Abstract
Mastocytosis is a rare condition described by clonal, neoplastic proliferation of abnormal mast cells. Infiltration of abnormal mast cells into the organ systems other than skin results in systemic mastocytosis (SM), which is frequently associated with a D816V (KIT) mutation. Bone involvement is a common manifestation of adult SM. It is often complicated by fragility fracture, especially vertebral fracture. However, SM-related bone manifestations range from asymptomatic infiltration to bone pain, bone loss including osteopenia and osteoporosis, and focal osteolytic lesions to osteosclerosis. The mechanisms of bone involvement in SM are due to not only the neoplastic infiltration of mast cells themselves but also the secretion of mediators and inflammatory markers that technically impair bone structure and cell function. Prevention of disease-related bone complications, especially fragility fracture, should be a concern for patients with SM. However, different treatments have been proposed for SM-related bone consequences, including antiresorptive medications (bisphosphonate and denosumab), ketotifen, cromolyn, antihistamines, sodium fluoride, interferon, and chemotherapeutic agents; controlling the underlying mastocytosis disease might be a better future approach.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Mastocytosis is caused by a neoplastic proliferation of abnormal mast cells (MC), driven by the binding of stem cell factor with the tyrosine kinase receptor KIT(CD117), in the mast cell progenitor, resulting in the activation and proliferation of mast cells. This accumulation and infiltration of mast cells in different tissues and organs lead to a heterogeneous group of diseases, ranging from cutaneous mastocytosis, involving the skin, to systemic mastocytosis (infiltrating deep organs). Cutaneous mastocytosis is usually seen during infancy and childhood and typically associated with a relatively good prognosis and spontaneous remission. Systemic mastocytosis, the most common form in adults, is generally more disturbing and associated with involvement of multiple organs and tissues other than skin, organ failure, and reduced life span. Furthermore, systemic mastocytosis (SM) is itself a heterogeneous group of diseases with variable prognoses. The clinical spectrum of SM varies from pre-diagnostic SM to mast cell leukemia. Other clinical varieties include indolent SM, smoldering SM, aggressive SM, and SM associated with hematologic malignancy or mast cell leukemia. Mast cell sarcoma (MCS) and extracutaneous mastocytoma are two other clinical conditions that have no SM criteria. Pre-diagnostic SM is the term for colonization of abnormal mast cells in bone marrow that does not fulfill the criteria of SM [1,2,3].
According to World Health Organization (WHO) classification, major criteria for SM are the presence of multifocal, dense infiltration of mast cells (aggregation of ≥15 mast cells) in biopsy of bone marrow or extracutaneous organs. Minor criteria include >25% mast cells with atypical or immature morphology; activating mutation D816V; presence of CD2- or CD25-positive mast cells in bone marrow, blood, or other extracutaneous organs; and tryptase level persistently >20 ng/ml. The presence of the major criterion and one minor criterion or at least three minor criteria support the diagnosis of SM. Serum tryptase level has a positive correlation with mast cell burden [2]. Other helpful tools for diagnosis include immunohistochemical staining against CD117 (KIT) and tryptase in bone marrow and analysis of urine histamine mediators [3].
Indolent SM is the most common type of SM that is usually associated with skin and gastrointestinal manifestations [4]. Disease progression is manifested by the appearance of B and/or C findings, which correlate with poorer prognosis. B findings include >30% infiltration of bone marrow by mast cells, serum total tryptase level >200 ng/mL, dysplasia or myeloproliferation in hematopoietic lineage other than mast cells, hepatomegaly with normal liver function, palpable splenomegaly with no signs of hypersplenism, and lymphadenopathy. C findings include cytopenia of one or more hematopoietic cell lineages without evident malignancy, palpable hepatomegaly associated with liver function abnormalities, ascites, portal hypertension, bone involvement manifested with large osteolytic lesions and/or pathological fractures, palpable splenomegaly accompanying with signs of hypersplenism, and malabsorption concomitant with weight loss [2].
