Abstract
Gastroparesis is a chronic neuromuscular disorder that can be disabling with significant impact on patient’s quality of life. Medical management is the cornerstone of treating patients with gastroparesis. The goals should focus on symptom control, maintenance of adequate weight, and prevention of nutritional deficiencies. Gastroparesis is part of a spectrum of functional diseases, which include functional dyspepsia, chronic unexplained nausea and vomiting, and cyclic vomiting syndrome. The delay in gastric emptying as a physiologic metric separates gastroparesis from these other functional illnesses. While gastric emptying as the disease-defining metric has been the traditional treatment target, the correlation between emptying delay and symptom severity is poor. Pharmacotherapy is thus shifting from an emphasis on prokinetics and changes in gastric emptying to agents that improve overall symptoms and quality of life independent of the underlying mechanism. There is only one FDA-approved medication for treatment of gastroparesis, and therefore most of the agents are used “off-label” for this condition. The components of medical management include nutritional and fluid management and pharmacotherapy with a combination of antiemetics, prokinetics, and neuromodulators. There is also renewed interest in non-pharmacologic treatments as well.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Medical management is the cornerstone of treating patients with gastroparesis. The goals should focus on symptom control, maintenance of adequate weight, and prevention of nutritional deficiencies. While gastric emptying as the disease-defining biomarker had been an additional treatment target, the correlation between emptying delay and symptom severity has been poor in cross-sectional and longitudinal studies [1,2,3,4,5]. Pharmacotherapy is thus shifting from an emphasis on prokinetics to approaches that improve symptoms independent of the underlying mechanism, a shift that also matches with FDA guidelines on clinical trials and endpoints in the management of functional GI disorders [6,7,8]. Recently published investigations reflect this development and typically use composite symptom indices as their primary outcome measures. As most of these trials recruit patients from many sites outside of the more specialized referral centers, they provide some insight into the short-term prognosis of this illness. An important insight for patients and clinicians alike is a relatively high response rate even during placebo interventions [9,10,11,12]. While this pattern complicates the design of trials and requires increasingly large sample sizes, it gives room for optimism about the prognosis of an illness that comes with concerning and lasting symptoms with tertiary care centers often reporting high and persistent symptom burdens with frequent need for more complex and invasive therapies [1, 13]. Presently there is only one FDA -approved medication for treatment of gastroparesis, and therefore most of the agents described below are used “off-label” for this condition. The components of medical management include nutritional and fluid management and pharmacotherapy with a combination of antiemetics, prokinetics, and neuromodulators. Finally, there is renewed interest in non-pharmacologic treatments as well.
Nutritional Management
Food intake with its link to gastric filling is a common trigger of symptoms. Dietary management can indeed limit such postprandial symptoms. In addition, nutritional needs must be addressed to prevent deficiencies that may otherwise develop. Considering the role of gastric filling and distension in the development of discomfort, limiting meal size and compensating by an increased meal frequency has been a cornerstone of gastroparesis management. Based on our understanding of factors that modulate gastric emptying [14] , most clinicians also recommend changes in consistency and composition of ingested food with an emphasis on small, low-fat, low-fiber, and low-residue meals 4–5 times per day. One well-designed trial has truly addressed such approaches and clearly demonstrated a benefit of a small particle-size diet defined as “food should be easy to mash with a fork into small particle size” or could be blenderized to consistency of mashed potatoes [15]. The fat content of ingested food or liquids significantly contributes to symptoms in gastroparesis and should be limited [16]. Because liquid emptying is often preserved in patients with delayed solid emptying, high calorie liquid formulas or homogenized meals can be added or substituted if solid food is not tolerated. Especially for persons relying on a more restricted diet, micronutrient supplementation should be considered. As many patients struggle with other illnesses, such as diabetes, detailed information and education by dieticians experienced in management of gastroparesis is of great benefit.
