Abstract
Nanotechnology is the engineering and art of manipulating matter at the nanoscale. The adaptability of physical and chemical properties of metal, semiconductor, noble, and composite nanoparticles renders them as promising materials in the fields ranging from optoelectronics to sensors. These nanoparticles or their self-assemblies are able to distinguish the mixtures of gases, volatile organic compounds, and others. Detection of pollutant, toxic, refining, and combustible gases is significant for framework and process control, safety monitoring, and environmental safeguard. In the last two decades, there have been essential improvements in two key areas that may make this guarantee a reality. First is the improvement of a diversity of excellent performing nanostructured metal oxide semiconductors (MOSs), the most commonly utilized materials for gas sensing. Second are advances in very low power loss reduced heater elements.
Here we review an overview about the principles and the technologies used in solid-state gas sensors. These devices work by measuring a physical property changed by adsorption/desorption processes and chemical reactions on the surface of a sensing element, i.e., a solid-state film of a gas-sensitive material. Some of the most used kinds of solid-state gas sensors are here described jointly with new sensor technologies in progress for commercial exploitation in the future. The analysis of different parameters of metal oxides and the search of criteria, which could be utilized through material chosen for solid-state gas sensor applications, were the main objectives of this chapter. Finally, the future horizons of such semiconductor nanomaterials for gas-sensing applications are also highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gas sensor
- Nanomaterials
- Metal oxide semiconductor
- Conducting polymers
- Environmental monitoring
- Sensing materials
- Sensitivity
- Gas sensors applications
10.1 Introduction and Historical Overview
In the last decade, the applications of nanomaterials have received increasingly magnificent attention in the field of nanotechnology , biotechnology, and bioanalytical chemistry . With regard to environmental applications, nanotechnology displays the potential of novel functional materials, procedures, and devices with unique effectiveness toward some contaminants, development of mobility in environmental media, and desired application elasticity.
Over the past six decades, there has been an increasing request for cheap, accurate, portable, and effective gas sensors that can distinguish between very low concentrations of analytes. Typically, gases of interest include NO, CO, CH4, CO2, NH4, SO2, NO2, and other hydrocarbons. These gases can be harmful to human health if present outside a specific concentration. Historically, gas sensors were first mostly used in coal mines where exact detection of hazardous gases has to be carried out continually. Readily gas sensors were also beginning to appear in the chemical industry, in environmental pollution sensing units, and in the human health strip. Some other significant applications of gas sensors include the analysis of organic vapors (methanol, toluene, benzene, etc.) for laboratory, industrial safety, diseases diagnosis, and breath analysis for traffic safety (Wang et al. 1995; Mitsubayashi et al. 2004, McEntegart et al. 2000). An electronic nose, based on arrays of gas sensors for testing aroma of food , perfumes and synthetic fragrances, etc., is one of the more recent personifications of gas sensing that has received much interest owing to its enhanced analytical power (Arshak et al. 2004).
Many fields of nanotechnology are based on physical and chemical interactions , including nanoparticles of specific size and shape. Nanoparticles (NPs) played a significant role in absorption/adsorption process of (volatile) organic molecules and gases due to their large specific surface area and high surface energy (Chen et al. 2006a, b). Nanoscale inorganic materials have received significantly more consideration because of their high chemical inertness, non-swelling influence, high purity, and hardness (El Kady et al. 2016). In order to use the nanomaterials as sensors, one has to understand the features of both the installation and interaction technique during the sensing action. In recent years, the interest of scientists and engineers to gas- and liquid-sensitive materials has grown extraordinarily because of the advances in nanotechnology. This attention is fundamentally connected to the promising electronic properties of nanomaterials, their size dependence, and the potential of controlling the material structure by using new experimental methods (El-Aassar et al. 2016). New generations of low-power, low-cost, and portable sensing devices are needed for monitoring of chemistry, medical, agriculture , and manufacturing environments . With the recent progress in nanoscience and nanotechnology, there is an urgent need for adaptable, mechanically durable, and environmentally stable chemical vapor sensors with a high efficiency and low power consumption. Among the major trends in the particle-gas-sensor nanotechnology, the invention of sensor arrays or electronic noses should be listed. Such multi-sensor systems can be fabricated on a single substrate, which can include gas sensors of various types. There are many models of sequential productions of their nanostructured prototypes, which are able to discriminate the mixtures of gases, volatile organic compounds, and odors (Font et al. 2011).
In recent years, great attention has been paid to inorganic nano-sized crystals because of their significant characteristics determined by the high surface areas and quantization of most electronic properties. Nanometer-sized inorganic particles potentially have unparalleled properties because of quantum confinement effects and their large surface area comparative to their volume. The versatility of physical and chemical properties of metal and semiconductor nanoparticles renders them as favorable materials in the fields extending from optoelectronics, sensors, to medicine. Till now, great research attentions have been involved in preparation nanoparticle assemblies because they represent a popular route toward the preparation of advanced functional materials as well as a central concept in nanoscience and nanotechnology (Elkady and Hassan 2015).
It has been known for a long time that electrical impedance of a semiconductor is very sensitive to the presence of defects in its volume or at the surface. At the beginning of the 1950s, Brattain and Bardeen gave the first proof that some semiconductor materials such as Ge change their resistance, depending on the atmosphere they are in contact with (Brattain and Bardeen 1952). Subsequently, Heiland furthermore found that metal oxides such as ZnO alter their semiconducting aspects with a change in the partial pressure of oxygen or other gases in the surrounding atmosphere (Heiland 1957). However, these results were not delicate further. The same properties were reported for SnO2 with higher stability. These results started further evolution of trade gas sensors.
Chemoresistive gas sensors were inserted for the first time 50 years ago. In 1962, Seiyama used ZnO thin film as a sensing layer and was able to confirm that gas sensing is possible with simple electrical devices (Seiyama and Kato 1962). He used an unpretentious chemoresistive device based on ZnO thin films operating at the temperature of 485 °C. The response of the sensing system to propane was about 100 times higher comparing with the thermal conductivity detector used at that time.
Then, in 1967 Shaver described the detection effects of oxide semiconductors with small additives of noble metals such as Pt, Pd, Ir, and Rh (Shaver 1967). Since that time, the sensitivity and selectivity of semiconductor sensing devices have been developed dramatically, and the search of new sensing materials has been concentrated.
At the beginning of the 1970s, Taguchi fabricated and patented the first chemoresistive gas sensor device for practical applications using tin dioxide (SnO2) as the sensitive material (Taguchi 1971). Certainly, after investigating many sensitive metal oxides, he found that SnO2 has many advantage aspects (e.g., higher sensitivity , low operating temperature, and thermal stability).
In the late 1980s, the field of semiconductor gas sensors underwent a considerable development and became one of the most attractive research areas within the sensor society. The request for high-performance gas sensors with high sensitivity and selectivity and faster response with low power consumption and reliability created intense efforts in order to improve novel sensing materials . The quick development of materials chemistry and the more extensive field of materials science have prompted to a dramatic increment in the number of new sensing materials (Seiyama 1988; Yamazoe 1991).
Seiyama probably would not have been able to predict that, half a century after the publication of his fundamental paper, research on gas sensor would consider a significant role in daily life. Certainly, the most recent five decades, due to their simplicity, small size, low cost, and ability to be integrated into electronic devices. Chemical sensors have seen an increase in their application to a variety of fields, including environmental monitoring , industrial emission control, vehicle emission control, domestic security, agricultural, biomedical, etc. (Shimizu and Egashira 1999; Yamazoe 2005).
Several decades after the first published paper on SnO2 sensors, researchers became the best-understood prototype of oxide-based gas sensors. It was well known that the sensor properties may be changed by modifying the crystal structures, doping, preparation techniques, operation temperatures, etc. (Neri 2015).
Now, the expansion of semiconducting sensing materials is depending on opportunities presented by new nanotechnology. Furthermore, emerging nanotechnologies promise dramatic changes in sensor designs and abilities.
10.2 Review of Solid-State Gas Sensors
Gas sensors can be categorized according to the operation mechanism (semiconductors, flame ionization, photoionization, electrochemical, thermal conductivity, oxidation, light scattering, catalytic photoionization, combustible, colorimetric, infrared or ultraviolet, absorption, etc.). In this section a review on the various kinds of sensors and their principle of operation will be discussed.
10.2.1 Catalytic Sensors
Catalytic sensors have been in use for almost a century to be used in detection of combustible gases. Jonson invented the first catalytic combustion-type sensor in 1923 (Firth et al. 1973) which was used for the detection of methane in mines.
10.2.1.1 Principle of Operation
Most kinds of metal oxides and their compounds have catalytic property. Combustible gas mixtures do not burn until they get a specific ignition temperature; nevertheless in the presence of a particular chemical process, the gas will begin to burn even at lower temperatures. This process is known as catalytic combustion. Catalytic gas sensor is a gas sensor made on the basis of catalytic principle. A Wheatstone bridge is used to measure the output of catalytic gas sensor. The catalytic gas sensor is divided into two, namely, pellistor type and thermoelectric type. The earliest catalytic gas sensor overview was simply a coil-shaped platinum wire (Fig. 10.1a). This was used to produce an effective heating and a strong signal for a gas sensor; however in spite of the perfect attributes of platinum, it is still a poor catalyst for combustion of hydrocarbon gases. The temperature needed for the sensing of hydrocarbons is between 900 °C and 1000 °C, but at this temperature, the platinum starts to evaporate, and as such resistance of the platinum wire increases.
Another disadvantage with the platinum wire is that at the temperature of 1000 °C, the platinum becomes tender. The solution to this problem is to overcoat the platinum with other metal oxides and then treat the sensor with a catalyst like palladium, platinum, or thoria compounds. Figure 10.1b shows a catalytic bead sensor with the metal oxide coating; the coating makes the sensor more stable, harder, and resistant to shock and vibrations.
Recently, microhotplates have been widely used in gas sensors instead of using platinum coil due to the high power consumption. These kinds of sensors always contain a catalytic surface coated on a hot plate with Pt resistor which heats up the catalyst to a very high temperature at which any flammable gas molecules can ignite. The concentrations of gases can be detected by measuring resistance change of the platinum resistance emerge from increase in temperature. This design was recently developed by Lei Xu et al. in which they design and construct a two-beam microplate for catalytic gas sensors (Karthikeyan et al. 2015). The two-beam microplate was designed using MEMS technology. Their design manifested low power consumption with a 30% power per active area as compared with other microhotplates, and a sensitivity of the sensor to 50% LEL methane was 2.4 mV/% methane.
10.2.2 Pellistor-Type Catalytic Gas Sensor
A pellistor-type catalytic gas sensor is depicted in Fig. 10.2a consisting of two platinum coils which have two functions. They serve as heater as well as resistance thermometer. It also consists of active and inactive beads. The active bead is activated with catalyst made from a metal like platinum or palladium. The inactive bead has no catalyst but usually plays as compensating element. A voltage source use for powering the circuit heats up the coils; so the beads are increased to a high temperature from a range of 300 °C to 500 °C depending on the target gas. This causes the gas to ignite and raises the temperature of the detector coil.
This increase in temperature raises the coil resistance and causes an imbalance in the voltage of the Wheatstone bridge which constitutes the detector signal. The output of the sensor is taken across the Wheatstone bridge circuit as shown in Fig. 10.2b. Recent researchers (Xu et al. 2010) enhanced a catalytic combustion-type methane detection sensor with a Pd-Pt catalyst working on pulse voltage mode. The sensor was fabricated by micromachining and sol-gel process on a silicon substrate. The output of the gas sensor is measured by a bridge circuit which consists of a resistive sensing element, microhotplate with a Pd-Pt-Al2O3 layer, a variable resistor R, and two fixed resistors R1 and R2.
The pellistor technology has witnessed significant development by using micro-electrochemical systems (MEMS) technology for their fabrication due to the advantage of integration, miniaturization, and reduced power consumption. Catalytic gas combustion gas sensors have been fabricated using thin-film or micromachining technologies. Recently, Lee et al. developed an integrated catalytic combustion H2 sensor using MEMS technology (Lee et al. 2011). The novelty of their design was that the gas sensors were fabricated with two sensing elements and two reference elements using MEMS technology.
10.2.3 Thermoelectric Gas Sensor
In 1985 McAleer invented the earliest thermoelectric gas sensor for the detection of combustible gas like H2 gas as reported by Shin et al. (Shin et al. 2003, 2004). Hydrogen detection using thermoelectric sensor is possible by generating an electrical signal based on the catalyzed exothermic oxidation reaction of hydrogen. Thermoelectric gas sensors work on the principle of the Seebeck effect. The Seebeck effect occurs as a result of a temperature change between two points of a conductor or semiconductor material which gives increase to a voltage difference between these two points. Thermoelectric sensors have been manufactured using micromachining techniques. A microelectric gas sensor for the detection of hydrogen and atomic oxygen was reported by Se-Chuk Park et al. using surface micromachining technique for the sensor fabrication (Kim et al. 2009). The sensor senses the gases by measuring the reaction temperature of the catalytic reaction between a novel metal catalyst using Cu-Bi thermopiles. Hydrogen recently reported thermoelectric sensors were used for the detection of volatile organic compounds (VOCs) by the use of SnO2 thin films. The precept of gas sensing using thermoelectric gas sensors is usually based on gas absorption. Nevertheless this technique usually slows down the response and recovery times. Seung-II Yoon et al. (Yoon et al. 2009) invented and fabricated a thermoelectric gas sensor based on the principle of gas adsorption instead of gas absorption. The sensor uses an embedded tin oxide catalyst for the detection of hydrogen and NOx gases. MEMS technology was employed for the sensor fabrication on a Pyrex substrate. To understand the principle of gas adsorption, sensing and reference thermopiles with Bi and Cr pairs were used. A thermopile was used so as to produce an electric potential that is proportionate to the temperature variation between the hot and cold junctions without the need of power consumption while a catalyst film is placed underneath the hot junctions of the thermopile.
A thermoelectric gas sensor for the detection of volatile organic compounds was fabricated and developed by S. Anuradha et al. (Anuradha and Rajanna 2006). The sensor was evaluated for response toward VOC, namely, ethanol, isopropyl alcohol, and hexane, in the temperature range of about 80–160 °C. The sensors developed with and without the metal films were tested for their response to acetone gas. Sensors with chromium metal showed good sensitivity to acetone as low as 28 ppm and were found to be selective toward acetone gas.
10.2.4 Thermal Conductivity Gas Sensor
Gases with thermal conductivities less than air are difficult to sense using this way due to interference of, for example, carbon dioxide and butane. Their principle of operation is based on the measured heat loss from a hotter body to the cold element through thermal conductivity. The first type of thermal conductivity gas sensor is called pellistor-like sensor, and it consists of two inert resistor beads with an implanted thermoresistor. The sensing device is usually located within a gas chamber which contains a reference gas. Comparable to a catalytic gas sensor, a Wheatstone bridge circuit is also utilized whereby the two beads are connected. The principle of the detection mechanism is such that when the resistor is exposed to the target gas mixture , heat is lost which is either higher or lower depending on the thermal conductivity of the target gas with respect to the reference gas. This leads to an increasing or decreasing temperature of the bead and furthermore a difference in its resistance which is measured as an imbalance in the Wheatstone bridge. The second type of sensor doesn’t need the use of reference cell because it is made up of a hot and cold element which has a known and a constant temperature variance. The heat is transferred from the hot element to the cold element by means of thermal conductivity of the investigated gas (Yunusa et al. 2014).
Tardy et al. (2004) developed a dynamic thermal conductivity sensor based on the transient response of a SiC microplate for the determination of CO, H2, and CH4. Simon and Arndt fabricated a simple micromachined thermal conductivity sensor. Experiments carried out showed good sensor performance predicted by the model. The sensor chips were used to build a hydrogen detector for automotive applications (Simon and Arndt 2002).