The most common mutation (found in 80–90% persons with systemic mastocytosis) is a gain-of-function mutation in the KIT receptor (D816V mutation) that leads to the neoplastic growth of MCs. The oncogene c-kit encodes c-Kit receptor, a class III receptor tyrosine kinase, which has five extracellular domains that are structurally like immunoglobulins, and a transmembrane portion. The gain-of-function mutation can potentiate the interaction of stem cell factor (SCF) with upper extracellular domains of receptor by inducing dimerization in lower extracellular domains. This interaction leads to a signaling transduction that plays a crucial role in facilitating angiogenesis, migration, cell survival, and proliferation of MCs [5, 6].
Pathogenesis and Etiology of Bone Disease in Mastocytosis
Bone is one of the major organ involvements in adult SM [1]. The exact mechanisms of bone involvement, including fragility, bone infiltration, bone loss, and sclerosis, in SM patients are not completely understood.
Osteoporosis and fracture occur more commonly in the lumbar spine than in the hip, demonstrating that the major underlying pathogenic process that leads to greater trabecular bone loss than cortical bone loss, in a similar pattern as most forms of osteoporosis. This preferential loss in the trabecular bone might be explained by the fact that neoplastic proliferation of abnormal mast cells occurs in bone marrow with higher metabolic activity [1, 3].
It is generally believed that neoplastic infiltration of mast cells, mast cell activation with release of different mediators (histamine, tryptase, and heparin), and inflammatory markers (TNF, growth factors, and ILs), all critically contribute to bone loss [3] (Fig. 8.1).
The bone histomorphometric information in SM patients with osteoporosis showed increase [7] or no change [8] in osteoclast number. However, the deterioration of bone health could be due to alteration of bone structure, increased bone turnover, increased osteoid tissue, fibrosis of peritrabecular area, and changes in trabecular structure [1, 3, 7, 9].
In addition, osteoclasts themselves express KIT on their surfaces that can also interact with SCF, but an increase in osteoclast activity due to this interaction is not proven definitively [10]. At the same time, KIT D816V mutation may increase oncostatin M, a mast cell secretion that stimulates proliferation of osteoblasts, endothelial cells, and fibroblasts and serves as a profibrogenic and angiogenic modulator [11]. However, the fraction of cells that acquire the KIT D816V mutation has no correlation with disease severity in ISM patients [12].
The process of mast cell activation has three steps, namely, degranulation, which occurs in a few seconds; synthesis and release of mediators originating from the lipid bilayer of the cell membrane in several minutes; and finally, within minutes to hours, synthesis of a mass of inflammatory cytokines [1, 3]. Mast cell products include [1] stored mediators in the granules such as tryptase, histamine, serotonin, heparin, and chymase, which can be secreted immediately; [2] newly synthesized biologic markers such as platelet-activating factor (PAF), prostaglandin D2 (PGD2), and leukotrienes (LTB4 and LTD4), produced after stimulation; and [3] different cytokines such as interleukins (IL-1, IL-3, IL-5, IL-8, and IL-10), TNF-α, TGF-β, GM-CSF, and VEGF. Thus, mast cells can secrete different biologic markers and have the ability to express variable receptors such as receptor for immunoglobulin, hormones, or Toll-like receptors, complement, chemokines and cytokines. The interaction between these highly complex structures of cells and biomarkers may augment or downregulate the immune response to allergens or antigens [6] (Fig. 8.1).
The bone remodeling process is a coordinated interaction between osteoblasts, osteoclasts, and osteocytes, which is, in turn, regulated by mechanical stimuli and diverse endocrine, paracrine, and autocrine biologic markers. PTH (parathyroid hormone) and the Wnt signaling pathway play crucial roles in osteoblast development and function. Receptor activator of nuclear factor-κB ligand (RANKL) is encoded by type 11 of tumor necrosis factor superfamily gene (TNFSF11) and leads to osteocyte formation and activation. Wnt activation also increases β-catenin levels, which increase osteoblast secretion of OPG (osteoprotegerin), which competitively blocks RANKL, blocking osteoclast stimulation [13, 14]. Sclerostin, a product of osteocytes stimulated by PTH, and DKK1 (Dickkopf-related protein 1), a soluble protein from osteoblasts, both act as endogenous inhibitors of the Wnt pathway [1].
