Abstract
Introduction
While delayed emptying is the defining criterion for gastroparesis, prokinetics often only have a limited impact on symptoms and have been associated with potentially serious adverse effects. The goal of this study was to determine how this information and regulatory changes affected gastroparesis management.
Methods
The electronic medical records of patients seen between 2003 and 2012 in the outpatient clinic of a large tertiary center were retrieved based on the billing diagnosis of gastroparesis. Demographic, clinical, and survival data were abstracted.
Results
A total of 709 patients were identified, with diabetes (21.2 %) and prior surgery (9.8 %) being the most common identifiable causes. The majority of patients (56 %) had idiopathic gastroparesis. The cohort was female predominant (79.5 %) with an average age of 45.4 ± 0.6 years. At the index encounter, 61.8 % received prokinetics. About one-third (37.7 %) used antiemetics at least intermittently. Between 2003 and 2012, prokinetic use dropped from 81 to 43 %, while the use of antiemetics increased from 14 to 41 %. Similarly, there was a significant increase in prescribed opioids and antidepressants. During the period of the study, 44 patients (6.2 %) died. Increasing age, a higher comorbidity burden, anxiety, and medication use were associated with higher mortality risks.
Conclusion
This large outpatient cohort suggests that treatment trends move away from prokinetics and focus on symptom-oriented therapy and/or confounding mood disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyspeptic symptoms are common, affecting 5–10 % of adults living in the USA [1, 2]. Altered gastric emptying can be found in up to one-third of the individuals with chronic dyspeptic symptoms and is thought to contribute to symptoms [3, 4], leading to the diagnosis of gastroparesis in this subgroup of patients. Prokinetic agents thus play a key role in the current management of gastroparesis [5]. However, recent studies show a poor correlation between symptom severity and documented abnormalities in gastric emptying [6]. Moreover, several clinical trials failed to show superiority of prokinetics compared to placebo in patients with gastroparesis [7–9]. Similarly, Janssen and colleagues performed a meta-analysis of clinical trials in patients with gastroparesis and concluded that symptomatic improvement did not correlate with acceleration or normalization of the delayed gastric emptying [10]. In the USA, only metoclopramide and erythromycin are available as approved medications with prokinetic effects on gastric emptying. Common drug–drug interactions with possible pro-arrhythmogenic properties and potentially irreversible and devastating neurological side effects increasingly raise concerns in patients and physicians about the use of these agents. A recent study by Ehrenpreis et al. [11] showed a decrease in metoclopramide prescriptions following the introduction of a warning label by the Federal Drug Administration (FDA). Thus, the question arises as to what therapy do patients and physicians shift to.
As doubts about the relevance of the delay in gastric emptying as the best biomarker and treatment target surfaced, several studies demonstrated the importance of affect, with depression correlating with symptom severity or perhaps even playing a role as underlying mechanism [12–16]. We thus hypothesized that pharmacotherapy of gastroparesis changed in the last decade, shifting from a focus on emptying to a symptom-oriented management or treatment of coexisting affective spectrum disorders, defined by listed diagnoses of anxiety or depression. The primary goal of this study was to quantify the use of prokinetics, antiemetics, and antidepressants in a large outpatient sample of patients with gastroparesis. As secondary goal, we examined factors determining survival in this cohort. Lastly, we have recently demonstrated a correlation between nutritional support and poor outcomes [17]. We therefore separately analyzed the use of enteral nutrition and associated complications.