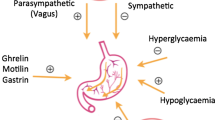
Optimal glycemic management is important for patients with diabetic gastroparesis as hyperglycemia inhibits gastric emptying and improved glycemic control may improve emptying and reduce symptoms. Medications (e.g., opiates, GLP-1 analogs, anticholinergics) can delay gastric emptying and may contribute to symptoms. This is especially relevant for the glucagon-like peptide-1 receptor agonists, which should be held to assess the relative role in patients with new or worsening symptoms of gastroparesis.
For most patients with gastroparesis, the oral route is preferred and more invasive approaches in the form of enteral or even parenteral nutrition are needed in only a small percentage of patients. Data from the National Inpatient Sample Database show that even in the skewed population of patients admitted with gastroparesis as primary diagnosis, feeding tube placement for initiation of nutritional support is listed in less than 2% of the hospitalizations [17, 18]. Considering the fact that only a small fraction of patients with this disorder will require inpatient management [19], this fraction is likely to be much lower in an outpatient cohort. While systematic studies are lacking, many patients will decide against long-term management with venting gastric or enteral tube due to dissatisfaction or side effects [8].
When enteral nutrition is required, a feeding jejunostomy that bypasses the stomach is preferred. This can be placed endoscopically, radiographically, or surgically depending on local expertise. Given the above issues, a trial of nasojejunal feeding to assess tolerance and patient satisfaction may be useful. Finally, enteral feeding is always preferred over parenteral nutrition due to cost, potential for complications, and ease of delivery.
Pharmacotherapy
Antiemetics
Nausea and vomiting are hallmark symptoms of gastroparesis, which often persist despite appropriate dietary or medical therapy. Thus, up to 70% of patients described in larger studies use antiemetics [8, 20]. While there is limited data for specific antiemetics for patients with gastroparesis, the use of many of these agents is extrapolated from treatment of chemotherapy-related nausea and motion sickness. Ondansetron (a 5-HT3 agonist) and phenothiazines (promethazine, prochlorperazine) are the most commonly prescribed agents due to their wide availability and coverage by insurance. Ondansetron is available as an orally disintegrating tablet, and promethazine is available in liquid and suppository formulation which may improve drug delivery in patients with oral intolerance. Other medications indicated for motion sickness including antihistamines (H1 receptor blockers) like meclizine and transdermal scopolamine (a cholinergic receptor antagonist) have been used off-label.
While intuitively appropriate, clinical investigations of antiemetics typically focus on chemotherapy-induced nausea, which conceptually differs from the chronic symptoms that characterize gastroparesis. Two small open-label studies of transdermal granisetron (another 5-HT3 receptor agonist) supported the approach and showed a moderate benefit in patients with otherwise refractory symptoms of gastroparesis [21, 22]. The NK1 receptor blocker aprepitant has both central and peripheral antiemetic effects and is approved for chemotherapy-induced nausea and vomiting. A placebo-controlled trial in patients with gastroparesis or gastroparesis-like symptoms did not meet its primary outcome in decreasing nausea severity but did demonstrate improvements in nausea, vomiting, and overall symptom scores [23]. Based on these admittedly limited data, it is reasonable to extrapolate from other scenarios with nausea or vomiting as defining manifestations and use agents effective in such settings. (See Table 4.1.)
Prokinetics
Dopamine Antagonists
Metoclopramide is both an antiemetic through centrally acting dopamine D2 receptor antagonist and 5-HT4 receptor agonist in the brain and prokinetic through 5-HT4 receptor agonist in the gut. Currently, metoclopramide remains the only FDA-approved medication for gastroparesis and is available in oral, oral dissolving tablet, liquid, intranasal, and parenteral formulations that may be administered intravenously, intramuscularly, or subcutaneously. However, its use is associated with significant extrapyramidal motor dysfunction including acute dystonias, Parkinson-type movements, and tardive dyskinesia [24, 25]. The tardive dyskinesia which occurs in <1% and is not always reversible has led to a FDA black box warning to limit its use to 12 weeks. Guidelines recommend starting at lowest effective dose (i.e., 5 mg TID before meals, maximum 40 mg/day) and dose reduction and/or drug holidays as able. Recently a nasally administered form of the agent was introduced and was similar in efficacy compared with the oral version of the drug [26]. In a post hoc analysis of a placebo-controlled trial in diabetic patients, gastroparesis-like symptoms showed marginal superiority over placebo. It should be noted that this finding was limited to female patients [27].