The underlying processes that have been involved in the impairment of bone health in SM patients are highly complex. Interactions between bone cells including osteoblasts, osteoclasts and osteocytes, immune cells, inflammatory mediators, and endocrine parameters determine the severity and type of bone involvement. Cytokines, including TNF-α, IL-1, and IL-6, can increase osteoclast activity and reduce osteoblast performance [10, 15, 16]. However, increase in the serum levels of bone formation markers such as OPG and bone-specific alkaline phosphatase and bone resorption markers including RANKL, SOST (Sclerostin gene), DKK1, and CTX (C-terminal telopeptide or carboxy-terminal collagen crosslinks) are also reported [17, 18], which perhaps means SM upregulates bone turnover with the dominancy of bone resorption over bone formation. The level and role of the Wnt inhibitors DKK1 and sclerostin are controversial. Rossini reported that serum levels of DKK1, but not sclerostin, were significantly higher in ISM patients and had positive correlation with PTH and bone turnover markers, CTX and bALP, but ISM patients with one or more vertebral fracture had lower serum DKK1 levels [18]. However, Rabenhorst found significant increase in serum levels of sclerostin, but not DKK1, in ISM patients [17]. RANKL is consistently elevated in SM patients in different studies, and to the best of our knowledge, there are no reports of decreased RANKL serum level in ISM patients. Additionally, treating ISM patients with denosumab (anti-RANKL human monoclonal antibody) for 1 year not only improves BMD and reduces bone turnover markers but may also decrease tryptase levels, which correlate with mast cell mass [19] (Fig. 8.1).
It seems that histamine can also modify the function of both osteoblasts and osteoclasts. Histamine serum levels have a positive correlation with osteoporosis in SM patients. Antihistamines (H1 blocker) can block differentiation of mesenchymal stem cells into osteoblasts [20]. However, regulating the gene for histamine synthesis by knocking out the histidine decarboxylase gene is associated with elevated calcitriol, alkaline phosphatase, and RANKL, while this suppresses PTH, which might explain protection from ovariectomy-induced bone loss [21]. Additionally, ketotifen (a mast cell degranulation inhibitor) improved bone pain, increased 1,25-dihydroxyvitamin D3 and osteocalcin levels, and normalized elevated plasma and urine histamine levels in a 59-year-old man with SM [22].
Clinical Bone Manifestations of Systemic Mastocytosis (SM)
Bone involvement can manifest with a varying clinical spectrum from asymptomatic to bone pain, with osteopenia, osteoporotic with fragility fractures, osteolytic lesions, osteosclerosis, and sometimes multiple conditions together in the same individual [1, 3]. Bone pain is often devastating and could be potentially due to bone marrow involvement, osteoporotic/pathologic fracture, osteolytic lesion, and/or anaphylaxis [1, 3, 23].
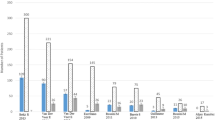
The incidence of fracture was variable in different studies (6–57%) [24, 25] (Table 8.1 and Fig. 8.2), and it was mainly fragility fracture. The source of the variability of fracture in different population groups could be due to sample size, population age, and other contributing risk factors such as duration of disease, disease progression, and medication history. As in postmenopausal osteoporosis, vertebral fracture occurs more than nonvertebral fracture (Table 8.1). The overall incidence of osteoporosis, which has been mainly reported according to WHO criteria, was between 12% and 60% in different studies (Table 8.2). It is noteworthy to mention that the incidence of fracture was higher than that of osteoporosis in some population groups (Tables 8.1 and 8.2). While low femoral and lumbar spine BMD are associated with an increased risk of fracture [26], it seems that DXA may underestimate the risk of fracture in SM patients (Tables 8.1 and 8.2), so we must consider risk factors other than osteoporosis determination by bone density to be able to predict and determine when to intervene to prevent fracture better.
