Abstract
Recalcitrant wounds pose a challenge to the dermatologist. In recent years, many skin substitutes have been developed and are broadly classified as either acellular or cellular. These skin substitutes are to be used in concert with standard of care to provide the stalled wound with a scaffold and key elements such as cytokines, growth factors, and extracellular matrix substances. Skin substitutes help initiate and accelerate wound healing through granulation, cell migration, re-vascularization, and re-epithelialization. Wounds of varying etiologies have been shown to benefit from the multitude of acellular and cellular skin substitutes that are available. This chapter provides clinically relevant background and practical guidance about skin substitutes to allow dermatologists to effectively incorporate these powerful tools into their wound healing armamentarium.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Skin substitute
- Wound healing
- Acellular matrix
- Cellular matrix
- Epidermal skin graft
- Dermal skin graft
- Composite graft
- Venous leg ulcer
- Diabetic foot ulcer
Introduction
Wounds are the most common skin disease. Therefore, dermatologists should develop a thorough understanding of the etiology, natural history, principles of diagnosis, and treatment of wounds. However, even under optimal expert care, some wounds do not heal at the appropriate rate. Fortunately, advanced wound therapy with skin substitutes and matrices can often correct the healing trajectory of a stalled wound. Therefore, dermatologists should be familiar with these products and develop basic understanding of the classes available, the way they are applied, and how they work since they are simple to use and often effective.
Historically, the first written account of skin substitutes occurred in the fifteenth century BCE in Ebers Papyrus, where frog skin was used as a xenograft [1]. Skin allografts were first reported 3000 years later in writings from the Branca family of Sicily in the first half of the fifteenth century [2]. In the early 1900s, amnion began to be used as a biological dressing for the management of burns [3]. Over the past 40 years, there have been breakthroughs in the ability to bioengineer tissue substitutes leading to a vast array of products. The first major breakthrough was in 1975 when Rheinwald and Green cultivated keratinocyte sheets from epidermal cells allowing for the production of large quantities of keratinocytes in vitro [4]. In 1981, the first artificial skin bilayer was used to treat burn wounds, and it was found that the host tissue utilized the bilayer to synthesize neoepidermis and neodermis [5, 6]. This paved the way for the first commercially available epithelial autografts in 1988 with the development of Epicel®, a cultured epidermal autograft. Since then, a multitude of matrices have been made available that allows the clinician to tailor to the unique needs of each clinical encounter.
Acellular and cellular matrices, also referred to as skin substitutes, are a form of advanced wound care utilized when standard wound care fails to heal a wound. Wound recalcitrance is often due to the “edge effect” whereby the epithelium fails to migrate across the granulation tissue. Utilization of skin substitutes may be used to overcome the edge effect by providing structural support for migration, tissue regeneration, growth factors, and cytokines and via restoration of the biochemical and moisture balance within the nonhealing wound [7]. Additionally, evidence suggests that skin substitutes revert the wound’s inflammatory environment back to an acute healing phenotype that promotes wound healing [5, 8]. Interestingly, these engineered tissues do not persist in the wound and are replaced by the patient’s tissues. These skin substitutes afford the clinician the ability to cover larger areas than what is usually allowed with a traditional skin graft [9]. Furthermore, skin substitutes obviate the need for autografts, thus avoiding a surgical procedure, painful surgical donor site, and additional scarring, which is often the most distressing aspect of a traditional skin graft [10].
There are multiple ways to classify skin substitutes. Most broadly, they can be classified as acellular or cellular skin equivalents [11]. Acellular matrices functionally act as a scaffold by transiently functioning as an extracellular matrix (ECM), which promotes host cellular migration leading to wound healing via replacement of the skin equivalent with endogenous host tissue. They may be biologically active or inert and are produced from natural sources, manufactured, or from a combination of both. On the other hand, cellular matrices contain functional cells that are embedded into an ECM. These cells are capable of secreting cytokines, growth factors, collagen, fibronectin, and glycosaminoglycans that promote angiogenesis, granulation, and re-epithelialization [12]. Generally, acellular matrices are less expensive and easier to produce, apply, and store [9].
Skin substitutes can be further divided into epidermal grafts, dermal replacements, and composite grafts. Epidermal grafts consist of autologous cultured keratinocytes, while dermal grafts consist of cellular or acellular dermal components, mainly collagen. Composite grafts are bilayered skin equivalents and consist of an epidermal layer of keratinocytes or synthetic material on top of a dermal layer. This categorization can be further divided into allogeneic, xenogeneic, or autologous grafts.
Characteristics of an ideal tissue-engineered skin substitute [11, 13, 14]:
-
Allow for endogenous cell adherence and migration
-
Nontoxic
-
Non-inflammatory
-
Non-immunogenic
-
Cost-effective
-
Widely available
-
Stored at room temperature
-
Prolonged shelf life
-
Durable
-
Malleable
-
Biodegradable
-
Prevent water loss
-
Provide coverage for unique wound characteristics including location, depth, and underlying etiology
Although no single skin equivalent meets all of these characteristics, the numerous products available afford the clinician the ability to tailor treatment to the unique clinical picture.
Principles of Selection and Use of Skin Equivalents
Skin substitutes should be applied according to manufacturer’s instructions, and typically the company can readily provide technical support. Since a myriad of products are available, each with some unique features and subtleties, an overview of the principles of selection and applications is provided.
