Abstract
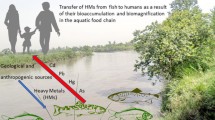
The presence of metals in aquatic environments, as a result of human activities, is common. Many water bodies have great importance for aquaculture, which produces and supplies organisms for the food market. Species consumed in Mexico include fish and shellfish from freshwater, brackish, and marine origin. Such is the case of tilapia and oysters that grow in Tenango dam and the estuary of Tecolutla, respectively. The presence of metals and its effects have not been studied in depth in these systems, or the information is restricted.
Biomarkers measurement as an ecotoxicological tool facilitates the diagnosis of the health status of organisms exposed to contaminants. An example of this is metallothioneins, which are biomarkers of exposure that are positively correlated with the concentration of metals present in individuals affected by metal pollution. Also, there are biomarkers of effect, which show biochemical, physiological, or behavioral alterations in organisms exposed to some contaminants; an example is the lysosomal membrane stability and other physiological parameters. Measurement of contaminant concentrations and related biomarkers can be used as an instrument to determine if an organism should be considered an acceptable resource for consumption. Finally, the bioavailability of toxic metals and their trophic transference are important due to the implicit risk to the species and human health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Metals
- Crassostrea virginica
- Oreochromis niloticus
- Biomarker
- Metallothionein
- Bioaccumulation
- Tenango dam
- Tecolutla estuary
12.1 Introduction
Wastewater frequently contains toxic metals and other contaminants that can be accumulated in the organisms that live in Mexico’s aquatic environments, which are exploitable resources. Their consumption can transfer contaminants to the highest trophic levels. Their effects on organisms result from direct contact with water, as well as from consumption of contaminated prey. This is also valid for people.
In the world, 97% of the water is marine, and, therefore, freshwater constitutes only a small portion, but it is very important as a potable water source and for other uses (Prescott et al. 2000). Lately, water quality has been compromised by the emission of contaminants. In the marine environment, pollution has increased especially in coastal areas. In continental areas, a variety of activities like mining, agriculture, and industry dump their wastes in the aquatic ecosystems. These toxic compounds have a direct impact on the aquatic organisms, and this depends on their chemical composition. Their effects can be manifested at the short, medium, or large term, which depends on their concentration, dispersion mechanisms, and toxicity level. Effects include physiological and biochemical alterations of the organism functions, increased susceptibility to illness (pathogen agents), as well as a decreased resistance to environmental factors. Contaminants can influence reproductive capacity and therefore species survival and, in severe situations, the result can be the extermination of wildlife populations (Dillon and Lynch 1981; Ramade 1989). Water pollution puts human beings at risk since their survival depends directly on water quality, which can have direct effects on their health and on organisms in general (Carabias and Landa 2006).
Water pollution studies have become relevant due to the growing demand for this resource, the economic activities that accompany human demographic growth, and the growing shortage of this resource stemmed from climate changes. As a consequence, there has been an increment in the exploration to create new wells for water supply and to increase storage through dams (Schmitz 1995).
A serious and recurring problem is the entry to aquatic ecosystems of domestic and industrial untreated wastewaters, which adds nutrients like phosphorus, nitrogen, and organic matter. All this modifies the ecological processes and may cause dissolved oxygen depletion, pH alterations, and introduction of coliform bacteria, among other consequences. Wastewaters frequently contain toxic metals and pesticides that are deposited and accumulated in the receptor aquatic system (Valdez and Vázquez 2003).
Toxic metals commonly found in aquatic ecosystems include lead, cadmium, chromium, and copper. The first two are not essential, and their toxic effects can hardly be compensated at the metabolic level. On the other hand, chromium and copper are essential, cells require small quantities, and therefore, there is some tolerance toward them. However, above certain quantities, these too can be toxic.
Lead is used in paints, ceramics, printing, batteries, gasoline, and other products. The main intake routes are pulmonary and gastrointestinal. It is distributed through the erythrocytes in which it binds to hemoglobin; it is accumulated in bone, kidney, liver, muscle, and brain tissues. Its toxic effects at the cellular level include the inhibition of ATPase activity, DNA and RNA syntheses, cellular respiration, and neural processes interference (Albert 2011).
Cadmium is used for alloys, welding, galvanizing, electroplating, paints, ceramics, batteries, pesticides, and fertilizers. It is absorbed through the pulmonary route, as well as gastrointestinal and dermal (Albert 2011). It is transported in the blood bound to proteins like metallothioneins (MT), hemoglobin, and albumin. It is deposited in the renal cortex, kidney, and testicles. Cd causes renal alterations, cellular damage, and tumors. Its toxic effects include the inhibition of cellular respiration, protein synthesis, and iron absorption; and it interferes the zinc and calcium metabolism. When Cd is incorporated into the cell, this stimulates the metallothioneins synthesis (Mencías and Mayero 2000).
Chromium is an abundant element in the earth’s crust, and it is used to generate stainless steel, metallic and plastic surface electrolytic covering, ceramic materials manufacturing, food supplements, pesticides, and leather dyes, among other things (Moreno 2003). Cr is absorbed through pulmonary, dermal, and oral routes. It is transported in transferrins, hemoglobin, and metallothioneins. Some of the effects that Cr can cause are gastrointestinal irritation and bleeding with nausea, vomit, diarrhea, aerial track irritation, and dermatitis (Mencías and Mayero 2000; Moreno 2003).
Copper is an essential nutrient widely distributed in nature. Humans use it to manufacture utensils, nutrients, coins, fertilizers, and pesticides, among other things. Cu is an important component of multiple enzymes, like oxidases which play a fundamental part in the cell’s energy metabolisms. It also a part of the superoxide dismutase enzyme, responsible for the reduction of the superoxide radical to peroxide hydrogen, a detoxification process that protects the cell against oxidation. Excess copper can cause an intoxication characterized by loss of function of the previously mentioned enzymes, as well as necrosis and hepatic lesions. The main absorption route is oral, and this metal is transported bound to albumin and metallothioneins (Moreno 2003).
Quantification of water contaminant levels is fundamental in environmental analysis, especially in those cases where there is legislation to protect aquatic and terrestrial ecosystems; however, determination of pollutants concentrations in the environment does not directly inform about the risks to populations in a particular site, and therefore, it is necessary to know the contaminant’s effects on the organisms that persist in the system. These effects can be evaluated through a series of responses that as a group is known as biomarkers .
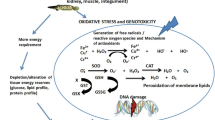
Biomarkers allow the estimation of early damage; they gather responses at different levels of organization, which permits a holistic vision of the toxic effects. The information that they provide is beyond the simple quantification of environmental concentrations (McCarthy et al. 1991). Biomarkers express in a quick, easy, and cheap way the consequences of exposure to one or more toxic agents impacting an environment and can be measured in the organisms (Zapata-Pérez et al. 2002; Gold-Bouchot and Zapata-Pérez 2004). Biomarkers evaluation can be done at different levels: biochemical and molecular (effects on enzymes, proteins, DNA, RNA, and others), cellular (membrane changes and damages), and histological and individual (effects on growth).
Biomarkers are classified into three categories:
-
(a)
Exposure: these allow the detection and measurement of chemical compounds and their metabolites, or register the interaction between a xenobiotic agent with certain molecule or cell, and are evaluated in an organism compartment.
-
(b)
Effect: these measure biochemical, physiological, and other modifications in tissues or fluids, which can be recognized and related to possible alterations in the health status of the organisms.
-
(c)
Susceptibility: these indicate the loss or acquisition of capacities to respond to changes caused by a specific toxic. These include genetic factors and changes in the susceptibility to such exposure (Van der Oost et al. 2003).
Biomarkers responses are generally considered as an intermediate reaction between the exposure to a xenobiotic and evident clinical effects in the individual. When these compensatory effects are activated, the organism’ survival probability declines and it will depend on its capability to adapt to environmental changes (Van Der Oost et al. 2003).
An example of an exposure biomarker associated with the effects of metals is metallothionein (MT). These are low molecular weight proteins that contain cysteine; they can bind a variety of biological important metals like zinc and copper. MT can also bind metals that do not have any known biological function; this is the case of Cd, Pb, and Hg. The concentration of these proteins rises when the concentration of metals increases in organs and tissues like the liver and muscle, among others. That is why it is possible to establish a direct relationship between metal exposure and MT concentration, which in turn allows for the determination of exposure in cases when there are no detectable metal levels in the tissues (Hamilton and Mehrle 1986; Dallinger et al. 1997).
