ND, as the long-time lead molecular causative mechanism—defects in synaptogenesis [23]. As genome-wide epigenome screens started becoming more affordable, efforts got underway to profile the epigenome (i.e., the DNA methylation profile, and histone modification profiles for example) in an attempt to search for etiological clues, with the focus shifting to characterization of epigenomic changes, regardless of the underlying genetics, as causative profiles for ND in general, and ASD in particular. Epigenetic profile alterations such as DNA methylation for example, have been suggested as key contributing factors for ASD development at the genome- [24] and gene-level [25]. Eshraghi et al. [26], provide a comprehensive summary of epigenomic profile changes associated with ASD. These include DNA methylation changes that are associated with maternal health conditions [27], DNA methylation changes in the placenta of subjects with ASD [28], histone acetylation changes in syndromic and non-syndromic ASD cases [29], and even RNAi signatures associated with ASD [30].
Box 2 Epigenetics Primer
The term Epigenetics comes from the Greek word “epi” for “over/above/on top of,” thus, epigenetics refer to heritable traits that are not encoded in the DNA sequence, rather they are formed by changes to factors that sit “on top” of the genome and thereby regulate gene expression. Epigenetic processes include three main components;
-
1.
DNA methylation
-
2.
Histone modification
-
3.
Chromatin remodeling
Together, all three processes cause genes to be either turned on, or shut down, as well as control fine-tuning of gene expression via non-coding RNA dependent mechanisms [31]. Particularly, histone modification and chromatin remodeling act in concert. DNA methylation at gene promoter sites which often contain CpG islands, is a driver of both histone modification and chromatin remodeling [20]. Figure 1 below is a reproduction of Figure 1 from the Zahir and Brown review paper [20] of the impact of epigenetic processes on neurodevelopment, and shows the cross-talk between the three processes above.
Modified reproduced Figure 1 from Zahir and Brown. Ped. Research. 2011. Caption for the image – Interactions between DNA methylation, histone modification and chromatin remodeling. The DNA strand is wrapped around histone protein cores to form repeating nucleosomes that make up chromatin. Histone tail modifications, are attached to histone tails, and DNA methylation marks are attached to the DNA strand. Epigenetic regulators that have DNA methylation, histone modification or chromatin remodeling interact with each other and display cross-recruitment
In addition a fourth component, RNAi (RNA interference) is a mechanism by which non-coding RNA interacts with some epigenetic components, or with gene transcripts directly in order to regulate genic expression, and is gaining prominence in regulation for neurodevelopmental and neuro-functional processes. It is often included as a fourth epigenetic process; RNA interference based gene expression regulation.
4 Environmentally Controlled Epigenetic Regulatory Impacts in utero and its Role in ASD
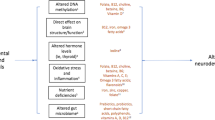
As noted previously, epigenetic regulation can be disrupted during the neurodevelopmental phase by an environmental stimulus either by altering the enzymes that lay down epigenetic marks, or by altering the substrate concentrations. In this regard, maternal ingestion of certain substances during pregnancy, such as drugs and nutritional supplements, that are suggested to induce epigenetic changes, is of prime importance in impacting embryonic and fetal neurodevelopment. We discuss the evidence of their influence below.
4.1 The Impact of Drugs Taken in utero on Epigenetic Regulation During Development
Epigenetic regulation is impacted by drugs primarily via disruption of the enzymatic processes involved, while nutritional supplements on the other hand, influence the concentration of substrate available (see Box 1). However, both drugs and supplements have been identified and used traditionally, based not on the mechanism of action, but on observed clinical or health outcomes. This is no longer the case, especially with respect to drugs. In the past few decades as synthetic drug development, fueled by the large and powerful pharmaceutical industry (so called “big pharma”), has grown to dominate drug provision world-wide, the focus has shifted to mechanism of drug action. This is primarily due to big pharma-led large scale efforts to develop advanced targeted synthetic therapeutics [32, 33]. During the process of synthetic drug development, the mechanism of action of traditionally prescribed drugs was studied and many well-known drugs were found to act via an epigenetic mechanism. This, in turn, fueled interest in a new wave of “epigenetic drugs” [32, 33]. In Table 1, we present an overview of well-known classes and examples for drugs that have been shown to act via epigenetic mechanisms.
As epigenetic deregulation has been highly implicated in the development of ND/ASD, it could be hypothesized that maternal intake of drugs that act via epigenetic mechanisms during pregnancy may be associated with adverse neurodevelopmental outcomes. While information is presently scarce due to the novelty of the field, some examples of maternal drug treatments during pregnancy that are associated with ASD have been suggested in the literature and are discussed below. However, we note that with the exception of valproate, there is very limited information on the role of possible epigenetic mechanisms that could be induced by the drugs discusse below and their association with ASD.
4.2 Associations of Maternal Drug Treatment in Pregnancy with the Diagnosis of ASD in the Prenatally Exposed Children
4.2.1 Thalidomide
In a report of 100 thalidomide embryopathy patients, four cases met the criteria of autism diagnosis (DSM 3), which was a high prevalence of the disorder at the time compared to the general population [44] (Table 2). However, with thalidomide not prevalently used during pregnancy, it would probably account for very few cases of ASD.