Assessment and preparation of the wound bed is the essential first step in preparing for advanced wound therapy. This is first accomplished by a thorough history and physical examination to determine the etiology of the wound. Any underlying causes for the delayed healing such as immunosuppression, poor nutrition, infection, or systemic illness should be addressed prior to initiation of advanced wound therapy [15].
The wound should then be inspected and measured for wound size, depth, color, undermining, edema, erythema, and exudate. Often, chronic wounds contain necrotic tissue and biofilms that impede healing and should be debrided. Wound edge should be assessed, and undermined tissue should be removed to allow for re-epithelialization. Similarly, debridement of callus is essential for relieving pressure. The wound surface should be cleansed of contaminants, bacteria, and remnants of previous dressings. If erythema, tenderness, and warmth are noted at the wound, infection should be suspected, and initiation of topical or systemic antimicrobials is essential. Moisture balance should be achieved with proper dressing choice to manage exudate and avoid excessive dryness or maceration at the skin edges [16]. The importance of meticulous wound bed preparation cannot be understated as healthy granulation tissue is crucial for the success of skin substitutes’ application [17, 18].
Typically, initiating treatment with advanced wound therapies should be considered when a wound fails to heal for at least a few weeks with appropriate standard wound care (i.e., multilayered compression for venous leg ulcers [VLUs] or offloading for diabetic foot ulcers [DFUs]) [9]. Contraindications to placing a skin substitute include wound infection; exposed muscle, tendon, or bone; or hypersensitivities to the matrix [19].
Following wound bed preparation, accurate measurements of the wound should be recorded to obtain a baseline by which the clinician can monitor the healing response to the skin substitute. Additionally, these measurements will help the clinician select and prepare the matrix to properly fit the wound. Careful preparation must be taken to ensure proper placement of the product as some come ready to be placed onto the wound, while others must be fenestrated to allow for exudate to permeate through the matrix [20]. Matrices that contain only one layer need not take into account orientation, while the bilayered composite grafts need to be carefully placed to ensure proper orientation [21]. Skin substitutes that are dehydrated require rehydration, and others matrices that are cryopreserved must be thawed with care according to the manufacturer’s instructions. Flowable matrices that are injected into wounds are usually hydrated before use [22]. The applied product should be secured with a preferred method such as Steri-Strips® or suture. Following placement of the matrix, application of a non-adherent contact layer helps to secure the product in place and protect during secondary dressing changes. A secondary dressing can then be placed to maintain moisture balance. Throughout these steps, aseptic technique should be strictly maintained. Once application is complete, standard of care should be completed as appropriate. Increase in exudate is common after application of skin substitutes, and the patient should be advised, and proper secondary dressing should be provided. However, the clinician should be vigilant for signs of infection.
Generally, it is advised that the primary dressing should not be disturbed for a week following placement of skin substitutes. However, clinicians should be familiar with the specific post-care instructions of each product as those may differ. Once the allotted time has passed, the clinician should remove the primary dressing and thoroughly inspect the wound for evidence of healing such as advancing wound edge. If unhealed, many of the wounds will retain some residue of the skin substitute in the wound, and it is generally advised to not remove it. In this case, necrotic tissue can be removed selectively, and new primary non-adherent dressing can be applied followed by standard of care. If the clinician is still concerned about the healing progress, reapplication may be considered. Guidelines for reapplication are generally derived from pivotal trials that classically utilize weekly applications. However, evidence on the most adventitious timing for reapplication is lacking.
Cellular Matrices (Table 15.1)
Cultured Epidermal Autografts (CEA, Epicel®, Vericel, Cambridge, MA)
Indications: deep dermal or full-thickness burns greater than or equal to 30% of total body surface area [40]
The in vitro capability of skin stem cells to expand has been leveraged to create an autologous skin graft from a biopsy taken from the patient [41]. In order to obtain autologous keratinocytes for culture, two 6 cm × 2 cm full-thickness biopsies are taken within 24–48 hours of admission from the axilla and/or groin. These biopsies are placed into biopsy media tubes provided by the manufacturer and sent to Epicel® for ex vivo expansion. The grafts mature over approximately 17 days to create 4800 cm2 sheets of keratinocytes that are 2–8 cell layers thick. This process allows expansion of a relatively small donor site into a graft that can cover a large body surface area. Additionally, if the use of the graft is not immediately necessary, the cultured autograft may be cryopreserved for future use [42].
Once the expanded cultured autograft is obtained, the graft should be arranged with the cell sheet facing down to preserve the basal-apical orientation of the keratinocyte sheets. The grafts should be placed as close together without overlap as possible and then stapled in place. Importantly, the graft material should not be stapled until the sheets are providing full coverage over the wound in case rearrangement is necessary. Once in place, the grafts should be covered with a primary nylon dressing and then an outer secondary dressing.
The disadvantages of CEAs include the high cost of the graft, sensitivity of the keratinocyte sheets to infection due to breakdown from bacterial proteases and cytotoxins, variable graft take rate, and the length of time it takes to culture and produce the epidermal autograft [43]. Some labs have reported using fibrin glue to decrease the culturing time to approximately 14 days. However, this has not been widely available [44]. The initial optimism for CEA has been hampered by reports of poor results from surgeons and various burn centers throughout the country [45]. Currently, its use is limited to the initial closing of the wound, but not for permanent closure [46, 47].