The acetylcholinesterase enzyme activity is a biomarker of effect which is usually used to evaluate neurological effects of diverse toxins like pesticides and metals like lead. This enzyme regulates nerve impulse transmission by hydrolyzing the accumulated acetylcholine in the cholinergic synapsis (Brewer et al. 2000). The inhibition of this enzyme can cause muscular paralysis, which in fish is manifested as disorientation, erratic swimming, and reflex lethargy (Dembele et al. 2000), which in turn causes hematomas due to the animal colliding with the rocks of the aquatic environment.
The study of biomarkers is carried out in test organisms that can be laboratory models (like the zebrafish), as well as in those that are found in contaminated sites (biomonitors). Biomonitor organisms represent an important tool to evaluate the consequences associated with the presence of toxins in the aquatic environment. The accumulation of some toxins occurs in specific organs like lungs, kidneys, and liver and in specific tissues like adipose, muscular, and bones. Pollutants accumulation analysis in organisms is a good indicator of the biological impact since it automatically takes into account their availability to living beings (Borgmann 2000; Chapman 1996).
Biomonitors can be used at the individual, community, and ecosystem level, while the damage they may present can be evaluated through biomarkers at the biochemical, histological, physiological, anatomical, genetic, and reproductive level (Capó 2002; Rosenberg et al. 2008; Prat et al. 2009). Biomonitors should be chosen carefully since the results of their investigation should allow inferring what happens with most of the species that inhabit a specific ecosystem. This type of analysis is denominated as ecotoxicological (Capó 2002). Tataruch and Kierdorf (2003) proposed the following characteristics for a biomonitor species: it should be distributed in one or more big areas so it can be considered representative; its collections should not represent a big effort, its manipulation and identification should be easily performed. Its population density should be high enough so that its sampling does not represent a problem. Its size should be enough to perform the necessary measurements and tissue and organ biopsies. It should be sensitive but at the same time resistant to the contaminants of the environment, so that it can manifest effects but without compromising its descendants. A long life cycle is convenient so that it can accumulate the contaminant and show evident effects along the time. Its importance as an exploitable resource will evidence if there is a risk for human health. An aquatic biological indicator is an organism whose presence and abundance signal a process or the state of the system in which it inhabits (Roldán 1999). Previous knowledge of the species function under normal circumstances is necessary, including life cycle, seasonality, and its natural variations, in a way that it is possible to compare before and after an environmental perturbation.
In Mexico, some species have the previously mentioned characteristics; such is the case of mollusks and fish that live in different environments, particularly of the oyster Crassostrea virginica and the tilapia Oreochromis niloticus which are two species that have been used as bioindicators.
The oyster Crassostrea virginica has demonstrated to be a good laboratory study model, due to the changes it manifests when exposed to contaminants, like behavioral, physiological, biochemical, and genetic modifications. C. virginica can be considered a good biomonitor since it has a wide distribution, it is a benthic sedentary organism in adult life, it resists contaminants, and it can accumulate metals (Baqueiro et al. 2007). Its role in the ecosystem includes the reduction of organic matter. In Mexico, it is distributed in the Gulf of Mexico, where it is extracted and has great commercial importance so that there are two close seasons per year established for its protection in the nation’s laws: NOM-015-SAG/PESC-2016 (SEGOB 2016). The uptake of metals in C. virginica occurs through direct contact of the animal with the metal in water, or through the oral route. This last route is more important when there are solid particles in the media and these are contaminated (Roesijadi 1996; Levine et al. 2006). Once inside the organism, the contaminated particles can interfere with a variety of processes. Metals can be accumulated, but in most benthic filtering organisms, like C. virginica , they hardly reach a dynamic equilibrium, resulting in a long-term presence of the contaminant in its tissues (Mok et al. 2015; Soto-Jiménez 2011). During stressful environmental conditions, the closing of the valves can last many hours, which implies that the filtration process is stopped. This represents a mechanism that minimizes the contact with the toxic. Additionally, when the metal is already in the organism, there are detoxification mechanisms like the induction of proteins specialized in the sequestering of metals, known as metallothioneins (Lemus et al. 2016).
The tilapia O. niloticus is an organism that was introduced to Mexico, and despite not being native, it has adapted itself to the conditions of the country, and it is an important food resource nowadays. There are three species of the Oreochromis genera in the territory: O. mossambicus, O. aureus, and O. niloticus (SAGARPA 2012). Its life cycle consists of various stages: egg, fry, young, juvenile, and adult. It develops between 20 °C and 30 °C. Males mature sexually between 4 and 6 months, while females do it between 3 and 5 months. When they are 6–12-month old, they can weigh between 250 and 500 g, with a length of 12–15 cm. Tilapia is highly accepted in the market due to its high nutritional value. Most commercialized fish in Mexico weigh around 150 and 500 g, and for fillet and exportation around 800 g to 1 kg (SAGARPA 2006, 2012; Saavedra 2006). A variety of toxic compounds affects its quality as a food resource and represents a human health risk; such is the case of Cd, Pb, Cr, and Cu. Tilapia is ideal to evaluate toxic effects because of its accelerated growth rate, continuous reproductive periods, high fecundity, high resistance to illnesses, its omnivore habits, as well as its tolerance to temperature variations, which is why it is distributed in semiwarm and warm waters. Like other fishes, tilapia can bioaccumulate metals in target organs and muscle and will manifest effects like morphometric, physiological, reproductive, and genetic modifications (Ramírez and Mendoza 2008). Numerous toxicological and biological studies have been performed in tilapia.
The objective of this chapter was to integrate information of effects caused by some metals present in Mexican aquatic ecosystems, on two ecologically and commercially important bioindicators, the oyster Crassostrea virginica (Gmelin 1791) and tilapia Oreochromis niloticus (Linnaeus 1758).
12.2 Method
The collection of oysters was carried out in the Gulf of Mexico (Tecolutla River, Veracruz, while tilapia was obtained from an artificial inland dam (Tenango, Puebla). The procedures applied for each species are described below.
12.2.1 C. virginica as a Bioindicator of Metal Trophic Transfer
Specimens of C. virginica were extracted from the Tecolutla estuary (20°28′48″ N, 97°06′40″ W), which is located in the state of Veracruz. This area has a beach expanding 64 km2; the weather is warm and humid with rains during the summer, the median annual temperature is 22 °C, and the average precipitation is 60 mm (H. Ayuntamiento de Tecolutla 2016). The primary economic activities of the region are agriculture, ranching, fishing, and tourism. The Tecolutla River provides most of the organisms that are locally commercialized, including shrimp (Litopenaeus sp.), fishes, crustaceans, and mollusks. The estuary is very important since it is associated with a mangrove forest that helps in the reproduction of the commercialized organisms (INEGI 2016).
Organisms were obtained from the pier of the Tecolutla River (Fig. 12.1, Site 2). In this site and other five located along the river, physicochemical parameters were registered in situ: pH, dissolved oxygen (DO), and temperature with a HANNA potentiometer (HI98128) and a YSI analog oximeter (model 54A). Salinity was measured with an ATC hand refractometer. Additionally, superficial water samples were taken at the same sites to analyze Cu and Cd concentrations.
Water samples were taken manually in 1 L plastic bottles previously washed with 10% HNO3. Samples were fixed to <2 pH with 0.5 mL of nitric acid (reagent grade) and put in a cooler at 4 °C, to be transported to the Ecotoxicology Laboratory in Mexico City where they were frozen to – 20 °C until metal analysis was performed.
Organisms were collected also manually, put in a black plastic bag, and transported to the laboratory in a cooler containing ice, where they were maintained in a water system that mimics the characteristics of its habitat, until the experimental phase.
When the organisms arrived at the laboratory, each oyster was washed under tap water with the help of a brush with plastic bristles to remove sediment, organic matter, and epibionts adhered to the shell; then, the oysters were submerged in a 70% alcohol solution for 10 min and rinsed with artificial seawater.
The maintenance system contained 800 L of artificial seawater (Kent marine), ripen a month in advance, that flowed continuously through 14 interconnected aquariums (to guarantee aeration). The system had a physical filter for the removal of particles, and an activated carbon filter, as well as a skimmer for the removal of proteins. Physicochemical parameters (pH, DO, temperature, and salinity) were evaluated every day. Initially, water salinity was prepared to be similar to that registered in the field, to avoid osmotic stress in the organisms. Later it was slowly increased to 22 ups.
The study of the effects of the metals on the oyster was performed in two phases. The first was to secure a Chlorella sp. culture to feed to the oysters as Cd- or Cu-contaminated food. It was necessary to test the conditions in which the algae could grow exposed to the metals, in order to generate the necessary cell concentration for feeding and, also, to guarantee metal accumulation. In the second phase, the oysters were exposed to contaminated food to evaluate the biomarkers .