Recently, micromachining have been used for hydrogen gas sensor due to miniaturization and reduced power consumption. De-Graaf and Wolffenbuttel (2012) recently developed a thermal conductivity gas sensing which uses MEMS technology for the fabrication of high-sensitivity thermal sensors for hydrogen detection. The analysis showed that the performance of surface-micromachined devices could be better than that of bulk-micromachined devices. It is made up of thermopile temperature sensors which depend upon the reduction in efficient thermal resistance between the sensitive area of the sensor and the substrate by the thermal conductance of the gas in the thin membrane . The heating element is a resistor which is set in the middle of the membrane. A gas chamber is located for the hydrogen sensing.
10.2.5 Electrochemical Gas Sensors
These types of sensors allow gases to diffuse through a porous membrane to an electrode where it is either reduced or oxidized at the electrode. Electrochemical sensors operate by reacting with a target gas and producing an electrical signal that is proportional to the gas concentration. A typical electrochemical gas sensor consists of a sensing electrode or working electrode and a counter electrode which are separated by a thin layer of electrolyte. Before the gas comes in contact with the sensor, it goes through a thin capillary-type opening and then diffuses through a hydrophobic barrier before finally reaching the electrode surface. The function of this membrane is to prevent liquid electrolyte from leaking out and generate enough electrical signal at the sensing electrode. It also consists of a reference electrode whose function is to maintain a stable and constant potential at the sensing electrode due to the continuous electrochemical reactions occurring on the electrode surface. The electrochemical reaction with the target gas generates a flow of current flow between the sensing and counter electrodes. The electrolyte is responsible for carrying the ionic charges across the electrode.
The earliest electrochemical cells were reported by Kohlraush in 1885 and Haber in the early 1900s (Hübert et al. 2011). Since after that a lot of researchers have worked on electrochemical gas sensors for detection of different gases. Currie et al. (1999) developed micromachined thin solid-state electrochemical sensor for simultaneous detection of CO2, NO2, and SO2 gases. Similarly, Sathiyamoorthi et al. (2004) developed an electrochemical sensor for the detection of fluorine and chlorine. In order to improve the sensitivity of the electrochemical gas sensor, Gan and Hu (2011) published a review paper on electrochemical sensors based on graphene materials; due to the fact that nanoscale materials are good candidates for gas-sensing elements due to high surface-to-volume ratio, they have reduced size and reduced power consumption and have been used for the detection of various gases as shown by Lu et al. (2009a, b). However, microelectronic systems (MEMS) have been employed for the design of electrochemical microsensors, and efforts have been made to improve their sensitivity as shown by Zhang et al. (2008).
They explained that improved sensitivity could be attained by coating nanosensors developed from carbon nanotubes with polymers. An electrochemical sensor can be used for measuring carbon monoxide by undergoing a chemical reaction as follows:
As shown in Eq. (10.1), oxidation reaction takes place at the sensing electrode, CO2 diffuses into the air, and the positively charged ions migrate into the electrolyte.
The oxidation reaction is balanced by a corresponding reduction reaction at the counter electrode as shown in Eq. (10.2). At one electrode, water is consumed, while electrons are generated, and at the other electrode, water is created, while electrons are consumed. The carbon monoxide generated diffuses in the air, and the positively charged hydrogen ions travel down to the electrolyte.
Similarly, for hydrogen the electrochemical reaction is shown in Eq. (10.3). Hydrogen gas diffuses and becomes oxidized at the sensing electrode. This reaction causes a change in the potential of the sensing electrode, and thus reduction of oxygen takes place as shown in Eq. (10.4):
The result of the flow of electrons from anode to the cathode constitutes an electric current that is proportional to the hydrogen gas concentration which obeys Faraday’s law:
where Z is the number of exchanged electrons/molecules, Q is the conversion rate of hydrogen in moles/second, and F is Faraday constant = 96486.7 As/mol. Electrochemical sensors are usually of three types, namely, amperometric, potentiometric, and conductometric, which are discussed below.
10.2.6 Amperometric Gas Sensor
The amperometric sensors work at a constant applied voltage, and the sensor signal is a diffusion-limited current. It usually consists of two electrodes, the working electrode and the counter electrode, and also a reference electrode which are immersed in the electrolyte solution and a potentiostat for maintaining constant voltage as shown in Fig. 10.3.
Amperometric sensors are usually built using two-electrode configuration, but due to the limits of the concentrations of reactant gas, they are built using a three-electrode scheme. In the three-electrode configuration, the current at the sensing electrode can be measured at a constant potential which gives a genuine thermodynamic potential for all reactions; in this case the reference electrode is not involved in the reaction. However, the current generated as a result of the target gas at the sensing or working electrode is measured as the sensor signal which can then be measured at either a fixed or variable electrode potential.
Amperometric sensors have been used for detecting various gases by changing the type of electrolyte. Xianbo Lu et al. (2005) developed a novel design for an amperometric gas sensor with the use of an yttria-stabilized zirconia (YSZ) porous layer which acts as an oxygen conductor and a gas diffusion barrier. The planar stack configuration was developed and allowed a deposition of YSZ layers. The device developed showed a linear output in the range of oxygen partial pressures. Ho and Hung (2001) also developed an amperometric NO2 gas sensor based on Pt/Nafion electrode; NO2 concentrations in the range of 0–485 ppm were detected. Similarly an amperometric hydrogen sensor was developed based on polymer electrolyte membrane with Nafion membrane as the conducting polymer . The response to hydrogen concentration was in the range of 260–11,500 ppm. Chao et al. (2005) developed an amperometric sensor using three different sensor designs for hydrogen and carbon monoxide sensing. The three different designs were tested under hydrogen and CO concentration, and devices II and III were found to have slower response times as compared to device I. Selectivity of the sensor under hydrogen concentrations was greatly improved by replacing Pt-air RE with modified Ag/AgCl RE and incorporating a semipermeable membrane. Amperometric gas sensors for the detection of hydrocarbon were reported by Dutta et al. (2005) for monitoring in exhaust pipes.
With the advent of microelectronics system (MEMS), microelectrodes with very small electrode surface area have been employed in the fabrication of electrochemical sensors due to their numerous advantages of having small size and weight, low cost, and faster response time without affecting the signal-to-noise ratio. Microamperometric sensors dated back in the 1980s consisted only of microfabricated electrodes on a suitable substrate; the earliest microamperometric sensor was developed by Sleszynski and Osteryoung in 1984 (La et al. 2011). Recently, techniques on how to improve the sensitivity of the sensors were reported by (Phawachalotorn et al. 2012).
10.2.7 Potentiometric Gas Sensors
Potentiometric gas sensors are used to determine the analytical concentration of some components of the analyte gas. They can measure the electric potential of an electrode without current flow. The signal is measured as the potential difference between the working electrode and the reference electrode. Potentiometric sensors have been used for oxygen detection. A typical potentiometric oxygen sensor is made up of an oxygen ion conducting solid electrolyte and two electrodes which are deposited on the two sides of the electrolyte. One of these is a reference electrode which is in contact with a known oxygen partial pressure, while the other is a working electrode which is in contact with an unknown oxygen partial pressure that needs to be measured. When the electrodes are in contact with two different oxygen partial pressures and isolated from each other, an EMF is developed by the sensor.
The electrodes are usually made from palladium, platinum, gold, or silver. Different electrolytes have been also used or a combination of two materials for the detection of different gases. Lee et al. (2001) developed a potentiometric CO2 gas sensor using lithium phosphorus oxynitride electrolyte, while recently Jiun-Chan Yang et al. (Yang and Dutta 2010) developed a high-temperature NO2 sensor fabricated with asymmetric reference and sensing electrode made with Pt and YSZ electrolyte. The combinations of these two materials have simplified the design and make it more compact. Similarly, Yan et al. (1995) developed a potentiometric sensor using stabilized zirconia for chlorine gas by combining MgO-stabilized zirconia tube with an auxiliary phase containing metal chloride with a sensitivity of 1–100 ppm of chlorine at 550–600 °C. With the advent of microfabrication technology, miniaturized sensors are produced so as to amplify the output of the potentiometric sensors. Radhakrishnan et al. (2005) fabricated a miniaturized series connected potentiometric sensor on a silicon fabricated electrode after using microfabrication techniques for oxygen detection.
10.2.8 Optical Gas Sensors
This type of sensors uses optical absorption/emission scattering of a gas species at defined optical wavelengths. An optical gas sensor consists of a light-emitting element, a photodetecting element, a gas-sensing element (the gas-sensing element responding to light), and a filter for picking up fluorescence or phosphorescence. Most optical sensors are usually based on thin films of palladium or chemochromic oxides coated along the length of an optical fiber. These types of fiber optic sensors are known as optodes. One of the most common optical gas sensors is infrared gas sensors which will be discussed later in more detail.
As shown by many authors, optical sensors have been used for many years in the detection of flammable gases like hydrogen. The first optical hydrogen gas sensor was reported by Butler (Hübert et al. 2011) in 1984 which consists of an optical fiber with palladium and titanium coatings. Detection of hydrogen was made using interferometry. Massie et al. (2006) also designed a low-cost portable optical sensor for methane detection with very good sensitivity ; the sensor can operate even in harsh environments. Acquaroli et al. (2010) designed an optical porous silicon gas sensor. The system was tested over a detection area of the porous silicon microcavity with isopropyl alcohol vapor, and even small changes in concentrations were detected. Manap et al. (2009) developed an optical fiber sensor for the monitoring of ammonia gas using an open optical path techniques. Cross sensitivity of CO2 and O2 was also tested to see their effect on ammonia gas. Okazaki et al. (2003) also developed a fiber optic hydrogen gas sensor using catalyst-supported tungsten trioxide (WO3). The sensor used platinic acid at 500 °C and showed good response toward hydrogen gas detection and can detect gas even at room temperature. Girschikofsky et al. (2012) recently reported an optical planar Bragg grating sensor which is capable of detecting substances like benzene, toluene, and xylene. Results obtained showed good sensitivity toward these gases.
10.2.9 Infrared Gas Sensor
Infrared sensors consist of a detector which converts electromagnetic radiation energy into electrical signals. The detectors are of different types, namely, thermoelectric, thermistor bolometer, pyroelectric detector, and photon detector. It also consists of an infrared source which could be a regular incandescent light or a heated wire filament which can be used for the detection of CO2, CO, and other hydrocarbons. Another component is an optical fiber which could be of two types: dispersive and nondispersive.
Nondispersive types use discrete optical band-pass filters and are mostly used for gas sensor applications, while the dispersive types use an optical device like a grating or prism. The last but not the least is the gas cell which allows the light path so as to interact with the target gas. Infrared gas sensors are used for detecting different gases like methane, ethane, propane, butane, benzene toluene, and xylene and other alcohols like methanol, ethanol, etc. Okajima et al. (2006) developed an infrared gas sensor using LED for the measurement of methane; absorption of gas samples between 0% and 97% was successfully measured. Garcia-R et al. (2012) developed a nondispersive infrared (NDIR) gas sensor for the measurement of CO2 gas concentration for wireless sensor networks with low power consumption. Similarly Chen et al. (2006a, b) designed a tunable diode laser absorption spectroscopy to produce a sensor that is miniaturized, and Zhang et al. (2010) developed a miniaturized CO2 sensor based on infrared absorption.
There are two types of optical structure which is used for the construction of infrared CO2 gas sensors, namely, time double beam and space double beams. The time double beam optical structure has only one infrared beam emitted from the infrared source, and the detector receives two infrared beams with different wavelengths and at different times, while the space double beam structure has one infrared beam emitted from the infrared source and simultaneously enters two parallel plate detectors. In this design, the space double beam is used so as to enhance the construction, and a cone-shaped air chamber is designed. The optical probe consists of an infrared source, an air chamber, an infrared receiving device, and two sapphire windows. The sensor showed an accuracy of 0.026% with CO2 gas concentration in the range of 0–3%. Kasai et al. (2011) investigated the ability of a system using a carbon infrared emitter and an infrared camera to detect combustible gas propane.
10.2.10 Acoustic Wave Gas Sensors
Acoustic wave sensors are so named because their detection mechanism is a mechanical or acoustic wave. As the acoustic wave propagates through or on the surface of the material, any changes to the characteristics of the propagation path affect the velocity and/or amplitude of the wave. Changes in velocity can be monitored by measuring the frequency or phase characteristics of the sensor and can then be correlated to the corresponding physical quantity being measured. An acoustic wave sensor contains a receptor which is an element that is sensitive to an analyte and a transducer, i.e., an element that converts the response into an electrical signal.
The first acoustic gas sensor was discovered by King in 1964 (King 1964) and was based on the measurement of bulk acoustic waves (BAW) in a piezoelectric quartz crystal resonator which is sensitive to mass changes. After intensive research studies in mid-1960, chemical sensors for industrial atmospheric pollutants were developed. Since piezoelectric quartz resonators were used, these types of sensors were called quartz microbalances (QMB).
There are different types of acoustic wave sensors which are based on the type of wave propagation. Acoustic wave sensors have a variety of applications as in temperature, pressure, mass, chemical, etc. In this paper the application will be for gas sensing. The principle of operation of acoustic chemical sensor is described as follows. When a receptor film is introduced unto the vibrating surface of a transducer that is activated by an electronic device, the characteristics of the receptor film such as its mass and thickness are changed when exposed to an analyte. This change directly affects the vibration frequency, amplitude, and phase. The shift is directly proportional to the analyte concentration.
As reviewed by several works on different gas sensors, the current trend has taken the direction of developing the gas sensors using microelectronics technology due to its advantage of miniaturization and low power consumption. However, acoustic wave sensors already possess this inherent characteristic and were used in gas sensors since 1964, so this makes it to be an attractive candidate over its gas-sensing counterparts. A review on acoustic waves will be made in this section of the paper with emphasis on surface acoustic wave sensors. Another advantage of surface acoustic wave technology is that the gas sensing can be made wirelessly as shown by (Lim et al. 2011), which makes real online monitoring of the gas sensor possible and eliminates the use of wired cables. This property makes it an attractive candidate for gas detection and makes it superior to other gas-sensing techniques. Also high selectivity and sensitivity have also been reported in many SAW gas-sensing applications (Dutta et al. 2005; Yang and Dutta 2010; Radhakrishnan et al. 2005).
10.2.10.1 Surface Acoustic Wave Gas Sensors
Surface acoustic wave technology refers to the use of the SAW device in several technological applications. Surface acoustic waves were first discovered by Lord Rayleigh in 1885. SAW sensors are developed based on Rayleigh waves. A Rayleigh SAW is made up of two mechanical displacement components in the sagittal plane, i.e., the plane containing the direction of propagation and the surface normal. For gas-sensing applications, the choice of piezoelectric substrate determines the type of SAW wave. Rayleigh waves propagate in a thin surface layer and can penetrate into the substrate at a distance of the order of a wavelength. The velocity of propagation of the wave depends on the substrate material, the crystal cut of the substrate, and the working frequency (Hübert et al. 2011).
Since after the discovery, a lot of potential applications have been exploited which among them are the sensor applications including chemical, optical, thermal, pressure, acceleration, torque, and biological. The main advantages of using SAW technology are high sensitivity , low power consumption, being wireless, and can be placed on moving or rotating parts and in hazardous environment. The SAW device is also technologically compatible because its fabrication process is similar to that of other microelectronic devices.
10.2.10.2 Principle of Operation
Surface acoustic wave sensors work based on the principle of transduction whereby the sensor converts an input electrical signal into a mechanical wave and reconverts back into electrical signal. This is made possible by means of the interdigitated transducer known as the IDT which uses the piezoelectric effect. The IDTs are made of electrodes made from aluminum, gold, or platinum. A typical SAW therefore consists of an input and output transducer with spacing between them called a delay line. The principle of gas sensing in SAW is realized by the application of a sensing material like a thin polymer across the delay line which selectively absorbs the gas or gases of interest as depicted in Fig. 10.4.