Available data around risk factors of fracture in SM patients are not consistent. Degboé reports age of disease onset, a skin pattern of telangiectasia macularis eruptiva perstans, symptoms of mast cell activation, digestive symptoms, and increased bone marrow tryptase predict increased fracture risk. Furthermore, higher bone marrow tryptase, low femoral neck bone density, and older age at the onset of disease could independently predict a higher risk of low trauma fracture [26]. Johansson states that moderately increased mast cell mass is associated with lower hip bone density and higher risk of vertebral fracture [35]. Van Der Veer indicates that fragility fracture happens more in older age, male, with a history of more anaphylactic reactions, fewer skin lesions (urticarial pigmentosa), higher bone and mastocytosis markers (higher methylimidazole acetic acid, osteocalcin and CTX levels), lower hip BMD, and history of more alcohol consumption at the time of diagnosis. Additionally, male sex, high CTX, lower hip BMD, absence of skin lesion (urticaria pigmentosa), and alcohol consumption at the time of diagnosis independently predict fracture [25]. Rossini reports that patients with low BMD or vertebral fracture are older and have lower osteocalcin serum levels [30]. To further confuse the biochemical markers, DKK1 was higher in ISM patients, but patients with vertebral fractures had lower DKK1 serum levels [18]. It seems that a comprehensive approach including fracture risk factors, DXA values, patient age, and associated conditions should be taken into account to institute appropriate management policies in preventing SM-related bone events.
Osteosclerosis occurs in 2–17% of SM population, mainly involving the vertebral spine and may be patchy or diffuse sclerosis (Tables 8.1 and 8.2). Paradoxically, osteosclerosis is associated with very high tryptase levels [18], more aggressive disease [33], increased bone turnover markers [30], and abnormal hematologic findings including anemia, thrombocytopenia, and eosinophilia [33]. Also, it seems that risk of fragility fractures is lower in SM patients with osteosclerosis [9].
Few studies report osteolytic lesions in SM patients. Sometimes, osteoporotic bone involvement is associated with concomitant osteosclerosis or osteolytic lesions [38] (Tables 8.1 and 8.2).
Treatment
The overall composite process of bone involvement in SM is bone resorption, which predisposes the patients to fragility fractures. Increased osteoclast activity is probably the main reason for bone resorption and bone loss, which occurs secondary to mast cell activation and proliferation. The concept of increased osteoclast activity and bone resorption might recommend antiresorptive therapies, such as bisphosphonates or denosumab, as the first line of treatment of osteoporosis in SM patients. However, Rossini and Rabenhorst report that elevated bone turnover (documented by both increased bone formation and resorption markers) is an important reason for SM-related bone events [17, 18]. While antiresorptive therapy can alleviate bone loss that is accompanied by increased bone turnover, this is not as effective as governing of underlying disease activity as adding interferon to pamidronate. This combination had better effects on BMD and could reduce tryptase level simultaneously [32, 39]. Therefore, it seems that management of the underlying disease might be the best way to prevent disease-related bone complications in the setting of increased bone turnover, in SM, similar to other bone disease with high turnover such as hyperthyroidism or hyperparathyroidism.
Bisphosphonates were shown to be effective in improving lumbar spine BMD but have lesser beneficial effects or even negative effects on femoral neck BMD [32, 39,40,41] (Table 8.3). They may also improve bone pain associated with osteopenia in SM patients [42]. While poor compliance is a well-documented problem with oral bisphosphonates, this could be addressed by recommending zolendronic acid yearly infusion to improve spine and hip BMD [36].
RANKL, the product of type 11 of tumor necrosis factor superfamily gene (TNFSF11), has quite an important role in bone biology and the immune system. It is secreted by osteoblasts and leads to osteoclastogenesis [13]. Elevation of serum RANKL levels has been reported in SM patients [17]. Additionally, denosumab, a human monoclonal antibody to RANKL, in SM patients was effective in improving lumbar spine and femoral neck BMD (increase in LS BMD > FN BMD) and also could reduce bone markers (CTX and bALP) and tryptase levels (Table 8.3) [19]. It seems that blocking RANKL could be fairly effective, not only in improving bone condition, but also in alleviating mast cell burden. However, denosumab is a monoclonal antibody, and some patients with SM are at higher risk of anaphylactic reaction to foreign antigens. But, it is important to mention that denosumab belongs to immunoglobulin of the IgG2 subclass [29], and it is generally agreed that infusion of IgG may cause mild reaction while chance of developing anaphylactic reaction is extremely rare [43]. The Freedom trial with denosumab in postmenopausal women with osteoporosis did not show a significantly higher risk of anaphylactic or even skin reaction to denosumab versus placebo (eczema 3.0% vs. 1.7%) [44]. Furthermore, a subcutaneous desensitization protocol in an eight-step escalating titration process is reported to be successful to make denosumab tolerable even in the patient with a history of anaphylaxis to denosumab [45]. However, there are only anecdotal reports of the use of denosumab in patients with mastocytosis, and these reports do not include patients with a history of anaphylaxis.