Chlorella culture was prepared in a mineral medium with a pH of 6 in artificial seawater (5 ups, Kent marine). Cell concentration counts were done using a Neubauer chamber and a Zeiss optical microscope. 0.01 mg of reagent grade (J.T. Baker) copper or cadmium salts (CuSO4 · 5H2O and CdCl2 · 2 1/2 H2O, respectively) was added to the medium, which implied a Cu concentration of 0.0254 mg/L or a Cd concentration of 0.0493 mg/L, as suggested by Cordero et al. (2005). Chlorella population growth was monitored every 24 h, until the exponential phase and a density of 30 × 106 cells per mL was reached (110 h). Culture media was centrifuged 3 minutes at 500 RPM in a Solbat centrifuge to separate the cell from the mineral medium. The supernatant was eliminated, and the cells were stored in 50 mL Falcon tubes added with artificial salty water at 5 ups, without a mineral medium, to feed the oysters.
Seventy-five organisms that had been fed every 24 h (30 × 106 cells/mL) were exposed either to clean food, Cu-contaminated food, or Cd-contaminated food. Biomarkers (lysosomal membrane stability and metallothioneins) evaluation in oysters was performed every 24 h for a 96 h period; for each time and concentration, five organisms were selected using an aleatory numbers table to avoid bias; morphometric data of each oyster were taken and its condition index calculated.
Lysosomal membrane stability was measured through the neutral red retention technique (Lowe et al. 1995). Slides were previously prepared with 10 μL of a solution containing 15 μL of poly-L-lysine (SIGMA-Aldrich) and 135 μL of distilled sterile filtered water, dried in a humid and dark chamber for 30 min. To extract hemolymph of each animal, its valves were moved away leaving the visceral mass exposed; then with a hypodermic needle containing 0.1 mL of saline physiological solution 0.9 NaCl (PISA), 0.1 mL of hemolymph was extracted from the venous sinus (heart). The needle was removed from the syringe, and the hemolymph was transferred to a silicone Eppendorf tube containing poly-L-lysine. Later 40 μL of the hemolymph and saline solution were dispensed over the slides to proceed to 30 min incubation in a wet chamber, to allow the cells to adhere to the slide. The excess solution was dried with a paper towel, and 40 μL of neutral red solution was added to each slide. The neutral red solution was prepared by diluting 20 mg of neutral red in 1 mL dimethyl sulfoxide; then, 10 μL of this mixture was diluted with 990 mL of saline solution.
Slides were placed in a humid dark chamber, and to observe the coloration, a cover glass was added before reviewing them under an optical microscope at 40×. Observations were done every 15 min during the first hour and then every 30 min for two more hours. To avoid the drying of the samples that can result in dye crystals formation, observation lasted less than 1 min.
Metallothionein analysis (Scheuhammer and Cherian 1986) required the previous hemolysis of heparinized rabbit’s blood; to this end, 10 mL of blood with 20 mL of KCl 1.15% were centrifuged at 500G for 5 min at 10 °C to sediment erythrocytes, which were resuspended with 20 mL of heparinized 1.15% KCl and centrifuged again under the same conditions twice more. Erythrocytes lysis was achieved by resuspending with 15 mL of a Tris 30 mM pH 8 buffer for 10 min. The supernatant was recovered in 1 mL Eppendorf tubes, which were preserved at −85 °C until their use.
MT analysis was performed in the digestive gland of the oysters, which was dissected from each organism analyzed using plastic knives, previously washed with 10% HNO3. The tissue samples were placed in Eppendorf tubes over ice. For each wet gram of tissue, 4 mL of cold sucrose 0.25 M solution was added, followed by a slow homogenization with Teflon pestle, to try to avoid bubble formation that could oxidize the tissues. Later the tissue was centrifuged at 2000g for 20 min at 4 °C. The supernatant was recovered and stored in 2 mL Eppendorf tubes. 375 μL of the supernatant was mixed with 825 μL glycine buffer to obtain a final volume close to 1200 μL. 500 μL of silver solution was added to each sample and incubated in darkness for 20 min, followed by the addition of 200 μL of hemolyzed rabbit’s blood to sweep along those metals not trapped by metallothioneins . Samples were then put in a double boiler for 2 min to eliminate undesirable molecules; then they were centrifuged at 4000 RPM. The supernatant was recovered and centrifuged at 13,000 RPM for 5 min. All samples were kept frozen at – 85 °C in a REVCO freezer until further analysis. MT concentration (μg/g wet weight) was obtained through the detection of silver (Ag) in atomic absorption spectroscopy with Varian AA20 equipment.
12.2.2 O. niloticus as a Bioindicator of the Presence of Metals in the Environment
The tilapia study took place in the Tenango dam (20°12′13″ N y 97°59′27″ W) which is located on the north mountain range of the State of Puebla, close to Tenango de Las Flores town in the Huachinango municipality (Fig. 12.2). It is located 1472 m ASL and it is part of the Necaxa River watershed. The dam belongs to the Necaxa hydroelectric system and has two main water sources the Acatlan and the Nexapa dams, which are connected through tunnels. In turn, the Tenango dam supplies water to the Necaxa dam for the generation of hydroelectric energy (INE-UACH 2007). There are touristic activities in the Tenango dam, like boat rides, camping, handicrafts, and ornate plants (chrysanthemums, gardenias, carnations, roses, poinsettias, orchids, cempasuchil, etc.) cultures; these last require the use of chemical fertilizers and pesticides. Among the fertilizers used are blue Nitrofosca, Chilean Nitrate, Urea 94515, Triple 20, and Multi-npk. In regard to the pesticides, Benomilo©, Folicure©, Talstar©, Tecto 60©, and Amistar are used to fight fungus, while Furadan©, Dioxinon©, Foley©, and Compo© are used to fight insects and Nemacur© and Etoprop© to fight nematodes. These pesticides are applied year round, but Benomilo© and Furadan© are the most used in the area. There is also raw wastewater deposition, coming from the homes located in the dam’s periphery; this is due to the lack of a drainage system. Solid wastes (bottles, clothing, food leftovers, and tires) are also deposited on the dam shores.
The study in Tenango took place along 1 year during which five field trips were accomplished (January, April, June, September, and November) in 2015. Water samples encompassed the whole dam; for metal quantification, APHA (1992) recommendations were applied: samples were taken in plastic bottles that were previously washed with Extran, followed by a 24 h soak in 10% HNO3 and a distilled water rinse. Samples pH was adjusted between 1 and 2 using a Hanna field pH meter (model HI981) and analytic grade Fermont HNO3. Afterward, samples were refrigerated at 4 °C and later transported in a cooler to the ecotoxicology laboratory where they were preserved at −20 °C until further processing.
With regards to fish, 30 tilapias were captured with the help of fishermen and a nylon monofilament net. Morphometric data (weight and length) of each specimen were taken, and a visual inspection was performed checking eye and gills appearance, wounds, lesions, hematomas, deformities and tumors presence, skin color, and general appearance as described in SAGARPA’s production of tilapia manual (2006), where quality standards for tilapia as a consumption product sold to restaurants and the general public are explained.
Muscle and liver samples were obtained from each specimen; one portion was for metals quantification and another for metallothionein evaluation. These were cut using plastic instruments to avoid metals traces contamination. Samples were placed in separate glass containers preciously washed as explained before (APHA 1992; Lozada-Zarate et al. 2006). Samples were transported in a cooler to the Ecotoxicology laboratory where they were frozen to −20 °C until further analysis.
Water samples were analyzed for the presence of Pb, Cd, Cr, and Cu with a Varian 220 FS atomic absorption spectrometer, following the technique explained in NMX-AA-051-SCFI-2001 (Secretaría de Economía 2001). Metal concentrations were compared with Mexican water quality criteria (QWC) for urban use and for the protection of aquatic life (CONAGUA 2016).
In Pb, Cd, Cr, and Cu analysis in fish tissues, liver and muscle samples were weighted and dried at 40 °C in a stove until constant weight was achieved. 0.5 g (dry weight) of each sample was placed in a Teflon cup, and 5 mL of analytic grade HNO3 was added before digesting the sample in a CEM MARSX5 microwave oven at 170 °C for 20 min (EPA 1995). The resulting sample was diluted to a volume of 30 mL with deionized water and analyzed in a Varian 220 FS atomic absorption spectrometer (Lozada-Zarate et al. 2006). Tissues metal levels were compared with the maximum allowed concentrations in norm NOM-242-SSA1-2009 (Secretaría de Salud 2011) and with Brazilian legislation for food for human consumption (LBMP 2017).
Metallothionein production was evaluated with Scheuhammer and Cherian (1986) technique previously described. In this case, tilapia tissues were homogenized with 4 volumes of 0.25 M sucrose (weight/volume = 1:4); the supernatant was frozen at – 85 °C in a REVCO freezer until further analysis.