4.2.2 Anti-Epileptic Drugs
Animal studies on rats have demonstrated that exposure to AEDs in utero may carry an increased risk of the development of autism [55]. In a study of 260 children exposed to an anti-epileptic medication (AED) during pregnancy, an increased risk of ASD was reported among mothers prenatally exposed to valproate alone, or in combination with other AEDs as well as among mothers exposed to carbamazepine alone or in combination with other AEDs [56]. However, the strongest association in this study was found with valproate exposure. Similarly, 6.3% of children prenatally exposed to valproate monotherapy had a diagnosis of ASD, compared to 0.9% of children of mothers who did not take antiepileptic medications during pregnancy [57]. In a larger, population-based Danish study of mothers prescribed with valproate monotherapy during pregnancy, elevated risks of ASD among school-aged children have also been demonstrated compared to children of mothers unexposed to valproate or another AED during pregnancy [58]. Recently, a large systematic review and meta-analysis of 29 cohort studies of maternal exposure to AED during pregnancy and neurological development of their children concluded that valproate monotherapy, or valproate in combination with other AEDs, showed the strongest association with adverse neurodevelopmental outcomes. On the other hand, oxcarbazepine and lamotrigine in utero exposure were mostly associated with risk to ASD in children compared to unexposed healthy mothers [46] (Table 2). In utero exposure to valproate is also a recognized risk factor for several developmental abnormalities, such as spina bifida, cardiac, skeletal, and craniofacial defects. The proposed mechanisms for valproate teratogenicity that have an epigenetic component include interference with folate metabolism and inhibition of histone deacetylases [59].
4.2.3 Selective Serotonin-Reuptake Inhibitors
Various studies on animals and humans have suggested that increased serotonergic activity during fetal brain development may be one of the causal pathways leading to the development of ASD [60,61,62]. As a result, the hypothesis that maternal treatment with a specific type of antidepressant termed selective serotonin reuptake inhibitors (SSRIs), during gestation may increase the risk of having a child with ASD, has emerged [48]. As SSRIs are the most commonly prescribed antidepressants, the literature has since been expanding with regard to studies suggesting that such an association probably exists [50, 63], specifically when mothers take an SSRI in the first trimester of pregnancy [49, 64, 65]. A recent, large systematic review of the literature and meta-analyses assessing such an association from preconception and across all trimesters of pregnancy included 10 studies, and concluded that a positive association between SSRI exposure and ASD risk is consistent across all trimesters [51]. However, the calculated odds ratio was 1.8, which is still considered small at the population level. When partially adjusted by controlling for the underlying maternal condition, the association remained significant but with an even lower odds ratio of 1.5. In conclusion, and in light of the current evidence, it remains difficult to separate the effect of the underlying disease and/or related comorbidities, lifestyle and other risk factors from the effect of the medication used [52]. Therefore, it is hard to attribute risk to one causal factor when it is more likely the interaction of the psychological status, pharmacological treatment, genetic factors, and other associated factors present in each individual case that determines the risk of an adverse outcome [66].
4.2.4 Other Suspected Drugs Associated with ASD
Acetaminophen is an over-the-counter drug that is currently recommended as a safe pain and fever treatment during pregnancy. However, in recent years, studies have suggested a possible association between maternal acetaminophen use during pregnancy and ASD, but evidence remains controversial [53, 67].
A few reports have indicated a possible association of maternal exposure to β-2 adrenergic receptor agonists, used for treatment of asthma, during pregnancy and risk of ASD [47, 54], but such results need to be replicated in future studies to establish a definite link.
4.3 Epigenetic Diet: The Impact of Nutritional Supplements on Epigenetic Regulation During Development
Nutritional supplements are categorized as part of complementary and alternative medicine (CAM) practices: a diverse group of medical and health care systems, practices, and products that are not generally considered part of conventional medicine or standard medical care. They include interventions such as massage, acupuncture and dietary supplements [68]. Currently, however, very little information is available about mechanisms of action for most CAM, precluding an in-depth discussion of possible links to ASD etiology.
Nutritional supplements that have epigenetic impacts (termed epigenetic diet nutrients) are however, gaining ground as an emerging area of research [69]. Epigenetic diets may influence epigenetic regulation by changing the concentration of available substrate for epigenetic regulatory reactions. Contrary to the scenario with drugs taken by pregnant women and the possible adverse effects upon the fetus discussed above, the effects on the developing fetus due to maternal epigenetic diet are predominantly positive [69, 70]. While a comprehensive review of an epigenetic diet is beyond the scope of this chapter, we shall highlight research foci showing associations of neurodevelopmental outcomes, especially with respect to ASD/ND, and maternal epigenetic diet.
4.3.1 Gestational Intake of Methyl Donors
The earliest report of maternal intake of methyl donors’ ability to alter fetal outcome was reported in 1998 by Wolff et al., in a seminal paper that paved the way for a new area of research. Wolff et al. [71], showed that feeding mice dams a methyl supplemented diet was able to alter the coat color of their pups. Albeit the experiment was conducted on a carefully controlled genetic background, it showed that maternal diet can impact fetal development via epigenetic mechanisms.