Domperidone is a peripherally acting dopamine antagonist with lower CNS penetration rates and associated with a less neurological side effects. Short-term studies show an effect on emptying and symptoms comparable to metoclopramide [28,29,30]. The agent has not been approved in the USA, but can be obtained through an FDA investigational drug application. The usual dose is 10 mg TID with maximum 20 mg TID and at bedtime. It does however interact with a potassium channel and can prolong cardiac repolarization phase, leading to long QT syndrome and potentially fatal arrhythmias. Thus, close monitoring with baseline and on-treatment electrocardiograms and potassium levels is essential. See Table 4.2.
Motilin Agonists
After earlier descriptions about its effect on motilin receptors and the resulting changes in gastrointestinal motility [31], clinical studies first described the prokinetic effect of erythromycin in patients with diabetic gastroparesis nearly 30 years ago [32]. Subsequent studies confirmed the accelerated emptying, which seemed to decline over time but remained significant compared to baseline with an associated improvement of symptom scores [33]. However, the reported benefits of motilin agonists were transient, likely due to receptor desensitization, and the observed symptomatic improvements were not superior to placebo [34]. Subsequent development of other motilin agonists without antibiotic effects similarly showed no benefit over placebo [35, 36]. The originally described approach with intravenous administration of erythromycin is still used in clinical practice when significant gastric retention or even bezoar formation contribute to acute worsening of symptoms, which is a rare but frustrating scenario for patients and physicians alike. Erythromycin may be given at 3 mg/kg IV every 8 hours in the inpatient setting and 50–100 mg before meals in the outpatient setting. However, interactions with many commonly used medications and development of tachyphylaxis limit its role for the agent in the chronic management of gastroparesis. See Table 4.2.
Ghrelin Agonist
The discovery of ghrelin, a peptide hormone produced in the stomach and acting on the growth hormone secretagogue receptor, triggered significant interest as it stimulates appetite, food intake, and a positive energy balance; it also acutely accelerates gastric emptying through vagally mediated pathways [37]. Acute administration of the ghrelin agonist TZP-101 indeed accelerated gastric emptying and improved symptoms [38, 39]. However, a follow-up investigation with an oral agent did not show benefit over placebo at 12 weeks of treatment [10, 11]. Relamorelin , another ghrelin agonist with greater potency and stability, administered subcutaneously improved symptoms of gastroparesis and enhanced gastric emptying in two large randomized trials but worsened diabetic control in about 15% of the patients [9, 40]. Phase III studies are underway with relamorelin in diabetic gastroparesis. Considering the fact that ghrelin signals through vagal pathways and that we likely face a high prevalence of autonomic neuropathy in patients with diabetic gastroparesis, it is possible that ghrelin agonists will have additional beneficial effects in patient groups with other causes of impaired gastric function.
Serotonin
Considering the importance of serotonin (5-HT) in gastrointestinal signaling and function, agents targeting these receptors have been tried in gastroparesis and in functional dyspepsia. The most commonly used drugs block the 5-HT3 receptor and play an important role as antiemetics. Their beneficial effect is likely due to a central effect on vagal pathways. In contrast, tegaserod and cisapride had agonistic properties on 5-HT4 receptors and stimulated contractions, resulting in accelerated gastric emptying [41]. Clinical studies indeed confirmed increased antral contractility and enhanced gastric emptying, but inconsistent symptomatic benefit [42,43,44]. Concerns about serious adverse events with cardiac arrhythmias due to QT prolongation or myocardial infarctions prompted the withdrawal of these agents from the market though cisapride is available for compassionate use. Tegaserod was just reapproved by the FDA for the treatment of irritable bowel syndrome in females 65 years and younger without a history of ischemic CV disease. Finally, another oral 5-HT4 agonist prucalopride was also recently FDA-approved for chronic constipation and has been shown to improve symptoms and gastric emptying in patients with idiopathic gastroparesis in recent small pilot study [45].