Results were statistically analyzed with IBM SPSS Statistics 23 and NCSS 2007 software; comparisons were examined with ANOVA or Kruskal-Wallis if data were not normal (Kolmogorov-Smirnov test) and had no homoscedasticity (Levene test). To analyze the variables relationships, Pearson and Spearman’s simple correlations were used according to each case (Sokal and Rohlf 2012). The significant level considered was P < 0.05 (Marques de Cantú 1991).
12.3 Results
12.3.1 Metal Trophic Transfer in C. virginica
The analyzed biomarkers responses indicated harmful effects due to Cu and Cd exposure through ingestion of contaminated Chlorella.
Lysosomal stability was lost, which was observed through the reduction in neutral red dye retention times (NRRT) in lysosomes (Fig. 12.3). Control organisms did not show changes in NRRT throughout the essay with a maximum retention time of 210 min. Adverse effects were observed since the first 24 h. By 72 h, organisms fed with Cu-contaminated cells presented a NRRT close to 24 min. By the end of the assay, those oysters fed with Cd-contaminated algae showed a NRRT of 9 min.
MT analysis in oyster’s digestive gland also showed deleterious effects after 24 h exposure to contaminated algae food. In the Cu assay, there was an increase in MT production with a subsequent reduction after 48 h. For Cd, the increase happened after 24 h of exposure and reached a maximum value at 72 h followed by a decrease at 96 h. Control oysters presented concentrations close to 60.0 μgMT/g tissue throughout the assay, except at 48 h where there was a significant increase (88.5 μgMT/g tissue, P < 0.05, Kruskal-Wallis test). Even when this concentration was higher than those obtained in the other days, the experimental groups (Fig. 12.4; Table 12.1) showed significant differences between them and controls; the MT were manifested in Cu-exposed animals after 24 h (115.6 μgMT/g tissue), and in Cd-exposed oysters, bigger concentration was at 72 h (115.6 μgMT/g tissue).
Other different values were not significantly different from controls. The absence of difference between exposed and control organisms may be due to wide data dispersion, which may mask the possible differences (Fig. 12.5). Standard deviations were always higher in exposed organisms, except at 96 h. In spite of not been able to demonstrate differences, the maximum values registered at 24 h indicate an important induction in oysters exposed to both metals, since they reached their highest values: 172.0 μgMT/g tissue for Cu, and 202.8 μgMT/g tissue for Cd, while the control only reached 107.4 μgMT/g tissue at 96 h.
Water metal pollution in Tenango dam showed significant differences among all collections (Table 12.2).
According to Mexican laws, Pb was the metal that exceeded in more occasions the WQC which occurred in four out of the five collections (January, April, June, and September) (Fig. 12.6). Cr exceeded the WQC in January, April, and September. Cu and Cd had similar behavior, exceeding the WQC for urban use and aquatic life protection in the month of June, while in November they only exceeded the WQC for aquatic life protection.
Metals average concentrations for each sampling. WQC (a) for urban use, 0.05 mgCr/L, and (b) for protection of aquatic life, 0.03 mgPb/L, 0.05 mgCr/L, 0.05 mgCu/L, 0.004 mgCd/L (CONAGUA 2016)
A total of 150 specimens of O. niloticus from the Tenango dam was analyzed; the morphological attributes considered for the visual quality inspection were fulfilled by all organisms, so they were suitable for commercialization (SAGARPA 2006). That is, eyes had black pupils and a crystalline cornea and were convex; gills were bright, pelvic and pectoral fins were present, and the animals did not show cuts, lesions, deformations, or mucus over the body; flesh was firm and elastic and finger pressure did not leave imprints; skin was red, gray, or black or combined as expected; scales loss was less than 15% on the body’s surface. Only in January and April, 4% of the specimens presented hematomas.
There were significant differences in length among collections, and in April and November, Tilapia size was less than acceptable for commercialization. Weight did not present significant differences; however, in all samplings, the organisms presented lower than acceptable values (Table 12.3). Metal concentrations in the liver and muscle presented significant differences among collections.
Pb presented the highest concentration in liver and muscle in January and April; these values exceeded the acceptable concentration in fishing products for human consumption, in accordance with Mexican law NOM-242-SSA1-2009 (Fig. 12.7). Cd presented the second highest concentrations and behavior similar to that of Pb, with higher concentrations in January and April and unacceptable values for human consumption. Cr also presented high values, exceeding the 0.1 mg/kg acceptable level for human consumption, also in January and April. Since Mexican laws don’t include Cu limits, Brazil’s regulation was used to evaluate its presence in fish tissues. It’s worth mentioning that neither FAO’s CODEX Alimentarius nor the European Union include Cu as a substance worth controlling in fishing products for human consumption. Cu was present in small concentrations, below the 30 mg/kg acceptable limit.
Metal concentrations in tilapia liver and muscle for each sampling. (a) Maximum limit (0.5 mg/kg) for fishery products; (b) maximum limit (0.1 mg/kg) for fishery products according to NOM-242-SSA1-2009 (Secretaría de Salud 2011)
Metallothionein concentrations in liver and muscle showed significant differences among collections. The highest concentrations of these proteins were observed in livers extracted in January, September, and November. The muscle MT concentrations presented a similar pattern with higher values in the previously mentioned months; however, these values were smaller than those in the liver, except for November where they were similar (Table 12.4).
O. niloticus weight and length had a significant correlation (P < 0.05) with tissue metal concentration; that is, the higher the size and weight, the higher the liver and muscle metal concentration. Likewise, MT levels and tissue metal concentration had a positive correlation, which implies that the higher the metal concentration in liver and muscle, the higher their MT concentrations. This was confirmed in all collections, except for April, where only muscle Pb and Cr had a positive correlation with MT (Table 12.5).
12.4 Discussion
Mexico has legislation to limit toxic substances concentrations, including some metals, in fresh and refrigerated fish that will be consumed by humans: NOM-242-SSA1-2009 (Secretaría de Salud 2011), and water quality criteria for various uses, including the protection of aquatic life (CONAGUA 2016). In spite of this, the degradation observed in many aquatic ecosystems demonstrates that these laws are not enforced. This situation includes freshwater bodies, as well as estuarine systems , where alimentary resources like Tilapia and oysters are found and caught. Metals presence in water is a determinant factor in their bioaccumulation , but the trophic transfer has not been analyzed in depth.
In the present study, trophic transfer of Cu and Cd in the oyster C. virginica resulted in deleterious effects on the evaluated biomarkers . Even though lysosomal stability is considered a nonspecific biomarker, because the lysosomal membrane can be affected by a variety of toxics, the results of this study demonstrate that the decrease in NRRT is related with the ingestion of contaminated Chlorella cells, which was evident after 24 h of exposure. This biomarker has been evaluated in different mollusk species. Matozzo et al. (2001) exposed Tapes philippinarum to Cu and Cd and observed that their highest concentrations (110 μgCu/L and 450 μgCd/L) resulted in the lowest NRRT, which were close to 5 min compared to 60 min in controls; even their lowest concentration (60 μg/l) reduced the NRRT to 30 min.
On the other hand, studies with C. angulata have shown that as Cu concentration increases, the digestive gland can eliminate the excess metal, when its uptake is through the trophic route. This is because this organ’s lysosomes sequester, neutralize, and eliminate contaminants (Rodríguez de la Rua et al. 2005). Lysosomes are the main cell organelle in charge of receiving and eliminating materials, either foreign or metals transported by MT for sequestering and elimination (Petrovic et al. 2001).
The differences in NRRT observed in the present study in C. virginica can be attributed to the type of metal (essential vs. nonessential). Cu is an essential metal that once inside the organism activates diverse processes related to homeostasis in the cells. In adequate amounts, it helps with the immune system maintenance, the generation of oxide-reduction enzymes, and the formation of molecules associated with genetic expression’s regulatory mechanisms (ATSDR 2016). However, excess Cu (in the food provided in the present study) causes a loss of balance and toxic effects appear in the lysosomes; which in this study were measured as a decrease in NRRT after 48 h. A similar effect was observed after 24 h in the Cd exposure. Lysosomal stability loss in this last experiment was due to the fact that Cd is not essential and is bioaccumulated rapidly, which damages the lysosomes during the first 24 h of exposure. Reduction in retention time has been linked to high levels of metal accumulation in the lysosomes which can cause oxidative stress and lead to an inhibition of the proton pump, in charge of maintaining acid in the internal lysosomal environment (Nassiri et al. 2000).