Subsequently several studies have shown that gestational intake of various forms of methyl donors are able to influence fetal development. A recent report from the Maternal Nutrition and Offsprings’ Epigenome (MANOE) study of 115 mother–infant pairs, found that maternal intake of methyl donor groups, via diet (in this case methionine, betaine, choline, folate) and via supplementation (folic acid), both before and during pregnancy, was able to significantly alter DNA methylation in cord-blood [72]. However, Boeke et al. [73] conducted a similar study in a folate-replete population, estimating maternal intake of methyl donors nutrients via vitamin B12, betaine, choline, folate, cadmium, zinc, and iron among mother–infant pairs, and found a negative association to DNA methylation levels in male offspring. This study is important for two reasons: firstly it shows that for a healthy population, there is likely no potentially adverse effect due to intake of methyl donor nutrients, and secondly, it also highlights a possible sex-specific signature that requires further study.
Additionally, work in animal models has shown that methyl donor supplementation exerts a protective effect; in chick embryos, it was found that maternal dietary zinc addition was able to protect the growing embryo against negative impacts of maternal heat-shock, by increasing antioxidant activity in the embryo [74]. While in rats, it has been shown that late pregnancy supplementation with folate is able to rescue structural and functional defects of the brain [75, 76].
4.3.2 Gestational Intake of Epigenetic Diet Nutrients of Unknown Mechanism
As epigenomic profiling techniques become more accessible, researchers are discovering that dietary elements are able to exert DNA methylation and histone acetylation profile changes, though the exact mechanism of doing so is unknown. While the reader is referred to more comprehensive reviews [77, 78], we highlight selected dietary nutrients for which epigenetic profile alterations important in neural development and function have been documented, following prenatal exposure.
4.3.2.1 Fish Oils
Fish oils have long been consumed during pregnancy as a dietary supplement. They contain polyunsaturated fatty acids (PUFA) whose role as epigenetic diet nutrients important for brain development is increasingly gaining attention [79]. In a large scale randomized control trial, Van Dijk et al. [76] investigated epigenetic profile changes upon the child following high dose maternal gestational supplementation with docosahexaenoic acid (DHA), a long-chain PUFA. They found a sex-specific increase in differentially methylated regions genome-wide in children of high-dose exposure mothers, interestingly with a higher impact on boys than girls [76]. Another group probed changes in DNA methylation due to prenatal DHA exposure, using a targeted approach. In two separate publications, they screened for DNA methylation differences in a cohort of pregnant Mexican women who were given dietary supplements of 400 mg of DHA daily. They showed small changes in DNA methylation of imprinted loci and repetitive elements [80, 81].
4.3.2.2 Curcumin
Curcumin (diferuloylmethane), is a component of turmeric (Curcuma longa), one of the most common Asian spices. Turmeric has been recognized and used for its many medicinal properties in both Ayurvedic and Traditional Chinese Medicine for several centuries, if not millennia. In the past decade, interest has grown exponentially in exploring the pharmacological benefits and pharmacoepigenomical effects of curcumin [82, 83]. Currently, epigenetic regulatory roles as a modulator of DNA methylation, histone acetylation and epigenetic programming via RNAi have been identified [84]. Curcumin health benefits are most documented for cancer. However, it also shows therapeutic potential for neurological and inflammatory disorders [84, 85]. Salehi et al. [86] have presented the most up to date review of clinical trials of curcumin, while Lopreseti [83] reviewed evidence for clinical and animal trials of curcumin in neuropsychiatric disease. The interested reader is referred to these papers. However, while we could find no specific information related to ASD, we note that the well-documented effects of curcumin as an anti-oxidative and anti-inflammatory agent, coupled with its ability to influence epigenomic programming broadly, suggests the hypothesis that curcumin may have an impact on ASD etiology, is plausible.
4.3.2.3 Others
Other important epigenetic dietary components that may impact fetal brain development via epigenetic mechanisms include polyphenols such as those found in green tea; which was shown to have a potential protective effect. In a mouse model of fetal alcohol spectrum disorder, pregnant dams fed on epigallocatechin-3-gallate (EGCG), the major anti-oxidative component of green tea [87], showed rescue of embryo size back to normal. Trans-resveratrol, another polyphenol, has been shown to be able to rescue aberrant epigenetic programming in rats following perinatal asphyxia [88], an important finding as perinatal asphyxia may cause ND [89].
5 Prevalence of Pregnant Women’s Exposure to Drugs with Potential for Adverse Fetal Outcomes, and Epigenetic Diet
Given the importance of maternal intake of drugs, that may exert an adverse effect on the developing embryo or fetus, and dietary supplements that induce epigenetic mechanisms influencing neurodevelopment, we discuss exposures below.
5.1 Drug Intake in Pregnant Women and Potential for Adverse Developmental Effects
Current evidence has suggested that 65–94% of women take at least one prescription drug during pregnancy [90,91,92]. Lupattelli et al. [93] showed that more than 80% of the pregnant women in the USA, Europe, and Australia use at least one prescribed drug during pregnancy. Importantly, the rate of pregnant women’s exposure to drugs has seen a rapid increase in the past few decades [94]. Furthermore, approximately 70% of women have been reported to be taking medication in the first trimester (encompassing the period of organogenesis, when the fetus’s important organs are developing) [92, 95], including both over the counter drugs and herbal medications [90, 92].