Similar products have recently been FDA approved such as RECELL®, which is a CEA spray [48]. There are many CEA products outside of the United States such as Celaderm, Laserskin, Autoderm, TransDerm, Myskin, Epidex, Lyphoderm, and Cryoceal, but they are not currently approved for use in the United States [49]. The level of evidence for the use and efficacy of CEAs is limited to smaller trials and case studies [44].
Bilayered Living Cellular Constructs (BLCC, Apligraf®, Organogenesis, Canton, MA, and OrCel, Ortec International, Atlanta, GA)
Indications: noninfected partial- and full-thickness VLUs that remain unhealed for greater than 1-month duration and full-thickness neuropathic DFUs that remain unhealed for greater than 3 weeks duration. In both cases, there should be no exposed tendon, muscle, or bone. Its successful use has also been reported in partial- and full-thickness burns, epidermolysis bullosa, surgical excisions, and pyoderma gangrenosum [20, 50,51,52,53].
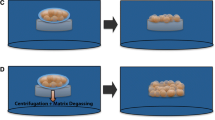
BLCCs are tissue-engineered composite skin equivalents that have been shown to decrease wound healing time [54]. Both Apligraf® and OrCel® contain epidermis from human neonatal foreskin on top of bovine collagen [55]. Thus, it possesses both allographic and xenographic features. Of the two, Apligraf® (Fig. 15.1) is the best trialed and consists of allogeneic neonatal keratinocytes and fibroblasts derived from neonatal foreskin on top of bovine type I collagen [50, 52,53,54,55]. Since it contains both keratinocytes and fibroblast, it allows for cross talk between the different cell types. Additionally, this graft can produce its own matrix proteins and growth factors. During engineering of the Apligraf®, the epidermal layer is exposed to air, allowing the keratinocytes to stratify and create a stratum corneum [56]. Apligraf® remains viable at room temperature for 10 days from the date of shipping.
Apligraf®, a BLCC, is shown to the left. It is important for the clinician to be prepared before graft placement as the BLCC is delicate. The easiest method to apply the Apligraf® is to place a gauze over the top of the matrix, which is contained in the center circle of the petri dish (black arrow). Wet the gauze with a few drops of saline to allow the membrane to adhere to the gauze. Then gently peel back the matrix and gauze together. If needed, fenestrate the membrane with a blade and cut to size while still attached to the gauze. Then place the matrix on the wound with the gauze side facing up so as to maintain the polarity of the matrix. The matrix can be gently separated from the gauze using a cotton-tipped applicator. It can then be affixed in place with Steri-Strips ™, a noncontact primary layer and a secondary dressing
After the wound bed is prepared, Apligraf® should be fenestrated with a blade. Fenestration allows the product to remain affixed in case of wound exudate. Care should be taken to maintain the orientation of the product such that the dermal layer is in contact with the wound bed. It should then be affixed with a preferred method such as wound glue or Steri-Strips™ at the periphery. The graft should then be covered with a primary non-adherent dressing and then covered with a secondary dressing. The wound should be inspected and redressed within 1 week [20].
OrCel® is comprised of keratinocytes derived from neonatal foreskin cross-linked to a bovine type I collagen sponge (epidermal side) that contains human dermal fibroblasts on the opposite side of the sponge (dermal side). This composite graft also produces necessary growth factors and matrix proteins. One of the benefits of OrCel® is that it can last for up to 9 months due to cryopreservation. OrCel® should be applied in a similar fashion as the Apligraf® [49].
These composite grafts promote fibrovascular ingrowth and re-epithelialization. Studies have demonstrated a lack of cultured cell DNA when the wound heals suggesting that it is the patient’s endogenous skin that heals the wound and the composite graft biodegrades [40]. The main disadvantage of the BLCCs is their high cost [57]. Contraindications to use include infected wounds or if the patient has known hypersensitivities to bovine collagen or components of the shipping medium [20].
Collagen Dermal Matrix (CDM, Dermagraft®, Organogenesis, Canton, MA TransCyte, Advanced BioHealing Inc., Westport, CT)
Indications: The best evidence is for Dermagraft®, and it has been approved for the treatment of full-thickness neuropathic DFUs that have failed to heal for more than 6 weeks without involvement of the tendon, muscle, bone, or joint capsule [29]. It has also been trialed, albeit with less success, in the treatment of VLUs and burns [58, 59]. TransCyte has been FDA approved for partial- and full-thickness burn wounds [31].
Dermagraft® is a CDM that is a bioabsorbable cryopreserved human fibroblast-derived dermal substitute. The dermal matrix is synthesized by culturing neonatal fibroblasts in a glycolic acid mesh. This mesh serves as a scaffold for the production of cytokines, growth factors, matrix proteins, and collagen into a three-dimensional matrix [29]. Aside from fibroblasts, Dermagraft® does not contain other skin cells like keratinocytes, endothelial cells, hair follicles, or white blood cells. When placed into a wound, the CDM stimulates fibrovascular growth and re-epithelialization [40].