Metallothioneins are considered exposure biomarkers and according to Capó (2002), they offer a fast answer to detect metal pollution in disturbed environments, which makes them very useful for environmental analysis. These proteins constitute a primary defense mechanism to avoid damage on the cell components in the presence of metals like enzyme-specific sites, structural proteins, lipid membranes, and even DNA (Giguére et al. 2003). They also comprise protection against oxidation and free radicals (Coyle et al. 2002).
MT role in the cells is to mobilize essential metals, regulating and directing them to the lysosome for deposition or elimination; therefore, a detoxification role is also attributed to them (Tanguy et al. 2001; Moulis 2010). Due to MT affinity to metals, when organisms are exposed to high concentrations, these proteins can be increased. In bivalve mollusks like C. virginica and C. gigas and other invertebrates, MT have been proven to help transport, regulate, and control Cu and Zn levels, which are essential elements, but also Cd that is not essential (Ettajani et al. 2001). Many of these investigations evaluate MT levels associated with metal concentrations in the water where these mollusks inhabit. In a study with Mytilus edulis, MT induction was observed in the digestive gland when exposed to low Cu concentrations for short periods of time; under these conditions, MT support metal transport. However, when Cu concentrations are raised (above 10 μg/L), or the exposure is prolonged, these proteins concentration gradually decreases, which prevents them to fulfill their functions (Perić et al. 2017). Research with Mytilus (Brown et al. 2004; Pytharopoulou et al. 2011; María and Bebianno 2011) demonstrated that under acute Cu exposure (50–100 μg/L), this metal is accumulated in the digestive gland, while MT induction is reduced. In this work, MT induction through metal trophic transfer was evident after 24 h and in the case of Cu, there was a significant reduction after 72 h. Serafim and Bebianno (2009) have related a MT reduction with a break in the MT/Cu complex, which indicates that when there is depuration, MT decrease. In the case of MT induction due to Cd-contaminated food, the highest concentrations were observed after 72 h (with a 141.2 μgMT/g tissue average and a maximum of 194.9 μgMT/g tissue). Decrease after 96 h (average of 109.5 μgMT/g tissue) did not reach levels as low as those associated with Cu feeding (average 83.8 μgMT/g tissue) and even less than those of controls (60.2 μgMT/g tissue). Amiard et al. (2006) and Ivanina et al. (2008) demonstrated that MT are expressed under stress conditions caused by Cd in C. virginica . When this metal is incorporated through trophic route, it is captured by the digestive gland, where MT act with other proteins, to capture and detoxify this metal. This is why MT can be expressed in a short time in exposed organisms; and, when Cd decreases in the individuals, MT are also reduced (Perić et al. 2017). Unlike these previously mentioned studies, in the present work, MT Cd induction occurred later than that of Cu.
MT response in different tissues is not the same; their induction is faster when exposure is through the gills since this organ is sensitive to environmental changes (Coyle et al. 2002). MT are more active in the gill than in the digestive gland (Serafim and Bebianno 2009). On the other hand, the digestive gland is an organ that under normal circumstances is in constant renewal of its functional units (Coyle et al. 2002). Barrera (2006) documented MT concentrations in C. virginica up to 471 μgMT/g tissue in digestive gland and 275 μgMT/g tissue in gills on oysters exposed to 110 μg/L and 211 μg/L of Cd. This is almost double than the levels detected in gills. In another work (Ivanina et al. 2008), where oysters were exposed to Cd, digestive gland MT levels after 4 h were double than the concentrations found in the gills. While Cd concentration increases in the medium, MT in the digestive gland are very effective at capturing most metal molecules; therefore, depuration is considered effective, and as a consequence gills MT report lower concentrations. Blackmore and Wang (2004) pointed out that once a particle moves in the digestive gland, intra- and extracellular digestion takes place in the tubules, so an excess of metallic particles can damage this organ compromising its function. Petrovic et al. (2001) mentioned that when metals are incorporated into the cell, MT spring into action, particularly if metal concentrations are high. In this case, the detoxification process is carried out by sequestration and transport of metals to the digestive gland lysosomes. These processes explain the obtained results since there was a MT increment in the digestive gland of C. virginica, more evident after 24 h of exposure for both metals, and highest after 72 h for Cd. In addition, NRRT decreased after 24 h and continued diminishing until the end of the experiment (96 h), which indicates that lysosomes were destabilized. Regardless of the metal type (essential or not essential), when MT transport metals to the lysosomes, these organelles’ integrity is compromised.
C. virginica is a species that lives in brackish environments, where metals tend to precipitate, and therefore, its uptake through particle matter is possible, as well as through trophic transfer; in contrast, freshwater species are more exposed to dissolved metals. O. niloticus is very well adapted to Mexican environments, and due to the lack of control over wastewater discharges to dams and other aquatic systems, it is convenient to find out if this fishing resource is affected by pollution.
To study in O. niloticus the relationship between morphological parameters and biomarkers in the presence of metals, monitoring was carried out in the Tenango dam during an annual cycle.
Metal concentrations in the Tenango dam appeared to be related to the management of the water that is allowed to enter and exit the system. The increase in Pb and Cr in January and April may be explained assuming that during these months the dam received water from the Nexapa dam when gates were open as a precautionary measure to lower the water level in this last dam after a period of intense rains, registered in the area during the samplings. This could have dragged metallic traces, detected as higher concentrations in these months. Cd and Cu presented higher concentrations in June, which is when rains started, and they probably dragged materials from the tourism and agricultural areas that border the dam. These activities discard batteries, tires, plastic bottles, fertilizer, and pesticide containers that may contain metallic traces in their manufacturing (Moreno 2003). Combined with the former, there are entries of residual waste water from the houses located in the periphery of the study site. These are characterized by their high metal content (González-Ramírez et al. 2009), so these discharges also contribute to the pollution of the site.
High water metal concentrations vary along the samplings and could have caused a variety of toxic effects on the local fishes, including O. niloticus . Pb reached an average of 3.2 mg/L and 2.9 mg/L in January and April, respectively. These concentrations are 100 times higher than the Mexican WQC for the protection of aquatic life. These values may cause deleterious effects like reduction in the fishes movement ability, oxidative stress, and erythrocyte damage, as has been demonstrated in previous studies of lead toxicity in tilapia and other fish species, at exposure concentrations of 0.8–1.6 mg/L (El-Badawi 2005; Ercal et al. 2001; Hou et al. 2011). Cr also presented elevated concentrations (eight times above the WQC) in the same months, 0.42 mg/L in January and 0.16 mg/L in April. Exposure to this metal may cause excess mucus secretion, respiratory and osmoregulatory capacity reduction, blood vessel congestion, and spermatozoid hypertrophy, as has been demonstrated in studies where tilapia and trout have been exposed to Cr concentrations of 0.01, 0.10, and 0.35 mg/L (Ackermann 2008; Arillo and Melodio 1988). Tilapia bioconcentration reflected the observed Pb and Cr increment, since their muscle concentrations were the highest in January and April (6.82mgPb/kg, 3.68 mgPb/kg, 0.89 mgCr/kg and 0.53 mgCr/kg). These were 14 and 9 times higher than the acceptable limits for Pb and Cr, respectively. It is necessary to emphasize that fish consumption with Pb concentrations like the ones found in this work has been associated with intoxications characterized by a decrease in protoporphyrins, erythrocyte pigmentation, abdominal pain, headaches, constipation, and nausea (Valle 2000). The registered Cr concentrations can be linked to gastrointestinal irritation, abdominal pain, vomit, and diarrhea (Mencías and Mayero 2000).
Cd concentrations in water were 30 times higher than WQC in June (0.13 mg/L average). Exposures to 0.1 mg Cd/L cause blood vessel congestion, hemoglobin concentration reduction, and cellular inflammation in tilapia (Dyk et al. 2007). Cu water concentrations were also 30 times higher than QWC in June (1.37 mg/L average). 0.02 mg Cu/L causes variation in methionine, histidine, and cysteine concentrations in the aquatic organism, due to the generation of oxygen reactive species (Harris and Githlin 1996; Grosell and Wood 2002). High Cd concentrations in tilapia did not coincide with high water concentrations; in contrast, Pb and Cr did match. High concentrations of Cd were observed in January and April (average 1.89 mg/kg and 2.06 mg/kg, respectively) and were four times higher than muscle acceptable levels. Concentrations like these have been related to renal tube small alterations in fish consumers (Pérez and Azcona 2012). In contrast, Cu concentrations did not exceed the limits defined by the criteria.
Except for Cd, metal concentrations were higher in the liver than in the muscle in O. niloticus , which coincides with Abdulali et al. (2012) results.