Methods
The study was designed as a retrospective analysis of patients seen in the outpatient clinic of the University of Pittsburgh Medical Center and had been approved by the institutional review board (PRO13030075). Patients seen at the Digestive Disorders Center between January 2003 and December 2012 were identified based on the billing code for gastroparesis. The electronic medical record was reviewed to abstract demographic information (age and sex), body mass index (BMI; only consistently available for index visits from May 2008 onward) data establishing the diagnosis and likely underlying etiology, comorbidities as listed in the medical record, current drug treatment at the time of the index visit, and survival status. Gastroparesis was operationally defined as established when confirmed by a gastric emptying test or wireless motility capsule test. As testing paradigms changed over time and evaluations were performed at different sites, the determination of an abnormal test result was based on the final assessment of the radiologist analyzing the study. If the diagnosis was based on the documented gastric retention of food after a prolonged fast, the diagnosis was considered to be probable gastroparesis [18]. Recorded comorbidities were used to calculate the Charlson Comorbidity Index [19]. A simple measure of health was extracted based on responses to a scale routinely used since 2008 to rate the overall quality of life using verbal descriptors excellent, very good, good, fair, or poor [20]. Considering the potential role of anxiety and depression in the development or subjective severity of gastroparesis, we separately noted their presence based on comorbidities listed in the electronic medical record. We extracted information about common functional disorders and chronic pain syndromes (fibromyalgia, irritable bowel syndrome, migraines, chronic back pain). We systematically determined the use of prokinetics (metoclopramide, erythromycin, domperidone, tegaserod, bethanechol). None of the patients received cisapride, which had been withdrawn from the market in the USA prior to the study period and is only available through special programs. In addition, we recorded the use of antiemetics (phenothiazine, ondansetron, granisetron, meclizine, scopolamine, dronabinol, aprepitant), antidepressants, benzodiazepines, and opioids. The drug treatment focused on active rather than prior therapies listed for the day of the index visit. If the index visit constituted a follow-up encounter, we abstracted age, BMI, and medication use for the time of this visit, but retrieved the initial encounter to obtain data on comorbid conditions and possible etiology of the gastroparesis. Patients receiving nutritional support with enteral or parenteral nutrition were separately assessed to determine the duration of therapy and potential complications.
In order to relate survival in the patient cohort to a reference population, we obtained crude death rates for Allegheny County in Pennsylvania, where the University of Pittsburgh is located. This information is publically available through the portal of the Department of Health (http://www.portal.state.pa.us). The data were normalized based on census records for the county.
Statistics
Descriptive information is presented as absolute frequencies and percentages for categorical variables, and as mean ± SEM for continuous data. Baseline characteristics for patients were compared between various subgroups with gastroparesis. We then compared dichotomous data using chi-square test statistic and analysis of variance for continuous variables with Sidak’s corrections for multiple comparisons. The Cochran–Armitage trend test was employed to assess temporal trends in the usage of opioids, antidepressants, prokinetics, and antiemetics. A value of Z > 0 denoted an increasing trend, while Z < 0 denoted a declining trend. The trend was considered significant when P was <0.05. The Kaplan–Meier method was used to estimate survival in patients across different categories. Differences between survival curves were analyzed by the log-rank test. Cox proportional hazards modeling was used to determine potential predictors of death. Initially, we performed a univariate Cox regression analysis using variables from baseline characteristics. Covariates with P values 0.2 or less on univariate testing were included in the multivariable Cox regression models based on an a priori decision rule. A Wald test-derived P value was reported for the overall variable. A P < 0.05 was considered significant. All results are reported by on two-tailed testing. Statistical analyses were performed with Stata version 12 (StataCorp, College Station, TX).
Results
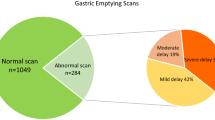
During the decade examined, a total of 1,031 individuals were seen at least once in the Digestive Disorders Center for dyspeptic symptoms with a billing code for gastroparesis. Out of this sample, 322 patients were excluded as they had normal gastric emptying studies (n = 100), did not meet the inclusion criteria for definite or probable gastroparesis due to a lack of confirmatory tests (n = 89), had chronic intestinal pseudo-obstruction (n = 25), cyclic vomiting syndrome (n = 19), a subtotal gastrectomy or gastric bypass surgery (n = 7), an eating disorder (n = 5), documented gastric outlet obstruction (n = 4), or a variety of other gastrointestinal disorders ranging from chronic pancreatitis to Crohn’s disease (n = 73). In the remaining 709 patients, a delay in gastric emptying was confirmed by an emptying study in 635 (89.4 %), endoscopic demonstration of retained food after at least 12 h of fasting (n = 73; 10.6 %) and wireless motility capsule in the remaining person. The index visit was the initial encounter in 81.4 %. Patients were evaluated by 37 different staff physicians with four providers accounting for 60.8 % of encounters. In patients with underlying conditions or disorders known to cause gastroparesis, confirmatory gastric emptying studies were less likely obtained compared to individuals with idiopathic or post-infectious syndromes (Table 1).