Unfortunately there has not been a concomitant increase in the amount of information available with respect to the potentially adverse effects of drugs on the developing embryo or fetus. This is because the effect of drugs on development cannot be studied in humans through clinical trials [91] becuase such studies are ethically unacceptable. According to the Automated Teratogen Information System (TERIS), the teratogenic risk in human pregnancy is undetermined for 92% of the drug treatments approved by the US FDA system between 1980 and 2000 [96]. In another review of the safety of 172 drugs approved by the US FDA between 2000 and 2010, it was found that the teratogenic risk in human pregnancy was undetermined for 98% of drugs, and for 74% there were no available data about the risk in pregnancy [97]. However, while clear clinical trials of drug safety in pregnant women is often unavailable, information on potentially adverse outcomes is usually gathered via a collection of animal and model organism studies and epidemiological investigations that usually occur several years after a drug has been put on the market.
Nevertheless, given the growing interest in drugs that act via epigenetic mechanisms (Table 1), and already emerging associations of certain drugs that may act via epigenetic mechanisms with ASD (Table 2), we raise a note of caution that further studies specific to possible teratogenic effects of drugs with epigenetic mechanisms of action are warranted.
5.2 Complementary and Alternative Medicine (CAM) Intake During Pregnancy
Parallel to the rise of drug intake among pregnant women, there is also a rising trend of CAM use, though it is less well documented. A literature review of CAM usage in industrialized countries in 2011, found that 1–87% of pregnant women use CAM [98]. On the other hand, a representative survey of pregnant women in the USA, published in 2008, found that over half of the respondents used CAM [99]. Furthermore, a European study found that approximately 60% of pregnant women use a dietary supplement [100].
Pregnant women use CAM to relieve specific pregnancy related problems such as nausea, vomiting, tiredness, and back pain [98, 101]. Examples include the use of ginger root, shown to be a safe and effective non-pharmacological option for nausea and vomiting during early pregnancy [102], and supplementing with Vitamin B6, also considered a safe alternative pharmacological treatment for nausea and vomiting [102, 103].
Thus, the widespread use of CAM during pregnancy, makes it clinically relevant [68, 104].
6 Conclusion
Epigenetic mechanisms are a molecular mechanism by which the environment is able to impact the genome. Recognizing ASD as a complex condition with a likely substantial causative environmental component [16], epigenetic modalities, by which the environment may cause disease, is receiving increased research attention. However, here we only discuss efforts in this area which focus on a key environment: that which the developing fetus is exposed to in utero. There are two main exposures in this regard: fetal exposure to drugs that may have adverse neurodevelopmental potential, and fetal exposure to an epigenetic diet, both of which are emerging areas of research.
Additionally, understanding epigenetic modes of action in disease causation accurately, is important as there the possibility of correcting them [59, 105]. Indeed, the entire field of epigenetic drugs is a direct result of efforts probing how epigenetic deregulation can be corrected back to the normal. Such precision-based medicine efforts, where treatment is based on directly addressing the molecular cause of disease, have seen success, especially in cancer research [106]. However, in this chapter, we drew attention to an overlooked area of epigenetic drugs: those that when injested by a pregnant mother, may impact ASD risk for their unborn child. We emphasize that more research is needed to specifically understand both their epigenetic mechanism of action and their potential to cause harm to the developing fetus.
On the other hand, encouragingly, the emerging area of epigenetic diet is gaining attention due to its potential for prevention and rescue from adverse effects for the developing fetus. Studies in epigenetic diet have focused on elucidating molecular mechanisms by which nutritional supplements act, and evidence is growing for possible epigenetic deregulation rescue outcomes of these “nutraceuticals.” Current reports, while few in number, predominantly detail protective and beneficial effects on neurodevelopment. Especially important in context of this discussion is the ability of epigenetic diet components to ameliorate known harmful environmental exposures for pregnant women; an area of research we highlight here and for which future studies hold promise.
In summary, the significant role epigenetics plays in ASD, as a molecular mechanism translating environment into genomic or genetic control, and the recognition that there are both drugs and diet that a pregnant woman can be exposed to, which act via epigenetic mechanisms, highlight the importance of more focused research on what such exposures in utero could mean for the baby. Thus, we end this chapter calling for further research to understand the epigenetic mechanisms underlying gestational exposures to drugs and diet, and highlighting the remarkable potential of epigenetic regulatory compounds to serve as therapeutics.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: Author.
Homberg, J. R., Kyzar, E. J., Scattoni, M. L., Norton, W. H., Pittman, J., Gaikwad, S., et al. (2016). Genetic and environmental modulation of neurodevelopmental disorders: Translational insights from labs to beds. Brain Research Bulletin, 125, 79–91.
Mpaka, D. M., Okitundu, D. L. E. A., Ndjukendi, A. O., N’situ, A. M., Kinsala, S. Y., Mukau, J. E., et al. (2016). Prevalence and comorbidities of autism among children referred to the outpatient clinics for neurodevelopmental disorders. The Pan African Medical Journal, 25, 82–82.
Vissers, L. E. L. M., Gilissen, C., & Veltman, J. A. (2015). Genetic studies in intellectual disability and related disorders. Nature Reviews Genetics, 17, 9.
Boyle, C. A., Boulet, S., Schieve, L. A., Cohen, R. A., Blumberg, S. J., Yeargin-Allsopp, M., et al. (2011). Trends in the prevalence of developmental disabilities in US Children, 1997–2008. Pediatrics, 127, 1034–1042.
Gupta, S., Venkatesan, S. P., Goswami, S., & Kumar, R. (2018). Emerging trends in the diagnosis and intervention of neurodevelopmental disorders. IGI Global.