Regarding tilapia quality, the morphological characteristics required for their sell did not show evident imperfections; therefore, from this point of view, tilapia has good quality for commercialization and consumption in the Tenango dam . The only characteristics out of criteria were length and weight, which were lower than desired. This may be a consequence of an excessive fishing effort on this resource that does not allow its recovery. Small size implies that young specimens are extracted. However, other studies point out that having a small size may be due to the deleterious effects of the presence of metals like Cd, Cr, and Cu, since growth and development problems have been reported in fish exposed to these metals (Shiau and Ning 2003; Abbas et al. 2007; Reid 2011).
Even though Cu concentrations exceeded WQC in June, tilapia’s levels did not surpass the criteria for their consumption in any specimen.
With regard to the observed statistical relationships, metal concentration-weight and metal concentration-length coincide with Authman’s study (2008), where tilapia metal uptake occurs gradually; so, bigger organisms tend to present the highest metal concentrations. This was the case even for Cu, which was not bioaccumulated in high concentrations. In addition, authors like Canli and Atli (2003) and Evans et al. (1993) stated that metal uptake in fish is favored by feeding and respiration, and in some cases metabolic regulation decreases as animals grow old and therefore, they tend to accumulate more metals.
Many metals can induce MT synthesis to regulate the concentrations of these molecules inside the organism; and, the higher the metal concentration, the higher MT concentration Atli and Canli 2003; Roesijadi 1992; Hamilton and Mehrle 1986). This was also observed in the present study since a direct relationship was found, indicating that MT were induced by the presence and the concentration of the metals. The highest metals and MT concentrations were found in the liver, which resembles the results of Gülüzar and Canli (2008), Hauser-Davis et al. (2014), Lim et al. (1998), Chatterjee et al. (2016), and Chandrasekera et al. (2008). In these studies, the liver accumulated more metals, and more MT were induced, with respect to other tissues.
At the international level, O. niloticus has been used in numerous studies to analyze human health risks due to fish consumption; to accomplish this, metal concentrations were quantified in fish muscle (Adazabra et al. 2014; Authman 2008; Cleoni Dos Santos et al. 2012; Muhammad et al. 2013; Mohamed and Osman 2014; Mulu and Mehari 2013; Pezo et al. 1992; Taweel et al. 2011; Yilmaz 2009).
In Mexico, tilapia (O. niloticus) has been analyzed in many studies. It was used as a biomonitor in the Metztitlan lagoon (Hidalgo State) to quantify metal concentrations (Lozada-Zarate et al. 2006); fish from the Fernando Hirirart Balderrama dam (also in Hidalgo State) were used to evaluate arsenic toxicity (Báez 2001). In the State of Tamaulipas, metals were quantified in specimens from the Laguito de Nuevo Laredo (Ramos et al. 2004); and in Chiapas State, metals and their effects were evaluated through biomarkers (Gold-Bouchot et al. 2006).
The importance of biomarkers studies in mollusks and fish from Mexican aquatic ecosystems relies on the fact that they are considered good-quality foods. If bad quality is demonstrated, even when morphological characteristics are acceptable, their consumption may imply a health risk. These types of studies are scarce in Mexico, which shows the importance of continuous monitoring in aquatic ecosystems to guarantee food quality and the ecosystem’s health, which today is not done.
12.5 Conclusions
Metal trophic transfer cause damages in the oyster C. virginica similar to those caused by water exposure.
The observed lysosomal membrane destabilization in the oyster indicated that there was oxidative stress related to trophic exposure to both metals after 24 h. Similarly, MT were expressed in the same period, in higher quantities in those organisms exposed to Cd. We propose that the higher activity of these proteins caused the rapid degradation of the lysosomes.
Metal water concentrations in the Tenango dam may be due to the entry of water from other dams that are connected to this study site; they can contribute with metal traces. Pb and Cr increases support this statement.
Cd and Cu increases in the dam’s water were related to potential runoffs, caused by rains, from the agricultural areas that may contain metal traces from fertilizers and pesticides.
Metal concentrations in the water of the Tenango dam prevent its exploitation for urban use and represent a risk for aquatic life.
The tilapia O. niloticus was a good biomonitor since it bioaccumulated the metals present in the dam’s water, but also it exhibited the deleterious effects expected from metals exposure.
Visually, tilapia fulfilled the quality specifications for its sale to the public; however, metal concentrations in the muscle tissue make it undesirable for human consumption. It is probable that the effects of the water metal concentrations are the reason for the fish small size and weight.
Pb and Cu are the metals that represent the highest risk for tilapia in the dam, since they contribute to the reduction of its quality as a food resource; in the future it could affect the fishermen’s economy.
The biomarkers evaluated in both species work out adequately and showed the exposure and effect associated with exposure to metals, either through trophic transfer or through contact with the contaminated water.
This study points to fact that the evaluated fishing resources are at risk due to metal exposure, and suggests that this may be the case of other Mexican aquatic ecosystems.
References
Abbas HHH, Hammada MM, Miller JD (2007) Vitamin C and cadmium toxicity in fish Oreochromis niloticus. Online J Vet Res 11(1):54–74
Abdulali KA, Shuhaimi-Othman M, Ahmad AK (2012) Analysis of heavy metal concentrations in Tilpia fish (Oreochromis niloticus) from four selected markets in Selangor, Peninsular Malaysia. J Biol Sci 12(3):138–145
Ackermann C (2008) A quantitative and qualitative histological assessment of selected organs of Oreochromis mossambicus after acute exposure to cadmium, chromium and nickel. University of Johannesburg, South Africa, M. Sc. dissertation
Adazabra AN, Kombat EO, Fletcher JJ (2014) Parameterization of non-essential heavy metals concentration in different tissues of inland commercial fish Oreochromis niloticus from Vea Dam, Bolgatanga, Northern Ghana. Int J Curr Res Acad Rev 2(7):247–258
Agencia para Sustancias Tóxicas y el Registro de Enfermedades (ATSDR) (2016) Reseña Toxicológica del Cobre. Atlanta: GA. Departamento de Salud y Servicios Humanos de EE. UU., Servicio de Salud Pública. Recuperado el 10 mayo del 2018. Disponible en https://www.atsdr.cdc.gov/es/phs/es_phs132.html
Albert LA (2011) Curso Básico de toxicología ambiental. Limusa, México
American Public Health Association (APHA) (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, D. C.
Amiard J, Amiard-Triquet C, Barka S, Pellerin J, Rainbow P (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76(2):160–202
Arillo A, Melodio F (1988) Effects of hexavalent chromium on trout mitochondria. Toxicol Lett 44:71–76
Atli G, Canli M (2003) Natural occurrence of metallothionein-like protein in liver of fish Orechromis niloticus and effects of cadmium, lead, copper, zinc, and iron exposures on their profiles. Bull Environ Contam Toxicol 70:619–627
Authman MMN (2008) Orechromis niloticus as a biomonitor of heavy metal pollution with emphasis on potential risk and relation to some biological aspects. Glob Vet 2(3):104–109
Báez ROA (2001) Toxicidad del arsénico de fuentes subterráneas naturales de agua potable y presa Fernando Hirirart Balderrama de Zimapán, Hgo. en Oreochromis niloticus. Tesis de maestría. Instituto Politécnico Nacional (IPN), D.F. México
Baqueiro CER, Borabe L, Goldaracena ICG, Rogríguez NJ (2007) Los moluscos y la contaminación. Una revisión. Revista Mexicana de Biodiversidad Suplemento 78:1–7
Barrera EG (2006) Toxicidad del Cromo y Cadmio en ostión Crassostrea virginica de la laguna de Mandinga, Ver. Tesis de Doctorado en Ciencias Biológicas. Universidad Autónoma Metropolitana. México, D.F. 229 pp.