Dermagraft® comes in a clear bag containing a single piece of 2 inch × 3 inch CDM. The product should be maintained at a temperature of −75 °C ± 10 °C. Additionally, Dermagraft® should not be kept at room temperature for more than 30 minutes. Dermagraft® should be removed from the foil packaging but kept in the clear packaging and submerged into a 34–37 °C water bath to thaw for 2 minutes. The clear bag should then be cut open, liquid should be removed, and the product should be rinsed three times with room temperature saline until ready for use. Once ready, the saline should be poured out, and the clear bag should be closed. The clear bag should then be placed over the ulcer, and a marker should be used to trace the ulcer. The bag should then be cut along the tracing, and CDM should be placed in the ulcer. After placement, it should be secured with a preferred method. It should then be covered with a non-adhesive primary dressing and then with a secondary dressing. The ulcer site should not be disturbed for 72 hours after placement of the CDM. Dermagraft® is contraindicated in infected wounds, wounds with sinus tracts, and patients that have hypersensitivity to bovine products [21].
TransCyte is synthesized from human newborn fibroblasts cultured onto a nylon mesh of Biobrane® (Dow B. Hickam, Inc., Sugarland, Tex). It is similar to Dermagraft®; however, the fibroblasts are not viable. It is prepared and applied in a similar fashion as Dermagraft®.
Dehydrated Human Amnion/Chorion Membrane (DHACM, Epifix®, MiMedx Group Inc., Marietta, GA)
Indications: VLUs , chronic vascular ulcers, DFUs, partial- and full-thickness wounds, pressure ulcers (PUs), trauma wounds, surgical wounds, and third-degree burns [28, 33, 36, 60,61,62]
Epifix® is an allographic cellular matrix that is composed of human amnion and chorion matrix. It contains a single layer of epithelial cells, basement membrane, and an avascular connective tissue matrix. This product is bioengineered in such a way that it removes blood products but leaves intact the amniotic membrane and extracellular matrix (ECM). As a result, the product contains cytokines, growth factors, and ECM proteins [36].
Epifix® can be stored in a clean, dry environment at room temperature for up to 5 years. Multiple sizes are available in sheets or mesh from 2 cm2 up to 49 cm2 allowing the clinician to meet the needs of a specific clinical scenario.
After the wound bed is prepared , remove the DHACM from the packaging and carefully cut to the size of the wound allowing no more than 1 mm of overlap of the wound margins. When placing the DHACM, use the embossed letters as a guide to maintain correct orientation. If the wound is exudative, the product can be fenestrated. Additionally, the product can be wet or dry depending on the clinical picture. To wet the DHACM, apply sterile saline after it has been placed in the wound. Place a non-adherent primary dressing followed by a secondary dressing and do not disturb the wound site for several days [63]. Epifix® is contraindicated in infected wounds [64]. The company also bioengineers other DHACM products such as AminoFix® (injectable DHACM) and EpiBurn® (DHACM for burn wounds) as well as other products derived from placental tissue and amniotic fluid. However, the evidence and efficacy of these products have not been extensively studied.
Cryopreserved Placental Membrane (CPM, Grafix®, Osiris Therapeutics, Inc., Columbia, MD)
Indications : The best evidence is for use in the management of DFUs [37, 38]. It has shown benefit in the treatment of VLUs, PUs, burns, surgical wounds, pyoderma gangrenosum, and epidermolysis bullosa [62, 65].
CPM is an allogeneic graft composed of growth factors, cytokines, ECM proteins, and cells including mesenchymal stem cells, epithelial cells, and neonatal fibroblasts [38].
CPM is available in multiple sizes and should be maintained at −80 °C. When maintained at that temperature, it has a shelf life of 2 years. After wound bed preparation, warm water that does not exceed 32 °C should be placed into a basin. Place the inner plastic bag into the basin with the label side up. Once no ice crystals are visible, remove the plastic bag and cut open. Using forceps, transfer the CPM to a second basin that is filled with saline. After the wound bed is prepared, remove the top cover from the CPM and place over the wound. Gently remove the back cover from the CPM to place the CPM on the wound. Using cotton tip applicators, arrange the CPM so that it is covering the entire wound including the wound edges. Cover with a non-adherent dressing and then a secondary dressing. CPM is contraindicated in wounds with acute or chronic infection or if the patient has known hypersensitivities to gentamicin, vancomycin, or amphotericin B [65].
Acellular Matrices (Table 15.2)
Porcine Derived (Oasis®, Smith and Nephew, Largo, FL, Biobrane®, UDL Laboratories, Inc., Rockford, Illinois)
Indications: Oasis® has been studied in the management of partial- and full-thickness wounds, DFUs, VLUs, PUs, chronic vascular ulcers, tunneled or undermined wounds, trauma wounds, second-degree burns, and surgical wounds [66, 67, 84,85,86,87]. The best evidence is for VLUs and DFUs [66, 67]. Biobrane® is indicated for donor sites and partial-thickness burns [69,70,71,72,73, 88,89,90].