Rayleigh SAW sensors are based on two types of acoustoelectronic devices, namely, delay line and resonator. The frequency of operation of Rayleigh wave’s sensors usually lies between 40 MHz and 600 MHz. These devices differ from each other in their design. A delay line has two receiving and transmitting interdigital transducers, whereas a resonator has one interdigital transducer placed at the resonator cavity . However, their mechanism of response is the same, and they also have similar output characteristics. A delay line is simpler to design compared with the resonator; that is why it is mainly used for practical applications. However, a delay line requires matching due to the insertion attenuation and is subjected to having oscillation frequency, but the resonators have smaller attenuations and do not require matching (Fischerauer et al. 1996). A resonator and a delay line could be either single- or two-port. A single-port delay line consists of a propagation path between one IDT and one or more interdigital reflectors. A two-port SAW delay line consists of a propagation path between two separate IDTs: the first serves as a transmitting transducer, and the second serves as a receiving transducer so as to convert the SAW back to electrical form.
10.2.11 SAW-Based Gas Sensors
Surface acoustic waves were the next generation of acoustic wave sensors after the advent of the quartz crystal microbalance (QCM) which was used to stabilize the frequencies of radio transmitters and later modified by the addition of sorptive film on the crystal so that it could be used for chemical sensing. Subsequently the device was analyzed and improved by some researchers in the 1950s. In the late 1970s, Wohltjen and Dessy (1979) realized that chemical vapor sensing could be accomplished with a device that was originally used for processing of electrical signals which is the SAW delay line.
Since then a lot of researchers have been working on SAW sensors for detection of different gases which are either toxic, harmful, or pollutants, while others can be used as fuel gases in industries and automobiles. Researchers tend to employ SAW resonator if their prime interest is to control the center frequency, whereas if the time response is of interest, they decide to use the delay line. SAW delay line is commonly used due to its simplicity in the design and fabrication. M. S. Nieuwenhuizen et al. (Nieuwenhuizen and Nederlof 1990) developed a SAW gas sensor for detection of CO2 and H2O using dual delay-line oscillators on a quartz substrate at a frequency of 40 MHz. Venema et al. (1987) also designed a SAW delay-line gas sensor using quartz substrate for the detection of NO2 gas. Subsequently, Anisimkin et al. (1995) also developed a SAW delay-line gas sensor for the detection of CO, NO, hydrogen, and oxygen gases, respectively. Beck et al. (1999) also developed a SAW delay line using lithium niobate substrate for the detection of NO2 and methane gases. A review paper was published recently by Wieslaw P. Jakubik on dual delay-line oscillator. However, Lee et al. (1997) designed SAW resonator gas sensor using lithium niobate for the detection of methane and hydrogen gas. Similarly, Fischerauer et al. (1996) also employed a SAW resonator for the detection of hydrocarbons. Due to the good results displayed by the use of SAW devices for gas sensing in terms of high sensitivity , selectivity, and good response times, a lot of researchers are working extensively so as to detect different gases using different configurations of SAWs and at different frequencies. Results obtained have produced excellent results in terms of high selectivity, high sensitivity, and good response times.
10.2.12 Semiconductor Gas Sensors
Semiconductor gas sensors are devices that are made up of heated metal oxides which are used for measurement of gas concentration of a target gas by measuring the electrical resistance of the device. They work on the principle of reversible gas adsorption process at the surface of the heated oxide, usually oxides of tin deposited on a silicon slide by chemical vapor deposition method. Absorption of the sample of gas on the oxide surface followed by catalytic oxidation results in a change of electrical resistance of the oxide material which is then related to the sample gas concentration which is monitored by the meter as shown in Fig. 10.5. The heater at the base is used for heating up the sensor to a constant temperature of about 200–250 °C so as to speed up the reaction rate (Hassan et al. 2013, 2014a, b, c).
An intrinsic n-type semiconductor is suitable for detecting reducing gases due to the high conductance change as a result of the injected electrons. Similarly, a p-type semiconductor is suitable for the detection of oxidizing gases. The oxides usually used for the n-type are mainly oxides: SnO2, ZnO In2O3, or WO3 (Hassan et al. 2014a, b, c). They are commonly used to detect hydrogen, oxygen, alcohol, and harmful gases like carbon monoxide. Gas sensors using metal oxide semiconductor were first proposed by Seiyama and Taguchi . When inflammable gases come into contact with metal oxides, they excite a new electron level within the solid and thus cause a change in electrical resistance of the gas-sensing elements. Jin Huang and Qing Wan published a review paper on the progress in gas sensors based on semiconducting metal oxide one-dimensional (1D) nanostructures (Dey 2018).
It was reported that due to the advent of microelectronics, new device structures such as the electronic nose and the low power consumption self-heated gas sensor have been designed, and their response has been evaluated (Huang and Wan 2009). Sensitivity and selectivity could be improved by the addition of small amounts of noble metals like Pd-added elements. Khodadadi et al. (2001) reported on improving the sensitivities of methane and carbon monoxide gases by adding 5% of K2O into SnO2 samples; the sensitivity was improved by 40%.
Similarly, 5% of Na2O in SnO2 layers showed reduction of sensor sensitivity to CO. Addition of platinum into the prepared samples improves the response to methane. However, improved sensitivity could also be obtained by the addition of doping agent in thick-film semiconductor gas sensor developed for the sensing of methane and butane. The semiconductor gas sensors have different configurations, one-electrode and two-electrode configuration.
10.2.12.1 Operation Principle of One-Electrode Semiconductor Gas Sensors
The one-electrode sensor configuration is depicted in Fig. 10.6; as reported by Korotcenkov (2007a, b), the metal resistor acts as both the heater and measuring electrode at the same time. One-electrode sensors are similar to pellistors or hot-wire sensors. The operation principle of one-electrode sensor is based on the shunting of the Pt wire by semiconductor oxide coating the metal spiral. The one-electrode sensors are typically incorporated in a Wheatstone bridge circuit, and they work under a stabilized constant current. The shunting semiconductor resistance should be ensured to have a resistance value of the same order of magnitude as that of the Pt resistor at the operation temperature. Due to the low heater resistance, only metal oxides with high conductivity are capable of changing the total resistance of sensors in the presence of gases detected. For one-electrode semiconductor sensor design, SnO2 and In2O3 have been used. However, the two-electrode configuration will not be discussed because it is not very common.
The gas-sensing materials for one-electrode sensors are deposited using chemical vapor deposition (CVD), physical vapor deposition, and sol-gel process. Recently, Shimanoe et al. (2011) developed a semiconductor nano-sized oxide for the detection of inflammable gases, odor gases, and other environmental-related gases using MEMS technology with low power consumption. Takada (2000) developed a new method for gas identification of different gases including hydrogen, carbon monoxide, methane, and volatile organic compounds. Recently, artificial intelligence techniques are integrated into gas sensors as shown by Byeongdeok Yea et al. (Konishi 1997).
In order to improve sensitivity of the sensor, different methods had been used including temperature modulation, synthesis of new sensor materials, designing new sensor constructions, adoption of new filter layers, and using of sensor array. Therefore, Halek et al. (2009) made a comparison of methods of selectivity improvements of semiconductor gas sensors and concluded that the kind of sensing material and filter layer has a strong influence on the sensor parameter.
10.2.12.2 Sensing Materials of Semiconductor Gas Sensor
From the first tin dioxide sensor developed in the 1970s for domestic gas alarms, there has been an increase in the demand for high-performance solid-state gas sensors. Results of a search study on metal oxide semiconductor (MOS) used as sensing materials for solid-state gas sensors, including both the n-type and p-type oxides, are summarized in the graph shown in Fig. 10.7 (Kim and Lee 2014).
Metal oxides stand out as one of the most common, diverse, and, most likely, largest class of materials due to their extensive structural, physical, and chemical properties and functionalities. The most common metal oxides utilized as sensing layer in gas sensor devices are binary oxides such as SnO2, ZnO , TiO2, etc.; however, ternary and more complex oxides are also applied in practical gas sensors (Huang and Wan 2009).
SnO2 is the most extensively studied metal oxide and is widely applied in practical commercial devices. Tin dioxide is a wide band gap (3.6 eV) semiconductor with interesting electrical properties (Ogawa et al. 1982). Due to its high sensitivities for different gas species, tin oxide-based sensors allow the detection of low concentration levels of gaseous species, though it unfortunately suffers from the lack of selectivity. Nevertheless, strategies devoted to enhance the SnO2-based device’s performance have been extensively studied (Korotcenkov 2005).
Zinc oxide is a II−VI semiconductor showing a wide band gap (3.37 eV), with the dominant defects identified as O vacancies (Mahmood et al. 2013). ZnO has attracted much attention in the gas-sensing field because of its high mechanical and chemical stability, suitability to doping, nontoxicity, and low cost. The sensing property of ZnO is strongly influenced by the nanostructural features, such as the grain size, geometry, and connectivity between the grains (Kashyout et al. 2010).
Titania is particularly attractive for gas sensors because of its lower cross sensitivity to humidity than other metal oxides (Tricoli et al. 2009). Among the other applications, TiO2 has been largely investigated as a sensing layer in resistive oxygen gas sensors operating at medium to high temperatures for automotive air/fuel ratio control. At medium temperatures (400–600 °C), oxygen detection is mainly due to reactions that takes place on the surface, whereas at high temperatures (700–1000 °C), oxygen detection is mainly due to diffusion of oxygen ions in the bulk of the material (Ramamoorthy et al. 2003).
A lot of factors affect the gas sensor performance of metal oxide which, in turn, is determined by the reception and transduction function along with the fabrication procedure (Yamazoe and Shimanoe 2009). The synthesis procedure, crystal size and shape, and the addition of foreign elements with the role of sensitizers or conductivity modifiers are some of the factors influencing the sensor response (Aleixandre and Gerboles 2012; Huang and Choi 2007; Jimenez-Cadena et al. 2007).
Doping the metal oxide layer with suitable promoters (metal particles, other metal oxide, and ions) is a common way of enhancing the sensing characteristics of gas sensitivity . The modification of the sensing properties of the sensing material by the introduced additives depends on the nature of the latter (Hassan et al. 2014a, b, c). For example, Pt is known to promote the gas-sensing reaction by the spillover of sample gas (chemical sensitization), whereas Pd is known to promote the gas-sensing reaction by electronic interaction between Pd and sensing materials (electronic sensitization) (Cabot et al. 2000).
The grain-size reduction at nanometric level is one of the main factors enhancing the detection properties of metal oxides. It is in fact well recognized that by reducing the particle size of the sensing material in the nanometer range, the sensitivity of gas sensors is greatly improved both for the large specific surface offered and for the influence in reducing the surface charge density (Dolbec et al. 2003; Korotcenkov 2008; Rothschild and Komem 2004a, b). Furthermore, in this size range, a large fraction of the atoms (up to 50%) are present at the surface or the interface region. Therefore, the chemical and electronic of nanoparticles are different from those of the bulk, consequently contributing to an increase in the sensing properties.
Over the last 50 years, novel sensing materials other than metal oxides have been proposed. In 1983, the gas-sensing properties of conducting polymers were first reported. The sensor involved the use of doped polypyrrole (PPy), functioning as an ammonia sensor. In fact, as the conductivity of the pure conducting polymer is rather low, in order to achieve the high conductivity suitable for sensing applications, a doping process was necessary (Fig. 10.8). A wide variety of polymers of this type is now available, including substituted polypyrroles, polythiophenes, polyindoles, and polyanilines (Hassan et al. 2015).
Organic materials are much more easily modified than inorganic materials with respect to such characteristics as sensitivity , working temperature, and selectivity. Long-term instability is a main drawback of the sensors based on conducting polymers as they are thermally unstable, so it is often impossible to use them at temperatures at which gas-solid interactions proceed rapidly and reversibly.
To fully use all the potential advantages of organic and inorganic materials, hybrid composites have also been introduced as sensing elements for resistive sensors (Jiang et al. 2013). These hybrid organic/metal oxide composites are not merely the sum of the individual components but rather new materials with new functionalities and properties. From the viewpoint of structure, organic support can induce the nucleation, growth, and formation of fine metal oxide nano-/microstructures with uniform dispersion and controlled morphology, thereby avoiding the agglomeration of metal oxides. If the organic component is a good electrical conductor (e.g., conductive carbon, carbon nanotubes , or graphene), the resulting composites can form a perfect integrated structure with a developed electron conductive network and shortened current transport paths, improving the poor electrical properties and charge transfer of pure metal oxides. Significant synergistic effects, such as room-temperature sensing capability when exposed to low-concentration gases such as NO2, H2, and CO, often occur in hybrid composites because of size effects and interfacial interactions in contrast to the high-temperature operation required for metal oxides alone.
The possibility of synthesized materials having dimensions in the order of nanometers has provided enormous advantages for gas sensing because their extremely high surface-to-volume ratio is ideal for gas molecule adsorption. One important example is that of carbon nanotubes (CNTs), which are currently receiving a great deal of interest in gas sensing. Dai et al. first demonstrated the variety that they can offer for practical applications in highly sensitive gas sensors (Kong et al. 2000). The high surface-to-volume ratio, high conductivity, and mechanical stability make CNTs very attractive for gas-sensing applications. Carbon nanotubes can now be synthesized by different methods in large quantities and with high purity, such as the chemical vapor deposition (CVD) technique, and can be doped with B and/or N groups, thus rendering them very sensitive to a wide range of gas vapors (Peng and Cho 2003). In a similar way, functionalizing the nanotube surface with the polar COOH group attached, the sensors will give stronger responses toward the volatile organic compounds (VOCs) as their absorption efficiency with these volatile organic molecules will be increased due to the dipole-dipole interactions (mainly hydrogen bonding) between the COOH and the polar organic molecules (Sin et al. 2007).
CNTs are also often used as carriers of supported metal oxide particles (Sun et al. 2002). CNTs provide high surface area and then help the dispersion of the sensing materials on the nanotube walls. The better performance of these hybrid sensors is also attributed to the effective electron transfer between the metal oxide particles and the highly conductive carbon nanotube network (Lu et al. 2009a, b; Sun et al. 2012; Willinger et al. 2008). CNTs could also be coated with metal oxides of controlled thickness (Marichy and Pinna 2013). By combining the nonaqueous sol-gel route with the atomic layer deposition, metal oxides have been grown from the respective metal alkoxide precursors at low temperatures. Moreover, the surface reaction leading to the M-O-M bond formation is self-limited and allows then the deposition of films with well-controlled thickness on the internal and external surface of CNTs.
The successful synthesis of nanoparticles, nanowires, nanotubes, and other shapes has generated a lot of work to use these nanostructured materials in gas sensing (Lu et al. 2006; Pan et al. 2001; Li et al. 2003; Comini 2006). In particular, low-dimensional structures of metal oxides were found to possess characteristics and enhanced gas-sensing properties. In 2002, the Sberveglieri and Yang groups initiated the investigation of gas-sensing properties of one-dimensional nanostructures (Comini et al. 2002; Law et al. 2002).
Hierarchical and hollow oxide nanostructures are also very promising gas sensor materials due to well-aligned nanoporous structures with less agglomerated configurations. The literature data clearly show that these peculiar nanostructures can increase both the gas response and response speed simultaneously and substantially (Lee 2009). This can be explained by the rapid and effective gas diffusion toward the entire sensing surfaces via the highly porous structures.