Baseline Characteristics
The entire cohort was female predominant (79.5 %) with a mean age around 45 years (Table 1). The Charlson Comorbidity Index shows a low burden of serious coexisting illnesses. However, about one-third (34.3 %) had previously been diagnosed with an affective spectrum disorder. Looking at other functional diseases (IBS, fibromyalgia and migraine), one-third of the cohort (n = 234; 33.0 %) reported at least one other illness with a prior diagnosis of irritable bowel syndrome (19.6 %), migraines (14.3 %), and/or fibromyalgia (7.9 %). Biometric data were available for about two-third of the cohort (Table 1). As shown in Fig. 1, 14.7 % had a BMI of less than 20, while nearly twice as many patients were obese or morbidly obese. In the majority of patients (56.0 %), no cause for the development of gastroparesis could be identified. Diabetes with secondary complications (21.2 %), prior surgery (9.8 %), or a post-infectious form of gastroparesis (5.9 %) were the most common etiologies of gastroparesis (Table 1). The remaining patients had scleroderma or related connective tissue disorders (n = 27), neurological diseases (n = 12), Parkinson’s disease (n = 4), inherited connective tissue disorders (n = 4), vascular abnormalities (n = 2), or a prior episode of acute pancreatitis (n = 1). When asked to rate their overall health, 257 of 448 (57.4 %) responding patients chose the descriptors fair or poor. As shown in Fig. 2, there were no changes in overall ratings during the time between 2008 and 2012.
Comparing the four major subgroups defined by etiology, patients with post-surgical gastroparesis were older (P < 0.01), those with post-infectious disease younger (P < 0.01) than the other groups (Table 1). While all subgroups showed a female predominance, there were relatively more men in patients with diabetic and post-surgical gastroparesis (P < 0.01). As all diabetic patients had secondary complications due to their disease, the comorbidity index was significantly higher compared to the rest of the group (P < 0.01). While confounded by age, post-infectious gastroparesis was associated with a lower, post-surgical disease with a higher burden of comorbid conditions (P < 0.05). The prevalence of affective spectrum disorders was similar across groups. Patients with idiopathic disease tended to have more functional syndromes associated with pain (P < 0.05).
Drug Treatment
While patients were routinely counseled on dietary management, instructions were neither standardized nor systematically recorded or captured. Focusing on pharmacotherapy, acid suppression with proton pump inhibitors was most commonly prescribed with 72.1 % of the patients receiving such an agent. At the time of the index visit, 438 patients (61.8 %) took at least one prokinetic drug; metoclopramide was used by 41.5 % followed by erythromycin (16.5 %), domperidone (8.2 %), and tegaserod (4.7 %), with the latter agent being only transiently available due to its withdrawal from the market (Table 1). Two patients had documented tardive dyskinesia, which had been attributed to the use of metoclopramide. One patient with idiopathic gastroparesis (female, 33 years old) had been treated in an outside institution for an unknown duration 7 years prior to the incident encounter. The second patient (female, 45 years old) suffered from diabetic gastroparesis and developed symptoms 26 months after intermittent treatment with metoclopramide with cumulative daily dosages between 20 and 40 mg; she was also started on aripiprazole during the same time period [21]. A total of 267 patients (37.7 %) had listed at least one antiemetic as part of their medical therapy with phenothiazines and/or ondansetron being taken by about one-fifth of the cohort. Other antiemetics, such as scopolamine, meclizine, dronabinol, aprepitant, or granisetron, were infrequently used (Table 1). Nearly half (46.6 %) of the cohort took antidepressants; about one quarter (25.8 %) used benzodiazepines and more than one-fifth (21.0 %) received prescription opioids (Table 1). Opioid users were significantly more likely to have one of the other diagnoses associated with pain (63.1 vs. 36.9 %; P < 0.01) or mood disorders (65.6 vs. 34.4 %; P < 0.01) compared to patients without prescription opioids. Patients taking opioids also more frequently received antidepressants (63.1 vs. 36.9 %; P < 0.01). Dividing the different subgroups based on etiology, patients with diabetic gastroparesis were significantly more likely to take opioids (P < 0.01), antidepressants (P < 0.01), and metoclopramide (P < 0.05; Table 1).