The 5-HT1a receptor agonist buspirone can mediate relaxation of the proximal stomach and thus enhances the accommodation after a meal. This property led to detailed mechanistic studies in patients with functional dyspepsia who often complain about significant postprandial fullness and discomfort. A small proof-of-concept study indeed confirmed the improved accommodation, which correlated with delayed emptying and decreased symptoms [46]. Buspirone use has not been formally studied in patients with gastroparesis, but may be useful in patients with postprandial fullness or bloating.
Antidepressants
Psychiatric comorbidities are not only common in functional gastrointestinal disorders, but they also play an important mechanistic role through somatization and/or hypervigilance and catastrophizing [47, 48]. Low-dose tricyclic antidepressants have been used extensively in clinical practice to treat nausea, vomiting, and abdominal pain in patients with functional GI disorders and gastroparesis. While results vary, meta-analyses concluded that the use of antidepressants in these patients provide a significant benefit over placebo. Based on presumed neuromodulatory effects, Parkman and colleagues performed a large randomized trial with nortriptyline in idiopathic gastroparesis [12]. Unfortunately, there was no benefit in overall symptoms over placebo including for abdominal pain. A more recent study of patients with dyspepsia with or without delayed gastric emptying demonstrated a benefit of TCA and SSRI therapy over placebo [49]. Taken together, both studies show that agents with anticholinergic effects thought to further slow-down emptying are not worsening symptoms and may convey a benefit for pain in these patients through their central effects.
Mirtazapine is a tetracyclic antidepressant with central antiemetic effects that has been used to treat functional dyspepsia and gastroparesis. It has shown benefit in a randomized trial of patients with functional dyspepsia and in a small open-label pilot study in gastroparesis [50, 51].
Alternative and Complementary
Questions regarding complementary and alternative medicine are routinely encountered by providers in clinical practice. Patients often view these treatments as low risk and attractive. Unfortunately, there are few rigorous clinical trials that assess the efficacy of these treatments in a wide spectrum of disease states including gastroparesis. Acupuncture is the most studied alternative therapy in the treatment for gastroparesis, and a recent Cochrane review concluded that there is very low-certainty evidence for short-term benefit from acupuncture and that the reported benefits should be interpreted with caution [52]. Iberogast STW 5 is an herbal preparation containing nine extracts that has been shown to improve symptoms in patients with functional dyspepsia. The effects of STW 5 were tested in 103 patients with functional dyspepsia and gastroparesis in a multicenter, placebo-controlled crossover trial. They showed that patients treated with STW 5 had an overall improvement in gastrointestinal symptom scores without effect on gastric emptying [53]. Ginger (1 g daily), hypnosis, and cognitive behavioral therapy have been shown to be effective in chronic and/or postoperative nausea and vomiting, but have not been appropriately studied in patients with gastroparesis [54].