10.2.12.3 Working Mechanism of Metal Oxide Semiconducting
Along with the improvement of the characteristics and properties of the devices, a great deal of effort has been also made during these years to improve knowledge of the sensing mechanism and understanding the related processes. The description of mechanisms of gas sensors based on metal oxides was first depicted through the application of electron theory of chemisorption and catalysis on semiconductors originally formulated by Wolkenstein (1961).
Later, Morrison , Yamazoe , and Gopel especially contributed to the description of conditions of transport of electric charges through the metal oxide semiconducting layer in the presence of oxygen and reactive gases (Morrison 1982; Yamazoe et al. 1983; Gopel and Schierbaum 1995). Following these theories, the sensing mechanism of MOS sensors relies on reactions which occur between adsorbed oxygen species and the probed gas on the surface of the sensing layer. Details on this subject can be found in many books and reviews (Azad et al. 1992; Barsan et al. 1999). In first approximation, oxygen adsorbed on the surface of n-type metal oxide semiconductors plays a key role, trapping free electrons because of its high electron affinity and forming a potential barrier at the grain boundaries. This potential barrier restricts the flow of electrons, causing the electric resistance to increase. When the sensor is exposed to an atmosphere containing reducing gases, e.g., CO, the gas molecules adsorb on the surface and react with active oxygen species, e.g., O−, which liberates free electrons in the bulk. This lowers the potential barrier allowing electrons to flow more easily, thereby reducing the electrical resistance. With oxidizing gases such as NO2 and ozone, the adsorption process increases instead the surface resistance (Leblanc et al. 2000). The converse is true for p-type oxides, where electron exchange due to the gas interaction leads either to a reduction (reducing gas) or an increase (oxidizing gas) in electron holes in the valence band (Li et al. 2002). However, to give a complete description of the gas-sensing mechanism for these sensors, it is necessary to take into account all elementary steps (adsorption, reaction, desorption, etc.) governing surface-gas target interactions leading to charge transfer (Yamazoe and Shimanoe 2008).
Yamazoe first demonstrated that reduction in crystal size would significantly increase the sensor performance (Yamazoe 1991). The sensing properties are partially associated with the depth of the surface space charge region which is affected by the gas adsorption and depends on the particle size. Specifically, the sensor’s response increases significantly if the crystallite size is about twice the adsorption depth, 2L (L is the depth of the space charge layer), of oxygen adsorbates. This means that the sensor performance improves not only when D decreases but also if L increases, since a major proportion of material takes place in the reaction of oxygen adsorbates with the target gas even if the grain size is not excessively small. For a typical sensing material , SnO2 with different grain sizes between 5 nm and 80 nm, simulations showed that the conductivity increases linearly with decreasing trapped charge densities and the sensitivity to the gas-induced variations in the trapped charge density increases too, in agreement with experimental findings (Rothschild and Komem 2004a, b).
When dealing with wire with lateral dimensions to the order of hundreds of nanometers, gas adsorption creates a surface depletion layer, consequently reducing the conducting channel thickness (Chen et al. 2013). The mobility dependence on surface coverage can be neglected because electron diffusion length (about 1 nm) is much shorter than the diameter (tens of nanometers). Electrical transport changes when the thickness of the wire is small like in nanowires with low lateral dimensions compared to the Debye length, the space charge region extends through the entire wire cross section, and all electrons are trapped in surface states (Zhu and Zeng 2017).
A model of the sensing mechanism of hybrid heterostructures such as metal oxide/CNTs has been attempted. The junctions existing between the nanotubes of the networking at their crossing points and the different depletion regions existing as a function of the presence of the target gas have been taken into account. Their role in the sensing mechanism has been established by several authors (Wei et al. 2004; Marichy et al. 2013).
10.2.12.4 Sensing Materials: Effect of Grain Size and Film Thickness
The most traditionally used materials for solid-state gas sensors are thick films of polycrystalline compressed or sintered MOS powders. In the case of compressed powders, the grain-boundary resistance dominates the response of the sensor device since the resistance at these intergranular contacts is much larger than the resistance across a single grain. Compressed powders are not very stable as the intergranular contact is pressure sensitive. In sintered powders, some of these grain boundaries disappear and form more stable neck-like structures as the grains sinter together. These necks often constitute the most sensitive sites of sensing materials as all the electrons from these narrow necks are potentially tied up with the adsorbed oxygen, giving rise to very sensitive completely electron-depleted highly resistive regions.
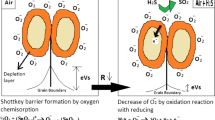
In Fig. 10.9, we illustrate the energy band model for an MOS compressed powder. A few grains are shown where chemisorbed oxygen has captured electrons from the material forming a depletion layer (also known as the space charge layer or the Debye layer). At the intergranular contact, two space charge layers back to back form a formidable barrier for electrons to cross, and this gives rise to the higher resistance values at those contact points. The thickness of the depletion layer or the Debye layer (L) is relatively small compared with the grain diameter (D) in most traditionally polycrystalline materials. A typical value for L is 100 nm for MOS films. Grain-boundary-controlled gas sensing, most important for relatively large-particle MOS sensors, has been extensively reviewed by Barsan and Weimar (2001, 2003). In this contribution, we use these early sensing models to gain an understanding about what to expect when particle size decreases to the nanometer range.
When an MOS gas sensor is exposed to reducing gases, such as H2, CO, and CH4, at a sufficiently high temperature, the chemisorbed oxygen reacts with the reducing gases, lowering the steady-state surface coverage of the oxygen while injecting electrons into the MOS crystallites and thus lowering the height of the potential barriers at the intergranular contacts (qVs in Fig. 10.1). The important reactions involved in this type of gas sensor may thus be summarized as follows.
Oxygen Reactions
As a result, electrons are extracted and resistance goes up.
Reducing Gas Reactions
Reducing gas molecules (R) react with the chemisorbed oxygen at the grain boundaries and/or necks:
As a result, electrons are injected and resistance goes down.
Also, oxidizing gases can be detected. The oxidizing reactions may be summarized as:
As a result, electrons are extracted and resistance goes up even further. Therefore, by measuring the change in the conductivity of the semiconductor oxide films, one can detect reducing and oxidizing gases in the atmosphere.
The above analysis of the MOS sensing mechanism, for the sake of clarity and brevity, is somewhat simplified. Indeed, not only a change in the number of free electrons (and thus boundary layer thickness modulation) but also changes in electron mobility may modulate the sensing response, as investigated by Ogawa et al. and recently reviewed by Tricoli et al. (Ogawa et al. 1982; Tricoli et al. 2010). Furthermore, there is still an ongoing debate in the MOS gas sensor community about the possibility of another sensing mechanism altogether. Gurlo and Riedel (2007) point out that, although it has been sought for a long time, there is not yet any convincing spectroscopic evidence for oxygen “ionosorption” as shown in Fig. 10.1. In their alternative model, there is no oxygen ionosorption on the MOS surface, and the model focuses instead on oxygen vacancies at the surface, which are considered to be “the determining factor in the chemiresistive behavior” (Zemel 1988). SnO2, the model gas-sensing material, is oxygen deficient and therefore acts as an n-type semiconductor, with oxygen vacancies that act as electron donors. Alternate reduction and reoxidation of the surface by gaseous oxygen control the surface conductivity and therefore the overall sensing behavior in this model.
Catalysts such as Pt enhance gas-sensing reactions of MOS via the spillover effect. Spillover refers to the process in which the catalyst dissociates a gas molecule, and then the atoms “spill over” onto the surface of the MOS. At the right temperature, a gas reactant first adsorbs onto the surface of the catalyst particles and then migrates to the oxide surface to react there with reactive surface oxygen species. For this process to be possible, the spilled-over species must be able to migrate to the interparticle contact (grain boundary or neck). As a consequence, for a catalyst to be effective, there must be a very good dispersion of the catalyst particles so that they are available near all contacts. Only then can the catalysts affect the interparticle contact resistance that controls the gas sensor sensitivity . Importantly, in the case of nanoparticles, there are several reports which indicate that the need for metal catalysts is somewhat alleviated (Rothschild and Komem 2004a, b; Cukrov et al. 2001).
Porosity, grain size, and film thickness all play an important role in the LOD and sensitivity of MOS gas sensors. Lee et al. (2000) carried out a comparative study of thick- and thin-film SnO2 gas sensors in order to understand the differences in gas-sensing characteristics as a function of the microstructure of the gas-sensing materials. Thin films, in their investigation, were deposited using metal organic chemical vapor deposition (CVD), which yielded a dense compact microstructure (Fig. 10.10). Thick porous films were derived from metal organic decomposition. These thick films were made up of a network of loosely connected crystallites that also featured several cracks (approx. 1 mm wide) as well as small pore channels at the crystallite surfaces that all help in the penetration of gas molecules throughout the material and thereby enhance the sensitivity (Fig. 10.10). Lee et al. confirmed a much improved gas sensitivity in the case of porous thick-film microstructures compared with compact thin layers of SnO2. In a porous MOS film, grain boundaries are present in all directions, and more surface area is available for reactions (R1)–(R3) to take place. In a compact film, on the other hand, only a limited exposed surface area is affected by the presence of analyte gases. Therefore, porous films are superior for gas-sensing purposes. In Fig. 10.2, we illustrate the surfaces that are likely to participate in the resistance change of these two types of MOS gas-sensitive material configurations.
Since the sensitivity of the high-surface-area porous gas sensors resides in the high resistivity grain-boundary contact points, one wants to make the number of these contact points as high as possible. So a thicker porous film is better. One also wants to make these contact points as stable as possible. A powder that is not sintered is pressure sensitive and quite unstable. In order to improve this, the MOS powders are sintered, giving rise to the formation of necks between the grains in the powder. The sensitivity of the film depends very much on the extent of the sintering of the material. One should not sinter so much that the film becomes one compact layer, reducing the porosity and thus the sensitivity of the sensor dramatically. In most sintered MOS films, both necks and grain contacts are present. Neck-grain models with different degrees of neck formation have been investigated by several research groups.
Xu et al. (1991) proposed a model with a chain of crystallites connected mostly by necks and sometimes by grain-boundary contacts. These authors assumed the neck size (X) 0.8 times the crystallite size (D) and suggested that, when D is larger than 2L, grain-boundary contacts display higher resistance and govern the electric gas sensitivity of the chain (grain-boundary control). As D becomes smaller and comparable to 2L, necks become most resistant, and therefore they start controlling the gas sensitivity (neck control). Finally, when D is smaller than 2L, the resistance of grains dominates the whole resistance of the chain, and the gas sensitivity in this case is controlled by grains themselves (grain control) and yields the largest gas sensor response.
Figure 10.11 demonstrates the three situations. Along the same line, Rothschild and Komem [19] showed that the gas-induced variations in the trapped charge density in the MOS are proportional to 1/D, where D is the average grain size. At the nanoscale, the grain size can be decreased to match the Debye layer (L) thickness (typical value is 100 nm – see above), or can even be made smaller, which leads to the extraction of all electrons by the chemisorbed oxygen present. Such nanostructures can thus be treated as gas sensor materials with uniformly changing resistance upon exposure to gases (see grain control in Fig. 10.11).
Ma et al. (2002) carried out a theoretical study to better understand the dependence of the grain-boundary potential barrier as a function of the density of electrons trapped at the surface (nt) and electron density in the bulk of the material (nb). These authors suggest a model for neck-grain boundary control in order to investigate the combined effect of neck and grain boundaries in a sintered powder. For a constant distribution of such donors, the height of the grain-boundary potential barrier (eVs; see Fig. 10.9) is
where e is the electron charge, n is the surface electron density, ΣoΣr is the permittivity, and nb denotes the free electron density in the grain body.
Assuming that the necks in a sintered ZnO powder are cylindrical, resistance values were calculated for both neck and grain boundaries employing Eq. 10.10. Like Xu et al. (1991), these authors concluded that gas sensitivity increases with decreasing grain size, which results from increased neck control. From this discussion, we can also appreciate that, in the case of a thin compact film, one likes the thickness to be small, perhaps in the range of L or less, so as to have a completely electron-depleted thin film.
We can conclude from the discussions so far on grain size, porosity, and thickness that, for the best gas sensor performance, we need a thick film of lightly sintered nanocrystalline, porous material. Nanocrystalline metal oxides offer other advantages as well; they can operate at lower temperatures and might obviate the need for expensive catalysts (Wang et al. 2010). Some words of caution are in order here with respect to the ultimate MOS grain size and the optimal film thickness.
Nanomaterials do indeed not come without disadvantages. Especially, thermal instabilities are worrisome since smaller MOS grains tend to agglomerate at lower temperatures, and this leads to changing sensor characteristics over time. Thermal degradation is responsible for temporal instabilities in device parameters and higher drift rates. According to Korotcenkov and Cho (2010), the larger the grain size, the wider the temperature range in which crystallites retain their size and shape without changes. Conversely, the smaller the grain size, the lower the temperature at which structural changes start taking effect; for example, films with a grain size of 2–3 nm start transforming at temperatures of approximately 200 °C. The same authors concluded that very small (approx. 20 nm) particles of SnO2 and In2O3 MOS materials are not quite suitable for gas sensing above 500 °C, as the particles melt and agglomerate at much lower temperatures than their respective bulk melting points. An optimum approach that balances a high sensitivity by decreasing the particle size with good stability, calibration frequency, and lifetime requirements of a commercial gas sensor is therefore recommended. Above, we advocated making porous, lightly sintered nanoparticles into a thick film for the ideal gas sensor characteristics. On the other hand, some reports have shown that reducing the film thickness does increase the sensor’s response considerably (Tricoli and Pratsinis 2010). This is in agreement with models of MOS sensor response to several analytes (Becker et al. 2001) and has been discussed in detail recently by Tricoli et al. (2010). Without more detailed studies, this is a hard argument to settle; indeed particle size, degree of sintering, and thus porosity are all intertwined. Depending on the porosity and particle size, either a thinner or a thicker film might be required.
MOS nanocatalysis can be considered as a bridge between homogeneous and heterogeneous catalysis. Because of their nanosize, i.e., high surface area, the contact between reactants, gas sensors, and nanoparticles is huge. Pinna et al. (2004), for example, demonstrated that gas sensors based on tin and indium oxide nanopowders exhibited high sensitivity and good recovery time at low temperature. Especially, indium oxide nanoparticles were highly sensitive toward NO2 with a detection limit of 1 ppb at low temperatures.
Nanobelts (nanowire-like structures but with flat cross sections), nanorods, and nanowires have all been successfully implemented as gas sensor materials and continue to be investigated for their potential advantages over traditional materials. These nanomaterial-based gas sensor devices are based either on mass assemblies of nanoparticles (e.g., porous mats) or on one or a set of individual nanoparticles (e.g., a small number of nanowires) (Sadek et al. 2007; Lee et al. 2008; Wang et al. 2008).
Sadek et al. (2007), for example, developed a conductometric H2, NO2, and hydrocarbon gas sensor using a ZnO nanobelt film as the sensitive layer. In Sadek et al.’s experiments, ZnO nanobelts display a structural morphology (wurtzite family) characterized by a rectangular cross section and a uniform long structure along their length. A mat of these nanobelts was deposited by radio frequency (RF) sputtering on a heater element.
The sensing elements in sensors fabricated by Lee et al. (2008) are thin films consisting of SnO2 nanorods. The nanorod films also were equipped with well-dispersed Pd catalyst nanoparticles with an average diameter of 3 nm. These thin-film nanorods were deposited using plasma-enhanced CVD (PECVD) with post-plasma treatment. The surface of these nanorod thin films was modified with Pd nanoparticles, and the sensing properties of the thus fabricated films were tested with H2 and ethanol vapors. Yeom et al. (2008) employed nanomembranes for pre-concentration of gases that were detected using a microfluidic sensor that resulted in much enhanced sensitivity .