Nutritional Support
Nutritional support with enteral (n = 37; 5.2 %) or parenteral nutrition (n = 1) was relatively uncommon in this outpatient cohort. As four patients were only seen once, we focus our analysis on the remaining 33 individuals, all of whom received enteral nutrition via feeding tubes and were followed for at least 6 months (mean follow-up period: 5.6 ± 0.6 years). As was true for the entire cohort, patients being treated with enteral nutrition were primarily women (88 %) around 40 years of age (39.3 ± 2.4 years). Biometric data prior to initiation of nutritional support were available for 16 patients, who had a BMI of 22.7 ± 1.9. The underlying etiology for gastroparesis were diabetes with secondary complications in 10 patients (30 %), post-surgical gastroparesis in 6 (18 %), and idiopathic in 15 (45 %), with the remaining 2 patients having neurological illnesses with dysautonomia. Mood disorders had been diagnosed in 64 %, which was significantly higher than in the remaining cohort (33 %; P < 0.01).
Definitive feeding tube placement was performed after a trial with naso-duodenal feeding tubes in nine patients. Most of these feeding tubes were inserted surgically, either as direct jejunostomy (J) tubes (n = 17) or as transgastric J-tubes (n = 8). In the remaining eight patients, gastrostomy (G) tube placement with insertion of a J-tube through the G-tube had been performed endoscopically. Within the first 30 days after tube placement, there were six readmissions (18 %) due to tube-related complications (severe pain around the tube site: n = 2; tube dysfunction: n = 2; infection: n = 1; accidental tube removal: n = 1). A total of nine tube revisions (27 %) in eight patients were needed within this time period. Additional late complications developed in nine patients within the first 12 months (infection around the tube site: n = 4; gastric ulcer due to the internal bumper: n = 2; severe pain at the tube site: n = 2; tube-related jejunal perforation: n = 1). At 12 months after insertion, feeding tubes had been removed in 15 patients (46 %) with one additional person not relying on the tube any longer. A total of 14 persons with feeding tubes were followed for at least 24 months, with four patients having tubes removed during this time period; complications requiring therapy were recorded in two individuals (cellulitis around the tube site). In three of eight patients, the tube was removed during the third year, with three patients having significant tube-related complications (cellulitis: n = 2; need for surgical closure of an enlarging and continuously draining gastric fistula: n = 1). Two patients were switched to parenteral nutrition due to intolerance of enteral feedings; both suffered at least one bloodstream infection during the first year. At the end of the study, 29 of this subgroup were still alive with two patients still using enteral nutrition and one person relying on parenteral nutrition, amounting to a discontinuation rate of 90 %.
Gastric Electrical Stimulation
Gastric electrical stimulation is not a part of the treatment offered at the institution. Therefore, we could not systematically assess the impact of this intervention on patients with gastroparesis. A total of seven patients had previously undergone implantation of a stimulator and did not derive any benefit; an additional four individuals subsequently received stimulators in outside institutions (total 1.5 %), with one being unchanged, one reporting improvement, one dying within less than 12 months from cardiovascular complications, and one dying from complications of subsequent gastric surgeries, performed due to ongoing and worsening symptoms attributed to refractory gastroparesis.