Christensen, D. L., Baio, J., Van Naarden Braun, K., Bilder, D., Charles, J., Constantino, J. N., et al. (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveillance Summaries, 65, 1–23.
Elsabbagh, M., Divan, G., Koh, Y.-J., Kim, Y. S., Kauchali, S., Marcín, C., et al. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research: Official Journal of the International Society for Autism Research, 5, 160–179.
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C. : 2002), 67, 1–23.
Xu, G., Strathearn, L., Liu, B., & Bao, W. (2018). Prevalence of autism spectrum disorder among us children and adolescents, 2014–2016. JAMA, 319, 81–82.
Bilbo, S. D., Jones, J. P., & Parker, W. (2012). Is autism a member of a family of diseases resulting from genetic/cultural mismatches? Implications for treatment and prevention. Autism Research and Treatment, 2012, 910946.
Bilbo, S. D., Nevison, C. D., & Parker, W. (2015). A model for the induction of autism in the ecosystem of the human body: The anatomy of a modern pandemic? Microbial Ecology in Health and Disease, 26, 26253.
Meerding, W. J., Bonneux, L., Polder, J. J., Koopmanschap, M. A., & Van Der Maas, P. J. (1998). Demographic and epidemiological determinants of healthcare costs in Netherlands: Cost of illness study. BMJ (Clinical research ed.), 317, 111–115.
El-Fishawy, P., & State, M. W. (2010). The genetics of autism: Key issues, recent findings, and clinical implications. The Psychiatric Clinics of North America, 33, 83–105.
Geschwind, D. H. (2011). Genetics of autism spectrum disorders. Trends in Cognitive Sciences, 15, 409–416.
Hertz-Picciotto, I., Schmidt, R. J., & Krakowiak, P. (2018). Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Research, 11, 554–586.
Lyall, K., Schmidt, R. J., & Hertz-Picciotto, I. (2014). Maternal lifestyle and environmental risk factors for autism spectrum disorders. International Journal of Epidemiology, 43, 443–464.
Grayson, D. R., & Guidotti, A. (2016). Merging data from genetic and epigenetic approaches to better understand autistic spectrum disorder. Epigenomics, 8, 85–104.
Loke, Y. J., Hannan, A. J., & Craig, J. M. (2015). The role of epigenetic change in autism spectrum disorders. Frontiers in Neurology, 6, 107.
Zahir, F. R., & Brown, C. J. (2011). Epigenetic impacts on neurodevelopment: Pathophysiological mechanisms and genetic modes of action. Pediatric Research, 69, 92R.
Bernier, R., Golzio, C., Xiong, B., Stessman, H. A., Coe, B. P., Penn, O., et al. (2014). Disruptive CHD8 mutations define a subtype of autism early in development. Cell, 158, 263–276.
O’Roak, B. J., Vives, L., Girirajan, S., Karakoc, E., Krumm, N., Coe, B. P., et al. (2012). Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature, 485, 246–250.
Zahir, F. R., Tucker, T., Mayo, S., Brown, C. J., Lim, E. L., Taylor, J., et al. (2016). Intragenic CNVs for epigenetic regulatory genes in intellectual disability: Survey identifies pathogenic and benign single exon changes. American Journal of Medical Genetics. Part A, 170, 2916–2926.
Keil, K. P., & Lein, P. J. (2016). DNA methylation: A mechanism linking environmental chemical exposures to risk of autism spectrum disorders? Environmental Epigenetics, 2, dvv012.
Elagoz Yuksel, M., Yuceturk, B., Karatas, O. F., Ozen, M., & Dogangun, B. (2016). The altered promoter methylation of oxytocin receptor gene in autism. Journal of Neurogenetics, 30, 280–284.
Eshraghi, A. A., Liu, G., Kay, S.-I. S., Eshraghi, R. S., Mittal, J., Moshiree, B., et al. (2018). Epigenetics and autism spectrum disorder: Is there a correlation? Frontiers in Cellular Neuroscience, 12, 78–78.
Gunawardhana, L. P., Baines, K. J., Mattes, J., Murphy, V. E., Simpson, J. L., & Gibson, P. G. (2014). Differential DNA methylation profiles of infants exposed to maternal asthma during pregnancy. Pediatric Pulmonology, 49, 852–862.
Ladd-Acosta, C., Hansen, K. D., Briem, E., Fallin, M. D., Kaufmann, W. E., & Feinberg, A. P. (2014). Common DNA methylation alterations in multiple brain regions in autism. Molecular Psychiatry, 19, 862–871.
Sun, W., Poschmann, J., Cruz-Herrera Del Rosario, R., Parikshak, N. N., Hajan, H. S., Kumar, V., et al. (2016). Histone acetylome-wide association study of autism spectrum disorder. Cell, 167, 1385–1397.e11.
Wu, Y. E., Parikshak, N. N., Belgard, T. G., & Geschwind, D. H. (2016). Genome-wide, integrative analysis implicates microRNA dysregulation in autism spectrum disorder. Nature Neuroscience, 19, 1463–1476.
Rosikiewicz, W., & Makalowska, I. (2016). Biological functions of natural antisense transcripts. Acta Biochimica Polonica, 63, 665–673.
Altucci, L., & Rots, M. G. (2016). Epigenetic drugs: From chemistry via biology to medicine and back. Clinical Epigenetics, 8, 56–56.