In another study by Liu et al. (2006), vanadium oxide nanobelts coated with MOS nanoparticles such as Fe2O3, TiO2, and SnO2 were employed as hybrid sensor nanostructures for sensing ethanol vapors. These vanadium pentoxide (V2O5) nanobelts coated with MOS nanoparticles showed a much improved sensitivity compared with the nanobelts alone. The authors suggest that the coated V2O5 nanobelts exhibit enhanced sensitivity owing to the synergy between electrical transport through the largely depleted nanobelts and the effective gas sensing on the high surface area MOS nanoparticles that inject electrons into the nanobelts. The V2O5 nanobelts were synthesized using a chemical process based on a mild hydrothermal reaction of 0.1 M ammonium metavanadate solution in dilute nitric acid, and the MOS nanoparticle coatings were fabricated from ferric nitrate, tetrabutyl titanate, and tin tetrachloride in ethanol, for depositing Fe2O3, TiO2, and SnO2, respectively. The sensing mechanism is clarified in Fig. 10.12 with a schematic drawing of a longitudinal cross section of nanobelts coated with MOS nanoparticles.
Upon exposure to ethanol, the electron-depleted surface layers in both the nanobelts and the nanoparticles are reduced because of the electron injection that accompanies the reaction of ethanol with the reactive adsorbed oxygen species. The authors believe that the boundaries between metal oxide grains on the V2O5 nanobelts dominate the sensor resistance.
As micromachined heater elements tend to be mechanically less sturdy than the ceramic substrate-based heaters in traditional gas sensors, we need to pay close attention to how the MOS nanoparticles are deposited. Techniques that do not exert mechanical pressure on the brittle micromachined heater substrates and result in porous nanoparticle films constitute the more attractive technologies. RF sputtering, for example, as used by Sadek et al. (2007), is a possible approach, but the process needs to be modified so as to generate more porous films.
As another example, Cukrov et al. (2001) synthesized tin oxide nanoparticles (average diameter 24 nm) and tested the O2 sensing properties of porous thin films prepared by mechanochemical processing and spin coating. The mechanochemical process uses a conventional ball mill in which the mechanical energy activates the necessary chemical reactions and induces structural changes. These particles were subsequently spin coated in a thin film on alumina substrates with interdigitated electrodes (measuring electrodes on the front side and the heater on the back side). These films were tested for their oxygen-sensing potential and were found to be extremely stable and repeatable. Although the nanomaterial is very interesting because it does not require any metal catalyst and because of its demonstrated stability, spin coating is not a method of choice to coat the MOS on fragile microelectromechanical system (MEMS) or nanoelectromechanical system (NEMS) structures. Screen printing of electrodes and sensing materials , as often employed in the construction of traditional gas sensors, is an even less attractive candidate as significant pressure is exerted on the substrate in the silk screen printing (Fine et al. 2010). During screen printing, pressure is exerted on the structures, and therefore it may not be a very useful technique for fabricating fragile nanogas sensors (Viricelle et al. 2006).
Another nanopowder gas sensor, based on the synthesis of Al-doped TiO2 nanoparticles (average diameter 100 nm), was reported by Choi et al. (2007). The nanopowder in this study was used to make thick-film gas sensors to measure CO selectively and sensitively in an O2 environment. A thick film of this material was prepared on an alumina substrate with interdigitated Pt-measuring electrodes. To integrate the material with the measuring electrodes, the nanopowder was mixed in an appropriate solvent (alpha teripineol) and, drop by drop, was deposited on the electrodes followed by drying and densifying, at 100 °C and 800 °C, respectively, on the thus deposited films. This chemical method as well as sol-gel techniques and physical vapor deposition (Fine et al. 2010) are all feasible approaches to integrate sensing materials on brittle MEMS and NEMS structures. An especially attractive approach to deposit MOS locally on a brittle heater structure is to use the heat of the heater element itself to initiate the CVD from a metallo-organic precursor. Such an approach was pioneered by Cavicchi et al. (Sharma and Madou 2012), who deposited films on the prefabricated gas heater structures employing thermally activated CVD.
In CVD, a controlled mixture of precursors is brought in contact with a heated substrate, which causes a chemical reaction between the vapor and the substrate and leads to the deposition of a layer of the desired compound on the substrate. A basic outline of the CVD method is illustrated in Fig. 10.13.
Commonly employed CVD techniques include atmospheric pressure CVD (APCVD), aerosol-assisted CVD (AACVD), PECVD, and rapid thermal CVD. An advantage of using CVD for gas sensor purposes is that the thickness and density of the film can be optimized by controlling the concentration of the reagents in the vapor and the time of deposition. A concentrated vapor precursor yields a compact film that may grow at a rate of up to 1 μmmin−1; a rarified mixture of reagents on the other hand deposits a more porous film at a considerably lower growth rate (Fine et al. 2010).
Two relevant CVD applications are by Shaw et al. and Ashraf et al. (Shaw et al. 2005; Ashraf et al. 2008). Shaw et al. employed AACVD for the deposition of tungsten oxide from W (OPh)6 (the precursor) in toluene at 600 °C. After a deposition time of 90 min, a 50–75-nm-thick fibrous film was obtained. The thus fabricated films can be made thicker by using an electrical field in the CVD process. The authors investigated the porosity of the tungsten oxide films, a feature, as we know, of key importance for the utility of these films in gas sensing, at different applied voltages. Ashraf et al. demonstrated that APCVD is an effective technique for depositing tungsten films as thick as 3.6–6.7 mm. They used WCl6 as the precursor vapor and operated the reactor at 625 °C in N2. The particles in these up to 6.7-mm-thick tungsten oxide films were thin needle-like crystals with a high surface area and high porosity. Significantly, Ashraf et al. found that the MOS films deposited this way are more sensitive than those fabricated by screen printing.
Carbon nanotube (CNT)-based miniaturized gas sensors constitute yet another innovation in nanogas sensing. A single-walled CNT is a one-molecule-thick layer of graphite rolled into a cylinder with a diameter of a few nanometers. Multi-walled CNTs are composed of several such graphite cylinders rolled up into one. The distribution of electrons on their outer surface only makes CNTs extremely sensitive to charge transfer and chemical doping effects by surrounding molecules (Zhang et al. 2008). When electron-withdrawing or electron-donating gas molecules come in contact with CNTs, it very dramatically changes the surface electron concentration. CNT-based gas sensors show faster response, higher sensitivity , and lower operating temperatures and detection ability for a wider variety of gases than any other gas-sensitive materials (Suehiro et al. 2003). Because of these extensively perceived advantages, the use of CNTs, both pristine and functionalized, continues to be studied extensively. Unfortunately, the difficulty in making electrical contact to CNTs and the tremendous variety of lengths, thicknesses, and conductivities they come in have hampered progress. Our approach to nanogas sensing with suspended CNWs (§5) constitutes a viable alternative to CNT-based gas sensing as we can control contact resistance, thickness, and resistivity of the nanowires very well.
10.2.12.5 Gas-Sensing Characteristics of Metal Oxides
-
(A)
Sensitivity
It is the response of a gas sensor per unit change in the gas concentration. Since metal oxide gas sensors are based on the principle of chemiresistivity, it is generally defined in terms of conductance or resistance. For n-type material in the presence of reducing gas and p-type material in the presence of oxidizing gas, sensitivity can be defined as:
where Ra and Rg are the stable values of the resistance of the material before and after exposure to gas.
-
(B)
Selectivity
A sensor should respond to only a particular molecule in a mixture of environment. The selectivity of a gas sensor toward a particular molecule is the ratio of its response toward it and that of another dominant interfering molecule in the atmosphere.
Selectivity of a gas sensor should be always greater than one.
-
(C)
Accuracy
Accuracy represents the degree of exactness of a measurement compared to the true value.
-
(D)
Speed of Response
The time required for a sensor to reach 90% of the total response of the signal such as resistance upon exposure to the target gas.
-
(E)
Recovery Time
The time required for a sensor to return to 90% of the original baseline signal upon removal of target gas.
-
(F)
Detection Limit
It is the lowest concentration of the gas that can be detected under given conditions, particularly at a given temperature.
-
(G)
Precision
It is a measure of repeatability of a gas sensor.
-
(H)
Error
It is the difference between the measurand value and true value.
-
(I)
Dynamic Range
The concentration range between the detection limit and the upper limiting concentration.
-
(J)
Linearity
The relative deviation of an experimentally determined calibration graph from an ideal straight line. Usually values for linearity are specified for a definite concentration range.
-
(K)
Resolution
The lowest concentration difference which can be distinguished when the composition is varied continuously. This parameter is important chiefly for detectors in flowing streams.
-
(L)
Stability
The ability of the sensor to maintain its performance for a certain period of time. As a measure of stability, drift values are used, e.g., the signal variation for zero concentration.
-
(M)
Life Cycle
The length of time over which the sensor will operate. The maximum storage time (shelf life) must be distinguished from the maximum operating life. The latter can be specified either for continuous operation or for repeated on-off cycles (Jimenez-Cadena et al. 2007; Korotcenkov 2007a, b; Wang et al. 2010).
Table 10.1 is summarizing the comparison of various kinds of the mentioned gas sensors and its most proper parameters that have been studied by Korotcenkov (2007a, b).
10.3 Applications of Gas Sensors
While the previous sections focused on the scientific breakthroughs achieved by constantly pushing the fundaments of sensing materials and devices, focus in the following is addressed toward the applicative aspects of chemoresistive gas sensors.
Rapid, comprehensive, and reliable information regarding the chemical state of a gaseous system is currently indispensable in many high-technology fields. Solid-state gas sensors are thus generating tremendous interest because of their widespread applications in industry, environmental monitoring , space exploration, biomedicine, and pharmaceutics. Generally, basic criteria for these practical gas-sensing devices are (i) high sensitivity and selectivity; (ii) fast response time and recovery time; (iii) low power consumption; (iv) low operating temperature and temperature independence; and (v) high stability (Hung et al. 2017).
For most of the practical applications, a relevant problem is not only to estimate the target gas concentration but also to identify it in the real mixture (outdoor and indoor ambient, exhaust gases, breath). Unfortunately, chemoresistive gas sensors are very sensitive but not selective. Such a task can therefore not be performed by a single sensor. E-nose, an artificial olfactory system consisting of an array of sensors, then needs to be used. Each sensor shows an individual response to all (or to a certain subset) of the target gases which are identified by pattern recognition of the sensor signals, e.g., that developed by Cyrano Sciences is based on the concept of using multiple semi-selective sensors combined with electronic computation, first proposed by Gardner and Bartlett (Wilson and Baietto 2009).
In the following, some of the most important applicative fields of semiconductor gas sensors are listed.
10.3.1 Environmental Applications
In environmental applications, field-portable monitors based on chemoresistive sensor devices are particularly suited to comply with the newly reinforced environmental regulations and give a valuable alternative to conventional analytical techniques; they are accurate but more expensive and time-consuming (Yamazoe and Miura 1994). Metal oxide semiconductor sensors are relatively inexpensive compared to other sensing technologies; they are also robust, lightweight, and long-lasting and benefit from high material sensitivity and quick response times. These properties could lead them to becoming ever more important tools in environmental monitoring with respect to the other type of sensors. They can be used to measure and monitor trace amounts of environmentally important gases such as carbon monoxide and nitrogen dioxide (Fine et al. 2010). Today, it is also highly desirable to develop simple and inexpensive sensors to measure atmospheric carbon dioxide in order to monitor indoor air quality (Marsal et al. 2003).
10.3.2 Automotive Applications
The ability of chemosensors based on refractory metal oxides to withstand high temperatures, the robust nature, efficient packaging procedures, and small size are strong technical merits for automotive applications. Indeed, usually high temperatures are reached in the exhausts, and sensor devices should operate in the range 600–900 °C in a harsh ambient requiring advanced packaging. Another requirement for these sensors is reaction measurement speed of the order of fraction of seconds and 5–10-year service life. As a result of the introduction of emergent technologies in the automotive industry, new vehicle diagnostic sensors are at present necessary to control motor functioning, monitor a range of emission gases (NO, NO2, CO, CO2, HC, O2, etc.), and detect high pollution levels in the vehicle cabin (Pijolat et al. 1999).
Chemoresistive sensors can be used to minimize the emission of CO, hydrocarbons, and nitrogen oxides coming from combustion engines. It is important for the engine to operate with the proper air-to-fuel ratio (A/F) so that combustion parameters are optimized. The first titania gas sensors were developed in the late 1970s and early 1980s. They were primarily used to detect the stoichiometric A/F (Cederquist et al. 1976). In these instances, the sensor resistance increased by orders of magnitude around the stoichiometric A/F, making it a very useful device for these applications.
Chemoresistive sensors will have high importance also in future cars. In fact, CO sensors have been identified as a critical need for proton exchange membrane (PEM) fuel cell systems, which offer a viable approach to improve efficiency of power generation from fossil fuels, while reducing emissions of pollutants and greenhouse gases (Holt et al. 2002). The primary uses of such sensors include measurement of the CO content of the reformate gas entering the PEM fuel cell (i.e., for fuel cell protection) and measurement of the CO content of the reformate gas at various catalytic stages of the fuel processor (for fuel processor feedback and control). A further requirement is that these sensors must not exhibit cross sensitivities to the gaseous components of the reformed gases (e.g., hydrogen, carbon dioxide, and/or humidity) or to other potential contaminants.
10.3.3 Biomedical Applications
Biomedical uses of chemoresistive gas sensors (e.g., in breath test) require instead high sensitivity to detect very low concentrations of target gases (in the ppt-ppm range), coming from biochemical processes occurring in the human body and used as markers for several pathologies (152 D’Amico et al. 2008). Furthermore, the target gas is to be analyzed and quantified in a complex mixture , i.e., in the presence of many other interferent gases and a high humidity content. Such sensors can complement or serve as an alternative to more sophisticated spectrometric systems for breath analysis and speciation, especially for clinical diagnostics and monitoring (Righettoni et al. 2015).
10.3.4 Health-Care Application
In the human gas breath, detection technique can be obtained; decreasing the cost of the system is essential for the spread of the breath-analyzing system. Sensor device or a detector that aimed to a high-performance breath gas detector at the medical diagnosis level was developed by Ohsawa et al. (2007).
The main gas composition of human breath consists of the following gases:
-
(i)
Nitrogen that is the most common gas in atmosphere
-
(ii)
Carbon dioxide produced by respiration
-
(iii)
Water vapor generated from bodily fluids
-
(iv)
Oxygen that was not consumed
Table 10.2 is a summary of the sensing methods and the relationships between the physical conditions or diseases and various biogases (Shin et al. 2015).
For such analysis or health screening, technologies are necessary to selectively detect various gas compounds in the breath and to measure the concentration of important gas species that are correlated with halitosis, metabolism, and disease (Shin et al. 2011).
Recently, the capacities to monitor health conditions and to predict disease noninvasively became easy without infringing the Medical Practitioners Act. One of the simple ways of detection is to make predictions based on biogas, just as in the previous times when the physicians made decisions by smelling the odor from patients. A representative example is alcohol detection tool in the breath, which will be nearly commercially available. However, drunkenness is caused by an external factor , and it is not an index of a human state. Also, there are devices of urea breath test to detect carbamide peroxide and other devices used for asthma patients to measure nitric oxide (Itoh et al. 2013).
Research is being conducted actively to clarify the relationships between various diseases and breath components, such as monitoring the liver function using ammonia, evaluation of cholesterol metabolism by isoprene, and monitoring the blood carboxyhemoglobin by carbon monoxide (Shin et al. 2015).