Treatment Time Trends
While the use of proton pump inhibitors varied between years, there was no significant change during the study period (data not shown). In contrast, we saw changes in prokinetic drugs, which remained relatively stable around 80 % until 2007, when it dropped and fell below 50 % in 2011 (Fig. 3a). The Cochrane–Armitage trend test had a P value of <0.01 for declining trend during the study period. Tegaserod had been approved for clinical use as a prokinetic for constipation in 2002. Off-label use for gastroparesis started rapidly and reached 33.3 % in our cohort, before the agent was removed from the market in 2007. The introduction of tegaserod correlated with a decline in metoclopramide use, taken by more than half of the cohort in 2003; this decline continued after withdrawal of tegaserod and was down to 26.0 % in 2012 (Fig. 3b). The fraction of patients taking erythromycin or domperidone varied over time but did not show a clear trend (Fig. 3b). In contrast to prokinetics, the prescription of antiemetics more than doubled during the decade (Fig. 2a). This change is largely due to a significant increase in ondansetron use (Fig. 3c), which started after 2006, the year the FDA approved the first generic version of the drug. In addition to these changes in practice patterns, we observed a significant increase in opioid and antidepressant use (Fig. 3a). The Cochrane–Armitage trend test for use of antiemetics, antidepressants, and opiods had a P value of <0.01 for increasing trend during the study period. The relatively small number of individuals receiving nutritional support did not allow a meaningful analysis of time trends.
Survival
During the study period, 44 patients (6.2 %) died. Patient survival separated by etiology of gastroparesis is presented in Fig. 4. Compared to patients with idiopathic or post-infectious gastroparesis, the diagnosis of diabetic and post-surgical gastroparesis was associated with higher mortality rates (Fig. 4). Crude annual mortality rates for Allegheny County during the 10 year period of the study were between 1.1 and 1.2 %. Thus, the diagnosis of idiopathic or post-infectious gastroparesis did not adversely affect survival compared to the reference population. We next identified potential predictors of mortality using univariate analyses, which demonstrated effects of the underlying etiology of gastroparesis, comorbid conditions, and treatment (Table 2). Controlling for potential confounders, mortality was independently associated with opioid use, coexisting anxiety, and higher Charlson’s Comorbidity Index. Conversely, PPI and benzodiazepine use correlated with lower risk of death (Table 3).
Discussion
This large cohort study of outpatients with gastroparesis has several important key messages. First, even though patients were seen in a tertiary referral center and thus likely represent a skewed population, the overall survival rate did not differ from the observed survival of a reference population. The observed deaths were largely due to complications of comorbid conditions, which are rare in patients with idiopathic gastroparesis, the predominant group in this and other large patient samples [18, 22, 23]. Second, treatment often targeted the underlying delay in gastric emptying. However, the use of prokinetics decreased within the last decade with a corresponding shift to symptom-oriented management. Third, opioid use was common with about one-fifth of the cohort at least intermittently receiving prescription opioids. Fourth, more aggressive interventions, most notably the use of enteral nutrition, were associated with high complication rates and were ultimately discontinued in the majority of patients.
Data on mortality in gastroparesis vary considerably and range from 2 to 38 % [22, 24–27]. Our findings are similar to those reported by the only published epidemiological study of patients with gastroparesis conducted in Olmsted County [18]. The excess mortality reported by Jung and coworkers was largely due to comorbid conditions seen in individuals with underlying diabetes mellitus. Consistent with these findings, patients with diabetic or post-surgical gastroparesis had a significantly higher risk of death compared to individuals with idiopathic or post-infectious gastroparesis, which did not adversely affect survival. These findings are important, as they should guide therapy and raise the threshold for interventions associated with morbidity or even mortality.
Medical treatment reflected common clinical practice and resembled data from a recently published multicenter trial with acid suppression, antiemetics, and prokinetics playing important roles in the management of gastroparesis [13]. Consistent with a report from Ehrenpreis [11], we noted a shift away from prokinetics, most notably metoclopramide. The emerging picture is complex and likely influenced by a variety of factors. The prescription of metoclopramide transiently dropped with the introduction of tegaserod, with apparent off-label use as less than 40 % of the patients receiving this medication had been diagnosed with irritable bowel syndrome or constipation (data not shown). After the FDA required a black box warning for metoclopramide in 2009, the fraction of patients on this agent dropped by more than 50 %. In contrast to Ehrenpreis, we did not see a shift to alternative prokinetic drugs [11]. The study design does not allow clear conclusions about the underlying reasons. However, the rapid rise of tegaserod use after its introduction in the USA suggests that the availability of presumably safe and potentially effective agents affected treatment decisions. Interestingly, there was, however, a parallel move toward symptom-oriented therapy with a significant increase in antiemetic use, largely driven by a steep rise in prescriptions for ondansetron, which had become available in a cheaper generic version in 2007. Despite these significant shifts in therapy, simple measures of health-related quality of life did not change.