As mast cell degranulation and proliferation may directly promote SM-related bone complications, it is suggested to use adding medication to block mast cell degranulation or their mediators potentially to improve bone health in SM patients. Graves et al. (1990) reported that ketotifen, an inhibitor of mast cell degranulation, administered for 3 months could reduce bone pain and histamine level; they also found no further bone loss in BMD after 6 and 14 months of therapy [22]. However, cromolyn, antihistamines, and sodium fluoride were effective. Moreover, even chemotherapeutic agents such as chlorambucil and mithramycin are recommended for refractory disease, but they were not superior to bisphosphonate (oral clodronate) regarding the SM-related bone circumstances [1, 46]. However, cytoreductive medications (interferon, 2-chlorodeoxyadenosine, or cladribine/2-CdA), which are currently recommended in advanced or aggressive forms of SM, may be used in treating osteoporosis secondary to ISM or SM [2, 3].
As PTH may stimulate mast cell proliferation and elicit histamine release from mast cells [47], teriparatide may increase symptomatology. Given the concerning data about osteosarcoma risk in rats and the understanding that mastocytosis may be a premalignant condition, we would recommend caution and further study, before consideration of teriparatide therapy for bone disease in this population.
Future Direction
Sclerostin , encoded by the SOST gene, is a glycoprotein secreted by osteocytes that downregulates bone formation. Romosozumab , a human monoclonal antibody against sclerostin, reduces fracture risk in postmenopausal women but is associated with increased adjudicated serious cardiovascular events [48]. However, the role of sclerostin in bone complications of SM is controversial (Tables 8.2 and 8.3) [17, 18]. Additionally, blocking sclerostin can lead to the activation of the Wnt pathway and increase in the β-catenin level, which might lead to malignant transformation or progression [49]. We could not find a study or abstract that reported effects of romosozumab on SM-induced osteoporosis.
Cathepsin K is a protease secreted by mature osteoclasts that destroys collagen and other matrix proteins. Cathepsin K inhibitor (odanactib) improves lumbar spine BMD and reduces clinical vertebral fractures (72%) and hip fractures (47%) versus placebo in postmenopausal women. However, it was associated with some complications such as skin lesions, atypical femoral fractures, and stroke [50]. Immunoreactivity to cathepsin-G in human mast cells with cutaneous mastocytosis has been reported [51]. Given systemic mastocytosis is associated with the increased osteoclastic activity and higher risk of vertebral fracture (Tables 8.2 and 8.3), the cathepsin K inhibitor (odanactib) might decrease SM-related bone loss. However, adverse vascular events associated with this drug present an important barrier to its usage.
Avapritinib (BLU-285) , in phase I trials for the treatment of advanced systemic mastocytosis, targets D816V mutant KIT and probably affects the activity of the disease and may improve bone damage also. Trials show a relatively good response rate (72%) without serious complications. However, the comparative cost and benefit of this medication should be investigated before being recommended. [52].
Summary
Bone consequences of systemic mastocytosis are heterogeneous, ranging from bone edema with or without pain, osteoporosis, lytic lesions, to osteosclerosis. Some patients may have one or more of these complications. In theory, controlling proliferation and activation of mast cells might also even prevent or delay bone disease in systemic mastocytosis. Additionally, applying antiresorptive therapy may help to improve bone density and reduce the risk of fracture. However, it is not known if anabolic agents for bone promote mast cell proliferation; this concern should be addressed with appropriate preclinical studies.