10.3.5 Miscellaneous Applications
During recent years, single or array of metal oxide semiconductor (MOS) sensors have found applications in many other different fields than those listed above, including odor landfill, chemical agent warfare detection, food quality control, etc. (Casalinuovo and Pierro 2006). These technologies enable rapid detection and identification of substances based on their chemical profile. They find applications in the monitoring of some medical conditions as well as industrial applications generally related to quality control or contamination detection. A new multichamber electronic nose (MCE-nose) which overcomes, to a great extent, one of the major disadvantages of the use of MOS technology for e-noses – the long recovery period needed after each gas exposure that severely restricts its use in applications where the gas concentrations may change rapidly – has been also proposed (G-Jimenez et al. 2011). It comprises several identical sets of MOS sensors accommodated in separate chambers which alternate between sensing and recovery states, providing, as a whole, a device capable of sensing more quickly changes in chemical concentrations.
10.3.6 New Opportunities
Although resistive gas sensors are still commercialized intensively as bulk devices, there is a strong demand for novel, smaller, integrated, high-performance, and reliable devices for advanced applications. Technological advances will assist gas sensor device scale of economies by exploring more efficient fabrication methods that may include material and component assembly into the final device.
At the material level, technological advances will arise from multidisciplinary contributions, which will hopefully open up new areas of nanoscience research. In the past few years, large efforts have been expended in the development of new synthetic approaches for sensing materials at the nanoscale. Advances in recent activities concerning the synthesis, characterization, and properties of novel materials with potential in sensing applications can be found in many reviews (Neri 2011; Carbone and Cozzoli 2010). Among the obvious challenges are improving reproducibly and control over nanoparticle structure, surface chemistry , and dispersion.
Future work will continue to focus also on generating improved component assembly and device fabrication strategies. For small- or medium-scale batch production , a hybrid design whereby component parts are created separately for subsequent assembly into a complete system is an attractive option, as it removes many of the restrictions imposed by the need for process compatibility.
Integrated gas sensor with a microhotplate (MHP) demonstrates better sensitivity , faster response, and lower power consumption than traditional thick-film devices. For the detection of a target gas, a gas-sensitive layer is applied on a suspended microhotplate that is mounted on top of a silicon chip. Any gas-induced change of the gas-sensitive layer’s surface potential is detected and digitally processed by the integrated electronics.
Sensor platforms robust enough for integration into standard, industrial weaving processes have been proposed (Ataman et al. 2013). Such sensors were fabricated on a thin, flexible plastic stripe and woven into a cotton fabric by using a standard weaving machine, to create a smart fabric able to detect ambient gas. The fabrication process is actually very simple and compatible with large-scale roll-to-roll fabrication. Inkjet printing can be used as an effective, low-cost alternative, suitable for the fabrication of long sensor stripes. Bending tests performed on the devices showed that they can be successfully woven within a cotton fabric using a standard weaving machine without being damaged.
From a processing point of view, employing modern microelectronics technology to manufacture both the sensing element and the signal conditioning circuitry on a single silicon chip is now possible to make low-power, low-cost smart gas sensors in high volume, and this should result in a new generation of miniaturized gas sensors (Gardner et al. 2010). Full integration of microelectronic and micro-mechanical components on a single wafer has been achieved commercially using silicon processing technology.
The sensing element is comprised of a sensing chip and an integrated heater fabricated on a silicon substrate using MEMS technology and a metal oxide semiconductor layer on the sensing chip. Due to miniaturization of the sensing chip, the sensor requires a heater power consumption of only 15 mW and is suitable for low-power equipment and battery-operated instruments. We expect many areas will benefit from these miniaturized sensors, including transportation (land, sea, air, and space), buildings and facilities (homes, offices, and factories), humans (especially for health and medical monitoring), and robotics of all types.
Recent technological advances are quickly changing the landscape of gas sensors. As these devices are becoming smaller in size, require less support infrastructure than currently used equipment, and are capable of operating autonomously, new opportunity for gas sensors could come from their integration into smartphones [86]. Chemical sensors, integrated within smartphones and other wireless and wearable devices, provide a vast array of functionality, from informing people about their conditions through breath analysis. For example, background apps on a smartphone can monitor CO or CO2 levels and provide safe/warning/alert indication to the user about possible dangerous changes in their environment.
Furthermore, monitoring devices can become simpler and cheaper as the computation is pushed to the cloud. This enables users to buy off-the-shelf devices and access customized monitoring applications via cloud-based services.
10.4 Conclusions
In this study, the historical progress of semiconductor gas sensors carried out during the past century has been briefly reviewed. As it follows from conducted analysis, the choice of an appropriate material for gas sensors should be based on good gas response time, high selectivity, low sensitivity to air humidity, low hysteresis, thermal cycling, high stability of parameters over the time, all range of operation temperatures, and on exposure to the various chemicals likely to be present in the environment. Therefore required efficiency of reactions, responsible for gas sensors’ sensitivity, is necessary to achieve, taking into account the necessity of an attainment of maximum chemical, structural, and long-term stability of the device’s parameters.
Such requirements drive the trends of R&D in gas sensors industry, which in turn fuels opportunities for technology progress that can open up new applications of gas sensors. Whereas many different approaches to gas detection are available, the R&D of solid-state gas sensors has extremely developed in recent years. Due to the diversity of solid-state gas sensors, it’s impossible to deal with all the different types of solid-state gas sensors. Hence, herewith we focused on the basics of some of the main solid-state gas sensors, discussing the principles of operation for each and the future trends of the scientific research. In particular, metal oxide gas sensors were discussed in more detailed with respect to the other types of solid-state gas sensors due to their comparative simplicity, low cost, and advantages that should work in their favor as new types of applications and technologies emerge.
In the next few days, there is no doubt that nanostructured metal oxides will constitute the key for the development of semiconducting gas sensors with improved gas-sensing properties. Not only in chemical gas sensors but also in solid-state gas sensors nanocrystalline materials will play a fundamental role in the gas sensors of new generation. In fact, nanoscience and nanotechnology are dedicating great efforts to developing of novel materials for gas sensor applications. This new classification of nanostructures shows a great potential for applications in ultrasmall sensors because the conductivity of these materials changes dramatically when gas or liquid molecules attach their surfaces. In addition, with the development of standard wireless communication protocols optimized for sensors, wireless sensor network is expected to gain a wide approval in applications such as homeland security, process monitoring, food monitoring, and medical instrumentation.
Creation of new sensitive, selective, and stable gas sensor materials is one of the most important and actual tasks of nanotechnology.
Abbreviations
- AACVD:
-
Aerosol-assisted chemical vapor deposition
- BAW:
-
Bulk acoustic waves
- CVD:
-
Chemical vapor deposition
- IDT:
-
Interdigitated transducer
- MEMS:
-
Micro-electrochemical systems
- MHP:
-
Microhotplate
- MOSs:
-
Metal oxide semiconductors
- NDIR:
-
Nondispersive infrared
- PECVD:
-
Plasma-enhanced chemical vapor deposition
- PEM:
-
Proton exchange membrane
- QCM:
-
Quartz crystal microbalance
- QMB:
-
Quartz microbalances
- SAW:
-
Surface acoustic wave
- VOC:
-
Volatile organic compounds
- YSZ:
-
Yttria-stabilized zirconia
References
Acquaroli LN, Urteaga R, Koropecki RR (2010) Innovative design for optical porous silicon gas sensor. Sensors Actuators B Chem 149:189–193. https://doi.org/10.1016/j.snb.2010.05.065
Aleixandre M, Gerboles M (2012) Review of small commercial sensors for indicative monitoring of ambient gas. Chem Eng Trans 30:169–174. http://publications.jrc.ec.europa.eu/repository/handle/JRC75718
Anisimkin VI, Penza M, Valentini A, Quaranta F, Vasanelli L (1995) Detection of combustible gases by means of a ZnO-on-Si surface acoustic wave (SAW) delay line. Sensors Actuators B Chem 23:197–201. https://doi.org/10.1016/0925-4005(94)01273-K
Anuradha S, Rajanna K (2006) Development of thermoelectric gas sensors for volatile organic compounds. In: Proceedings of the 5th IEEE conference on sensors, Daegu, pp 716–718. https://doi.org/10.1109/ICSENS.2007.355561
Arshak K, Moore E, Lyons GM, Harris J, Clifford S (2004) A review of gas sensors employed in electronic nose applications. Sens Rev 24:181–198. https://doi.org/10.1108/02602280410525977
Ashraf S, Blackman CS, Naisbitt SC, Parkin IP (2008) The gas sensing properties WO3−x thin films deposited via the atmospheric pressure chemical vapor deposition (APCVD) of WCl6 with ethanol. Meas Sci Technol 19:025203. https://doi.org/10.1088/0957-0233/19/2/025203
Ataman C, Kinkeldei T, Mattana G, V-Quintero A, Molina-Lopez F, Courbat J, Cherenack K, Briand D, Tröster G, de Rooij NF (2013) A robust platform for textile integrated gas sensors. Sensors Actuators B Chem 177:1053–1061. https://doi.org/10.1016/j.snb.2012.11.099
Azad AM, Akbar SA, Mhaisalkar SG, Birkefeld LD, Goto KS (1992) Solid-state gas sensors: a review. J Electrochem Soc 139:3690–3704. https://doi.org/10.1149/1.2069145
Barsan N, Weimar U (2001) Conduction model of metal oxide gas sensor. J Electroceram 7:143–167. https://doi.org/10.1023/A:1014405811371
Bârsan N, Weimar U (2003) Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J Phys Condens Matter 15:813–839. https://doi.org/10.1088/0953-8984/15/20/201
Barsan N, Schweizer-Berberich M, Göpel W (1999) Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: a status report. Fresenius J Anal Chem 365:287–304. https://doi.org/10.1007/s002160051490
Beck K, Kunzelmann T, Von Schickfus M, Hunklinger S (1999) Contactless surface acoustic wave gas sensor. Sensors Actuators A 76:103–106. https://doi.org/10.1016/S0924-4247(98)00359-8
Becker T, Ahlers S, Bosch V, Braunmuhl C, Muller G, Kiesewetter O (2001) Gas sensing properties of thin-and thick-film tin-oxide materials. Sensors Actuators B Chem 77:55–61. https://doi.org/10.1016/S0925-4005(01)00672-4
Brattain WH, Bardeen J (1952) Surface properties of germanium. Bell Syst Tech J 32:1–41. https://doi.org/10.1002/j.1538-7305.1953.tb01420.x
Cabot A, Arbiol J, Morante JR, Weimar U, Barsan N, Gopel W (2000) Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol-gel nanocrystals for gas sensors. Sensors Actuators B Chem 70:87–100. https://doi.org/10.1016/S0925-4005(00)00565-7
Carbone L, Cozzoli PD (2010) Colloidal heterostructured nanocrystals: synthesis and growth mechanisms. Nano Today 5:449–493. https://doi.org/10.1016/j.nantod.2010.08.006
Casalinuovo I, Pierro D (2006) Application of electronic noses for disease diagnosis and food spoilage detection. Sensors 6:1428–1439. https://doi.org/10.3390/s6111428
Cederquist A, Gibbons E, Meitzler A (1976) Characterization of Zirconia and Titania engine exhaust gas sensors for air/fuel feedback control systems. SAE Tech Pap. https://doi.org/10.4271/760202
Chao Y, Yao S, Buttner WJ, Stetter JR (2005) Amperometric sensor for selective and stable hydrogen measurement. Sensors Actuators B Chem 106:784–790. https://doi.org/10.1016/j.snb.2004.09.042
Chen D, Liu W, Zhang Y, Liu J, Kan R, Wang M, Chen J, Cui Y (2006a) H2S detection by tunable diode laser absorption spectroscopy. In: Proceedings of the IEEE international conference on information acquisition, Shandong, 20–23 August, pp 754–758. https://doi.org/10.1109/ICIA.2006.305823
Chen H, Liu X, Muthuraman H, Zou J, Wang J, Dai Q (2006b) Direct laser writing of microtunnels and reservoirs on nanocomposite materials. Adv Mater 18:2876–2879. https://doi.org/10.1002/adma.200601560
Chen X, Wong CKY, Yuan CA, Zhang G (2013) Nanowire-based gas sensors. Sensors Actuators B Chem 177:178–195. https://doi.org/10.1016/j.snb.2012.10.134
Choi YJ, Seeley Z, Bandyopadhyay A, Bose S, Akbar SA (2007) Aluminum-doped TiO2 nano-powders for gas sensors. Sensors Actuators B Chem 124:111–117. https://doi.org/10.1016/j.snb.2006.12.005
Comini E (2006) Metal oxide nano-crystals for gas sensing. Anal Chim Acta 568:28–40. https://doi.org/10.1016/j.aca.2005.10.069
Comini E, Faglia G, Sberveglieri G (2002) Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl Phys Lett 81:1869–1871. https://doi.org/10.1063/1.1504867
Cukrov LM, McCormick PG, Galatsis K, Wlodarski W (2001) Gas sensing properties of nanosized tin oxide synthesized by mechanochemical processing. Sensors Actuators B Chem 77:491–495. https://doi.org/10.1016/S0925-4005(01)00751-1
Currie JF, Essalik A, Marusic J-C (1999) Micromachined thin film solid state electrochemical CO2, NO2 and SO2 gas sensors. Sensors Actuators B 59:235–241. https://doi.org/10.1016/S0925-4005(99)00227-0