Oasis® is three-dimensional dermal substitute that is derived from porcine small intestinal mucosa. It provides a collagen scaffold as well as other ECM components such as glycosaminoglycans, proteoglycans, fibronectin, and growth factors [66]. It is available in multiple sizes as either a single- or tri-layered matrix. It can be stored at room temperature for up to 2 years. After the wound bed is prepared, the Oasis® sheet should be cut to the size and shape of the wound. After placement, it should be hydrated with sterile saline, and a non-adhesive primary dressing should be placed followed by a secondary dressing. Its use is contraindicated in those with known porcine hypersensitivities [91]. Biobrane® is a bilaminar nylon mesh that is filled with porcine collagen type I. The product is available in multiple sizes and is placed in a similar fashion but should be secured in place with a preferred method under tension [92].
Dermal Regeneration Template (DRT, Integra®, LifeSciences, Plainsboro, NJ)
Indications : Partial- and full-thickness wounds, PUs, VLUs, repair of scar contractions, surgical wounds, chronic vascular ulcers, and second-degree burns [74, 75, 93,94,95,96,97]
Integra® is an acellular three-dimensional DRT that consists of a porous bilayer matrix. The temporary epidermal layer is made of a thin layer of silicone, and the dermal layer consists of a cross-linked bovine tendon collagen and glycosaminoglycan (chondroitin-6-sulfate) biodegradable matrix. This provides a scaffold for cellular migration and angiogenesis [74, 97].
It is available in sheet, mesh, fenestrated, or flowable form. These products may be stored at room temperature. Once the wound bed is prepared, remove the DRT from the packaging and peel off the cover sheet. Place the DRT in a basin with sterile saline. Cut the DRT to fit the wound and carefully place it on the wound ensuring direct contact. Care must be taken to maintain the orientation so that the dermal layer is in direct contact with the wound. This can be verified by black threads in the silicone layer, which should be facing outward (away from the wound bed). It should be affixed with a preferred method, and a non-adherent primary dressing should be placed followed by a secondary dressing [97]. For tunneled wounds, the flowable form should be utilized. This can be prepared by drawing up sterile saline into an empty syringe and connecting the syringe to the syringe that contains collagen via a Luer Lock Connector. Depress the plungers back and forth to mix together. Once mixed, disconnect the two syringes and attach the flexible injector. The product can then be injected into the wound. Place a primary dressing and then a secondary dressing over the wound [22]. DRMs are contraindicated in infected wounds and those with known hypersensitivities to bovine collagen or chondroitin materials. Newer products such as PriMatrix® (Dermal Repair Scaffold, Integra®, LifeSciences, Plainsboro, NJ) are available, but their evidence and efficacy have not been extensively studied. Additional DRMs such as Matriderm® are available outside of the United States.
Cadaveric Allograft (CA, AlloDerm® Regenerative Tissue Matrix, LifeCell, Branchburg, NJ)
Indications: replacement of damaged or inadequate integument including surgical wounds, burns, soft tissue defects, and sinus tracts [77, 98,99,100,101,102].
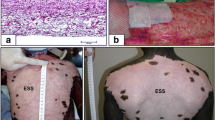
CA is an acellular dermal allograph that is processed to remove all cellular components so as to minimize the risk of tissue and graft rejection while preserving the three-dimensional structure of the dermis as well as other biological components [100]. It is available as a sheet or in an injectable form (Cymetra® Micronized AlloDerm® Tissue) (Fig. 15.2) [103].
The CA should be stored at room temperature. Before the wound bed is prepared, remove the tissue from the packaging and place it into a basin filled with 37 °C saline for 5 minutes. Transfer the tissue into the second basin filled with rehydration fluid for approximately 40 minutes. Once it is rehydrated, place onto the wound making sure the “L” that is in the mesh pattern on the tissue is facing outward, assuring that the dermal layer is in contact with the wound. Cover with a non-adherent primary dressing and then a secondary dressing [98].
CA is contraindicated in known hypersensitivities to antibiotics listed on the packaging (gentamicin, cefoxitin, lincomycin, polymyxin, and vancomycin) or to Polysorbate 20.
Future Research
Many new acellular and cellular skin substitutes are currently being developed, and it is hoped that new grafting sources and multipurpose products will deliver better outcomes; however, clinical evidence is still premature. For example, an acellular xenogeneic dermal matrix derived from fish skin (Kerecis® Omega3 wound matrix, Isafjordur, Iceland) is being trialed to heal various wound etiologies and has initially demonstrated efficacy in the stimulation of granulation tissue and re-epithelialization. Additionally, it has been reported to have antinociceptive and analgesic properties [104]. Hyalomatrix PA® (Fidia Advanced Biopolymers, Padua, Italy) that is a bilayer of esterified hyaluronan scaffold beneath a silicone membrane has been shown to provide a favorable environment for cellular migration and wound healing [105]. PuraPly ® Antimicrobial Wound Matrix is a collagen sheet, similar to Oasis®, that is coated with the antimicrobial agent polyhexmethylenebiguanide. It was released in 2016 and indicated for the management of wounds of multiple etiologies and is intended to provide a scaffold for cellular migration as well as protect against bacterial overload.