Abbreviations
- BMD:
-
Bone mineral density
- DKK1:
-
Dickkopf-related protein 1
- DXA:
-
Dual-energy X-ray absorptiometry
- FN:
-
Femoral neck
- IL:
-
Interleukin
- IL:
-
Interleukins
- ISM:
-
Indolent systemic mastocytosis
- LS:
-
Lumbar spine
- LT:
-
Leukotrienes
- OPG:
-
Osteoprotegerin
- P1CP:
-
Propeptide of type I C-terminal procollagen
- P1NP:
-
Propeptide of type I N-terminal procollagen
- PAF:
-
Platelet-activating factor
- PGD2:
-
Prostaglandin D2
- PTH:
-
Parathyroid hormone
- PTH-rP:
-
Parathyroid hormone-related peptide
- RANK:
-
Receptor activator of nuclear factor-κB (NF-κB)
- RANKL:
-
Receptor activator of nuclear factor-κB (NF-κB) ligand
- SCF:
-
Stem cell factor
- SM:
-
Systemic mastocytosis
- TGF-β:
-
Transforming growth factor-beta
- TH:
-
Total hip
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
References
Greene LW, Asadipooya K, Corradi PF, Akin C. Endocrine manifestations of systemic mastocytosis in bone. Rev Endocr Metab Disord. 2016;17(3):419–31.
Pardanani A. Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification and management. Am J Hematol. 2016;91(11):1146–59.
Rossini M, Zanotti R, Orsolini G, Tripi G, Viapiana O, Idolazzi L, Zamò A, Bonadonna P, Kunnathully V, Adami S, Gatti D. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporos Int. 2016;27(8):2411–21.
Scherber RM, Borate U. How we diagnose and treat systemic mastocytosis in adults. Br J Haematol. 2018;180(1):11–23.
Vaes M, Benghiat FS, Hermine O. Targeted treatment options in mastocytosis. Front Med (Lausanne). 2017;4:110.
Komi DEA, Rambasek T, Wöhrl S. Mastocytosis: from a molecular point of view. Clin Rev Allergy Immunol. 2018;54(3):397–411.
Fallon MD, Whyte MP, Teitelbaum SL. Systemic mastocytosis associated with generalized osteopenia. Histopathological characterization of the skeletal lesion using undecalcified bone from two patients. Hum Pathol. 1981;12(9):813–20.
Delling G, Ritzel H, Werner M. Histological characteristics and prevalence of secondary osteoporosis in systemic mastocytosis. A retrospective analysis of 158 cases. Pathologe. 2001;22(2):132–40.
Seitz S, Barvencik F, Koehne T, Priemel M, Pogoda P, Semler J, Minne H, Pfeiffer M, Zustin J, Püschel K, Eulenburg C, Schinke T, Amling M. Increased osteoblast and osteoclast indices in individuals with systemic mastocytosis. Osteoporos Int. 2013;24(8):2325–34.
Chiappetta N, Gruber B. The role of mast cells in osteoporosis. Semin Arthritis Rheum. 2006;36(1):32–6.
Hoermann G, Cerny-Reiterer S, Perné A, Klauser M, et al. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am J Pathol. 2011;178(5):2344–56.
Broesby-Olsen S, Kristensen T, Vestergaard H, Brixen K, Møller MB, Bindslev-Jensen C, et al. KIT D816V mutation burden does not correlate to clinical manifestations of indolent systemic mastocytosis. J Allergy Clin Immunol. 2013;132(3):723–8.
Nagy V, Penninger JM. The RANKL-RANK story. Gerontology. 2015;61(6):534–42. https://doi.org/10.1159/000371845. Epub 2015 Feb 14
Amirhosseini M, Madsen RV, Escott KJ, Bostrom MP, Ross FP, Fahlgren A. GSK-3β inhibition suppresses instability-induced osteolysis by a dual action on osteoblast and osteoclast differentiation. J Cell Physiol. 2018;233(3):2398–408.