Heerboth, S., Lapinska, K., Snyder, N., Leary, M., Rollinson, S., & Sarkar, S. (2014). Use of epigenetic drugs in disease: An overview. Genetics & Epigenetics, 6, 9–19.
Yang, X., Lay, F., Han, H., & Jones, P. A. (2010). Targeting DNA methylation for epigenetic therapy. Trends in Pharmacological Sciences, 31, 536–546.
Gnyszka, A., Jastrzębski, Z., & Flis, S. (2013). DNA methyltransferase inhibitors and their emerging role in epigenetic therapy of cancer. Anticancer Research, 33, 2989–2996.
Ahuja, N., Sharma, A. R., & Baylin, S. B. (2016). Epigenetic therapeutics: A new weapon in the war against cancer. Annual Review of Medicine, 67, 73–89.
Eckschlager, T., Plch, J., Stiborova, M., & Hrabeta, J. (2017). Histone deacetylase inhibitors as anticancer drugs. International Journal of Molecular Sciences, 18, 1414.
Goey, A. K., Sissung, T. M., Peer, C. J., & Figg, W. D. (2016). Pharmacogenomics and histone deacetylase inhibitors. Pharmacogenomics, 17, 1807–1815.
Dekker, F. J., Van Den Bosch, T., & Martin, N. I. (2014). Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discovery Today, 19, 654–660.
Forster, V. J., Mcdonnell, A., Theobald, R., & Mckay, J. A. (2017). Effect of methotrexate/vitamin B(12) on DNA methylation as a potential factor in leukemia treatment-related neurotoxicity. Epigenomics, 9, 1205–1218.
Williams, K., Brignell, A., Randall, M., Silove, N., & Hazell, P. (2013). Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews, Cd004677.
Ahmadvand, M., Noruzinia, M., Fard, A. D., Zohour, M. M., Tabatabaiefar, M. A., Soleimani, M., et al. (2014). The role of epigenetics in the induction of fetal hemoglobin: A combination therapy approach. International Journal of Hematology-Oncology and Stem Cell Research, 8, 9–14.
Mahajan, S. S., Leko, V., Simon, J. A., & Bedalov, A. (2011). Sirtuin modulators. Handbook of Experimental Pharmacology, 206, 241–255.
Stromland, K., Nordin, V., Miller, M., Akerstrom, B., & Gillberg, C. (1994). Autism in thalidomide embryopathy: A population study. Developmental Medicine and Child Neurology, 36, 351–356.
Christensen, J., Grønborg, T. K., Sørensen, M. J., Schendel, D., Parner, E. T., Pedersen, L. H., et al. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA, 309, 1696–1703.
Veroniki, A. A., Rios, P., Cogo, E., Straus, S. E., Finkelstein, Y., Kealey, R., et al. (2017). Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: A systematic review and network meta-analysis. BMJ Open, 7, e017248.
Croen, L. A., Connors, S. L., Matevia, M., Qian, Y., Newschaffer, C., & Zimmerman, A. W. (2011). Prenatal exposure to beta2-adrenergic receptor agonists and risk of autism spectrum disorders. Journal of Neurodevelopmental Disorders, 3, 307–315.
Harrington, R. A., Lee, L. C., Crum, R. M., Zimmerman, A. W., & Hertz-Picciotto, I. (2013). Serotonin hypothesis of autism: Implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Research, 6, 149–168.
Harrington, R. A., Lee, L.-C., Crum, R. M., Zimmerman, A. W., & Hertz-Picciotto, I. (2014). Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics, 133, e1241–e1248.
Gidaya, N. B., Lee, B. K., Burstyn, I., Yudell, M., Mortensen, E. L., & Newschaffer, C. J. (2014). In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 44, 2558–2567.
Mezzacappa, A., Lasica, P. A., Gianfagna, F., Cazas, O., Hardy, P., Falissard, B., et al. (2017). Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: A systematic review and meta-analysis. JAMA Pediatrics, 171, 555–563.
Morales, D. R., Slattery, J., Evans, S., & Kurz, X. (2018). Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: Systematic review of observational studies and methodological considerations. BMC Medicine, 16, 6.
Bauer, A. Z., Kriebel, D., Herbert, M. R., Bornehag, C. G., & Swan, S. H. (2018). Prenatal paracetamol exposure and child neurodevelopment: A review. Hormones and Behavior, 101, 125–147.
Gidaya, N. B., Lee, B. K., Burstyn, I., Michael, Y., Newschaffer, C. J., & Mortensen, E. L. (2016). In utero exposure to beta-2-adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics, 137, e20151316.
Ingram, J. L., Peckham, S. M., Tisdale, B., & Rodier, P. M. (2000). Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicology and Teratology, 22, 319–324.
Rasalam, A. D., Hailey, H., Williams, J. H., Moore, S. J., Turnpenny, P. D., Lloyd, D. J., et al. (2005). Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Developmental Medicine and Child Neurology, 47, 551–555.
Bromley, R. L., Mawer, G., Clayton-Smith, J., & Baker, G. A. (2008). Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology, 71, 1923–1924.
Christensen, J., Gronborg, T. K., Sorensen, M. J., Schendel, D., Parner, E. T., Pedersen, L. H., et al. (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA, 309, 1696–1703.
Grafodatskaya, D., Chung, B., Szatmari, P., & Weksberg, R. (2010). Autism spectrum disorders and epigenetics. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 794–809.