Summary
Gastroparesis is a chronic neuromuscular disorder that can be disabling with significant impact on patient’s quality of life. Medical management is the foundation of treatment for gastroparesis though admittedly present treatment options are limited. It is used initially in all patients, is the sole treatment in most patients, and is used in conjunction with other nonmedical therapies in the most severe patients. Gastroparesis is part of a spectrum of functional diseases, which include functional dyspepsia, chronic unexplained nausea and vomiting, and cyclic vomiting syndrome. The delay in emptying as a biomarker separates it from other functional illnesses of the stomach. However, the degree in emptying delay poorly correlates with symptoms, suggesting that other factors contribute to the clinical picture. Because of this, regulatory agencies and the pharmaceutical industry have moved to focus on overall improvement in symptoms and quality of life as a metric of treatment success rather than focusing on improvement in objective gastric emptying time. As there is only one FDA-approved medication (metoclopramide) for gastroparesis, medications that address gastroparesis pathophysiology and/or symptoms (i.e., functional dyspepsia, chemotherapy-induced nausea and vomiting, abdominal pain) are used off-label. Drug therapy should focus on the patient’s primary or most disabling symptoms. It is also important for the clinician to be aware of the alternative medication delivery routes in these patients who may have limitations with oral intake including use of disintegrating tablets and intranasal, liquid, and rectal formulations. Transdermal routes include patches, subcutaneous, intramuscular, and intravenous routes. Other pharmacologic agents, most notably ghrelin agonists, are currently in phase II and III testing and may become available in the near future. In addition, novel endoscopic and surgical therapies detailed in other chapters of this book explore alternative options that may benefit patients who fail to respond to dietary and medication management.
References
Koch KL, Hasler WL, Yates KP, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil. 2016;28:1001–15.
Franck-Larsson K, Hedenstrom H, Dahl R, et al. Delayed gastric emptying in patients with diffuse versus limited systemic sclerosis, unrelated to gastrointestinal symptoms and myoelectric gastric activity. Scand J Rheumatol. 2003;32:348–55.
Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–22.
Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464–70.
Janssen P, Scott Harris M, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382–91.
Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–91.
Zhao X, Mashimo H. Current and emerging medical therapies for gastroparesis. Curr Treat Options Gastroenterol. 2015;13:452–72.
Dudekula A, Rahim S, Bielefeldt K. Time trends in gastroparesis treatment. Dig Dis Sci. 2014;59:2656–65.
Camilleri M, McCallum RW, Tack J, et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology. 2017;153:1240–1250.e2.
Ejskjaer N, Wo JM, Esfandyari T, et al. A phase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e140–50.
McCallum RW, Lembo A, Esfandyari T, et al. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e705–17.
Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the norig randomized clinical trial. JAMA. 2013;310:2640–9.
Pasricha PJ, Yates KP, Nguyen L, et al. Outcomes and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology. 2015;149:1762–1774.e4.
Hunt JN, Stubbs DF. The volume and energy content of meals as determinants of gastric emptying. J Physiol. 1975;245:209–25.
Olausson EA, Storsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109:375–85.
Homko CJ, Duffy F, Friedenberg FK, et al. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2015;27:501–8.
Bielefeldt K. Factors influencing admission and outcomes in gastroparesis. Neurogastroenterol Motil. 2013;25:389–e294.
Bielefeldt K. Regional differences in healthcare delivery for gastroparesis. Dig Dis Sci. 2013;58(10):2789–98.
Jung H-K, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33.
Parkman HP, Yamada G, Van Natta ML, et al. Ethnic, racial, and sex differences in etiology, symptoms, treatment, and symptom outcomes of patients with gastroparesis. Clin Gastroenterol Hepatol. 2019;17(8):1489–1499.e8.
Simmons K, Parkman H. Granisetron transdermal system improves refractory nausea and vomiting in gastroparesis. Dig Dis Sci. 2014;59:1231–4.
Midani D, Parkman HP. Granisetron transdermal system for treatment of symptoms of gastroparesis: a prescription registry study. J Neurogastroenterol Motil. 2016;22:650.
Pasricha PJ, Yates KP, Sarosiek I, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology. 2018;154:65–76.e11.
Shaffer D, Butterfield M, Pamer C, et al. Tardive dyskinesia risks and metoclopramide use before and after U.S. market withdrawal of cisapride. J Am Pharm Assoc (2003). 2004;44:661–5.
Bielefeldt K. From harmful treatment to secondary gain: adverse event reporting in dyspepsia and gastroparesis. Dig Dis Sci. 2017;62:2999–3013.
Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil. 2014;26:521–8.
Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray reduces symptoms of gastroparesis in women, but not men, with diabetes: results of a phase 2B randomized study. Clin Gastroenterol Hepatol. 2015;13:1256–1263.e1.