Blackmore G, Wang W-X (2004) The transfer of cadmium, mercury, methylmercury, and zinc in an intertidal rocky shore food chain. J Exp Mar Biol Ecol 307(1):91–110
Borgmann U (2000) Methods for assessing the toxicological significance of metals in aquatic ecosystems: bioaccumulation-toxicity relationships water concentrations and sediment spiking approaches. Aquat Ecosyst Health Manag 3:277–289
Brewer SK, Little EE, Delonay AJ, Beauvais SL, Jones SB, Ellersieck MR (2000) Behavioral dysfunctions correlate to altered physiology in rainbow trout (Oncorhynchus mykiss) exposed to cholinesterase-inhibiting chemicals. Arch Environ Contam Toxicol 40:70–76
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66(3):267–278
Canli M, Atli G (2003) The relationships between heavy metals (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121(1):129–136
Capó MA (2002) Principios de Ecotoxicología. In: Diagnóstico, tratamiento y gestión del medio ambiente. España, McGraw-Hill
Carabias J, Landa R (2006) Agua, medio ambiente y sociedad. Colegio de México, México
Chandrasekera LWHU, Pathiratne A, Pathiratne KAS (2008) Effects of water borne cadmium on biomarker enzymes and metallothioneins in Nile tilapia, Oreochromis niloticus. J Natl Sci Found 36(4):315–322
Chapman D (1996) Water quality assessments: a guide to the use of biota, sediments and water in environmental monitoring. Chapman Hill, Londres, p 626
Chatterjee S, Datta S, Das TK, Veer V, Mishra D, Chakraborty A, Chattopadhyay B, Datta S, Mukhopadhyay KS, Gupta KD (2016) Metal accumulation and metallothionein induction in Orechromis niloticus grown in waster fed fishponds. Ecol Eng 90(16):405–416
Cleoni Dos Santos C, Aline BV, Sobreiro SH, Gaeta EE, Narciso FM (2012) Biomarker responses as indication of contaminant effects in Orechromis niloticus. Chemosphere 89:60–69
Comisión Nacional del Agua (CONAGUA) (2016) Ley federal de derechos. Disposiciones aplicables en materia de aguas nacionales. Diario Oficial de la Federación, México. Noviembre 13
Cordero J, Guevara M, Morales E, Lodeiros C (2005) Efecto de metales pesados en el crecimiento de la microalga tropical Tetraselmis chuii (Prasinophyceae). Rev Biol Trop 53:325–330
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59(4):627–647
Dallinger R, Egg M, Kock G, Hofer R (1997) The role of metallothionein in cadmium accumulation of Arctic char (Salvelinus alpinus) from high mountain lakes. Aquat Toxicol 38:47–66
Dembele K, Haubruge E, Gaspar C (2000) Concentration effects of selected insecticides on brain acetylcholinesterase in the common carp (Cyprinus carpio L.). Ecotoxicol Environ Saf 45:49–54
Dillon TM, Lynch MP (1981) Physiological response with determinative stress in marine and estuarine organisms. In: Barret W, Rosemberg R (eds) Stress effect on natural ecosystems. Wiley, Chichester, pp 56–134
Dyk JCV, Pieterse GM, Van Vuren JHJ (2007) Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol Environ Saf 66:432–440
El-Badawi AA (2005) Effect of lead toxicity on some physiological aspects of Nile tilapia fish, Oreochromis niloticus. In: International conferences of the veterinary research division. NRC, Cairo
EPA (1995) Method 3015A. Microwave assisted acid digestion of aqueous sample an extract. https://www.epa.gov/sites/production/files/2015-12/documents/3015a.pdf
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539
Ettajani H, Berthet B, Amiard JC, Chevolot L (2001) Determination of cadmium partitioning in microalgae and oyster’s contribution to the assessment of trophic transfer. Arch Environ Contam Toxicol 40:209–221
Evans DW, Dodoo DK, Hanson PJ (1993) Trace elements concentrations in fish livers implications of variations with fish size in pollution monitoring. Mar Pollut Bull 26(6):329–334
Giguére A, Couillard Y, Campbell PG, Perceval O, Hare L, Pinel-Alloul B, Pellerin J (2003) Steady-state distribution of metals among metallothionein and other cytosolic ligands and links to cytotoxicity in bivalves living along a polymetallic gradient. Aquat Toxicol 64(2):185–200
Gold-Bouchot G, Zapata-Pérez O (2004) Contaminación, ecotoxicología y manejo costero. In: Rivera Arriaga E, Villalobos GJ, Adeath IA, May FR (eds) El Manejo Costero en México, vol 654. Universidad Autónoma de Campeche, SEMARNAT, CETYS-Universidad, Universidad de Quintana Roo, pp 277–286
Gold-Bouchot G, Zapata-Pérez O, Rodríguez-Fuentes G, Ceja-Moreno V, Rio-García M, Chan-Cocom E (2006) Biomarkers and pollutants in the Nile Tilapia, Orechromis niloticus, in four lakes from San Miguel, Chiapas, Mexico. Int J Environ Pollut 26(1–3):129–141
González-Ramírez CA, Prieto-García F, Prieto-Méndez J, Román-Gutiérrez AD (2009) Contaminación y fitotoxicidad en plantas por metales pesados provenientes de suelos y agua. Trop Subtrop Agroecosyst. Sin mes:29–44
Grosell M, Wood CM (2002) Copper uptake across rainbow trout gills: mechanisms of apical entry. J Exp Biol 205:1179–1188
Gülüzar A, Canli M (2008) Responses of metallothionein and reduced glutathione in a freshwater fish Oreochromis niloticus following metal exposures. Environ Toxicol Pharmacol 25:33–38
H. Ayuntamiento de Tecolutla 2014–2017 (2016). Disponible en http://www.tecolutla.gob.mx
Hamilton SJ, Mehrle PM (1986) Metallothionein in fish: a review of its importance in assessing stress from metal contaminants. Trans Am Fish Soc 115:596–609
Harris ZL, Githlin JD (1996) Genetic and molecular basis for cooper toxicity. Am J Clin Nutr 63:836S–841S
Hauser-Davis RA, Bastos FF, Tuton B, Chávez RR, Saint PT, Ziolli LR, Arruda MAZ (2014) Bile and liver metallothionein behavior in copper-exposed fish. J Trace Elem Med Biol 28:70–74
Hou JL, Zhuang P, Zhang LZ, Feng L, Zhang T, Lium JY (2011) Morphological deformities and recovery, accumulation and elimination of lead in body tissues of Chinese sturgeon, Acipenser sinensis, early life stages: a laboratory study. J Appl Ichthyol 27:514–519
Instituto Nacional de Ecología-Universidad Autónoma de Chihuahua (INE-UACH) (2007) Estudio de Ordenamiento Ecológico Territorial de las Cuencas Hidrológicas de los ríos Necaxa y Laxaxalpa. https://www.agua.org.mx/biblioteca-tematica/manejo-de-cuencas/95%2D%2Dsp942/676-ordenamiento-de-las-cuencas-de-los-rios-necaxa-y-laxaxalpa. Consultado el 15 de mayo de 2017
Instituto Nacional de Estadística y Geografía (INEGI) (2016) Catálogo Nacional de Indicadores. Disponible en: http://www3.inegi.org.mx
Ivanina AV, Cherkasov AS, Sokolova IM (2008) Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters Crassostrea virginica Gmelin. J Exp Biol 211(4):577–586
Legislación brasileña de metales pesados (LBMP) (2017) Metales pesados en materia de alimentos. http://paguicidas.comercio/MetalPesado.pdf. Consultado el 15 de mayo de 2017
Lemus M, Salazar R, Lapo B, Chung K (2016) Metalotioneínas en bivalvos marinos. Lat Am J Aquat Res 44(2):202–215
Levine JF, Law M, Corsin F (2006) Bivalvos. In: Lewbart GA (ed) Medicina de los invertebrados. Editorial Acribia, S.A. Zaragoza, España, pp 111–140
Lim LK, Wai KP, Ka-Yee J, Ming CK (1998) Metal toxicity and metallothionein gene expression estudies in common Carp and Tilapia. Mar Environ Res 46(1–5):563–566
Lowe DM, Soverchia C, Moore MN (1995) Lysosomal membrane responses in the blood and digestive cells of mussels experimentally exposed to fluoranthene. Aquat Toxicol 33(2):105–112
Lozada-Zarate EJ, Monks S, Pulido-Flores G, Gordillo-Martínez AJ, Prieto-García F (2006) Determinanción de metales pesados en Oreochromis niloticus en la laguna de Metztitlan, Hidalgo, México. https://www.researchgate.net/profile/Alberto_Martinez15/publication/209827913_Determinacion_de_metales_pesados_en_Cyprinus_carpio_en_la_Laguna_de_Metztitlan_Hidalgo_Mexico/links/0046353bd88c3a19d8000000/Determinacion-de-metales-pesados-en-Cyprinus-carpio-en-la-Laguna-de-Metztitlan-Hidalgo-Mexico.pdf. Consultado el 24 de abril de 2018
María VL, Bebianno MJ (2011) Antioxidant and lipid peroxidation responses in Mytilus galloprovincialis exposed to mixtures of benzo (a) pyrene and copper. Comp Biochem Physiol 154(1):56–63
Marques de Cantú MJ (1991) Probabilidad y estadística para ciencias quimicobiológicas. McGraw-Hill, México, 657 p