D’Amico A, di Natale C, Paolesse R, Macagnano A, Martinelli E, Pennazza G, Santonico M, Bernabei M, Roscioni C, Galluccio G, Bono R, Agrò EF, Rullo S (2008) Olfactory systems for medical applications. Sensors Actuators B Chem 130:458–465. https://doi.org/10.1016/j.snb.2007.09.044
de Graaf G, Wolffenbuttel R (2012) Surface-micromachined thermal conductivity detectors for gas sensing. In: Proceedings of the IEEE international instrumentation and measurement technology conference (I2MTC), Graz, 13–16 May, pp 1861–1864. https://doi.org/10.1109/I2MTC.2012.6229412
Dey A (2018) Semiconductor metal oxide gas sensors: a review. Mater Sci Eng B 229:206–217. https://doi.org/10.1016/j.mseb.2017.12.036
Dolbec R, el Khakani MA, Serventi AM, Saint-Jacques RG (2003) Influence of the nanostructural characteristics on the gas sensing properties of pulsed laser deposited tin oxide thin films. Sensors Actuators B Chem 93:566–571. https://doi.org/10.1016/S0925-4005(03)00229-6
Dutta A, Nishiguchi H, Takita Y, Ishihara T (2005) Amperometric hydrocarbon sensor using La(Sr)Ga(Fe)O3 solid electrolyte for monitoring in exhaust gas. Sensors Actuators B Chem 108:368–373. https://doi.org/10.1016/j.snb.2004.10.042
El Kady M, Shokry H, Hamad H (2016) Effect of superparamagnetic nanoparticles on the physicochemical properties of nano hydroxyapatite for groundwater treatment: adsorption mechanism of Fe(II) and Mn(II). RSC Adv 85:82244–82259. https://doi.org/10.1039/c6ra14497g
El-Aassar MR, El-Kady MF, Hassan HS, Al-Deyab SS (2016) Synthesis and characterization of surface modified electrospun poly (acrylonitrile-co-styrene) nanofibers for dye decolorization. J Taiwan Inst Chem Eng 58:274–282. https://doi.org/10.1016/j.jtice.2015.05.042
Elkady MF, Hassan HS (2015) Equilibrium and dynamic profiles of azo dye sorption onto innovative nano-zinc oxide biocomposite. Curr Nanosci 11:805–814. https://doi.org/10.2174/1573413711666150415003115
Fine GF, Cavanagh LM, Afonja A, Binions R (2010) Metal oxide semiconductor gas sensors in environmental monitoring. Sensors 10:5469–5502. https://doi.org/10.3390/s100605469
Firth JG, Jones A, Jones T (1973) The principles of the detection of flammable atmospheres by catalytic devices. Combust Flame 20:303–311. https://doi.org/10.1016/0010-2180(73)90021-7
Fischerauer G, Dickert F, Forth P, Knauer U (1996) Chemical sensors based on SAW resonators working at up to 1 GHz. In: Proceedings of the IEEE ultrasonics symposium, 1:439–442. https://doi.org/10.1109/ULTSYM.1996.584007
Font X, Artola A, Sánchez A (2011) Detection, composition and treatment of volatile organic compounds from waste treatment plants. Sensors 11:4043–4059. https://doi.org/10.3390/s110404043
Gan T, Hu S (2011) Electrochemical sensors based on graphene materials. Microchim Acta 175:1–19. https://doi.org/10.1007/s00604-011-0639-7
Garcia-R D, Martínez A, Azcona C (2012) NDIR-based CO2 monitor system for wireless sensor networks, IEEE Latin American symposium on circuits and systems LASCAS, Playa Del Carmen, 29 February–2 March, 1–4. https://doi.org/10.1109/LASCAS.2012.6180326
Gardner JW, Guha PK, Udrea F, Covington JA (2010) CMOS interfacing for integrated gas sensors: a review. IEEE Sensors J 10:1833–1848. https://doi.org/10.1109/JSEN.2010.2046409
Girschikofsky M, Rosenberger M, Belle S, Brutschy M, Waldvogel SR, Hellmann R (2012) Optical planar Bragg grating sensor for real-time detection of benzene, toluene and xylene in solvent vapour. Sensors Actuators B Chem 171–172:338–342. https://doi.org/10.1016/j.snb.2012.04.046
Gonzalez-Jimenez J, Monroy JG, Blanco JL (2011) The multi-chamber electronic nose-an improved olfaction sensor for mobile robotics. Sensors 11:6145–6164. https://doi.org/10.3390/s110606145
Gopel W, Schierbaum K (1995) SnO2 sensors: current status and future prospects. Sensors Actuators B Chem 26/27:1–12. https://doi.org/10.1016/0925-4005(94)01546-T
Gurlo A, Riedel R (2007) In situ operando spectroscopy for assessing mechanisms of gas sensing. Angew Chem Int Ed 46:3826–3848. https://doi.org/10.1002/anie.200602597
Halek G, Malewicz M, Teterycz H (2009) Methods of selectivity improvements of semiconductor gas sensors. In: Proceedings of the international students and young scientists workshop on photonics and microsystem’, Wernigerode, 25–27 June, pp 31–35. https://doi.org/10.1109/STYSW.2009.5470313
Hassan SH, Kashyout AB, Soliman HMA, Uosif MA, Afify N (2013) Effect of reaction time and Sb doping ratios on the architecturing of ZnO nanomaterials for gas sensor applications. Appl Surf Sci 277:73–82. https://doi.org/10.1016/j.bjbas.2014.10.007
Hassan SH, Kashyout AB, Morsi I, Nasser AAA, Ali I (2014a) Synthesis, characterization and fabrication of gas sensor devices using ZnO and ZnO: in nanomaterials. J Basic Appl Sci 3:216–221. https://doi.org/10.1016/j.bjbas.2014.10.007
Hassan SH, Kashyout AB, Morsi I, Nasser AAA, Raafat A (2014b) Fabrication and characterization of gas sensor micro-arrays. Sens Bio-Sens Res 1:34–40. https://doi.org/10.1016/j.sbsr.2014.04.001
Hassan SH, Kashyout AB, Morsi I, Nasser AAA, Raafat A (2014c) Fabrication and characterization of nano-gas sensor arrays. AIP Conf Proc 1:34–40. https://doi.org/10.1063/1.4914233
Hassan SH, Kashyout AB, Morsi I, Nasser AAA, Abuklill H (2015) Development of polypyrrole coated copper nanowires for gas sensor application. Sens Bio-Sens Res 5:50–54. https://doi.org/10.1016/j.sbsr.2015.07.004
Heiland G (1957) Zum einfluss von wasserstoff auf die elektrische leitfähigkeit von Zinkoxydkristallen. Z Phys 138:459–464. https://doi.org/10.1007/BF01327362
Ho K-C, Hung W-T (2001) An amperometric NO2 gas sensor based on Pt/ Nafion® electrode. Sensors Actuators B Chem 79:11–16. https://doi.org/10.1016/S0925-4005(01)00782-1
Holt CT, Azad A-M, Swartz SL, Rao RR, Dutta PK (2002) Carbon monoxide sensor for PEM fuel cell systems. Sensors Actuators B Chem 87:414–420. https://doi.org/10.1016/S0925-4005(02)00290-3
Huang X-J, Choi Y-K (2007) Chemical sensors based on nanostructured materials. Sensors Actuators B Chem 122:659–671. https://doi.org/10.1016/j.snb.2006.06.022
Huang J, Wan Q (2009) Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 9:9903–9924. https://doi.org/10.3390/s91209903
Hübert T, Boon-Brett L, Black G, Banach U (2011) Hydrogen sensors –a review. Sensors Actuators B Chem 157:329–352. https://doi.org/10.1016/j.snb.2011.04.070
Hung CM, Le DTT, Hieu NV (2017) On-chip growth of semiconductor metal oxide nanowires for gas sensors: a review. J Sci 2:263–285. https://doi.org/10.1016/j.jsamd.2017.07.009
Itoh T, Nakashima T, Akamatsu T, Izu N, Shin W (2013) Nonanal gas sensing properties of platinum, palladium, and gold-loaded tin oxide VOCs sensors. Sensors Actuators B Chem 187:135–141. https://doi.org/10.1016/j.snb.2012.09.097
Jiang T, Wang Z, Li Z, Wang W, Xu X, Liu X, Wang J, Wang C (2013) Synergic effect within n-type inorganic–p-type organic nano-hybrids in gas sensors. J Mater Chem C 1:3017–3025. https://doi.org/10.1039/C3TC00370A
Jimenez-Cadena G, Riu J, Xavier Rius F (2007) Gas sensors based on nanostructured materials. Analyst 132:1083–1099. https://doi.org/10.1039/b704562j
Karthikeyan S, Pandya HM, Sharma MU, Gopal K (2015) Gas sensors-a review. J Environ Nanotechnol 4:1–14. https://doi.org/10.13074/jent.2015.12.153163
Kasai N, Tsuchiya C, Fukuda T, Sekine K, Sano T, Takehana T (2011) Propane gas leak detection by infrared absorption using carbon infrared emitter and infrared camera. NDT E Int 44:57–60. https://doi.org/10.1016/j.ndteint.2010.09.006
Kashyout AB, Soliman HMA, Hassan SH, Abousehly AM (2010) Fabrication of ZnO and ZnO:Sb nanoparticles for gas sensor applications. J Nanomater 2010:1–8. https://doi.org/10.1155/2010/341841
Khodadadi A, Mohajerzadeh SS, Mortazavi Y, Miri AM (2001) Cerium oxide / SnO2-based semiconductor gas sensors with improved sensitivity to CO. Sensors Actuators B Chem 80:267–271. https://doi.org/10.1016/S0925-4005(01)00915-7
Kim H-J, Lee J-H (2014) Highly sensitive and selective gas sensors using p-type oxide semiconductors: overview. Sensors Actuators B Chem 192:607–627. https://doi.org/10.1016/j.snb.2013.11.005
Kim Y-J, Park SC, Yoon S, Ch L (2009) A micro-thermoelectric gas sensor for the detection of hydrogen and atomic oxygen. Analyst 134:236–242. https://doi.org/10.1039/B807882C
King WH (1964) Piezoelectric sorption detector. Anal Chem:1735–1739. https://doi.org/10.1021/ac60215a012
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Science 287:622–625. https://doi.org/10.1126/science.287.5453.622
Konishi R (1997) The concentration-estimation of inflammable gases with a semiconductor gas sensor utilizing neural networks and fuzzy inference. Sensors Actuators B Chem 41:121–129. https://doi.org/10.1016/S0925-4005(97)80284-5
Korotcenkov G (2005) Gas response control through structural and chemical modification of metal oxide films: state of the art and approaches. Sensors Actuators B Chem 107:209–232. https://doi.org/10.1016/j.snb.2004.10.006
Korotcenkov G (2007a) Practical aspects in design of oneelectrode semiconductor gas sensors: status report. Sensors Actuators B Chem 121:664–678. https://doi.org/10.1016/j.snb.2006.04.092
Korotcenkov G (2007b) Metal oxides for solid-state gas sensors: what determines our choice? Mater Sci Eng B 139:1–23. https://doi.org/10.1016/j.mseb.2007.01.044
Korotcenkov G (2008) The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater Sci Eng B 61:1–39. https://doi.org/10.1016/j.mser.2008.02.001
Korotcenkov G, Cho B (2010) Grain size effect in structural stability of SnO2 and In2O3 films aimed for gas sensing application. In: Proceedings of 2nd international conference on computer research and development, Kuala Lumpur, Malaysia, 7–10 May, pp 461–464. https://doi.org/10.1109/ICCRD.2010.100
La D-D, Kim CK, Jun TS, Jung Y, Seong GH, Choo J, Kim YS (2011) Pt nanoparticle-supported multiwall carbon nanotube electrodes for amperometric hydrogen detection. Sensors Actuators B Chem 155:191–198. https://doi.org/10.1016/j.snb.2010.11.045
Law M, Kind H, Messer B, Kim F, Yang PD (2002) Photochemical sensing of NO2 with SnO2 nanoribbon nanosensors at room temperature. Angew Chem Int Ed 41:2405–2408. https://doi.org/10.1002/1521-3757(20020703)114:13<2511::AID-ANGE2511>3.0.CO;2-N
Leblanc E, Perier-Camby L, Thomas G, Gibert R, Primet M, Gelin P (2000) NOx adsorption onto dehydroxylated or hydroxylated tin dioxide surface. Application to SnO2-based sensors. Sensors Actuators B Chem 62:67–72. https://doi.org/10.1016/S0925-4005(99)00376-7
Lee J-H (2009) Gas sensors using hierarchical and hollow oxide nanostructures: overview. Sensors Actuators B Chem 140:319–336. https://doi.org/10.1016/j.snb.2009.04.026
Lee H, Han D, Ahn H (1997) Design and fabrication of SAW gas sensor with resonator structure. In: Proceedings of the 5th international conference on properties and applications of dielectric materials, Seoul, 25–30 May, vol 2, pp 1058–1061. https://doi.org/10.1109/ICPADM.1997.616629
Lee SW, Tsai PP, Chen H (2000) Comparison study of SnO2 thin and thick film gas sensors. Sensors Actuators B Chem 67:122–127. https://doi.org/10.1016/S0925-4005(00)00390-7
Lee C, Akbar SA, Park CO (2001) Potentiometric CO2 gas sensor with lithium phosphorous oxynitride electrolyte. Sensors Actuators B Chem 80:234–242. https://doi.org/10.1016/S0925-4005(01)00902-9
Lee YC, Huang H, Tan OK, Tse MS (2008) Semiconductor gas sensor based on Pddoped SnO2 nanorod thin films. Sensors Actuators B Chem 132:239–242. https://doi.org/10.1016/j.snb.2008.01.028
Lee E-B, Hwang I-S, Cha J-H, Lee H-J, Lee W-B, Pak JJ, Lee J-H, Ju B-K (2011) Micromachined catalytic combustible hydrogen gas sensor. Sensors Actuators B Chem 153:392–397. https://doi.org/10.1016/j.snb.2010.11.004
Li Y, Wlodarski W, Galatsis K, Moslih SH, Cole J, Russo S, Rockelmann N (2002) Gas sensing properties of p-type semiconducting Cr-doped TiO2 thin films. Sensors Actuators B Chem 83:160–163. https://doi.org/10.1016/S0925-4005(01)01031-0
Li C, Zhang D, Liu X, Han S, Tang T, Han J, Zhou C (2003) In2O3 nanowires as chemical sensors. Appl Phys Lett 82:1613–1615. https://doi.org/10.1063/1.1559438
Lim C, Wang W, Yang S, Lee K (2011) Development of SAW-based multi-gas sensor for simultaneous detection of CO2 and NO2. Sensors Actuators B Chem 154:9–16. https://doi.org/10.1016/j.snb.2010.02.057
Liu J, Wang X, Peng Q, Li Y (2006) Preparation and gas sensing properties of vanadium oxide nanobelts coated with semiconductor oxides. Sensors Actuators B Chem 115:481–487. https://doi.org/10.1016/j.snb.2005.10.012
Lu X, Wu S, Wang L, Su Z (2005) Solid-state amperometric hydrogen sensor based on polymer electrolyte membrane fuel cell. Sensors Actuators B Chem 107:812–817. https://doi.org/10.1016/j.snb.2004.12.022
Lu JG, Chang P, Fan Z (2006) Quasi-one-dimensional metal oxide materials-synthesis, properties and applications. Mater Sci Eng R 52:49–91. https://doi.org/10.1016/j.mser.2006.04.002
Lu G, Ocola LE, Chen J (2009a) Room-temperature gas sensing based on electron transfer between discrete tin oxide nanocrystals and multiwalled carbon nanotubes. Adv Mater 21:1–5. https://doi.org/10.1002/adma.200803536
Lu GH, Ocola LE, Chen JH (2009b) Reduced grapheme oxide for room-temperature gas sensors. Nanotechnology 20:Article ID: 445502. https://doi.org/10.1088/0957-4484/20/44/445502
Ma Y, Wang WL, Liao KJ, Kong CY (2002) Study on sensitivity of nano-grain ZnO gas sensors. J Wide Bandgap Mater 10:112–120. https://doi.org/10.1177/1524511X02043537
Mahmood MR, Soga T, Mamat MH, Khusaimi Z, Nor AM (2013) A review on zinc oxide nanostructures: doping and gas sensing. Adv Mater Res 667:329–332. https://doi.org/10.4028/www.scientific.net/AMR.667.329
Manap H, Muda R, O’Keeffe S, Lewis E (2009) Ammonia sensing and a cross sensitivity evaluation with atmosphere gases using optical fiber sensor. Procedia Chem 1:959–962. https://doi.org/10.1016/j.proche.2009.07.239
Marichy C, Pinna N (2013) Carbon-nanostructures coated/decorated by atomic layer deposition: growth and applications. Coord Chem Rev 257:3232–3253. https://doi.org/10.1016/j.ccr.2013.08.007
Marichy C, Russo PA, Latino M, Tessonnier J-P, Willinger M-G, Donato N, Neri G, Pinna N (2013) Tin dioxide-carbon heterostructures applied to gas sensing: structure-dependent properties and general sensing mechanism. J Phys Chem C 117:19729–19739. https://doi.org/10.1021/jp406191x