Anderson, G. M., Freedman, D. X., Cohen, D. J., Volkmar, F. R., Hoder, E. L., Mcphedran, P., et al. (1987). Whole blood serotonin in autistic and normal subjects. Journal of Child Psychology and Psychiatry, 28, 885–900.
Cook Jr., E. H., Leventhal, B. L., & Freedman, D. X. (1988). Free serotonin in plasma: Autistic children and their first-degree relatives. Biological Psychiatry, 24, 488–491.
Vorhees, C. V., Acuff-Smith, K. D., Schilling, M. A., Fisher, J. E., Moran, M. S., & Buelke-Sam, J. (1994). A developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in rats. Fundamental and Applied Toxicology, 23, 194–205.
Rai, D., Lee, B. K., Dalman, C., Golding, J., Lewis, G., & Magnusson, C. (2013). Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: Population based case-control study. BMJ, 346, f2059.
Croen, L. A., Grether, J. K., Yoshida, C. K., Odouli, R., & Hendrick, V. (2011). Antidepressant use during pregnancy and childhood autism spectrum disorders. Archives of General Psychiatry, 68, 1104–1112.
Sorensen, M. J., Gronborg, T. K., Christensen, J., Parner, E. T., Vestergaard, M., Schendel, D., et al. (2013). Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clinical Epidemiology, 5, 449–459.
Alwan, S., Friedman, J. M., & Chambers, C. (2016). Safety of selective serotonin reuptake inhibitors in pregnancy: A review of current evidence. CNS Drugs, 30, 499–515.
Andrade, C. (2016). Use of acetaminophen (paracetamol) during pregnancy and the risk of autism spectrum disorder in the offspring. The Journal of Clinical Psychiatry, 77, e152–e154.
Steel, A., Adams, J., Sibbritt, D., & Broom, A. (2015). The outcomes of complementary and alternative medicine use among pregnant and birthing women: Current trends and future directions. Women’s Health, 11, 309–323.
Li, Y., Saldanha, S. N., & Tollefsbol, T. O. (2013). Impact of epigenetic dietary compounds on transgenerational prevention of human diseases. The AAPS Journal, 16, 27–36.
Bianco-Miotto, T., Craig, J. M., Gasser, Y. P., Van Dijk, S. J., & Ozanne, S. E. (2017). Epigenetics and DOHaD: From basics to birth and beyond. Journal of Developmental Origins of Health and Disease, 8, 513–519.
Wolff, G. L., Kodell, R. L., Moore, S. R., & Cooney, C. A. (1998). Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. The FASEB Journal, 12, 949–957.
Pauwels, S., Ghosh, M., Duca, R. C., Bekaert, B., Freson, K., Huybrechts, I. A. S., et al. (2016). Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation. Epigenetics, 12, 1–10.
Boeke, C. E., Baccarelli, A., Kleinman, K. P., Burris, H. H., Litonjua, A. A., Rifas-Shiman, S. L., et al. (2012). Gestational intake of methyl donors and global LINE-1 DNA methylation in maternal and cord blood: Prospective results from a folate-replete population. Epigenetics, 7, 253–260.
Zhu, Y., Liao, X., Lu, L., Li, W., Zhang, L., Ji, C., et al. (2017). Maternal dietary zinc supplementation enhances the epigenetic-activated antioxidant ability of chick embryos from maternal normal and high temperatures. Oncotarget, 8, 19814–19824.
Geoffroy, A., Kerek, R., Pourié, G., Helle, D., Guéant, J.-L., Daval, J.-L., et al. (2017). Late maternal folate supplementation rescues from methyl donor deficiency-associated brain defects by restoring let-7 and miR-34 pathways. Molecular Neurobiology, 54, 5017–5033.
Van Dijk, S. J., Zhou, J., Peters, T. J., Buckley, M., Sutcliffe, B., Oytam, Y., et al. (2016). Effect of prenatal DHA supplementation on the infant epigenome: Results from a randomized controlled trial. Clinical Epigenetics, 8, 114–114.
Hardy, T. M., & Tollefsbol, T. O. (2011). Epigenetic diet: Impact on the epigenome and cancer. Epigenomics, 3, 503–518.
Meeran, S. M., Ahmed, A., & Tollefsbol, T. O. (2010). Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clinical Epigenetics, 1, 101–116.
Schuchardt, J. P., Huss, M., Stauss-Grabo, M., & Hahn, A. (2010). Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. European Journal of Pediatrics, 169, 149–164.
Lee, H.-S., Barraza-Villarreal, A., Biessy, C., Duarte-Salles, T., Sly, P. D., Ramakrishnan, U., et al. (2014). Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. Physiological Genomics, 46, 851–857.
Lee, H.-S., Barraza-Villarreal, A., Hernandez-Vargas, H., Sly, P. D., Biessy, C., Ramakrishnan, U., et al. (2013). Modulation of DNA methylation states and infant immune system by dietary supplementation with ω-3 PUFA during pregnancy in an intervention study. The American Journal of Clinical Nutrition, 98, 480–487.
Aggarwal, B. B., & Harikumar, K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. The International Journal of Biochemistry & Cell Biology, 41, 40–59.
Lopresti, A. L. (2017). Curcumin for neuropsychiatric disorders: A review of in vitro, animal and human studies. Journal of Psychopharmacology, 31, 287–302.