Conclusion
Acellular and cellular skin substitutes are an important adjunctive treatment for nonhealing wounds. These products provide key elements and scaffolding that promote healing and may be used to treat varying wound etiologies. Many different skin substitutes are available on the market, and each product has unique characteristics, benefits, and disadvantages. For example, non-exudating wounds may benefit from sheet matrices, while exudative wounds may benefit from fenestrated or mesh forms. Additionally, flowable matrices are advantageous in tunneled or sinus wounds. Some may be able to cover wounds with exposed tendon, bone, or muscle, while others may not. Although some have been extensively trialed, further research is necessary to demonstrate the full efficacy of many of the matrices currently available. It is imperative that clinicians are familiar with the many differing products available in order to best tailor the product to the unique clinical presentation. Correct patient and product selection combined with meticulous wound bed preparation is key to successful use of these products. Future studies are needed to better understand the mechanism of action of these products, and large, well-designed, randomized controlled clinical trials are warranted to compare between products. Given the high cost of these grafts, evidence of the best application timing, number, and spacing of applications will help to develop efficient guidelines for use.
Abbreviations
- BLCCs:
-
Bilayered living cellular constructs
- CA:
-
Cadaveric allograft
- CDM:
-
Collagen dermal matrix
- CEAs:
-
Cultured epidermal autografts
- CPM:
-
Cryopreserved placental membrane
- DFU:
-
Diabetic foot ulcer
- DHACM:
-
Dehydrated human amnion/chorion membrane
- DRT:
-
Dermal regeneration template
- ECM:
-
Extracellular matrix
- PU:
-
Pressure ulcer
- VLU:
-
Venous leg ulcer
References
Ramanujam CL, Zgonis T. An overview of autologous skin grafts and advanced biologics for the diabetic foot. Clin Podiatr Med Surg. 2012;29(3):435–41.
Mazzola IC, Mazzola RF. History of reconstructive rhinoplasty. Facial Plast Surg. 2014;30(3):227–36.
Halim AS, Khoo TL, Mohd Yussof SJ. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg. 2010;43(Suppl):S23–8.
Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43.
Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194(4):413–28.
Yannas IV, Burke JF, Orgill DP, Skrabut EM. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215(4529):174–6.
Woo K, Ayello EA, Sibbald RG. The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care. 2007;20(2):99–117; quiz 8–9.
Stone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, et al. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med. 2017;9(371).
Kallis PJ, Friedman AJ, Lev-Tov H. A guide to tissue-engineered skin substitutes. J Drugs Dermatol. 2018;17(1):57–64.
Sinha S, Schreiner AJ, Biernaskie J, Nickerson D, Gabriel VA. Treating pain on skin graft donor sites: review and clinical recommendations. J Trauma Acute Care Surg. 2017;83(5):954–64.
Nicholas MN, Jeschke MG, Amini-Nik S. Methodologies in creating skin substitutes. Cell Mol Life Sci. 2016;73(18):3453–72.
Clark RA, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127(5):1018–29.
Nathoo R, Howe N, Cohen G. Skin substitutes: an overview of the key players in wound management. J Clin Aesthet Dermatol. 2014;7(10):44–8.
Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493–508; quiz 9–10.
Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–93.
Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14(6):449–59.
Morton LM, Phillips TJ. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74(4):589–605; quiz −6.
Childs DR, Murthy AS. Overview of wound healing and management. Surg Clin North Am. 2017;97(1):189–207.
Daugherty S, Spear M. Skin and skin substitutes–an overview. Plast Surg Nurs. 2015;35(2):92–7.
Apligraf Label. Available from: http://www.apligraf.com/professional/pdf/prescribing_information.pdf.
Dermagraft label. Available from: http://www.dermagraft.com/home/wp-content/uploads/sites/1/Dermagraft_Directions_for_Use.pdf.
Integra Flowable Matrix Label. Available from: http://occ.integralife.com/products%2Fpdfs%2Fflowable_wound_matrix_ifu.pdf.
Gardien KL, Marck RE, Bloemen MC, Waaijman T, Gibbs S, Ulrich MM, et al. Outcome of burns treated with autologous cultured proliferating epidermal cells: a prospective randomized multicenter Intrapatient comparative trial. Cell Transplant. 2016;25(3):437–48.
Hickerson WL, Remmers AE, Recker D. Twenty-five years’ experience and beyond with Cultured Epidermal Autografts (CEA) for coverage of large burn wounds in adult and pediatric patients, 1989–2015. J Burn Care Res. 2019;40(2):157–65.
Sood R, Roggy D, Zieger M, Balledux J, Chaudhari S, Koumanis DJ, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31(4):559–68.
Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7(4):201–7.
Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8(1):11–8.
Zelen CM, Serena TE, Gould L, Le L, Carter MJ, Keller J, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272–82.
Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–5.
Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19(4):350–4.
Noordenbos J, Dore C, Hansbrough JF. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J Burn Care Rehabil. 1999;20(4):275–81.
Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial-thickness burns: a prospective, randomized trial using Transcyte. ANZ J Surg. 2004;74(8):622–6.
Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724–32.
Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502–7.
Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):688–93.
Bianchi C, Cazzell S, Vayser D, Reyzelman AM, Dosluoglu H, Tovmassian G. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix((R))) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114–22.
Lavery L, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al. Open-label extension phase of a chronic diabetic foot ulcer multicenter, controlled, randomized clinical trial using cryopreserved placental membrane. Wounds. 2018;30(9):283–9.
Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al. The efficacy and safety of Grafix((R)) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554–60.