Marsal A, Dezanneau G, Cornet A, Morante JR (2003) A new CO2 gas sensing material. Sensors Actuators 95:266–270. https://doi.org/10.1016/S0925-4005(03)00443-X
Massie C, Stewart G, McGregor G, Gilchrist JR (2006) Design of a portable optical sensor for methane gas detection. Sensors Actuators B Chem 113:830–836. https://doi.org/10.1016/j.snb.2005.03.105
McEntegart CM, Penrose WR, Strathmann S, Stetter JR (2000) Detection and discrimination of coliform bacteria with gas sensor arrays. Sensors Actuators B Chem 70:170–176. https://doi.org/10.1016/S0925-4005(00)00561-X
Mitsubayashi K, Matsunaga H, Nishio G, Ogawa M, Saito H (2004) Biochemical gas sensor (Bio-sniffer) for breath analysis after drinking. In: SICE Annual Conference, Sapporo, Japan, 4–6 August 2004
Morrison RS (1982) Semiconductor gas sensors. Sensors Actuators 2:329–341. ISBN: 978-0-85709-236-6
Neri G (2011) Better sensors through chemistry: some selected examples. Lect Notes Electr Eng 91:19–30. https://doi.org/10.1007/978-94-007-1324-6
Neri G (2015) First fifty years of chemoresistive gas sensors. Chemosensors 3:1–20. https://doi.org/10.3390/chemosensors3010001
Nieuwenhuizen MS, Nederlof AJ (1990) A SAW gas sensor for carbon dioxide and water. Preliminary experiments. Sensors Actuators B Chem 2:97–101. https://doi.org/10.1016/0925-4005(90)80017-T
Ogawa H, Nishikawa M, Abe A (1982) Hall measurement studies and an electrical conduction model of tin oxide ultrafine particle films. J Appl Phys 53:4448–4455. https://doi.org/10.1063/1.331230
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694. https://doi.org/10.1038/nm1577
Okajima H, Kakuma S, Uchida K, Wakimoto Y, Noda K (2006) Measurement of methane gas concentration using infrared LED. In: Proceedings of the international joint conference SICE-ICASE, Busan, 18–21 Oct, pp 1656–1659. https://doi.org/10.1109/SICE.2006.315585
Okazaki S, Nakagawa H, Asakura S, Tomiuchi Y, Tsuji N, Murayama H, Washiya M (2003) Sensing characteristics of an optical fiber sensor for hydrogen leak. Sensors Actuators B Chem 93:142–147. https://doi.org/10.1016/S0925-4005(03)00211-9
Oletic D, Bilas V (2013) Empowering smartphone users with sensor node for air quality measurement. J Phys Conf Ser 450:012028. https://doi.org/10.1088/1742-6596/450/1/012028
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291:1947–1949. https://doi.org/10.1126/science.1058120
Peng S, Cho K (2003) Ab initio study of doped carbon nanotube sensors. Nano Lett 3:513–517. https://doi.org/10.1021/nl034064u
Phawachalotorn C, Sanguanruang O, Ishihara T (2012) Highly selective amperometric sensors for carbon monoxide detection in exhaust gas. Sensors Actuators B Chem 161:635–640. https://doi.org/10.1016/j.snb.2011.10.081
Pijolat C, Pupier C, Sauvan M, Tournier G, Lalauze R (1999) Gas detection for automotive pollution control. Sensors Actuators B Chem 59:195–202. https://doi.org/10.1016/S0925-4005(99)00220-8
Pinna N, Neri G, Antonietti M, Niederberger M (2004) Nonaqueous synthesis of nanocrystalline semiconducting metal oxides for gas sensing. Angew Chem Int Ed 43:4345–4349. https://doi.org/10.1002/anie.200460610
Radhakrishnan R, Virkar AV, Singhal SC, Dunham GC, Marina OA (2005) Design, fabrication and characterization of a miniaturized series-connected potentiometric oxygen sensor. Sensors Actuators B 105:312–321. https://doi.org/10.1016/j.snb.2004.06.014
Ramamoorthy R, Dutta PK, Akbar SA (2003) Oxygen sensors: materials, methods, designs and applications. J Mater Sci 38:4271–4282. https://doi.org/10.1023/A:1026370729205
Righettoni M, Amann A, Pratsinis SE (2015) Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater Today 18:163–171. https://doi.org/10.1016/j.mattod.2014.08.017
Rothschild A, Komem Y (2004a) The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J Appl Phys 95:6374–6380. https://doi.org/10.1063/1.1728314
Rothschild A, Komem Y (2004b) On the relationship between the grain size and gas-sensitivity of chemo-resistive metal-oxide gas sensors with nanosized grains metal oxide gas sensors with nanosized grains. J Electroceram 13:697–701. https://doi.org/10.1007/s10832-004-5178-8
Sadek AZ, Choopun S, Wlodarski W, Ippolito SJ, Kalantar-Zaden K (2007) Characterization of ZnO nanobelt-based gas sensor for H2, NO2 and hydrocarbon sensing. IEEE Sensors J 7:919–924. https://doi.org/10.1109/JSEN.2007.895963
Sathiyamoorthi R, Chandrasekaran R, Mathanmohan T, Muralidharan B, Vasudevan T (2004) Study of electrochemical based gas sensors for fluorine and chlorine. Sensors Actuators B Chem 99:336–339. https://doi.org/10.1016/j.snb.2003.11.031
Seiyama T (1988) Chemical sensors-current status and future outlook. In: Seiyama T (ed) Chemical sensor technology. Elsevier, Amsterdam
Seiyama T, Kato A (1962) A new detector for gaseous components using semiconductor thin film. Anal Chem 34:1502–1503. https://doi.org/10.1021/ac60191a001
Sharma S, Madou M (2012) A new approach to gas sensing with nanotechnology. Phil Trans R Soc A 370:2448–2473. https://doi.org/10.1098/rsta.2011.0506
Shaver PJ (1967) Activated tungsten oxide gas detectors. Appl Phys Lett 11:255–257. https://doi.org/10.1063/1.1755123
Shaw G, Parkin IP, Pratt KFE, Williams DE (2005) Control of semiconducting oxide gas-sensor microstructure by application of an electric field during aerosol-assisted chemical vapor deposition. J Mater Chem 15:149–154. https://doi.org/10.1039/b411680a
Shimanoe K, Yuasa M, Kida T, N. Yamazoe (2011) Semiconductor gas sensor using nano-sized oxide for high-sensitive detection of environment-related gases. In: Proceedings of the IEEE international conference on nanotechnology materials and devices, October, pp 38–43. https://doi.org/10.1109/NMDC.2011.6155309
Shimizu Y, Egashira M (1999) Basic aspects and challenges of semiconductor gas sensors. MRS Bull 24:18–24. https://doi.org/10.1557/S0883769400052465
Shin W, Matsumiya M, Izu N, Murayama N (2003) Hydrogen-selective thermoelectric gas sensor. Sensors Actuators B Chem 93:304–308. https://doi.org/10.1016/S0925-4005(03)00225-9
Shin W, Matsumiya M, Qiu F, Izu N, Murayama N (2004) Thermoelectric gas sensor for detection of high hydrogen concentration. Sensors Actuators B Chem 97:344–347. https://doi.org/10.1016/j.snb.2003.08.029
Shin W, Nishibori M, Izu N, Itoh T, Matsubara I, Nose K, Shimouchi A (2011) Monitoring breath hydrogen using thermoelectric sensor. Sens Lett 9:684–687. https://doi.org/10.1166/sl.2011.1591
Shin W, Itoh T, Izu N (2015) Health care application of gas sensors. Synthesiol Engl Ed 8:211–219. https://doi.org/10.5571/syntheng.8.4_211
Simon I, Arndt M (2002) Thermal and gas-sensing properties of a micromachined thermal conductivity sensor for the detection of hydrogen in automotive applications. Sensors Actuators A 97–98:104–108. https://doi.org/10.1016/S0924-4247(01)00825-1
Sin MLY, Chow GCT, Wong GMK, Li WJ, Leong PHW, Wong KW (2007) Ultralow-power alcohol vapor sensors using chemically functionalized multiwalled carbon nanotubes. IEEE Trans Nanotechnol 6:571–577. https://doi.org/10.1109/TNANO.2007.900511
Suehiro J, Zhou G, Hara M (2003) Fabrication of a carbon nanotube based gas sensor using dielectrophoresis and its application for ammonia detection be impedance spectroscopy. J Phys D Appl Phys 36:109–114. https://doi.org/10.1088/0022-3727/36/21/L01
Sun YP, Fu KF, Lin Y, Huang WJ (2002) Functionalized carbon nanotubes: properties and applications. Acc Chem Res 35:1096–1104. https://doi.org/10.1021/ar010160v
Sun Y-F, Liu S-B, Meng F-L, Liu J-Y, Jin Z, Kong L-T, Liu J-H (2012) Metal oxide nanostructures and their gas sensing properties: a review. Sensors 12:2610–2631. https://doi.org/10.3390/s120302610
Taguchi N (1971) Gas detecting devices. U.S. Patent 3,631,436
Takada T, Fukunaga T, Maekawa T (2000) New method for gas identification using a single semiconductor sensor. Sensors Actuators B Chem 66:22–24. https://doi.org/10.1016/S0925-4005(98)00254-8
Tardy P, Coulon J-R, Lucat C, Menil F (2004) Dynamic thermal conductivity sensor for gas detection. Sensors Actuators B Chem 98:63–68. https://doi.org/10.1016/j.snb.2003.09.019
Tricoli A, Pratsinis SE (2010) Dispersed nanoelectrode devices. Nat Nanotechnol 5:54–60. https://doi.org/10.1038/nnano.2009.349
Tricoli A, Righettoni M, Pratsinis SE (2009) Minimal cross-sensitivity to humidity during ethanol detection by SnO2-TiO2 solid solutions. Nanotechnology 20:315502. https://doi.org/10.1088/0957-4484/20/31/315502
Tricoli A, Righettoni M, Teleki A (2010) Semiconductor gas sensors: dry synthesis and application. Angew Chem Int Ed 49:7632–7659. https://doi.org/10.1002/anie.200903801
Venema A, Nieuwkoop E, Ghijsen WJ, Barendsz AW, Nieuwenhuizen MS (1987) NO2 Gas-concentration measurement with a SAW chemosensor. IEEE Trans Ultrason Ferroelectr Freq Control 34:148–155. https://doi.org/10.1987/ITUFF.34.148V
Viricelle JP, Pijilat C, Riviere B, Rotureau D, Briand D, DeRooij NF (2006) Compatibility of screen printing technology with microhotplate for gas sensor and solid oxide micro fuel cell development. Sensors Actuators B Chem 118:263–268. https://doi.org/10.1016/j.snb.2006.04.031
Wang X, Carey WP, Yee SS (1995) Monolithic thin film metal oxide gas sensor arrays with application to monitoring of organic vapors. Sensors Actuators B Chem 28:63–70. https://doi.org/10.1016/0925-4005(94)01531-L
Wang W, Huang H, Li Z, Zhang H, Wang Y, Zheng W, Wang C (2008) Zinc oxide nanofiber gas sensors via electrospinning. J Am Ceram Soc 91:3817–3819. https://doi.org/10.1111/j.I551-2916.2008.02765.x
Wang C, Yin L, Zhang L, Xiang D, Gao R (2010) Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10:2088–2016. https://doi.org/10.3390/s100302088
Wei B-Y, Hsu M-C, Su P-G, Lin H-M, Wu R-J, Lai H-J (2004) A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sensors Actuators B Chem 101:81–89. https://doi.org/10.1016/j.snb.2004.02.028
Willinger MG, Neri G, Rauwel E, Bonavita A, Micali G, Pinna N (2008) Vanadium oxide sensing layer grown on carbon nanotubes by a new atomic layer deposition process. Nano Lett 8:4201–4204. https://doi.org/10.1021/nl801785b
Wilson AD, Baietto M (2009) Applications and advances in electronic-nose technologies. Sensors 9:5099–5148. https://doi.org/10.3390/s90705099
Wohltjen H, Dessy R (1979) Surface acoustic wave probe for chemical analysis. II. Gas chromatography detector. Anal Chem 51:1458–1478. https://doi.org/10.1021/ac50045a025
Wolkenstein FF (1961) Effect of ionizing radiation on the adsorptive and catalytic properties of semiconductors. Discuss Faraday Soc 31:209–218. https://doi.org/10.1039/DF9613100209
Xu C, Jun Tamaki J, Miura N, Yamazoe N (1991) Grain size effects on gas sensitivity of porous SnO2-based elements. Sensors Actuators B Chem 3:147–155. https://doi.org/10.1002/anie.200903801
Xu L, Li T, Gao X, Wang, Y, Zheng R, Xie L Lee L (2010) Behavior of a catalytic combustion methane gas sensor working on pulse mode. In: Proceedings of the IEEE international conference on sensors, Kona, HI, USA, 1–4, 391–394. https://doi.org/10.1109/ICSENS.2010.5690213
Yamazoe N (1991) New approaches for improving semiconductor gas sensors. Sensors Actuators B Chem 5:7–19. https://doi.org/10.1016/0925-4005(91)80213-4
Yamazoe N (2005) Toward innovations of gas sensor technology. Sensors Actuators B Chem 108:2–14. https://doi.org/10.1016/j.snb.2004.12.075
Yamazoe N, Miura N (1994) Environmental gas sensors. Sensors Actuators B Chem 20:95–102. https://doi.org/10.1016/0925-4005(93)01183-5
Yamazoe N, Shimanoe K (2008) Theory of power laws for semiconductor gas sensors. Sensors Actuators B Chem 128:566–573. https://doi.org/10.1016/j.snb.2007.07.036
Yamazoe N, Shimanoe K (2009) Receptor function and response of semiconductor gas sensor. J Sens 2009:875704. https://doi.org/10.1155/2009/875704
Yamazoe N, Kurokawa Y, Seiyama T (1983) Effects of additives on semiconductor gas sensors. Sensors Actuators B Chem 4:283–289. https://doi.org/10.1016/0250-6874(83)85034-3
Yan Y, Miura N, Yamazoe N (1995) Potentiometric sensor using stabilized zirconia for chlorine gas. Sensors Actuators B Chem 24:287–290. https://doi.org/10.1016/0925-4005(95)85062-7
Yang J-C, Dutta PK (2010) High temperature potentiometric NO2 sensor with asymmetric sensing and reference Pt electrodes. Sensors Actuators B Chem 143:459–463. https://doi.org/10.1016/j.snb.2009.09.023
Yeom J, Oh I, Field C, Radadia A, Ni Z, Bae B, Han J, Masel RI, Shannon MA (2008) Enhanced toxic gas detection using a MEMS pre-concentrator coated with metal organic framework absorber. In: Proceedings of MEMS, Tucson, AZ, 13–17 January 2008. https://doi.org/10.1109/MEMSYS.2008.4443635
Yoon S, Lee C, Kim Y (2009) A thermoelectric gas sensor based on an embedded tin oxide catalyst for detecting hydrogen and NOx gases. In: Proceedings of the IEEE 22nd international conference on micro electro mechanical systems (MEMS ’09), Sorrento, pp 272–275. https://doi.org/10.1109/MEMSYS.2009.4805371
Yunusa Z, Hamidon MN, Kaiser A, Awang Z (2014) Gas sensors: a review. Sens Transducers 168:61–75
Zemel J (1988) Theoretical description of gas–film interaction on SnOx. Thin Solid Films 163:189–202. https://doi.org/10.1016/0040-6090(88)90424-5
Zhang T, Mubeen S, Myung VN, Deshusses MA (2008) Recent progress in carbon nanotube-based gas sensors. Nanotechnology 19:Article ID: 332001. https://doi.org/10.1088/0957-4484/19/33/332001
Zhang G, Li Y, Li QA (2010) Miniaturized carbon dioxide gas sensor based on infrared absorption. Opt Lasers Eng 48:1206–1212. https://doi.org/10.1016/j.optlaseng.2010.06.012
Zhu L, Zeng W (2017) Room-temperature gas sensing of ZnO-based gas sensor: a review. Sensors Actuators A 267:242–261. https://doi.org/10.1016/j.sna.2017.10.021
Acknowledgement
The authors wish to thank Dr. Aya Gomaa for technical assistance.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hassan, H.S., Elkady, M.F. (2020). Semiconductor Nanomaterials for Gas Sensor Applications. In: Dasgupta, N., Ranjan, S., Lichtfouse, E. (eds) Environmental Nanotechnology Volume 3. Environmental Chemistry for a Sustainable World, vol 27. Springer, Cham. https://doi.org/10.1007/978-3-030-26672-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-26672-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-26671-4
Online ISBN: 978-3-030-26672-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)