Soykan I, Sarosiek I, McCallum RW. The effect of chronic oral domperidone therapy on gastrointestinal symptoms, gastric emptying, and quality of life in patients with gastroparesis. Am J Gastroenterol. 1997;92:976–80.
Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699–706.
Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–4.
Tomomasa T, Kuroume T, Arai H, et al. Erythromycin induces migrating motor complex in human gastrointestinal tract. Dig Dis Sci. 1986;31:157–61.
Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322:1028–31.
Richards R, Davenport K, McCallum R. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol. 1993;88:203–7.
Talley NJ, Verlinden M, Geenen DJ, et al. Effects of a motilin receptor agonist (ABT-229) on upper gastrointestinal symptoms in type 1 diabetes mellitus: a randomised, double blind, placebo controlled trial. Gut. 2001;49:395–401.
McCallum RW, Cynshi O, Team I. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis – a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;15:1121–30.
Russo A, Stevens JE, Giles N, et al. Effect of the motilin agonist KC 11458 on gastric emptying in diabetic gastroparesis. Aliment Pharmacol Ther. 2004;20:333–8.
Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45.
Ejskjaer N, Vestergaard ET, HellstrÖM PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–87.
Ejskjaer N, Dimcevski G, Wo J, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2010;22:1069–e281.
Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151:87–96.e6.
Taniyama K, Nakayama S, Takeda K, et al. Cisapride stimulates motility of the intestine via the 5-hydroxytryptamine receptors. J Pharmacol Exp Ther. 1991;258:1098–104.
Jian R, Ducrot F, Ruskone A, et al. Symptomatic, radionuclide and therapeutic assessment of chronic idiopathic dyspepsia. A double-blind placebo-controlled evaluation of cisapride. Dig Dis Sci. 1989;34:657–64.
de Caestecker JS, Ewing DJ, Tothill P, et al. Evaluation of oral cisapride and metoclopramide in diabetic autonomic neuropathy: an eight-week double-blind crossover study. Aliment Pharmacol Ther. 1989;3:69–81.
Testoni PA, Bagnolo F, Fanti L, et al. Long term oral cisapride improves interdigestive antroduodenal motility in dyspeptic patients. Gut. 1990;31:286–90.
Carbone F, Rotondo A, Andrews C, et al. A controlled cross-over trial shows benefit of Prucalopride for symptom control and gastric emptying enhancement in idiopathic gastroparesis. Gastroenterology. 2016;150:S213–4.
Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239–45.
Van Oudenhove L, Vandenberghe J, Geeraerts B, et al. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57:1666–73.
Van Oudenhove L, Vandenberghe J, Vos R, et al. Abuse history, depression, and somatization are associated with gastric sensitivity and gastric emptying in functional dyspepsia. Psychosom Med. 2011;73:648–55.
Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology. 2015;149:340–9.e2.
Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol. 2016;14:385–392.e4.
Malamood M, Roberts A, Kataria R, et al. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Devel Ther. 2017;11:1035–41.
Kim KH, Lee MS, Choi TY, et al. Acupuncture for symptomatic gastroparesis. Cochrane Database Syst Rev. 2018;12:Cd009676.
Braden B, Caspary W, Börner N, et al. Clinical effects of STW 5 (Iberogast®) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterol Motil. 2009;21:632–e25.
Chaiyakunapruk N, Kitikannakorn N, Nathisuwan S, et al. The efficacy of ginger for the prevention of postoperative nausea and vomiting: a meta-analysis. Am J Obstet Gynecol. 2006;194:95–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bielefeldt, K., McKenzie, P., Fang, J.C. (2020). Medical Management of Gastroparesis. In: Ibele, A., Gould, J. (eds) Gastroparesis. Springer, Cham. https://doi.org/10.1007/978-3-030-28929-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-28929-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28928-7
Online ISBN: 978-3-030-28929-4
eBook Packages: MedicineMedicine (R0)