Theoharides TC, Boucher W, Spear K. Serum interleukin-6 reflects disease severity and osteoporosis in mastocytosis patients. Int Arch Allergy Immunol. 2002;128(4):344–50.
Brockow K, Akin C, Huber M, Metcalfe DD. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115(2):216–23.
Rabenhorst A, Christopeit B, Leja S, et al. Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J Allergy Clin Immunol. 2013;132(5):1234–7.
Rossini M, Viapiana O, Zanotti R, Tripi G, Perbellini O, Idolazzi L, et al. Dickkopf-1 and sclerostin serum levels in patients with systemic mastocytosis. Calcif Tissue Int. 2015;96(5):410–6.
Orsolini G, Gavioli I, Tripi G, Viapiana O, Gatti D, Idolazzi L, Zanotti R, Rossini M. Denosumab for the treatment of mastocytosis-related osteoporosis: a case series. Calcif Tissue Int. 2017;100(6):595–8.
Pochampally RR, Ylostalo J, Penfornis P, Matz RR, Smith JR, Prockop DJ. Histamine receptor H1 and dermatopontin: new downstream targets of the vitamin D receptor. J Bone Miner Res. 2007;22(9):1338–49.
Fitzpatrick LA, Buzas E, Gagne TJ, Nagy A, Horvath C, et al. Targeted deletion of histidine decarboxylase gene in mice increases bone formation and protects against ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2003;100(10):6027–32.
Graves L 3rd, Stechschulte DJ, Morris DC, Lukert BP. Inhibition of mediator release in systemic mastocytosis is associated with reversal of bone changes. J Bone Miner Res. 1990;5(11):1113–9.
Hermine O, Lortholary O, Leventhal PS, Catteau A, et al. Case-control cohort study of patients’ perceptions of disability in mastocytosis. PLoS One. 2008;3(5):e2266.
Guillaume N, Desoutter J, Chandesris O, Merlusca L, Henry I, Georgin-Lavialle S, Barete S, Hirsch I, Bouredji D, Royer B, Gruson B, Lok C, Sevestre H, Mentaverri R, Brazier M, Meynier J, Hermine O, Marolleau JP, Kamel S, Damaj G. Bone complications of mastocytosis: a link between clinical and biological characteristics. Am J Med. 2013;126(1):75.e1–7.
Van der Veer E, Arends S, van der Hoek S, Versluijs JB, de Monchy JGR, Oude Elberink JNG, van Doormaal JJ. Predictors of new fragility fractures after diagnosis of indolent systemic mastocytosis. J Allergy Clin Immunol. 2014;134(6):1413–21.
Degboé Y, Eischen M, Nigon D, Apoil PA, Mailhol C, Tournier E, Laurent C, Hanssens K, Hermine O, Paul C, Laroche M, Bulai-Livideanu C. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone. 2017;105:219–25.
Artuso A, Caimmi C, Tripi G, Viapiana O, Bonifacio M, Idolazzi L, Gavioli I, Gatti D, Zanotti R, Rossini M. Longitudinal evaluation of bone mineral density and bone metabolism markers in patients with indolent systemic mastocytosis without osteoporosis. Calcif Tissue Int. 2017;100(1):40–6.
Alpay Kanıtez N, Erer B, Doğan Ö, Büyükbabani N, Baykal C, Sindel D, Tanakol R, Yavuz AS. Osteoporosis and osteopathy markers in patients with mastocytosis. Turk J Haematol. 2015;32(1):43–50.
Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–92.
Rossini M, Zanotti R, Bonadonna P, Artuso A, Caruso B, Schena D, Vecchiato D, Bonifacio M, Viapiana O, Gatti D, Senna G, Riccio A, Passalacqua G, Pizzolo G, Adami S. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone. 2011;49(4):880–5.
van der Veer E, van der Goot W, de Monchy JG, Kluin-Nelemans HC, van Doormaal JJ. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy. 2012;67(3):431–8.
Laroche M, Livideanu C, Paul C, Cantagrel A. Interferon alpha and pamidronate in osteoporosis with fracture secondary to mastocytosis. Am J Med. 2011;124(8):776–8.