The Oxford 2011 Levels of Evidence: Oxford Centre for Evidence-Based Medicine. Available from: http://www.cebm.net/index.aspx?o=5653.
Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12(9):2407–24.
Leary T, Jones PL, Appleby M, Blight A, Parkinson K, Stanley M. Epidermal keratinocyte self-renewal is dependent upon dermal integrity. J Invest Dermatol. 1992;99(4):422–30.
Epicel Guidelines. Available from: https://www.epicel.com/pdfs/Epicel%20SurgicalGuide%202018%20DIGITAL.pdf.
Ronfard V, Rives JM, Neveux Y, Carsin H, Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70(11):1588–98.
Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32(4):395–401.
Chester DL, Balderson DS, Papini RP. A review of keratinocyte delivery to the wound bed. J Burn Care Rehabil. 2004;25(3):266–75.
Williamson JS, Snelling CF, Clugston P, Macdonald IB, Germann E. Cultured epithelial autograft: five years of clinical experience with twenty-eight patients. J Trauma. 1995;39(2):309–19.
Atiyeh BS, Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33(4):405–13.
Holmes Iv JH, Molnar JA, Carter JE, Hwang J, Cairns BA, King BT, et al. A comparative study of the ReCell(R) device and autologous spit-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39(5):694–702.
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, et al. Advances in skin regeneration using tissue engineering. Int J Mol Sci. 2017;18(4).
Duchini G, Itin P, Arnold A. A case of refractory pyoderma gangrenosum treated with a combination of Apligraf and systemic immunosuppressive agents. Adv Skin Wound Care. 2011;24(5):217–20.
Trent JT, Kirsner RS. Epidermolysis bullosa: identification and treatment. Adv Skin Wound Care. 2003;16(6):284–90.
Hayes DW Jr, Webb GE, Mandracchia VJ, John KJ. Full-thickness burn of the foot: successful treatment with Apligraf. A case report. Clin Podiatr Med Surg. 2001;18(1):179–88.
Shealy FG Jr, DeLoach ED. Experience with the use of apligraf to heal complicated surgical and nonsurgical wounds in a private practice setting. Adv Skin Wound Care. 2006;19(6):310–22.
Dinh TL, Veves A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast Reconstr Surg. 2006;117(7 Suppl):152S–7S; discussion 8S–9S.
Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13.
Zaulyanov L, Kirsner RS. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging. 2007;2(1):93–8.
Schonfeld WH, Villa KF, Fastenau JM, Mazonson PD, Falanga V. An economic assessment of Apligraf (Graftskin) for the treatment of hard-to-heal venous leg ulcers. Wound Repair Regen. 2000;8(4):251–7.
Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J. 2013;10(2):132–7.
Purdue GF, Hunt JL, Still JM Jr, Law EJ, Herndon DN, Goldfarb IW, et al. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18(1 Pt 1):52–7.
Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 2014;11(6):711–7.
Miranda EP, Friedman A. Dehydrated human amnion/chorion grafts may accelerate the healing of ulcers on free flaps in patients with venous insufficiency and/or lymphedema. Eplasty. 2016;16:e26.
Hughes OB, Rakosi A, Macquhae F, Herskovitz I, Fox JD, Kirsner RS. A review of cellular and acellular matrix products: indications, techniques, and outcomes. Plast Reconstr Surg. 2016;138(3 Suppl):138s–47s.
Epifix instructions. Available from: https://www.woundsource.com/print/product/epifix-dehydrated-human-amnionchorion-membrane-allograft.
Epifix Product Overview. Available from: https://mimedx.com/epifix/.
About Grafix. Available from: http://www.osiris.com/grafix/healthcare-professionals/#about.
Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41(5):837–43.
Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5 Pt 1):258–66.
Cazzell SM, Lange DL, Dickerson JE Jr, Slade HB. The management of diabetic foot ulcers with porcine small intestine submucosa tri-layer matrix: a randomized controlled trial. Adv Wound Care. 2015;4(12):711–8.
Schulz A, Depner C, Lefering R, Kricheldorff J, Kastner S, Fuchs PC, et al. A prospective clinical trial comparing Biobrane((R)) Dressilk((R)) and PolyMem((R)) dressings on partial-thickness skin graft donor sites. Burns. 2016;42(2):345–55.
Prasad JK, Feller I, Thomson PD. A prospective controlled trial of Biobrane versus scarlet red on skin graft donor areas. J Burn Care Rehabil. 1987;8(5):384–6.
Feldman DL, Rogers A, Karpinski RH. A prospective trial comparing Biobrane, Duoderm and xeroform for skin graft donor sites. Surg Gynecol Obstet. 1991;173(1):1–5.
Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg. 2000;105(1):62–5.
Gerding RL, Emerman CL, Effron D, Lukens T, Imbembo AL, Fratianne RB. Outpatient management of partial-thickness burns: Biobrane versus 1% silver sulfadiazine. Ann Emerg Med. 1990;19(2):121–4.
Driver VR, Lavery LA, Reyzelman AM, Dutra TG, Dove CR, Kotsis SV, et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23(6):891–900.
Campitiello F, Mancone M, Della Corte A, Guerniero R, Canonico S. To evaluate the efficacy of an acellular Flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: a randomized clinical trial. Updat Surg. 2017;69(4):523–9.
Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21(4):243–8.