It is unclear whether the focus on symptoms also led to the increased number of patients taking opioids. While the fraction of patients receiving prescription opioids was lower in our cohort than previously reported by others [13], it still reached about 20 %. Pain is common in gastroparesis and typically does not improve with dietary or other medical interventions [28, 29]. Thus, opioid use has previously been described by others [30, 31] and fits a national trend of rising opioid use for benign conditions, including those associated with chronic abdominal pain [32, 33]. We also saw an increase in the fraction of prescription opioid users between 2003 and 2012, which corresponds with a nationwide trend [34]. While we did not record pain prevalence in our cohort, our findings argue against gastroparesis being the only or even primary target of pain management, as coexisting pain syndromes and psychiatric problems significantly contributed to chronic opioid use in our cohort.
Chronic opioid use in benign disorders is controversial considering concerns about abuse and adverse effects as well as limited evidence of clinically relevant functional improvements [35–37]. Our data point at another concerning aspect, an increased mortality, which has previously been reported in a longitudinal study of patients with Crohn’s disease [38]. The negative predictive power remained significant after controlling for potential confounders, pointing at an inherent risk of opioids. Prior studies have clearly demonstrated a correlation between opioid prescriptions, drug abuse, emergency medical encounters, and death related to unintentional overdoses [39, 40]. The risk of adverse events is dose dependent [41], a factor that was not assessed in our cohort. Our study also did not systematically capture risk factors for substance abuse. However, mood disorders and concomitant use of benzodiazepines, both markers of higher substance abuse potential [34], were more commonly seen in opioid users. Considering the rising concerns about the negative impact of opioid use, these data should prompt providers to review the appropriateness and dosing of these potent analgesics [42]. Interestingly, meclizine was also associated with a higher likelihood of death. The agent is widely available in the USA as “over the counter” preparation with an acceptable safety profile. Prior studies have demonstrated a sedating effect with minor cognitive impairment [43]. The small number of patients using this medication does not enable us to further assess the relevance of this effect or other potential confounders. Conversely, proton pump inhibitors and benzodiazepine use seemed to have a protective effect in our study. The apparently beneficial effect of benzodiazepines is difficult to reconcile with large epidemiological studies showing increased mortality in individuals using sedating agents [44]. While proton pump inhibitors decrease the risk of gastrointestinal bleeding in patients on aspirin or non-steroidal anti-inflammatory drugs [45], we did not identify such complications as a common problem in our cohort. Thus, the observed impact of opioids is consistent with a growing body of the literature as mentioned above. The effects of other agents are statistically significant, but cannot easily be explained in terms of underlying mechanisms. As published data are limited or even point in the opposite direction, we will need to wait for prospective trials or use case–control studies with appropriately balanced confounders to assess the true relevance of these findings.
One additional goal was the assessment of more aggressive interventions, most notably nutritional support. Using administrative data sources, we recently reported that placement of feeding tubes or institution of nutritional support was associated with worsening outcomes in patients hospitalized for gastroparesis [17, 46]. Relatively little is known about the chronic use of nutritional support in large patient groups with gastroparesis. Soykan et al. [22] reported that 21 % of their patients at least transiently received some form of nutritional support. Rates were lower in a large multicenter study of idiopathic gastroparesis, but still reached 11 % of the recruited patients [47]. Similarly, about 10 % of patients undergoing implantation of a gastric electrical stimulator have undergone feeding tube insertion prior to this surgery [24]. Thus, our numbers are lower with only about 5 %, but are likely less skewed as patients were not exclusively seen by specialists with a focus on functional gastrointestinal disorders. Consistent with our findings based on the Nationwide Inpatient Sample [17], we observed a high rate of complications with a nearly 20 % readmission rate within 30 days after placement and with a total complication rate of 34 % within the first year. These results are consistent with prior reports that even included procedure-related mortality [26, 48, 49]. Interestingly, tube removal and maintenance of oral nutrition was eventually possible in about 90 % of the cases, which matches prior reports about the decreasing need for nutritional support, which in that series was attributed to benefits of gastric electrical stimulation [27]. Thus, the findings obtained in our cohort and the correlation between nutritional support and worsening outcomes both point at potential harm, which is important to consider in view of the overall lack of any excess mortality of the disorder per se.