Barete S, Assous N, de Gennes C, Grandpeix C, Feger F, Palmerini F, Dubreuil P, Arock M, Roux C, Launay JM, Fraitag S, Canioni D, Billemont B, Suarez F, Lanternier F, Lortholary O, Hermine O, Francès C. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann Rheum Dis. 2010;69(10):1838–41.
Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, Jara-Acevedo M, Teodósio C, García-Cosío M, Bellas C, Orfao A. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124(3):514–21.
Johansson C, Roupe G, Lindstedt G, Mellström D. Bone density, bone markers and bone radiological features in mastocytosis. Age Ageing. 1996;25(1):1–7.
Rossini M, Zanotti R, Viapiana O, Tripi G, Idolazzi L, Biondan M, Orsolini G, Bonadonna P, Adami S, Gatti D. Zoledronic acid in osteoporosis secondary to mastocytosis. Am J Med. 2014;127(11):1127.e1–4.
Kushnir-Sukhov NM, Brittain E, Reynolds JC, Akin C, Metcalfe DD. Elevated tryptase levels are associated with greater bone density in a cohort of patients with mastocytosis. Int Arch Allergy Immunol. 2006;139(3):265–70.
Rossini M, Zanotti R, Viapiana O, Tripi G, Orsolini G, et al. Bone involvement and osteoporosis in mastocytosis. Immunol Allergy Clin N Am. 2014;34(2):383–96.
Laroche M, Bret J, Brouchet A, Mazières B. Clinical and densitometric efficacy of the association of interferon alpha and pamidronate in the treatment of osteoporosis in patients with systemic mastocytosis. Clin Rheumatol. 2007;26(2):242–3.
Lim AY, Ostor AJ, Love S, Crisp AJ. Systemic mastocytosis: a rare cause of osteoporosis and its response to bisphosphonate treatment. Ann Rheum Dis. 2005;64(6):965–6.
Marshall A, Kavanagh RT, Crisp AJ. The effect of pamidronate on lumbar spine bone density and pain in osteoporosis secondary to systemic mastocytosis. Br J Rheumatol. 1997;36(3):393–6.
Escribano L, Akin C, Castells M, Schwartz LB. Current options in the treatment of mast cell mediator-related symptoms in mastocytosis. Inflamm Allergy Drug Targets. 2006;5(1):61–77.
Bonilla FA. Intravenous and subcutaneous immunoglobulin G replacement therapy. Allergy Asthma Proc. 2016;37(6):426–31.
Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756.
Gutiérrez-Fernández D, Cruz MJ, Foncubierta-Fernández A, Moreno-Ancillo A, et al. Monoclonal antibody desensitization in a patient with a generalized urticarial reaction following denosumab administration. Allergy Asthma Clin Immunol. 2015;11:29.
Cundy T, Beneton MN, Darby AJ, Marshall WJ, Kanis JA. Osteopenia in systemic mastocytosis: natural history and responses to treatment with inhibitors of bone resorption. Bone. 1987;8(3):149–55.
Harvey JA, Anderson HC, Borek D, Morris D, Lukert BP. Osteoporosis associated with mastocytosis confined to bone: report of two cases. Bone. 1989;10(4):237–41.
Cosman F, Crittenden DB, Adachi JD, Binkley N, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–43.
McDonald MM, Delgado-Calle J. Sclerostin: an emerging target for the treatment of cancer-induced bone disease. Curr Osteoporos Rep. 2017;15(6):532–41.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907.
Ribatti D, Nico B, Finato N, Crivellato E, Beltrami CA. Co-localization of tryptase and cathepsin-G in mast cells in cutaneous mastocytosis. Cancer Lett. 2009;279(2):209–12.
Rapid responses to avapritinib (BLU-285) in mastocytosis. Cancer Discov. 2018;8(2):133.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Asadipooya, K., Greene, L.W. (2020). Systemic Mastocytosis and Bone-Related Events. In: Akin, C. (eds) Mastocytosis. Springer, Cham. https://doi.org/10.1007/978-3-030-27820-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-27820-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27822-9
Online ISBN: 978-3-030-27820-5
eBook Packages: MedicineMedicine (R0)