Boyanapalli, S. S. S., & Kong, A.-N. T. (2015). “Curcumin, the King of Spices”: Epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Current Pharmacology Reports, 1, 129–139.
Zhu, L.-N., Mei, X., Zhang, Z.-G., Xie, Y.-P., & Lang, F. (2019). Curcumin intervention for cognitive function in different types of people: A systematic review and meta-analysis. Phytotherapy Research, 33(3), 524–533.
Salehi, B., Stojanovic-Radic, Z., Matejic, J., Sharifi-Rad, M., Anil Kumar, N. V., Martins, N., et al. (2018). The therapeutic potential of curcumin: A review of clinical trials. European Journal of Medicinal Chemistry, 163, 527–545.
Long, L., Li, Y., Wang, Y. D., He, Q. Y., Li, M., Cai, X. D., et al. (2010). The preventive effect of oral EGCG in a Fetal Alcohol Spectrum Disorder Mouse Model. Alcoholism: Clinical and Experimental Research, 34, 1929–1936.
Isac, S., Panaitescu, A. M., Spataru, A., Iesanu, M., Totan, A., Udriste, A., et al. (2017). Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neuroscience Letters, 653, 308–313.
Van Handel, M., Swaab, H., De Vries, L. S., & Jongmans, M. J. (2007). Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: A review. European Journal of Pediatrics, 166, 645–654.
Ayad, M., & Costantine, M. M. (2015). Epidemiology of medications use in pregnancy. Seminars in Perinatology, 39, 508–511.
Mosley 2nd, J. F., Smith, L. L., & Dezan, M. D. (2015). An overview of upcoming changes in pregnancy and lactation labeling information. Pharmacy Practice (Granada), 13, 605.
Temming, L. A., Cahill, A. G., & Riley, L. E. (2016). Clinical management of medications in pregnancy and lactation. American Journal of Obstetrics and Gynecology, 214, 698–702.
Lupattelli, A., Spigset, O., Twigg, M. J., Zagorodnikova, K., Mårdby, A. C., Moretti, M. E., et al. (2014). Medication use in pregnancy: A cross-sectional, multinational web-based study. BMJ Open, 4, e004365.
Mitchell, A. A., Gilboa, S. M., Werler, M. M., Kelley, K. E., Louik, C., & Hernandez-Diaz, S. (2011). Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. American Journal of Obstetrics and Gynecology, 205, 51.e1–51.e8.
Mitchell, A. A., Gilboa, S. M., Werler, M. M., Kelley, K. E., Louik, C., Hernández-Díaz, S., et al. (2011). Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. American Journal of Obstetrics and Gynecology, 205, 51.e1–51.e518.
Lo, W., & Friedman, J. (2002). Teratogenicity of recently introduced medications in human pregnancy. Obstetrics & Gynecology, 100, 465–473.
Adam, M. P., Polifka, J. E., & Friedman, J. M. (2011). Evolving knowledge of the teratogenicity of medications in human pregnancy. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 157, 175–182.
Hall, H. G., Griffiths, D. L., & Mckenna, L. G. (2011). The use of complementary and alternative medicine by pregnant women: A literature review. Midwifery, 27, 817–824.
Wade, C., Chao, M., Kronenberg, F., Cushman, L., & Kalmuss, D. (2008). Medical pluralism among American women: Results of a national survey. Journal of Women’s Health (2002), 17, 829–840.
Holst, L., Wright, D., Haavik, S., & Nordeng, H. (2011). Safety and efficacy of herbal remedies in obstetrics-review and clinical implications. Midwifery, 27, 80–86.
Steel, A., Adams, J., Sibbritt, D., Broom, A., Gallois, C., & Frawley, J. (2012). Utilisation of complementary and alternative medicine (CAM) practitioners within maternity care provision: Results from a nationally representative cohort study of 1,835 pregnant women. BMC Pregnancy and Childbirth, 12, 146.
Thomson, M., Corbin, R., & Leung, L. (2014). Effects of ginger for nausea and vomiting in early pregnancy: A meta-analysis. Journal of American Board of Family Medicine, 27, 115–122.
Firouzbakht, M., Nikpour, M., Jamali, B., & Omidvar, S. (2014). Comparison of ginger with vitamin B6 in relieving nausea and vomiting during pregnancy. Ayu, 35, 289–293.
Birdee, G. S., Kemper, K. J., Rothman, R., & Gardiner, P. (2014). Use of complementary and alternative medicine during pregnancy and the postpartum period: An analysis of the National Health Interview Survey. Journal of Women’s Health (2002), 23, 824–829.
Siu, M. T., & Weksberg, R. (2017). Epigenetics of autism spectrum disorder. Advances in Experimental Medicine and Biology, 978, 63–90.
Moran, S., Martinez-Cardus, A., Boussios, S., & Esteller, M. (2017). Precision medicine based on epigenomics: The paradigm of carcinoma of unknown primary. Nature Reviews. Clinical Oncology, 14, 682–694.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bastaki, K.N., Alwan, S., Zahir, F.R. (2020). Maternal Prenatal Exposures in Pregnancy and Autism Spectrum Disorder: An Insight into the Epigenetics of Drugs and Diet as Key Environmental Influences. In: Essa, M., Qoronfleh, M. (eds) Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management. Advances in Neurobiology, vol 24. Springer, Cham. https://doi.org/10.1007/978-3-030-30402-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-30402-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30401-0
Online ISBN: 978-3-030-30402-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)