In addition to these key findings, our results show that patients with gastroparesis reflect an admittedly female-predominant cross section of the general population and thus include an increasing fraction of obese individuals, which has recently been reported by others as well [50]. As is true for other functional illnesses, gastroparesis is commonly associated with other functional and affective spectrum disorders, which may also contribute to the high number of persons using antidepressants and anxiolytics [47, 51].
While this is the largest cohort with gastroparesis described, the study comes with several important limitations. The retrospective nature makes it impossible to use standardized diagnostic criteria for the primary disease, symptom severity, and comorbid conditions. We required evidence of delayed gastric emptying and used previously established criteria to identify definite and probable gastroparesis [18]. Even though nearly 90 % of the patients had undergone a gastric emptying study, the tests were not uniformly performed, as approaches varied between imaging centers and over time. The results were obtained in a single tertiary referral center and may thus not be representative for patients seen in rural areas, different regions of the country, or in general practice. However, we focused on outpatients to avoid the inherent bias associated with selection of patients who are either more severely ill and/or higher resource utilizers. Overall, key demographic and clinical data are consistent with several prior reports from different areas of the country [13, 18, 22, 52]. In addition, the high number of physicians involved in their care, the relatively low rate of more aggressive interventions, and the low mortality argue against significant skewing due to tertiary referral bias. Dietary management was often emphasized in the medical record, but also not standardized or routinely described, not allowing us to see whether the apparent shift in pharmacotherapy was also associated with an increasing emphasis of dietary treatment. Similarly, we were only able to extract the BMI as routine measure for a subset of patients. Micronutrient assessments were not consistently obtained, thus not enabling us to see how and when deficiencies played a role in treatment decisions, such as the institution of nutritional support.
In conclusion, treatment of gastroparesis is changing. The limited correlation between symptoms and gastric emptying delay and increasing concerns about adverse drug effects have led to shift away from prokinetics to symptom-oriented and dietary treatment. While the disease can be a significant burden for the affected patients, profoundly affecting the quality of life, gastroparesis by itself does not carry a relevant mortality. This fact stands in contrast to the risk of more aggressive interventions, which often lead to complications, may only be used transiently and should thus be avoided whenever possible.
References
Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580.
Olafsdottir LB, Gudjonsson H, Jonsdottir HH, et al. Natural history of functional gastrointestinal disorders: comparison of two longitudinal population-based studies. Dig Liver Dis. 2012;44:211–217.
Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783–788.
Talley NJ, Locke GR III, Lahr BD, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933–939.
Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37.
Sachdeva P, Malhotra N, Pathikonda M, et al. Gastric emptying of solids and liquids for evaluation for gastroparesis. Dig Dis Sci. 2011;56:1138–1146.
McCallum RW, Cynshi O, Team I. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis—a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;15:1121–1130.
Talley NJ, Verlinden M, Geenen DJ, et al. Effects of a motilin receptor agonist (ABT-229) on upper gastrointestinal symptoms in type 1 diabetes mellitus: a randomised, double blind, placebo controlled trial. Gut. 2001;49:395–401.
McCallum RW, Lembo A, Esfandyari T, et al. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e705–e717.
Janssen P, Scott Harris M, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382–1391.
Ehrenpreis ED, Deepak P, Sifuentes H, et al. The metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuits. Am J Gastroenterol. 2013;108:866–872.
Bielefeldt K, Raza N, Zickmund SL. Different faces of gastroparesis. World J Gastroenterol. 2009;15:6052–6060.
Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1056–1064.
Van Oudenhove L, Vandenberghe J, Geeraerts B, et al. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57:1666–1673.
Van Oudenhove L, Vandenberghe J, Vos R, et al. Abuse history, depression, and somatization are associated with gastric sensitivity and gastric emptying in functional dyspepsia. Psychosom Med. 2011;73:648–655.