Sinha UK, Shih C, Chang K, Rice DH. Use of AlloDerm for coverage of radial forearm free flap donor site. Laryngoscope. 2002;112(2):230–4.
Sinha UK, Chang KE, Shih CW. Reconstruction of pharyngeal defects using AlloDerm and sternocleidomastoid muscle flap. Laryngoscope. 2001;111(11 Pt 1):1910–6.
Kellner DS, Fracchia JA, Voigt E, Armenakas NA. Preliminary report on use of AlloDerm for closure of intraoral defects after buccal mucosal harvest. Urology. 2007;69(2):372–4.
Pushpoth S, Tambe K, Sandramouli S. The use of AlloDerm in the reconstruction of full-thickness eyelid defects. Orbit. 2008;27(5):337–40.
Deneve JL, Turaga KK, Marzban SS, Puleo CA, Sarnaik AA, Gonzalez RJ, et al. Single-institution outcome experience using AlloDerm(R) as temporary coverage or definitive reconstruction for cutaneous and soft tissue malignancy defects. Am Surg. 2013;79(5):476–82.
Park JY, Lee TG, Kim JY, Lee MC, Chung YK, Lee WJ. Acellular dermal matrix to treat full thickness skin defects: follow-up subjective and objective skin quality assessments. Arch Craniofac Surg. 2014;15(1):14–21.
Bolton WD, Ben-Or S, Hale AL, Stephenson JE. Reconstruction of a long-segment tracheal defect using an AlloDerm conduit. Innovations. 2017;12(2):137–9.
Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care. 2010;23(1):34–8.
Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. Int Wound J. 2007;4(1):3–7.
Lev-Tov H, Li CS, Dahle S, Isseroff RR. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
Oasis Wound Types. Available from: https://www.oasiswoundmatrix.com/wound-type.
Lal S, Barrow RE, Wolf SE, Chinkes DL, Hart DW, Heggers JP, et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock. 2000;14(3):314–8; discussion 8–9.
Cassidy C, St Peter SD, Lacey S, Beery M, Ward-Smith P, Sharp RJ, et al. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: a prospective, randomized trial. Burns. 2005;31(7):890–3.
Wood F, Martin L, Lewis D, Rawlins J, McWilliams T, Burrows S, et al. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane(R) synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns. 2012;38(6):830–9.
Oasis Application. Available from: https://www.oasiswoundmatrix.com/application.
Biobrane Application. Available from: https://www.woundsource.com/product/biobrane.
Heimbach DM, Warden GD, Luterman A, Jordan MH, Ozobia N, Ryan CM, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24(1):42–8.
Jeschke MG, Rose C, Angele P, Fuchtmeier B, Nerlich MN, Bolder U. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg. 2004;113(2):525–30.
Branski LK, Herndon DN, Pereira C, Mlcak RP, Celis MM, Lee JO, et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med. 2007;35(11):2615–23.
Campitiello F, Della Corte A, Guerniero R, Pellino G, Canonico S. Efficacy of a new Flowable wound matrix in tunneled and cavity ulcers: a preliminary report. Wounds. 2015;27(6):152–7.
Integra Label. Available from: https://www.integralife.com/file/general/1459196235.pdf.
AlloDerm label. Available from: https://allergan-web-cdn-prod.azureedge.net/actavis/actavis/media/allergan-pdf-documents/labeling/alloderm/adm_ifu_121p0541f_t3.pdf.
Sclafani AP, Romo T 3rd, Jacono AA, McCormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg. 2000;2(2):130–6.
Bochicchio GV, De Castro GP, Bochicchio KM, Weeks J, Rodriguez E, Scalea TM. Comparison study of acellular dermal matrices in complicated hernia surgery. J Am Coll Surg. 2013;217(4):606–13.
Cazzell S, Vayser D, Pham H, Walters J, Reyzelman A, Samsell B, et al. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen. 2017;25(3):483–97.
Hinchcliff KM, Orbay H, Busse BK, Charvet H, Kaur M, Sahar DE. Comparison of two cadaveric acellular dermal matrices for immediate breast reconstruction: a prospective randomized trial. J Plast Reconstr Aesthet Surg. 2017;70(5):568–76.
Banta MN, Eaglstein WH, Kirsner RS. Healing of refractory sinus tracts by dermal matrix injection with Cymetra. Dermatol Surg. 2003;29(8):863–6.
Dorweiler B, Trinh TT, Dunschede F, Vahl CF, Debus ES, Storck M, et al. The marine Omega3 wound matrix for treatment of complicated wounds: a multicenter experience report. Gefasschirurgie. 2018;23(Suppl 2):46–55.
Gravante G, Sorge R, Merone A, Tamisani AM, Di Lonardo A, Scalise A, et al. Hyalomatrix PA in burn care practice: results from a national retrospective survey, 2005 to 2006. Ann Plast Surg. 2010;64(1):69–79.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cahn, B., Lev-Tov, H. (2020). Cellular- and Acellular-Based Therapies: Skin Substitutes and Matrices. In: Alavi, A., Maibach, H. (eds) Local Wound Care for Dermatologists. Updates in Clinical Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-28872-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-28872-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28871-6
Online ISBN: 978-3-030-28872-3
eBook Packages: MedicineMedicine (R0)