Matozzo V, Ballarin L, Pampanin DM, Marin MG (2001) Effects of copper and cadmium exposure on functional responses of hemocytes in the clam, Tapes philippinarum. Mar Arch Environ Contam Toxicol 41:163–170
McCarthy JF, Halbrook RS, Shugart LR (1991) Conceptual strategy for design, implementation, and validation of a biomarker-based biomonitoring capability. Oak Ridge National Lab, Oak Ridge
Mencías RE, Mayero FLM (2000) Manual de toxicología básica. Díaz de Santos. Madrid, Madrid
Mohamed EH, Osman AR (2014) Heavy metals concentration in water, muscles and gills of Orechromis niloticus collected from the sewage-treated water and the White Nile. Int J Aquac 4(6):36–42
Mok JS, Yoo HD, Kim PH, Yoon HD, Park YC, Lee TS, Kwon JY, Son KT, Lee HJ, Ha KS, Shim KB, Kim JH (2015) Bioaccumulation of heavy metals in oysters from the southern coast of Korea: assessment of potential risk to human health. Bull Environ Contam Toxicol 94(6):749–755
Moreno GMD (2003) Toxicología ambiental. México, McGraw Hill
Moulis JM (2010) Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 23(5):877–896
Muhammad JL, Shehu RA, Bilbis LS, Dangoggo SM (2013) Estimation of some heavy metals and mineral elements in tissues of Orechromis niloticus collected from Goronyo Dam and its two tributaries in North-Western sub-Sahara Nigeria. J Environ Sci Toxicol Food Technol 3(5):46–52
Mulu BD, Mehari MW (2013) Distribution of trace metals in two commercially important fish species (Tilapia zilli and Oreochromis niloticus) sediment and water from lake Gubdahri, Eastern Tigrar of Northern Ethiopia. Int J Sci Res Publ 3(9):1–7
Nassiri Y, Rainbow PS, Amiard-Triquet C, Smith BD, Rainglet F (2000) Trace-metal detoxification in the ventral caeca of Orchestia gammarellus (Crustacea: Amphipoda). Mar Biol 136(3):477–484
Pérez GPE, Azcona CMI (2012) Los efectos del cadmio en la salud. Revista de especialidades Médico-Quirúrgicas 17(3):199–205
Perić L, Nerlović V, Žurga P, Žilić L, Ramšak A (2017) Variations of biomarkers response in mussels Mytilus galloprovincialis to low, moderate and high concentrations of organic chemicals and metals. Chemosphere 174:554–562
Petrovic S, Ozretic B, Krajnovic-Ozretic M, Bobinac D (2001) Lysosomal membrane stability and metallothionein in digestive gland of mussels (Mytilus galloprovincialis Lam.) as biomarkers in a field study. Mar Pollut Bull 42(12):1373–1378
Pezo DR, Paredes AH, Bedayán ANY (1992) Determinación de metales pesados bioacumulables en especies ícticas de consumo humano en la Amazonia peruana. Folia Amazonica 4(2):171–181
Prat N, Ríos B, Acosta R, Rieradevall M (2009) Los macroinvertebrados como indicadores de calidad de las aguas. In: Domínguez E, Fernández HR (eds) Macroinvertebrados bentónicos sudamericanos. Sistemática y biología. Fundación Miguel Lillo, Tucumán, pp 631–654
Prescott LM, Harley JP, Klein DA (2000) Microbiologia. McGraw Hill, España, pp 683–702
Pytharopoulou S, Grintzalis K, Sazakli E, Leotsinidis M, Georgiou CD, Kalpaxis DL (2011) Translational responses and oxidative stress of mussels experimentally exposed to Hg, Cu and Cd: one pattern does not fit at all. Aquat Toxicol 105(1–2):157–165
Ramade F (1989) The pollution of the hydrosphere by global contaminants and its effects on aquatic ecosystems. In: Boudou A, Rybeyre F (eds) Aquatic ecotoxicology: fundamental concepts and methodologies. CRC Press Inc, Boca Raton, pp 152, 352 p–183
Ramírez RP, Mendoza CA (2008) Ensayos toxicológicos para la evaluación de sustancias químicas en agua y suelo. La experiencia en México. SEMARNAT-INE, México
Ramos O, Guevara N, Macías B, Ortiz Y (2004) Evaluación de riesgo a la salud por la presencia de metales pesados en pescado. Toxicología 1:2–4
Reid SD (2011) Molybdenum and chromium. Academic Press, New York
Rodríguez de la Rua A, Arellano J, González M, Blasco J, Sarasquete C (2005) Acumulación de cobre y alteraciones histopatológicas en el ostión Crassostrea angulata. Cienc Mar 31(3):455–466
Roesijadi G (1992) Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat Toxicol 22:81–114
Roesijadi G (1996) Environmental factors: response to metals. In: Kennedy VS, Newell RIE, Eble AF (eds) The eastern oyster Crassostrea virginica. Maryland Sea Grant College, College Park, pp 515–537
Roldán PG (1999) Los macroinvertebrados y su valor como indicadores de la calidad del agua. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 23(88):375–387
Rosenberg DM, King RS, Resh VH (2008) Use of aquatic insects in biomonitoring. In: Merritt RW, Cummins KW, Berg M (eds) An introduction to the aquatic insects of North America, 4th edn. Kendall/Hunt, Dubuque, pp 123–137
Saavedra MA (2006) Manejo del cultivo de tilapia. CIDEA-Coastal Resources Center, Nicaragua
Scheuhammer AM, Cherian MG (1986) Quantification of metallothionein by silver saturation methods. Toxicol Appl Phamarcol 82(3):417–425
Schmitz RJ (1995) Introduction to water pollution biology. Gulf Publishing Co., Huston
Secretaria de Agricultura, Ganadería, Desarrollo regional, Pesca y Alimentación (SAGARPA) (2012) Criterios técnicos y económicos Para la producción sustentable de Tilapia en México. SAGARPA, México
Secretaría de Agricultura, Ganadería, Desarrollo rural, Pesca y Alimento (SAGARPA) (2006) Manual de producción de tilapia con especificaciones de calidad e inocuidad. SAGARPA, México
Secretaría de Economía (2001) NMX-AA-051-SCFI-2001. In: Determinación de metales por absorción atómica en aguas naturales, potables, residuales y residuales tratadas. México, Diario oficial de la federación
Secretaría de Gobernación (SEGOB) (2016) NOM-015-SAG/PESC-2016. Para regular el aprovechamiento de ostión (Crassostrea virginica) en los sistemas lagunarios estuarinos del Estado de Tabasaco. Diario Oficial de la Federación, México
Secretaria de Salud (2011) NOM-242-SSA1-2009. Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. Diario Oficial de la Federación, México
Serafim A, Bebianno MJ (2009) Metallothionein role in the kinetic model of copper accumulation and elimination in the clam Ruditapes decussatus. Environ Res 109(4):390–399
Shiau SY, Ning YC (2003) Estimation of dietary copper requirements of juvenile tilapia Oreochromis niloticus & O. aureus. J Anim Sci 77:287–292
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research, 4th edn. W. H. Freeman and Co, New York
Soto-Jiménez MF (2011) Transferencia de elementos traza en tramas tróficas acuáticas. Hidrobiológica 21(3):239–248
Tanguy A, Mura C, Moraga D (2001) Cloning of a metallothionein gene and characterization of two other sequences in the Pacific oyster Crassostrea gigas (CgMT1). Aquat Toxicol 55:35–47
Tataruch F, Kierdorf H (2003) Mammals as bioindicators. In: Bioindicators and biomonitors: principles, concepts and applications. Elsevier, Oxford
Taweel A, Shuhaimi-Othman M, Ahmad AK (2011) Heavy metals concentration in different organs of tilapia fish (Orechromis niloticus) from selected areas of Bangi, Selangor, Malaysia. Afr J Biotechnol 10(55):11562–11566
Valdez C, Vázquez GA (2003) Ingeniería de los sistemas de tratamiento y disposición de agua residuales. Fundación ICA, A.C., México
Valle VP (2000) Toxicología de Alimentos. Instituto Nacional de Salud Pública, México
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk. Assess Environ Toxicol Pharmacol 13:57–149
Yilmaz F (2009) The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Orechromis niloticus) inhabiting Koycegiz Lake-Mugla. Turk J Sci Technol 4(1):7–15
Zapata-Pérez O, Gold-Bouchot G, Ortega A, López T, Albores A (2002) Effect of pyrene on hepatic cytochrome P450 1A (CYP1A) expression in Nile tilapia (Oreochromis niloticus). Arch Environ Contam Toxicol 42:477–485
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guzmán, F.A.M., Nájera, M.A.M., Escorcia, G.B., Romero, P.R. (2019). Effects Induced by the Presence of Metals in Species of Economic and Ecological Importance in Mexican Aquatic Environments. In: Gómez-Oliván, L. (eds) Pollution of Water Bodies in Latin America. Springer, Cham. https://doi.org/10.1007/978-3-030-27296-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-27296-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27295-1
Online ISBN: 978-3-030-27296-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)