Clauwaert N, Jones MP, Holvoet L, et al. Associations between gastric sensorimotor function, depression, somatization, and symptom-based subgroups in functional gastroduodenal disorders: are all symptoms equal? Neurogastroenterol Motil. 2012;24:1088-e565.
Bielefeldt K. Factors influencing admission and outcomes in gastroparesis. Neurogastroenterol Motil. 2013;25:389-e294.
Jung H-K, Choung RS, Locke GR III, et al. the incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, From 1996 to 2006. Gastroenterology. 2009;136:1225–1233.
Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
Lackner JM, Gudleski GD, Zack MM, et al. Measuring health-related quality of life in patients with irritable bowel syndrome: can less be more? Psychosom Med. 2006;68:312–320.
Brown R, Taylor M, Geddes J. Aripiprazole alone or in combination for acute mania. Cochrane Database Syst Rev. 2013;12:CD005000.
Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404.
Hasler WL, Parkman HP, Wilson LA, et al. Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol. 2010;105:2357–2367.
Keller D, Parkman H, Boucek D, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620–626.
Chang J, Rayner CK, Jones KL, et al. Prognosis of diabetic gastroparesis—a 25-year evaluation. Diabet Med. 2013;30:e185–e188.
Fontana R, Barnett J. Jejunostomy tube placement in refractory diabetic gastroparesis: a retrospective review. Am J Gastroenterol. 1996;91:2174–2178.
McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314.e1–319.e1.
Cherian D, Sachdeva P, Fisher RS, et al. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676–681.
Olausson EA, Storsrud S, Grundin H, et al. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109:375–385.
Uppalapati SS, Ramzan Z, Fisher RS, et al. Factors contributing to hospitalization for gastroparesis exacerbations. Dig Dis Sci. 2009;54:2404–2409.
Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078.
Boudreau D, Von Korff M, Rutter C, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175.
Dorn SD, Meek PD, Shah ND. Increasing frequency of opioid prescriptions for chronic abdominal pain in US outpatient clinics. Clin Gastroenterol Hepatol. 2011;9:1078.e1–1085.e1.
Olfson M, Wang S, Iza M, et al. National trends in the office-based prescription of schedule II opioids. J Clin Psychiatry. 2013;74:932–939.
Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47.
Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459.
Eriksen J, Sjøgren P, Bruera E, et al. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125:172–179.
Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT[trade] registry. Am J Gastroenterol. 2012;107:1409–1422.
Wisniewski A, Purdy C, Blondell R. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis. 2008;27:1–11.
Braden JB, Russo J, Fan MY, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–1432.
Bohnert AB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321.
Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–ES38.
Paul M, MacLellan M, Gray G. Motion-sickness medications for aircrew: impact on psychomotor performance. Aviat Space Environ Med. 2005;76:560–565.
Weich S, Pearce H, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. Br Med J. 2014;348:g1996.
Iwamoto J, Saito Y, Honda A, et al. Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy. World J Gastroenterol. 2013;19:1673–1682.
Bielefeldt K. Regional differences in healthcare delivery for gastroparesis. Dig Dis Sci. 2013;58:2789–2798. doi:10.1007/s10620-013-2643-8.
Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101.e10–115.e10.
Maple JT, Petersen BT, Baron TH, et al. Direct percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attempts. Am J Gastroenterol. 2005;100:2681–2688.
Poulose B, Kaiser J, Beck W, et al. Disease-based mortality after percutaneous endoscopic gastrostomy: utility of the enterprise data warehouse. Surg Endosc. 2013;1–5.
Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585.
Whitehead W, Palsson O, Jones K. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156.
Coleski R, Anderson M, Hasler W. Factors associated with symptom response to pyloric injection of botulinum toxin in a large series of gastroparesis patients. Dig Dis Sci. 2009;54:2634–2642.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dudekula, A., Rahim, S. & Bielefeldt, K. Time Trends in Gastroparesis Treatment. Dig Dis Sci 59, 2656–2665 (2014). https://doi.org/10.1007/s10620-014-3369-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3369-y